Note: Descriptions are shown in the official language in which they were submitted.
WO 95/22565 -. PCT/EP95/00544
_ ~ ~.
The present invention relates to a continuous process for
the gas phase polymerization of olefins of formula CHZ=CHR, in
which R is hydrogen or an alkyl, cycloalkyl or aryl radical
having 1 to 12 carbon atoms, carried out in one or more
reactors having a fluidised bed, in the presence of a high
activity catalyst preferably comprising a titanium compound
having at least one Ti-halogen bond supported on magnesium
dichloride in active form.
Processes for the continuous polymerization of one or
more olefins, such as ethylene or propylene, carried out in
gas phase in fluidised bed reactors are well known in the art.
The polymerizzation is generally carried out in the presence
of a catalyst based on a transition metal compound belonging
to the groups IV, V or VI of the periodic table, in particular
in the presence of a Ziegler-Natta type catalyst or a chromium
oxide based catalyst.
The reactor generally consists of a reaction zone, in
which the polymer particles are maintained in a fluidised
state by passing a gaseous reaction mixture containing
olefins) and optionally an inert gas through a bed of polymer
particles, and a gas velocity reduction zone, where most of
the polymer particles entrained in the fluidisation gas fall
in the underlying reaction zone. The catalyst is introduced in
the reactor continuously and the polymer constituting the
- 2 -
WO 95/22565 PCT/EP95/00544
fluidised bed is also removed continuously.
A gas distribution grid placed in the lower part of the
reactor under the reaction zone is the means through which the
fluidisation gas is sent through the polymer bed and is used
to support the bed itself when the polymerization is
discontinued.
The gaseous mixture, comprising monomers, comonomers,
inert gas and molecular weight regulators, leaving the top of
the reactor is sent to the reactor at a point below the gas
distribution grid through a recycling line. Devices for the
compression and cooling of the gases are generally arranged on
said recycling line.
Make-up monomers are usually fed in the gas recycling
line in such a way to have a certain homogeneity of the
gaseous mixture inside the reactor.
It is in fact known that small variations in the
operating conditions during the polymerization, resulting for
example from small variations in the quality of the catalyst
or of the olefin used in the reaction or from the
dishomogeneity in the composition and in the flow rate of the
gaseous mixture, can bring about changes in behaviour and
catalytic activity of the polymer particles and produce a
particularly adverse effect on the gas phase polymerization
process. These small variations may cause an unexpected rising
of the amount of-heat produced in the reaction, which can not
- 3 -
WO 95122565 PCTIEP95/00544
~'~~~I~3''t
be removed in a sufficiently quick and efficient manner by
the gaseous reaction mixture passing through the bed.
As a result, hot spots can be generated in the bed with
the consequent formation of aggregates of melted polymer.
When these hot spots are formed in the bed, it is in
general too late to prevent the formation of aggregates.
Nevertheless if the reaction conditions are promptly
corrected, especially by reducing the temperature or the
pressure of polymerization, or by reducing the velocity at
which the catalyst is fed into the reactor in order to avoid
the negative effect of undesirable superactivity, the quantity
and size of the aggregates could be reduced to a certain
extent.
In the industrial practice these operations are not
generally carried out, in that they bring about a reduction in
the polymer production and a deterioration of the quality of
the obtained polymer.
In order to avoid these drawbacks, general polymerization
conditions are usually selected with a safety margin such to
not allow local rising of the temperature and the consequent
formation of aggregates. For example catalysts are used having
a reduced activity.
The use of these conditions inevitably results either in
a substantial reduction in the production or in a
deterioration of the quality of the polymer produced.
- 4 -
WO 95/22565 PCT/EP95/00544
'' ' ' -:
U.S. patent 3,709,853 describes a process for the
polymerization of ethylene using chromium catalysts which are
directly fed into the polymerization bed. The make-up feed
stream is used, partially or totally, to carry the catalyst
into the reactor; preferably only a part of the feeding
monomers is used as the carrier for the catalyst since the
injection of large amounts of gas into the bed will cause the
formation of preferential channels and a consequent loss in
the fluidisation. It should be kept in mind that the
introduction of the catalyst and the gas directly into the
bed, even if in small quantities, is in any case an
inconvenience for the fluidisation of the bed.
For this reason feeding fresh monomers into the recycle
line is generally preferred, so that the only gas stream that
enters into the bed is the fluidisation gas.
U.S. patent 4,855,370 describes a gas phase process for
the polymerization of ethylene in which the monomers are fed
into the recycle line together with a suitable amount of H~O
in such a way to neutralise the electrostatic charges which
are formed inside the reactor and which cause the adhesion of
the catalyst and polymer particles to the reactor walls. The
feeding point of the ethylene is close to the reactor inlet
point, downstream of the heat exchanger placed on the recycle
line. In these conditions there is an insufficient gas
homogeneity and the local difference of the reactive monomer
- 5 -
WO 95!22565 PCT/EP95/00544
concentrations in the gaseous mixtures can give rise to the
previously described problems. In addition, when the. gaseous
mixture comprises heavy comonomers, at least a portion of
these comonomers is introduced in the reactor in liquid form,
with the consequent problems of dishomogeneity and
agglomeration in the lower part of the bed.
U.S. patent 5,034,479 describes a gas phase process for
the polymerization of ethylene and mixtures thereof with other
cx-olefins in which the monomers, an inert gas and hydrogen are
fed in the recycle line at a point upstream of the heat
exchanger in order to neutralise the negative effects of the
impurities present in the gaseous feed mixture.
One of the problems that are found when the make-up
monomers are fed in the gas recycle line derives from the
presence of solid particles, entrained in the gas exiting the
reactor, in the gaseous mixture which is recycled. The
composition of the recycling gaseous mixture is generally
similar to the composition of the gas mixture present in the
reactor and also comprises, besides the polymerisable
monomers, inert gas and molecular weight regulators. The
reactivity of the entrained solids in this environment is
relatively low. Nevertheless, close to the feeding point of
the monomers, the solid particles are locally in a highly
reactive environment in that they are practically pelted by a
stream of monomers; the polymerization then continues also in
- 6 -
WO 95!22565 _ 7 i ' PCT/EP95/00544
the recycle line with the consequential problems of fouling of
the pipe and of the devices placed along the line itself.
These problems are particularly highlighted when the feeding
is carried out in a point comprised between the compressor and
the heat exchanger, which is generally the point where there
is the highest temperature and therefore the reactivity is
extreme. The problems are worsened when the monomers are fed
in the liquid form.
Direct feeding of make-up monomers in the reactor is
usually carried out in gas phase processes in which the
polymer bed is mechanically stirred, in that the problems
connected with the non-uniform distribution of the gas inside
the bed can be avoided by means of this system for stirring.
Now it has been found that it is possible to solve the
problems of reactor fouling and fouling of the devices for
transferring and discharging the polymer and the gas, by
feeding the make-up monomers directly in the fluidised bed
reactor at one or more points above said bed. Additionally it
has been surprisingly observed that, even though the monomers
are fed in the upper part of the reactor generally counter
current to the fluidisation gas, no interruptions or
inconveniences to the maintenance of the homogenous
fluidisation of the polymer bed result; in addition the
problems of fouling and clogging of pipes and devices, which
take place when the monomer feeding is carried out in the
WO 95!22565 s . .~ ° ' PCT/EP95/00544
~~ #d~~~ ~'
recycle line, are overcome with consequent improvements of the
operation conditions of the plant and of the quality of the
polymer. The feeding of the monomer in the upper part of the
reactor additionally allows an improved homogeneity of the
monomers in the fluidising gas stream; at the same time the
possible post-polymerization of fresh monomers with the
particles of catalyst and/or polymer containing catalyst,
entrained in the gas velocity reduction zone, does not have
negative consequences on the polymer properties. For point or
points in the upper part of the fluidised bed, it is meant any
point or points situated in the velocity reduction zone of the
gas stream, in which zone the polymer particles entrained in
the gas stream have the possibility of falling back in the
fluidised bed. For make-up monomers, the monomers are intended
which are fed into the reactor in order to compensate for the
monomers used during the polymerization reaction.
Therefore, the object of the present invention is a
continuous process for the gas phase polymerization of one or
more olefins CH,=CHR, where R is hydrogen or an alkyl,
cycloalkyl or aryl radical having 1 to 12 carbon atoms, using
a catalyst comprising the reaction product of the following
components: (A) a titanium or vanadium compound having at
least one Ti-halogen or V-halogen bond respectively, a
magnesium halide and optionally an electron donor; (B) an
alkyl-aluminium compound; (C) optionally, an electron donor
_ g _
WO 95/22565 PCT/EP95/00544
~ , .. st y ~
compound. Said polymerization is carried out in one or more
fluidised bed reactors comprising a polymerization zone,
including the fluidised bed, and a gas velocity reduction zone
situated above the bed, said fluidised bed reactor being
joined to a recycle line, comprising compression and cooling
devices, by means of which the gas exiting at the top of the
reactor is compressed, cooled and again sent to the reactor at
a point below the reaction zone. The process of the invention
is characterised by the fact that the make-up monomer or
monomers are directly sent to said fluidised bed reactor in
one or more points above the fluidised bed.
Preferably the polymerization process is carried out in
the presence of an inert gas selected from the alkanes having
3 to 5 carbon atoms, among which propane is particularly
preferred. Other inert gases, such as for example nitrogen,
methane and ethane, can be used.
The preferred catalyst comprises the reaction product of
a titanium compound, containing at least one Ti-halogen bond,
supported on activated magnesium halide with a trialkyl
aluminium compound.
In order to improve the production efficiency the
catalyst components, before being introduced in the reactor in
the gas phase, maybe subjected to the following treatments:
(a) precontacting the catalyst components in the absence
of polymerizable olefins or in the presence of said
- 9 -
WO 95/22565 ' '' '~' ~ . PCT/EP95/00544
olefins in amounts smaller than 5 grams per gram of solid
catalyst component (A);
(b) prepolymerization of one or more olefins CHZ=CHR, in
amounts ranging from 10 g per g of solid component (A) up
to 10~ of the final catalyst yield.
In this case the make-up monomer or monomers may be fed
into the reactor together with the stream exiting from the
prepolymerization reactor; said stream is also sent to the
polymerization reactor at a point placed above the fluidised
bed.
Moreover it has been seen that by operating as previously
described, it is possible to feed the reactor with monomers in
condensed form, without the known inconveniences of
agglomeration of the bed and loss of fluidisation. Besides the
previously described advantages, the liquid monomer feed
- contributes, at least partially, to the polymerization heat
removal with a consequent improvement in the operating
conditions.
The gas phase polymerization is generally carried out at
a temperature lower than the polymer sintering temperature.
Generally the temperature is between 50°C and 120°C and
preferably between 70°C and 100°C.
The total pressure is generally between 1.5 and 3 MPa.
In the gas phase reactor the fluidisation is obtained
with a high speed flow of the recycle gas towards and through
- to -
WO 95/22565 PCT/EP95/00544
the bed, typically of the order of about 50 times the flow
speed of the feed gas being introduced.
The make-up monomer or monomers are fed to the bed in an
amount approximately equal to the amount of polymer produced.
In order to ensure complete fluidisation, the recycle gas
is resent to the reactor at a point below the bed. A gas
distribution plate, positioned above the inlet point of the
recycle gas, ensures an appropriate distribution of the gas
and additionally acts as a support of the resin bed when the
gas flow is stopped.
Hydrogen may be used as a chain transfer agent in order
to regulate the molecular weight of the polymer.
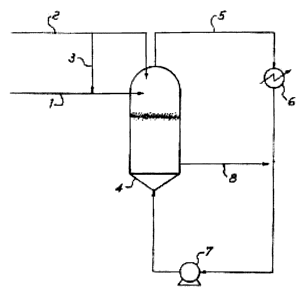
A typical simplified scheme of the process is shown in
enclosed Figure 1. The reference number (1) indicates the line
through which the catalyst, optionally subjected to the
previously described precontacting and prepolymerization
treatments, is fed to the gas phase reactor (4) at a point
above the fluidised bed. The fresh make-up monomers are sent
to the gas phase reactor (4), in one or more points above the
fluidised bed, by means of a line (2) . Part of said monomers
may be conveniently sent, by means of line (3), to the
catalyst feed line (1) and then into the gas phase reactor
(4). The system also comprises a gas recycle line (5) on which
are placed a heat exchanger (6) and a compressor (7) which
provide for the cooling and compression of the recycle gas.
- 11 -
CA 02160876 2005-12-09
WO 95122565 PCT/EP95/00544
The polymer is discharged along line (8) and sent to the
subsequent process step.
Another embodiment of the process, comprising a
precontacting step of the catalyst components, a
prepolymerization step and two gas phase polymerization steps,
1401
is indicated in enclosed Figure 2. The reference number (1)
indicates the apparatus in which the components of the
1201
catalyst system are precontacted. The loop reactor (2) is the
prepolymerizer. The gas phase reactors are indicated by
X401 101
numbers (4) and (6), the separators of the solid from the
(~oI ISo1 1~0J
fluids with the numbers (3), (5) and (7). The catalyst
( 10!
components are fed into the precontacting raactor (1), as
X80)
indicated by the arrow ( 8 ) . The activated catalyst is fed to
(z~~ 1901
the reactor loop (2) as indicated by the arrow (9). The
prepolymer-catalyst system produced is fed to the gas phase
reactor (4) or, in the case that it is desirable to separate
(30~
the solids from the liquids, to separator (3) and from there
~~+OJ (~t0~
to the gas phase reactor (4). The polymer exiting reactor (4),
( 5 0l
after having passed through the separator (5), is introduced
1601
in reactor (6). The polymer is then discharged from reactor
l~ol 101
(6) to separator (7). The make-up monomers are fed into
lsr.01 1
reactor (4) and (6) at a point above the fluidi5ed bed through
loo) 01101
lines (10) and (11). If the process requires a single gas
phase step the polymer produced is collected at the exit of
( 50/
the separator (5).
- 12 -
WO 95/22565 PCT/EP95/00544
. : F~ ~~ ~
The process of the invention can be used for preparing a
large number of different olefin polymers such as, for
example, high density polyethylene (HDPE; density higher than
0.940) among which are ethylene homopolymers and copolymers of
ethylene with alpha olefins having 3 to 12 carbon atoms;
linear low density polyethylene (LLDPE; density lower than
0.940) and very low or ultra low density polyethylene (VLDPE
or ULDPE; density less than 0.920 and as low as 0.880)
consisting of a copolymer of ethylene and one or more alpha
olefins having 3 to 12 carbon atoms and with a content of
ethylene units of higher than 80~ by mole; elastomeric
terpolymers of ethylene, propylene and diene and elastomeric
copolymers of ethylene and propylene having a content of
ethylene units comprised between 30 and 70~ by weight;
isotactic polypropylene and crystalline copolymers of
propylene and ethylene and/or other alpha olefins, having a
content of units deriving from propylene of higher than 85°s by
weight; impact resistant propylene polymers obtained by
sequential polymerization of propylene and a mixture of
propylene with ethylene, containing up to 30~ by weight of
units deriving from ethylene; copolymers of propylene and 1-
butene having a content of units deriving from 1-butene
between 10 and 40g by weight.
The following examples can further illustrate the present
invention. Naturally, variations can be carried out without
- 13 -
i, ~ .
CA 02160876 2005-04-22
WO 95/22565 PCT/EP95/00544
departing from the scope of the present invention.
The .properties indicated have been determined according
to the following methods:
- MIE Melt flow index: ASTM-D 1238, condition E;
..
- MIF Melt flow index: ASTM-D 1238, condition F;
- MIL Melt flow index: ASTM-D 1238, condition L;
- Bulk densitv: DIN-53194;
- Xylene soluble fraction: determined at 25°C;
Comonomer content: percentage by weight of comono-
mer determined by IR spectra;
- Real Density: ASTM-D 792.
EXAMPLE 1
Preparation of the solid catalyst component
Into a stirred reactor 28.4g of MgCl2, 49.5 g of
anhydrous ethanol, 10 ml of Vaseline* oil ROL OB/30, 100 ml of
silicone oil having a viscosity of 350 cs were added. The
mixture was heated at 120°C until MgCl, was dissolved. The hot
reaction mixture Was then transferred to a 1.5 1 reactor
having a Ultra Turrax T-45 N stirrer, containing 150 cm3 of
vaseline oil and 150 cm3 of silicone oil. The temperature was
maintained at 120°C whilst stirring for 3 minutes at 2000 RPM.
The mixture was then discharged in a 2 litre stirred tank,
containing 1 1 of anhydrous n-heptane cooled to 0°C, whilst
stirring at a speed of 6 m/sec for about 20 minutes,
maintaining the temperature at 0°C. The particles so obtained,
* trade mark
- 14 -
WO 95/22565 PCT/EP95/00544
-$ ,
after washing with n-hexane, were subjected to a thermal
treatment in a nitrogen stream, at temperatures ranging from
50 to 150°C, until spherical particles, having a residual
alcohol content of about 35~°s by weight, were obtained. 300 g
of this product were charged into a 5000 cm3 reactor in
suspension with 300 cm3 of anhydrous hexane. Whilst stirring
at room temperature, 130 g of triethyl aluminium (TEAL) in
hexane solution was slowly added. The mixture was heated to
60°C for 60 minutes and then stirring was stopped; the mixture
was left to settle and the clear phase was separated. The
treatment with TEAL was repeated two more times under the same
conditions; the obtained solid was then washed with hexane and
dried at 50°C. 260 g of so obtained support was charged into a
reactor together with 3 litres of anhydrous hexane; whilst
stirring 242 g of Ti(OBu)4 was fed at room temperature. The
mixture was stirred for 30 minutes and then 350 g of SiCl4,
diluted with 250 cm3 of hexane, was fed over 30 minutes and at
room temperature. The mixture was heated to 65 °C and
maintained under stirring for 3 hours; the liquid phase was
then separated by settling and siphoning. Then, 7 washings
with hexane were carried out followed by drying the compound
obtained at 50°C under vacuum.
Polymerization
A plant having the set up of Figure 2 was used to produce
LLDPE through copolymerization of ethylene with hexene. The
- 15 -
WO 95/22565 ~ PCTIEP95/00544
.: f.-~~ °A~= ~v ~h~ . .
'~1~GO~~I~i
solid component prepared according to the above described
process and a solution of TEAL in n-hexane were fed to a
precontacting reactor and from this to a slurry
prepolymerization reactor wherein ethylene was polimerized.
The suspending liquid was liquid propane. The propanic slurry
containing the prepolymer was continuously discharged from the
prepolymerization reactor to the first gas phase reactor.
Hydrogen was also fed to the prepolymerization reactor in
order to control the molecular weight of the prepolymer. To
the first and second gas phase reactors propane was added for
a better control of the reactivity of the system.
Main operating conditions
Precontact step
- Temperature (°C) - 20
- Residence time (min) - 10
- TEAL/Ti ~ (mol) - 30
Prepolymerization step
- Temperature (°C) - 25
- Residence time (min) - 30
First gas phase reactor
- Temperature (°C) _ 85
- Pressure (barg) - 20
-. Hydrogen/Ethylene (mol) - 0.14
- Hexene/(hexene + ethylene) (mol) - 0.15
- Propane (~mol) - 80.0
- 16 -
WO 95/22565 PCTJEP95/00544
a .
Second phase reactor
- Temperature (°C) - 85
- Pressure (barg) - 20
- Hydrogen/Ethylene (mol) - 0.14
- Hexene/(hexene + ethylene) (mol) - 0.15
- Propane (%mol) - 50.0
Characteristics of the end product
- Final Yield (Kg/gr cat) - 10.4
- Real Density (Kg/1) - 0.918
- Melt Index ~~E~~ (gr/10 min) - 1
- Bulk Density (Kg/1) - 0.380
The test lasted about 15 days. At the end, the inspection
of the reactors revealed that they were perfectly clean: the
walls were not covered in polymer and there was neither
formation of chunks nor caking.
EXAMPLE 2
Preparation of the solid catalyst component
Into a stirred reactor 28.4g of MgCl2, 49.5 g of
anhydrous ethanol, 10 ml of vaseline oil ROL OB/30, 100 ml of
silicone oil having a viscosity of 350 cs were added. The
mixture was heated at 120°C until MgCl2 was dissolved. The
hot reaction mixture was then transferred to a 1.5 1 reactor
equipped with a Ultra Turrax T-45 N stirrer, containing 150
cm3 of vaseline oil and 150 cm3 of silicone oil. The
temperature was maintained at 120°C whilst stirring for 3
- 17 -
WO 95/22565 - ~ ~ ~~ 'r ~ f PCT/EP95/00544
~.~~~fi'n 8'~ 6
minutes at 3000 RPM. The mixture was then discharged in a 2
litre stirred tank containing 1 1 of anhydrous n-heptane
cooled to 0°C whilst stirring at a speed of 6 m/sec for about
20 minutes, maintaining the temperature at 0°C. The particles
so obtained, after washing with n-hexane, were subjected to a
thermal treatment in a nitrogen stream, at temperatures
ranging from 50 to 150°C until spherical particles were
obtained having a residual alcohol content of about 35°~ by
weight. 25 g of this product were charged into a stirred
reactor containing 625 ml of TiCl4 at 0°C and under stirring.
The mixture was then heated to 100°C for one hour and then
left to cool. When the temperature of 40°C was reached,
diisobutyl phthalate was added in quantities such to give a
molar ratio of magnesium to phthalate of 8. The mixture was
heated to 100°C for 2 hours under stirring and then the solid
was left to settle. The hot liquid was removed by syphoning.
Then 500 ml of TiCl4 was added and the mixture was heated to
120°C for 1 hour whilst stirring. After settling, the hot
liquid was removed by syphoning and the solid was washed with
n-hexane.
Polymerization
A plant having the set up of Figure 2 was used to produce
LLDPE modified through copolymerization of butene/propylene in
the first gas phase reactor and copolymerization of
ethylene/butene in the second gas phase reactor. The solid
- 18 -
WO 95/22565 PCT/EP95/00544
.~ ~~ fi a_ 8 °~ ~
component prepared according to the above described process, a
solution of TEAL in n-hexane and cyclohexylmethyl-dimethoxy-
silane were fed to the precontacting reactor and from this to
the prepolymerization reactor in liquid propylene. The
propylene slurry containing the prepolymer was continuously
discharged from the prepolymerization reactor to the first gas
phase reactor. Hydrogen was also fed to the prepolymerization
reactor in order to control the molecular weight of the
prepolymer. To the first and second gas phase reactors propane
was added for a better control of the reactivity of the
system.
Main oneratinaconditions
Precontact ste
- Temperature (C) - 20
- Residence time (min) - g
- TEAL/Ti (mol) - 120
- TEAL/silane (mol) - 20
Prepolymerization step
- Temperature (C) - 50
- Residence time (min) _ 80
First gas phase reactor
- Temperature (C) - 60
- Pressure (barg) - 18
- Ha/CsHe (mol ) - 0 . 010
- CQH$/ ( C4Hg + C3H6) (mol ) - 0 . 115
- 19 -
WO 95/22565 ~ ~ , ~ ~ , PCT/EP95/00544
,i . ; ~ r
- Propane (~mol) - 80.0
Second qas phase reactor
- Temperature (C) - 90
- Pressure (barg) - 17.5
- Ha/CzHa (mol) - 0.27
- C4H$/ ( C~H$ + CZH4 ) (mol ) - 0 . 2 0
- Propane (gmol) - 44
Characteristics of the final product
- Real Density (Kg/1) - 0.916
- Bound butene ( s wt ) _ 7
- Melt Index "E" (gr/10 min) - 1.1
The test lasted about 20 days. At the end, the
inspection
of the reactors revealed that they were perfectl y clean:
the
walls were not covered in polymer and there was neither
formation of chunks nor caking.
EXAMPLE 3
A heterophasic copolymer of propylene was prepared
by
sequential polymerization of propylene and a mixture of
propylene with ethylene, by using a plant of the
type
described in figure 2 comprising:
- a precontact step;
- a prepolymerization step;
- a gas phase polymerization step carried out using three
reactors connected in series.
The solid catalyst components prepared according
to the
- 20 -
WO 95/22565 PCT/EP95/00544
method described in example 2, a TEAL solution in n-hexane and
dicyclopentyl-dimethoxy-silane were fed to the precontacting
reactor which was maintained at a constant temperature of
30°C. The product discharged from this reactor was fed to the
prepolymerization reactor to which also propylene and propane
were fed. The residence time in the prepolymerization reactor
was about 20 minutes and the temperature was maintained
constant at 20°C. The prepolymer was then fed to the first of
the three gas phase reactors connected together in series. In
the first reactor polypropylene homopolymer was produced,
while in the second and third reactors ethylene/propylene
copolymer was produced. In all the gas phase reactors the
make-up monomers were directly fed into the reactors at a
point placed above the fluidised bed.
Main operating conditions
Precontact step
- Temperature (°C) - 30
- Residence time (min) - g
- TEAL/Ti (mol) - 80
- TEAL/silane (mol) - 20
Prepolymerization step
- Temperature (°C) - 20
- Residence time (min) - 20
First aas phase reactor
- Temperature (°C)
- 21 -
WO 95/22565 PCT/EP95/00544
s.,
- Pressure (barg) - 16
- Hz/CsHe (mol ) - 0 . 17
- Propane (~mol) - 60.0
Second aas phase reactor
- Temperature (C) - 60
- Pressure (barg) - 16
- Hz/C2Ha (mol ) ~- 0 . 11
- CZH4/ ( CZH4 + C3H6 ) (mol ) - 0 . 3 3
- Propane (~mol) - 50
Third aas phase reactor
- Temperature (C) - 60
- Pressure (barg) - 16
- Hz/CZHa (mol ) - 0 . 10
- CzHa/ ( CZH4 + C3H6 ) (mol ) - 0 . 32
- Propane (~mol) - 30
Characteristics of the final roduct
p
- Final Yield (Kg/gr cat) - 10.4
- Melt Index L~~ (gr/10 min) - 2.8
- Bound ethylene (~ weight) - 28.6
- Xylene solubility (a weight) - 46
The plant ran continuously As an indicative
for 8 days.
parameter for the arising of reactor fouling,
the heat
exchange coefficient of the heat exchanger
placed in the
recycle line was measured: the whole test this coefficient
for
remained constant (700 Kcal/hr.mz.K)
- 22 -
WO 95/22565 PCT/EP95/00544
..
For comparative purposes, the make-up monomers were fed
in the recycle line at a point placed upstream of the heat
exchanger: after 3 days of running the heat exchange
coefficient of the heat exchanger had reduced 25~, indicating
a build up of polymer on the surfaces of the haat exchanger.
- 23 -