Note: Descriptions are shown in the official language in which they were submitted.
~EC 40320/50059
- 21~1279
PYROTEC~NIC ~ H 1~.~; l MATERIAL
Field of Invention
This invention relates to gas-generating pyrotechnic sheet material which is especially
useful in igniferous booster charges (hereafter termed ignition elements or igniters) for
propellant compositions. In vehicle occupant restraint safety systems the material may be
5 used advantageously in ignition elements for gas-generating compositions for gas-bag ("air
bag") infiation and heating elements for heating stored gas in hybrid inflators. The invention
also relates to the method of m~nllf~cturing said pyrotechnic sheet material.
Back~round of Invention
Pyrotechnic sheet material consisting of one or more substrate layers of oxidizing
polymeric film having a layer of oxidizable material, preferably metal, on at least a portion
of at least one surface of the, or each, substrate layer, the polymeric film and the oxidizable
material being conjointly capable of reacting together exothermically on ignition, has been
described in PCT International Publications Nos WO 90/10611 and WO 90/10724.
15 Improved pyrotechnic sheet material having enhanced burning rate has been described in
United Kingdom patent specification No. C~B 2,282,136A.
The use of the aforesaid pyrotechnic sheet material to ignite a gas-generating
propellant charge for air bag inflation has been described in European patent publication no.
505024.
The pl~relled oxidizing polymeric film is halogenated film such as
polytetrafiuoroethylene containing little if any hydrogen and the pl erell ed oxidizable material
of the aforedescribed pyrotechnic sheet material comprises a metal selected from the group
consisting of lithium, sodium, m~gn~ m, beryllium, calcium, strontium, barium, aluminium,
titanium, zirconium, and alloys of any one or more thereof, the most preferred metal being
magnesium. Advantageously the metal is vapour-deposited on the film by known methods,
the
amount of metal being preferably subst~nti~lly stoichiometric at the location of the film
underlying the metal. On ignition this pyrotechnic sheet material produces substantially only
solid products, any gases such as metal fluoride produced in the combustion zone
21~ 1279
inct~nt~neously ct nden.~ing to solid form. Such gasless material is advantageous in certain
applications where blast effects must be avoided, but in applications where the material is
primarily required to propagate ~ame to a further charge of combustible material the absence
of gaseous products can be a disadvantage.
s Summary of Invention
We have now found that the ease of igr~ition and flame tr~n.~mi~sion properties of the
aforedescribed pyrotechnic sheet material can be enhanced by providing the sheet with a
cont~ctin~: layer of gas-generating de~agrating material. The res-~ltin~ pyrotechnic sheet has
the fast burning rate of the original material and also produces gas which rapidly travels to,
0 and penetrates, any ignitable gas-generating main charge in contact with the sheet material,
thereby accelerating the ignition of the main charge. In addition to Pnh~ncin~ the flame
tr~n~mi~ci~n capability, the layer of deflagrating material can also act as a protective barrier
material to prevent or retard oxidation of a layer of oxitli7~ble material such as m~gnPsillm
which oxidizes at a significant rate under normal atmospheric conditions. When Pnh~nced
5 ease of ignition only is required this effect can be achieved by the application of the
deflagration material over a small portion of the pyrotechnic sheet material adjacent to an
ignition point and the amount of deflagrating material need not be sufficient to produce a
significant amount of gaseous products on combustion.
Thus, in accordance with this invention pyrotechnic sheet material comprises a substrate of
20 oxidizing m~tPri~l; a coating layer of oxidizable material on at least a portion of at least one
surface of said substrate, the said substrate and the said layer of oxi(li7.~ble material being
conjointly capable of reacting together exothermically on ignition; and a layer of gas-
generating deflagrating material overlying at least a portion of the surface area of the
substrate and/or the layer of oxidizable material, said deflagrating material being in ignition
25 ~l~n.~",;c~ion relationship with said substrate and oxidizable material. Deflagrating material
in this context refers to material capable of sustained rapid burning without reaction with
further oxidizing or red~lcing material.
The gas-generating deflagrating material may be applied as an adhering layer to the
substrate and/or the layer of oxidizable material or it may be provided as a separate layer over
30 a free surface ofthe substrate and/or the oxi(li7~hle m~P.n~l, for example as a co-rolled sheet.
21fi 127~ -
~_ 3
The substrate may advantageously be coated on both sides with oxidizable material and at
least a portion of at least one of the layers of oxidizable material may advantageously be
covered with a layer of gas-generating deflagrating material.
The gas-generating deflagrating material may, for example, comprise any gas-
5 generating propellant material. A nitrocellulose based propellant is advantageous andconvenient and may, for example, be applied to the film or oxidizable material as a separate
sheet in laminar pyrotechnic sheet material of the invention, or as a solution in a solvent, for
example ~cetonP, which is subsequently removed to leave an adherent layer of nitrocellulose
propellant material on the film or oxidizable material. Other gas-generating propellant
0 materials which may be used include deflagrating materials such as black powder, sodium
a_ide/oxidizer compositions, potassium perchlorate/~ minil~m compositions or other solid
pyrotechnic gas-genel~Ling composition. These may be applied over the coated substrate as
a solution or dispersion in a carrier liquid which can be removed, or in a curable liquid
polymer or in a polymer solution, such as polymelhylLlin~loroethylene in acetone or
1S polyvinyl~cet~te in water, from which the solvent can subsequently be removed.
The layer of gas-genel~Ling de~agrating material is conveniently from 3-100 microns
thick and preferably from 10-40 microns thick.
The plerelled oxi~i7.ing substrate comprises polymeric film preferably co~ g
atoms chemically bound therein selected from the group con~i~ting of halogens (especially
20 fluorine), oxygen, sulphur, nitrogen and phosphorous. One prerel,ed film substrate
comprises fluoropolymer such as polytetrafluoroethylene (PTFE) which produces a high
energy pyrotechnic sheet, but other suitable polymeric films include those comprising
polychlorotrifluoroethylene, polyhexafluoropropylene, copolymers of trifluoroethylene and
hexafluoropropylene, copolymers of trifluoroethylene and tetrafluoroethylene, copolymers
25 of hexafluoropropylene and tetrafluoroethylene,
copolymers of hexafluoropropylene and vinylidene fluoride,
copolymers of tetrafluoroethylene and partially fluorinated propylene, copolymers of
chlorotrifluoroethylene and vinylidene fluoride, homopolymers of partially fluorinated
propylene, copolymers of partially fluorinated propylene and vinylidene fluoride,
- ~`J.61279
trichloroethylene homopolymers, copolymers of trichloroethylene and vinylidene fluoride,
mixtures oftwo or more of such polymers or mixtures of any one or more of such polymers
with PTFE.
The polymeric film may optionally be a porous film, the pores advantageously
occupying 6-95% of the film volume (i.e. porosity of 6-95%). Preferably the pores are
interconnecting vapour-permeable pores having at least part of the oxitli7.~ble material
vapour-deposited therein. Pyrotechnic sheet material comprising such porous film generally
has faster burning rates than that cont~ining only solid polymeric film.
A preferred pyrotechnic sheet of the invention has discontinuous portions in the0 oxidizing substrate and/or the layer of oxidizable material, preferably in the oxidizable
material, these portions having flame-permeable apertures through which the interface
between the o~ in~ substrate and the oxidizable material is exposed as described in United
Kingdom patent specific~tion no.GB 2,282,136A which is incorporated herein by reference.
Such exposure of portions of the interface enhances the ease of ignition and rate of
combustion of the pyrotechnic sheet. In an especially ple~lled pyrotechnic sheet the
substrate and the oxidizable material are permanently deformable and have di~lenl strains
for rupture value thereby enabling one ofthe materials to be ruptured by stretching to expose
flame-permeable apertures at the interface. The stretching may advantageously be effected
by st~mping protrusions (embossing) on the contacting substrate and layer of oxidizable
material, the protrusions subsequently serving as spacer elements to ~nh~nce the rate of
combustion of the pyrotechnic sheet material.
The oxidizable material may advantageously comprise metal selected from the group
consisting of lithium, sodium, magnesium, beryllium, calcium, strontium, barium, ~ minillm,
titanium, zirconium and alloys thereof, which metal may be advantageously be vapour-
2s deposited on the substrate. A metal layer is especially advantageous as it significantly
enhances the dimensional stability of the pyrotechnic sheet and is easily ruptured. A most
preferred metal for high heat generation is magnesium or an alloy thereof preferably coated
on to a substrate film comprising fluoropolymer. Preferably the ratio of metal to the
substrate of ~itli7in~ polymeric film is substantially stoichiometric or there is a small excess
- 2161273
s
of metal at the location of the film underlying the metal. The reaction between PT~E and
magnesium can be represented empirically as
(C2F4)n - 2nMg --> 2nMgFz + 2nC
This reaction releases 5.98 megajoules/kilogram of reactant pyrotechnic material.
s The rate of energy release on ignition varies inversely with the thickness and directly
with the porosity of the pyrotechnic sheet material and, accordingly, the thickness and
porosity will be chosen to attain the desired energy release. Thus the pr~rel,~;d polymeric
film will generally have an areal mass of 10 to l50g/m2, typically 25-75g/m2 and the total
amount ofthe oxidizable m~t~.ri~l will be equivalent to a laminar thickness of 2 to 30 microns,
0 typically 4 to l S microns.
A typical pyrotechnic sheet comprises a film of halogenopolymer 3 to S0 microns,(preferably 10 - 30 microns) thick having on each side a vapour-deposited layer of
magnesium 2 to 40 microns (preferably 4 - 15 microns) thick each m~gnesi~lm layer being
overlaid with a cont~cting layer of gas-ge~ li"g defiagrating material 10 - 40 microns thick.
The invention also includes a method of m~nl-f~cturing a pyrotechnic sheet material
which comprises depositing a layer of oxidizable material on at least a portion of at least one
surface of a substrate of oxidizing material, the substrate and the oxidizable material being
conjointly capable of reacting together exothermically on ignition, and applying to at least a
portion of the surface of the oxidizable material and/or the substrate an overlying layer of
gas-generating deflagrating material in ignition tr~n~mi~sion relationship with said substrate
and oxidizable material.
Ple~l~bly the o~i~li7~ble material is vapour-deposited at low pressure on the polymer
substrate by direct evaporation or magnetron sputtering.
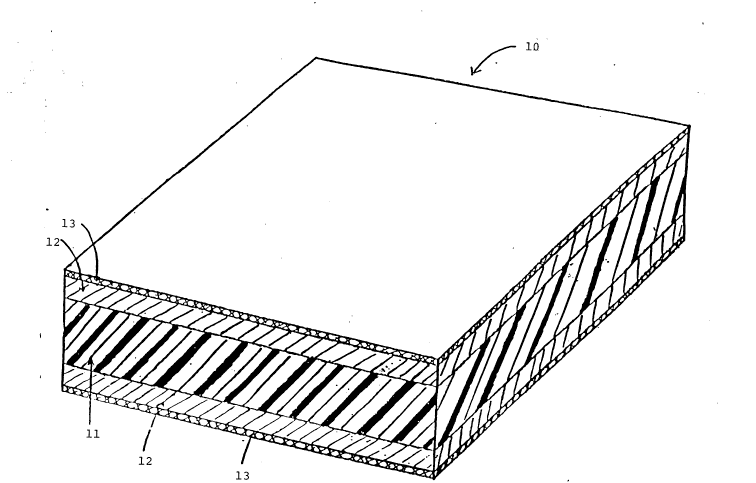
Brief description of the drawing
The invention is further described by way of example only with reference to the
accompanying drawing which is a diagrammatic perspective, part-sectional view ofpyrotechnic sheet material of the invention.
~Z1612~ -
Detailed description of the drawin~
Referring to the drawing, pyrotechnic sheet material design~ted generally by thenumber 10 consists of a substrate 11 of oxidizing polymeric film, for example ofpolytetrafiuoroethylene, coated on each side with a vapour-deposited layer of oxidizable
5 metal for example magnesium 12. Each layer of oxidizable metal is coated with a layer of
gas-generating deflagrating material 13 .
Specific Examples
The m~nl-f~ctllre of the pyrotechnic sheet material of the invention is further
described in the following specific Examples wherein parts and percentages are given by
0 weight.
Example 1
A 25 micron thick solid sheet of P lF~ was coated on each side with an 8.5 micron
thick vapour-deposited layer of m~gn~.~illm (appluxil"ately stoichiometric proportions). The
coated sheet was embossed with regular rows of dimples by passing the sheet between a
15 patterned metal roll and a plain rubber roll. The dimples were spaced at 3rnm centres in each
direction and each dimple was approximately 0.75mm square at the base, O.5mm square at
the top, and 0.25mm high. The upper layer of magnesium was thereby ruptured around the
periphery of the top of the dimples to expose the oxidizing polymeric film at the
m~gnesillm/PTFE upper interface, the width ofthe exposed areas being up to 10 microns.
A sample portion of the embossed sheet having an area of 164 cm2 was coated witha solution in acetone of nitrocellulose, having a nitrogen content of 12.2%, and the acetone
was evaporated offto leave a continuous contacting film of nitrocellulose over the layers of
m~gnesium.
2s The sample was rolled into a helically wound charge assembly by winding around a
central tubular phenolic resin former having an internal diameter of 6mm and 6mm wall
thickness. Three longi~l~in~l slits e~P.n~ing to within lOmm from each end of the charge
were cut through the spiral section of the pyrotechnic sheet. The assembled charge was
ignited by a squib in a combustion test vessel (ballistic bomb) having a volume of 3 Scc.
21612~9
The pressure in the vessel after ignition was recorded. A sample coated with 0. lg
nitrocellulose gave a maximum pressure of 22.1MPa in 2.02 milliseconds and a sample
coated with 0.21g nitrocellulose gave a maximum pressure of 27.9MPa after 1.84
milliseconds. In a compa,~live test a sample without a nitrocellulose coating gave a
s maximum pressure of 20MPa after 2.03 milliseconds.
These results clearly demonstrate the improved pressure (gas production) obtained
with the nitrocellulose coated material.
Example 2
0 A pyrotechnic sheet comprising 25 micron thick PT~E film coated on each side with
an 8.5 micron thick vapour-deposited layer of m~gn~.sjllm was prepa~ed and embossed as
described in Example 1. A 10.61g sample of the embossed sheet was cut to the shape of a
trapesium with opposite parallel sides having respective lengths of 150mm and 118mm and
a length of 0.8 metres between opposite equal sides. The sample was coated on each side
with a slurry co~ i";,lg 4 parts sodium azide, 0.065 parts carbon black, 1.81 parts vinylidene
fluoridefhPx~fl-lolopl~ylene copolymer (binder and oxidizer for the sodium azide) available
under the registered trade mark ~ITON and 8.37 parts ethyl acetate. The ethyl acetate was
removed by evaporation leaving 3.09g of residual coating material. The sample was wound
around a 12mm .I;~ ler tubular phenolic resin former with the shorter parallel edge on the
side and inserted into a 28mm tli~met~.r x 11.7mm long thin steel tube leaving about 15mm
at each end of the sample protruding beyond the steel tube. Each protruding end was
'petalled' by making 6 equally spaced longit~l~in~l cuts around the circumference, each cut
extending to lcm from the end for a length of 15mm.
Two such wound samples were placed in proxi"Ji~y in a ballistic bomb having a
volume of 7100 cc and ignited .~imlllt~neously at the end of one sample. A m~im~lm
pressure of 1.6MPa was reached in 81.7 milliseconds.
In a comparative test two 16.51g samples of the uncoated pyrotechnic sheet
generated a pressure of 1.4MPa in 103 milliseconds.