Note: Descriptions are shown in the official language in which they were submitted.
ICICAN 816 2162~11
EmulsifYing Aqent for Use in Explosive Compositions
Field of the Invention
This invention relates to an emulsifying agent
suitable for use in water-in-fuel and melt-in-fuel
explosives.
Description of the Related Art
Water-in-fuel and melt-in-fuel emulsion explosives are
well known in the explosives industry and are routinely
used in civilian excavation, mining and quarrying. Water-
in-fuel emulsions comprise a discontinuous phase of
droplets of an oxygen supplying component such as an
aqueous oxidiser salt solution dispersed in a continuous
phase of oils and/or waxes in the presence of one or more
emulsifying agents. The oxygen-supplying discontinuous
phase of a melt-in-fuel emulsion comprises only a small
proportion of water or adventitious water only. The
discontinuous phase may be a eutectic composition, that is
the melting point of the composition is either at the
eutectic or in the region of the eutectic of the component
salts of the discontinuous phase. Where used herein the
term emulsion refers to both water-in-fuel and melt-in-fuel
emulsions.
In general the emulsions suitable for use in emulsion
explosives are relatively inert until mixed with
sensitizing agents such as self-detonable compounds (e.g.
nitroglycerine) or void material such as glass
microballoons, gas bubbles or the like.
Water-in-fuel emulsion explosives compositions were
first disclosed by Bluhm in United States Patent 3,447,978
and comprise (a) a discontinuous aqueous phase comprising
ICICAN 816
-
21~2~11
discrete droplets of an aqueous solution of inorganic
oxygen-releasing salts; (b) a continuous water-immiscible
organic phase throughout which the droplets are dispersed
and (c) an emulsifier which forms an emulsion of the
S droplets of oxidizer salt solution throughout the
continuous organic phase. They may also include
sensitizing agents such as a discontinuous gaseous phase.
Subsequently, numerous documents have been published which
provide modifications and/or improvements over the
formulations originally described by Bluhm.
A key component in the formulation of an emulsion
explosive composition is the selection of the proper
emulsifying agent. The type of emulsifying agent chosen
will have an effect on many properties of the emulsion
including, for example, the ease of formation of the
emulsion, the discontinuous phase droplet size and the
tendency of the droplets to crystallise or coalesce. These
properties are particularly relevant to the storage
stability of the emulsion and ultimately, the performance
of the composition as an explosive.
Australian patent application no. 40006/85 (Cooper &
Baker) discloses emulsion explosive compositions in which
the emulsifier is a reaction product of a poly[alk(en)yl]
species (e.g. an alkylated succinic anhydride) and amines
such as ethylene diamine, diethylene triamine and mono- and
di-ethanolamines.
McKenzie in US patent No. 4,931,110 describes the use
of a bis(alkanolamine or polyol) amide and/or ester
derivatives of, for example, polyalk(en)yl succinic
anhydride compounds as suitable emulsifying agents.
Polyalk(en)yl succinic anhydride compounds are described by
Baker in Canadian patent No. 1,244,463.
Forsberg et al. in US patent No. 4,840,687 describe an
emulsion explosive composition wherein the emulsifier is a
nitrogen-containing emulsifier derived from at least one
carboxylic acylating agent, a polyamine and an acidic
compound.
ICICAN 816
-
2152411
--3--
Additionally, Chattopadhyay, in Canadian patent
application No. 2,076,987 describes the use of a mixed
emulsifying agent system for emulsion explosives comprising
a surfactant and co-surfactant, each having branched chain
hydrocarbon tails.
However, the identification of additional emulsifying
agents is still desirable in order to lead to improved
emulsifying agent or emulsion properties.
Emulsifying agents perform several functions during
the formation and subsequent stabilisation of an emulsion
explosive composition. As an emulsion is being formed the
emulsifying agent must be able to lower the interfacial
tension between the discontinuous and continuous phases and
thus stabilize the interfaces between the oxidiser salt
droplets and the fuel. The emulsifying agent must also
form a structured bilayer at the interface to aid
suppression of droplet coalescence and inhibit the
crystallisation of salts in the droplets. It is also
important that the emulsifying agent be able to preserve
bilayer integrity dynamically when an emulsion explosive is
subjected to shear stress, such as the shear stress which
occurs during pumping.
Providing a single emulsifying agent which satisfies
all these criteria is not straightforward. Accordingly, an
emulsifier system is often employed which contains a
mixture of emulsifying agents; each of which satisfies
different criteria. A particularly preferred mixed
emulsifier system of the prior art is described in the
aforementioned Cooper & Baker and Chattopadhyay references
and by Yates et al. in US patent No. 4,710,248 which
describes the use of a derivatized polyisobutene succinic
anhydride surfactant in combination with a co-surfactant
such as sorbitan monooleate. The emulsifying agents of the
prior art are relatively effective in performing some
functions but improvements are still sought.
For example, it has proved particularly difficult to
identify suitable emulsifying agents for certain types of
emulsions for use in emulsion explosives compositions. In
ICICAN 816 2152411
particular, emulsifying agents of the prior art have proved
inadequate for the formation of emulsions which can be
formed into stable, oxygen balanced emulsion explosives
compositions. In the field of emulsion explosives, a
S composition is said to be oxygen balanced at the point at
which adequate oxygen from the components is made available
for the explosive detonation to go to completion, leaving
no unreacted material. Oxygen balanced compositions are
particularly desirable because they provide maximum energy
and minimum fume.
Many emulsion explosive compositions currently in use
are oxygen negative, that is there is insufficient oxidizer
salt present to achieve total reaction of all materials. In
these oxygen negative formulations, the ratio of oxidiser
phase to fuel oil phase is commonly between 90:10 and 94:6
and the detonation of these materials tends to produce
toxic NOX fumes. Ideally, maximum energy release and
minimum fume would be provided by an oxygen balanced
composition. Typically, these oxygen balanced compositions
have an oxidiser phase:fuel oil phase ratio of between
about 95:5 and 96:4. However oxygen balanced compositions
can be difficult to prepare, in practice, because of the
lack of suitable emulsifying agents which can stabilize
such low fuel phase-content compositions.
It is thus an object of the present invention to
provide an improved emulsifying agent capable of providing
the desired properties described hereinabove.
Summary of the Invention
It has now been found that a particular class of
emulsifier is particularly suitable for use in emulsion
explosive compositions, and in particular, for use in
oxygen balanced emulsion explosive compositions.
Accordingly, the present invention provides an emulsifying
agent suitable for use in an emulsion explosive composition
comprising a hydrophilic species, a first lipophilic chain
ICICAN 816 ~ 4 ~ ~
-
which is attached to said hydrophilic species by a first
linking moiety, and a second lipophilic chain which is
attached to said hydrophilic species by a second linking
moiety, and wherein said second lipophilic chain has more
than one olefinic unsaturated bond in its hydrocarbon
chain.
As is known by those skilled in the art, the degree of
olefinic unsaturation for the second lipophilic chain may
be an average value for the individual lipophilic
molecules. Accordingly, the level of unsaturation of the
second lipophilic chain may approach the value of 1, but
preferably, the second lipophilic chain comprises at least
two olefinic unsaturated bonds in its hydrocarbon chain,
and preferably these two unsaturated bonds are separated by
at least one saturated carbon bond (e.g. -CH2-).
Further, the current invention also provides an
emulsion suitable for use in an emulsion explosive
composition having a continuous hydrocarbon phase, a
discontinuous aqueous salt or eutectic phase, and at least
one emulsifying agent, wherein said emulsifying agent is an
emulsifying agent as described hereinabove with respect to
the present invention.
Description of the Preferred Embodiments
In a particularly preferred embodiment the emulsifying
agent is as described hereinabove with respect to the
current invention, and has the structure shown for formula
I;
L1 \ /Rx
M1 ~ N Formula I
\(CHz)m - M2 L2
wherein L1 is a first lipophilic chain,
L2 is a second, unsaturated lipophilic chain
having an olefinic unsaturated level greater
ICICAN 816
21~2411
--6--
than 1,
R is hydrogen or a hydrophilic group, or a
direct bond to M1 when x is 0,
M1 is an ester, amide or imide linkage,
M2 is an ester linkage, and
m is 2 O, and x is O or 1.
The first lipophilic chain (L1) may be either monomeric
or polymeric in nature. The chain structure should
incorporate a backbone sequence of at least 10 and
preferably not more than 500 linked atoms. These linked
atoms may be entirely carbon atoms or they may be
predominantly carbon atoms interrupted by hetero atoms such
as oxygen or nitrogen. A preferred type of first
lipophilic chain is a saturated or unsaturated hydrocarbon
chain derived, for example, from a polymer of a mono-olefin
wherein the polymer chain contains from 40 to 500 carbon
atoms. Suitable polyolefins include those derived from
olefins containing from 2 to 6 carbon atoms, in particular
ethylene, propylene, butene-l and isoprene, but especially
isobutene.
It has been observed, that during selection of the
second lipophilic chain, a higher degree of olefinic
unsaturation generally results in an increasing stability
of the emulsion formed, and generally leads to a smaller
droplet size in the emulsion. Accordingly, it is preferred
that the second lipophilic chain (L2) be preferably a
hydrocarbon chain comprising greater than 1, and more
preferably greater than 2 or more preferably 3, olefenic
unsaturated bonds. It is also preferred that the second
lipophilic chain comprise a long chain hydrocarbon
comprising between 8 - 36 carbon atoms, preferably 10 - 26
carbon atoms and most preferably between 16 and 22 carbon
atoms. A preferred material of use as the second lipophilic
chain is based on the residual of a polyunsaturated fatty
acid. Polyunsaturated fatty acids include fatty acids
having an average olefinic unsaturation level of greater
than 1 (e.g. 1.1), and can include mixtures of fatty acids.
ICICAN 816 21S2411
Examples of these fatty acids include linoleic acid
(cis,cis-9,12-octadecadienoic acid), linolenic acid
(cis,cis,cis-9,12,14-octadecatrienoic acid), or mixtures
thereof and therebetween, and rusic acid.
Further, it is preferred that the second lipophilic
chain be an aliphatic chain, which preferably is not
significantly branched.
The hydrophilic species (R) of the preferred
emulsifying agent of the current invention is hydrogen or a
hydrophilic group. However, when x is 0, R may be a second
direct link to M1. When R is a hydrophilic group, it is
preferred that the group be polar in character and suitably
comprise an organic residue having a molecular weight not
exceeding 450 and preferably not exceeding 200. In
determining the aforementioned molecular weights any
contribution from an ionic moiety is to be disregarded.
The organic residue is desirably monomeric although
oligomeric groupings - containing, for example, not more
than about 10 repeat units - may be employed provided that
the molecular weight thereof is within the aforementioned
limit. In a preferred embodiment suitable monomeric
groupings may be chosen from the group comprising hydroxyl,
amino hydroxyl, alkyl hydroxy pyridine, alkyl hydroxy
pyrimidine and polyhydroxy carboxylic acid.
A preferred formula for R is -(CH2)jOH wherein j is 1,
2 or 3.
Preferably, either of the two linking moieties (M1 or
MZ) may comprise hydroxyl, amino, carboxylic acid or
carboxylic acid anhydride groups, and each acts to link
either the first and second lipophilic chains to the
hydrophilic moiety.
Conveniently, the first linking moiety (M1) and the
first lipophilic chain (L1) may be present in the same
species. For example, the first linking moiety and the
first lipophilic chain may both be a poly[alk(en)yl]
succinic anhydride based compound (or its acid form) in
which a lipophilic carbon chain is terminated by a succinic
anhydride linking moiety. A preferred material of use in
ICICAN 816
~1~2~1
--8--
this embodiment is a polyisobutylene succinic anhydride
based material.
Similarly, the second linking moiety (M2) and the
hydrophilic species may be combined into a common species.
For example, the second linking moiety and the hydrophilic
species may form a material, such as, a dialkanolamine.
It is preferred that the emulsifying agent of the
current invention comprises ester linkages between the
second linking moiety (M2) and the second lipophilic chain
(L2). It is also preferred that the emulsifying agent
comprises ester, amide or imide linkages between the first
linking moiety (M1) and the first lipophilic chain (L1).
Preferably, M1 has one of the following formulas:
=~ \ _ O -- ( CH2 ) n
OH O
when x is 0
~ O in Formula I
or
o
OH O
Preferably, M2 has the formula:
-- O -- C
\
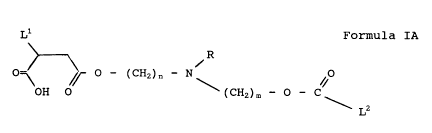
One particularly preferred emulsifying agent has the
formula IA:
L1 Formula IA
~ R
O=\ \j~ O ~ ( CH2 ) n ~ N /O
OH O (CH2) m ~ O ~ C
L2
ICICAN 816
216~
g
where L1, L2 and R are as defined hereinabove, and n is
2 1, and m 2 0.
Further, one particularly preferred emulsifying agent
has the formula IA shown above, wherein L1 and M1 combined
are the residual of polyisobutylene succinic anhydride
having a backbone structure of less than 500 carbon atoms
in the polyisobutylene portion, n and m are 1, R is
hydrogen or -(CH2)jOH wherein j is 1, 2 or 3, and L2 is
linoleic acid or linolenic acid, or mixtures thereof, or
rusic acid.
Formation of the emulsifying agent of the current
invention may be effected by conventional procedures
depending upon the chemical nature of the lipophilic chains
and hydrophilic species involved. Commonly this would
involve (1) reaction of the first lipophilic chain with the
first linking moiety, (2) condensation of the hydrophilic
species containing the second linking moiety, with the
first linking moiety and (3) derivatization of the second
lipophilic chain with the second linking moiety.
For example where the first lipophilic chain and first
linking moiety together comprise a poly[alk(en)yl] succinic
anhydride based compound and the hydrophilic species/second
linking moiety together are a diaIkanolamine the anhydride
group can be caused to react with the hydroxyl or amino
group by heating the two components together in a suitable
solvent, in the presence of a catalyst if desired. Where
the succinic anhydride and amino groupings are present in a
1:1 molar ratio there is imide/ amide formation. The
compound so formed may then be heated with a cis-
polyunsaturated fatty acid to promote esterification.
ICICAN 816 ~1624~1
--10--
This reaction sequence may be represented as follows:
-_D + ~O (C z)~ ~ (C~2)
L~ ,R
O~r ~ O -_ (C~Z~ 0
v ~ L cr~o H
C)--~ O-~C~ C~Z~--O-C~\LZ
wherein L1, L2, R, m and n are as previously defined.
The emulsifying agents of the present invention as
described hereinabove, are suitable for use in emulsion
explosive compositions. These compositions comprise a
continuous water-immiscible, hydrocarbon phase, a
discontinuous aqueous salt or eutectic phase and at least
one emulsifying agent, wherein the emulsifying agent is as
described hereinabove.
Typically the total emulsifier component of the
emulsion comprises up to 5% by weight of the emulsion. A
higher proportion of the emulsifier component may be used
and may serve as a supplemental fuel for the composition,
but in general it is not necessary to add more than 5% by
weight of emulsifier component to achieve the desired
effect. Stable emulsions can be formed using relatively
low levels of emulsifier component and for reasons of
economy it is preferable to keep to the minimum amounts of
emulsifier necessary to achieve the desired effect. The
preferred level of emulsifier component used in the
practise of the present invention is preferably in the
range of from 0.4 to 3.0% by weight of the emulsion, and
more preferably in the range of between 1.5 to 2.5% by
weight.
ICICAN 816 21~2~1
The remaining components of the emulsion explosive
composition are described in detail in the prior art.
However, the following describes, in general, typical
formulation parameters for emulsion explosives.
The oxidizer salt for use in the discontinuous phase
of the emulsion is preferably selected from the group
consisting of ammonium and alkali and alkaline earth metal
nitrates and perchlorates and mixtures thereof. It is
particularly preferred that the oxidiser salt is ammonium
nitrate or a mixture of ammonium nitrate and sodium nitrate
or calcium nitrate.
The oxidiser salt for use in the discontinuous phase
of the emulsion may further contain a melting point
depressant. Suitable melting point depressants for use
with ammonium nitrate in the discontinuous phase include
inorganic salts such as lithium nitrate, sodium nitrate,
potassium nitrate; alcohols such as methyl alcohol,
ethylene glycol, glycerol, mannitol, sorbitol,
pentaerythritol; carbohydrates such as sugars, starches and
dextrins; aliphatic carboxylic acids and their salts such
as formic acid, acetic acid, ammonium formate, sodium
formate, sodium acetate, and ammonium acetate; glycine;
chloracetic acid; glycolic acid; succinic acid; tartaric
acid; adipic acid; lower aliphatic amides such as
formamide, acetamide and urea; urea nitrate; nitrogenous
substances such as nitroguanidine, guanidine nitrate,
methylamine nitrate, and ethylene diamine dinitrate; and
mixtures thereof.
Typically the discontinuous phase of the emulsion
comprises 60 to 97% by weight of the emulsion explosive,
and preferably 86 to 95% by weight of the emulsion
explosive.
The continuous water-immiscible phase of the emulsion
comprises an organic fuel. Preferred organic fuels for use
in the continuous phase include aliphatic, alicyclic and
aromatic compounds and mixtures thereof which are in the
liquid state at the formulation temperature. Suitable
organic fuels may be chosen from fuel oil, diesel oil,
ICICAN 816
2162411
-12-
distillate, furnace oil, kerosene, naphtha, waxes (e.g.
microcrystalline wax, paraffin wax and slack wax), paraffin
oils, benzene, toluene, xylenes, asphaltic materials,
polymeric oils such as the low molecular weight polymers of
olefins, animal oils, fish oils, vegetable oils, corn oil
and other mineral, hydrocarbon or fatty oils, and mixtures
thereof. Preferred organic fuels are liquid hydrocarbons
generally referred to as petroleum distillate, such as
gasoline, kerosene, fuel oils and paraffin oils.
Typically, the continuous water-immiscible fuel phase
of the emulsion (including emulsifier) comprises more than
3 to less than 30% by weight of the emulsion, and
preferably from 5 to 15% by weight of the emulsion. For
acceptable production properties, however, the total
continuous water-immiscible fuel phase of the prior art
emulsions typically comprised greater than 5% by weight of
the emulsion in order to effectively process the emulsion
in a "Jet" mixer commonly used for production.
Because of the improved emulsifying efficiency (the
ability of emulsifying agent to form an emulsion) of the
emulsifiers of the present invention, however, it is now
possible to reduce the emulsifying agent level of the
emulsion, and thus lower the level of total fuel phase
required for production processing of the emulsion.
Accordingly, with the emulsifiers of the present invention,
the amount of fuel oil required for acceptable production
properties is reduced, thereby creating emulsions having a
lower total fuel oil phase content. This permits the
practical production of emulsion explosives which are
essentially oxygen balanced (e.g. having less than 5% total
fuel oil phase content), or which are not significantly
oxygen deficient.
Accordingly, in a preferred embodiment, the emulsion
comprises a combined total level of the continuous water-
immiscible hydrocarbon phase and the emulsifying agent ofless than or equal to 5% by weight of the emulsion.
If desired, optional additional fuel materials
hereinafter referred to as secondary fuels may be mixed
ICICAN 816 2162411
into the emulsion. Examples of such secondary fuels
include finely divided materials such as: sulphur,
aluminium, carbonaceous materials such as gilsonite,
comminuted coke or charcoal, carbon black, resin acids such
as abietic acid, sugars such as glucose or dextrose and
other vegetables products such as starch, nut meal, grain
meal and wood pulp; and mixtures thereof.
Typically, the optional secondary fuel component of
the emulsion is used in an amount up to 30% by weight based
on the weight of the emulsion.
The emulsion may be sensitised to provide an emulsion
explosive by combination with a self-explosive compound or
composition or by the addition of finely dispersed voiding
agents. Voiding agents may also be used to vary the
density and/or the sensitivity of an explosive composition.
For example, the explosive composition may comprise a
discontinuous gaseous component as a voiding agent.
Methods of incorporating a gaseous component and the
enhanced sensitivity of explosive compositions comprising
gaseous components are well known to those skilled in the
art. The gaseous components may, for example, be
incorporated into the explosive composition as fine gas
bubbles dispersed through the composition, as hollow
particles which are often referred to as microballoons or
microspheres, as porous particles of e.g. perlite or
mixtures thereof.
A discontinuous phase of fine gas bubbles may be
incorporated into the explosive composition by mechanical
agitation, injection or bubbling the gas through the
composition or by chemical generation of the gas in situ.
Suitable chemicals for the in situ generation of gas
bubbles include peroxides, such as hydrogen peroxide,
nitrites, such as sodium nitrite, nitrosoamines, such as
N,N'-dinitrosopentamethylenetetramine, alkali metal
borohydrides, such as sodium borohydride, and carbonates,
such as sodium carbonate. Preferred chemicals for the in
situ generation of gas bubbles are nitrous acid and its
salts which decompose under conditions of acid pH to
ICICAN 816 2162~1~
produce nitrogen gas bubbles. Preferred nitrous acid salts
include alkali metal nitrites, such as sodium nitrite.
These can be incorporated as an aqueous solution, a pre-
emulsified aqueous solution in an oil phase or as a water-
in-oil micro emulsion comprising oil and nitrite solution.
Catalytic agents such as thiocyanate or thiourea may be
used to accelerate the decomposition of a nitrite gassing
agent. Suitable small hollow particles include small
hollow microspheres of glass or resinous materials, such as
phenol-formaldehyde, urea-formaldehyde and copolymers of
vinylidene chloride and acrylonitrile. Suitable porous
materials include expanded minerals such as perlite and
expanded polymers such as polystyrene.
Gas bubbles may also be added to the emulsion as a
preformed foam of air, carbon dioxide, nitrogen or nitrous
oxide in liquid, preferably an oil phase.
As described hereinabove, preferred emulsion explosive
compositions formed using the emulsion of the current
invention are preferably oxygen balanced or not
significantly oxygen deficient. Additional components may
be added to the explosive composition to control the oxygen
balance of the explosive composition such as solid
particulate ammonium nitrate as powder or porous prill.
The emulsion may also be blended with ANFO.
The emulsions and emulsion explosives of the present
invention are, preferably made by preparing a first premix
of water and inorganic oxidiser salt and a second premix of
fuel/oil and a mixture of the surfactant and co-surfactant
(if desired) in accordance with the present invention. The
aqueous premix is heated to ensure dissolution of the salts
and the fuel premix is heated as may be necessary to
provide liquidity. The premixes are blended together and
emulsified. Common emulsification methods use a mechanical
blade mixer, rotating drum mixer, or a passage through an
in-line static mixer. Thereafter, the property modifying
materials such as, for example, glass microspheres, may be
added along with any auxiliary fuel, e.g. aluminium
particles or any desired particulate ammonium nitrate.
ICICAN 816 21 6 2 411
Accordingly in further aspect, the present invention
provides a method of manufacturing an emulsion explosive
comprising emulsifying an oxidiser salt phase into an
emulsifier/fuel mixture using the emulsifying agent of the
current invention and then adding a sensitising agent.
In a further aspect, the present invention also
provides a method of blasting comprising placing an
emulsion explosive as described hereinabove in operative
contact with an initiating system including a detonator and
initiating said detonator and thereby said emulsion
explosive.
Examples
The emulsifier invention of the current application
will now be further explained with reference to the
following examples:
Example l
Triethanolamine (32 parts by volume) was added to
polyiso-butylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Linoleic acid (55 parts) was slowly added to the
hot reaction mixture and stirring continued at 100~C for a
further 1 hour until completion of the reaction. Paraffin
oil (120 parts by volume) was then added to the reaction to
form a 50% active emulsifying agent.
Example 2
Diethanolamine (32 parts by volume) was added to
polyiso-butylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Linoleic acid (55 parts) was slowly added to the
hot reaction mixture and stirring continued at 100~C for a
further 1 hour until completion of the reaction. Paraffin
oil (120 parts by volume) was then added to the reaction to
form a 50% active emulsifying agent.
ICICAN 816 21624~1
.
-16-
Example 3
Aminopropylene glycol (20 parts by volume) was added
to polyisobutylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Linoleic acid (55 parts) was slowly added to the
hot reaction mixture and stirring continued at 100~C for a
further 1 hour until completion of the reaction. Paraffin
oil (120 parts by volume) was then added to the reaction to
form a 50% active emulsifying agent.
Example 4
Triethanolamine (32 parts by volume) was added to
polyiso-butylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Linolenic acid (55 parts) was slowly added to
the hot reaction mixture and stirring continued at 100~C
for a further 1 hour until completion of the reaction.
Paraffin oil (120 parts by volume) was then added to the
reaction to form a 50% active emulsifying agent.
Comparative Example 5 (CE 5)
Triethanolamine (32 parts by volume) was added to
polyiso-butylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Oleic acid (55 parts) was slowly added to the
hot reaction mixture and stirring continued at 100~C for a
further 1 hour until completion of the reaction. Paraffin
oil (120 parts by volume) was then added to the reaction to
form a 50% active emulsifying agent.
Comparative Example 6
Triethanolamine (32 parts by volume) was added to
polyiso-butylene succinic anhydride (300 parts by volume;
ex-Mobil) at 100~C and the components stirred together for
one hour. Stearic acid (55 parts) was slowly added to the
hot reaction mixture and stirring continued at 100~C for a
further 1 hour until completion of the reaction. Paraffin
oil (120 parts by volume) was then added to the reaction to
ICICAN 816 216241 i
-17-
form a 50% active emulsifying agent.
Example 7
A water-in-oil emulsion was formed by slowly adding
the emulsifying agent of Example 1 and fuel oil mixture to
an aqueous ammonium nitrate solution. The emulsion formed
was of the following composition:
Component Proportion (wt/%)
Example 7A Example 7B
ammonium nitrate 79.05 79.05
water 16.20 16.20
emulsifying agent:
as per Example 1 2.30
as per Example 4 2.30
fuel oil 2.45 2.45
~5 Comparative Example 8
A water-in-oil emulsion was formed by slowly adding
the emulsifying agent of Comparative Example 5 and fuel oil
mixture to an aqueous ammonium nitrate solution. The
emulsion formed was of the following composition:
Component ProPortion (wt/%)
ammonium nitrate 79.05
water 16.20
emulsifying agent:
as per CE 5 2.30
fuel oil 2.45
ICICAN 816
21~2411
-18-
Comparative Example 9
A water-in-oil emulsion was formed by slowly adding
the emulsifying agent of Comparative Example 6 (CE6) and
fuel oil mixture to an aqueous ammonium nitrate solution.
The emulsion formed was of the following composition:
Component Proportion (wt/%)
ammonium nitrate 79.05
water 16.20
emulsifying agent 2.30
(as per CE6)
fuel oil 2.45
The water-in-oil emulsions of Examples 7A and 7B, and
Comparative Examples 8 and 9 were subjected to microscopic
and physical examination and the results are recorded in
Table 1.
ICICAN 816
~162~11
--19--
TABLE 1
Example No.
Criterion 7A 7B CE 8 CE 9
Unsaturation 2 3 1 0
level of L2
in Formula 1
Droplet Size<2 <2 approx >3
(micron) 2
Viscosity 272,000300,000 252,000247,000
(cps)
Conductivity<100 <100 >1000 >2000
(pmho/m)
Interfacial approx. approx. approx. approx.
Tension16.5 16.5 16.5 16.5
(dynes/cm)
Stability2 v. good v. good good poor
(at 6 weeks)
Stability good at good at fair at poor at
with AN36 weeks 6 weeks 4 weeks 1 week
Notes: (1 - viscosity measured at 20~C using a number 7
brookfield spindle at 50 rpm)
(2 - stability measured by microscopically examining
emulsion stored at ambient temperature)
(3 - stability of emulsions mixed with 30% by weight
ammonium nitrate prill)
ICICAN 816
- 2162411
-20-
The emulsions of Examples 7A and 7B comprise
emulsifiers having a second lipophilic chain (L2) derived
from polyunsaturated fatty acids, having olefinic
unsaturation, such as linoleic acid or linolenic acid. The
emulsion of Comparative Examples 8 and 9 comprise an
emulsifier having a second lipophilic chain (L2) derived
from a mono-unsaturated fatty acid (oleic acid) or a
saturated fatty acid (stearic acid). The results recorded
in Table 1 illustrate the superior storage stability of
emulsions of the current invention even though the three
examples all have essentially constant interfacial tension.
Comparative Example 10
A water-in-oil emulsion was formed by slowly adding an
emulsifying agent of the prior art and fuel oil mixture to
an aqueous ammonium nitrate solution. The emulsifying
agent of the prior art comprised a condensation product of
the reaction of succinic anhydride with an alpha-olefin
mixed with sorbitan mono-oleate. The emulsion formed was
of the following composition:
ComponentProportion (wt/%)
ammonium nitrate76.20
water 19.05
emulsifying agent 2.30
fuel oil 2.45
Comparative Example 11
A water-in-oil emulsion was formed by slowly adding an
emulsifying agent of the prior art comprising a
condensation product of the reaction of succinic anhydride
with an alpha-olefin and fuel oil mixture to an aqueous
ammonium nitrate solution. The emulsion formed was of the
following composition:
ICICAN 816 ~16~;41~
-21-
ComponentProportion (wt/%
ammonium nitrate 73.90
water 18.50
emulsifying agent 2.30
fuel oil 5.30
The emulsion of Example 7A which comprises the
emulsifying agent of the current invention were compared
with the emulsions of Comparative Examples CE10 and CE11
which comprise emulsifying agents of the prior art. The
emulsions were hand mixed with ammonium nitrate prills and
then subjected to a higher shear stress by mixing at about
60 rpm in a Hobart mixer at 60~C for 5 minutes. The mixed
material was stored at ambient temperature and periodically
subjected to photomicroscopic examination. The results are
shown in Table 2. It can be seen that the emulsion of
Example 7 showed greater stability than the emulsion of
either CE10 or CEll.
Table 2: Stability with Ammonium Nitrate
Rate of Emulsion Formulation*
Crystallization**
Ex. 7 CE 10 CE 11
Time: 2 days 1 4.5 1.5
1 week 1.5 5 1.5
2 weeks 1.5 5 2
* - Photomicroscopically examined emulsion mixed with
30% by weight ammonium nitrate prill
** - Rate of Crystallization (0 = no crystallization,
5 = completely crystallized)
Having described specific embodiments of the present
invention, it will be understood that modifications thereof
may be suggested to those skilled in the art, and it is
intended to cover all such modifications as fall within the
scope of the appended claims.