Note: Descriptions are shown in the official language in which they were submitted.
WO 94t29309 PCT/US94/0~45
2 1 63q95
TITLE OF THE INVENTION
SPIRO-SUBSTITUTED AZACYCLES AS NEUROK~IN
ANTAGONISTS
5 BACKGROUND OF THE INVENTION
The invention disclosed herein is directed to certain spiro-
substituted azacycles useful as tachykinin receptor antagonists. In
particular, the compounds disclosed herein are neurokinin receptor
antagonists.
The tachykinins, substance P (SP), neurokinin A (NKA)
and neurokinin B (NKB), are structurally similar members of a family
of neuropeptides. Each of these is an agonist of the receptor types,
neurokinin-1 receptor (NK-1), neuorokinin-2 receptor (NK-2) and
neuorokinin-3 receptor (NK-3), which are so defined according to their
5 relative abilities to bind tachykinins with high affinity and to be
activated by the natural agonists SP, NKA and NKB respectively.
The tachykinins are distinguished by a conserved carboxyl-
terminal sequence Phe-X-Gly-Leu-Met-NH2. More specifically,
substance P is a pharmacologically-active neuropeptide that is produced
20 in mamm~ls and possesses a characteristic amino acid sequence:
Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2
Neurokinin A possesses the following amino acid sequence:
His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu-Met-NH2.
Neurokinin B possesses the following amino acid sequence:
3 Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2.
(Chang et al., Nature New Biol. 232, 86 (1971); D.F. Veber et al., U.s.
Patent No. 4,680,283).
WO 94/29309 PCTIUS94/05545
21 639~5
The neurokinin receptors are widely distributed throughout
the m~mm~lian nervous system (especially brain and spinal ganglia), the
circulatory system and peripheral tissues (especially the duodenum and
jejunum) and are involved in regulatmg a number of diverse biological
processes. This includes sensory perception of olfaction, vision,
audition and pain, movement control, gastric motility, vasodilation,
salivation, and micturition (B. Pernow, Pharmacol. Rev., 1983, 35, 85-
141). The NK1 and NK2 receptor subtypes are implicated in synaptic
transmission (Laneuville et al., Life Sci., 42: 1295-1305 (1988)).
Substance P acts as a vasodilator, a depressant, stimulates
salivation and produces increased capillary permeability. It is also
capable of producing both analgesia and hyperalgesia in animals,
depending on dose and pain responsiveness of the ~nim~l (see R.C.A.
Frederickson et al., Science, 199, 1359 (1978); P. Oehme et al., Science,
208, 305 (1980)) and plays a role in sensory transmission and pain
perception (T.M. Jessell, Advan. Biochem. Psychopharmacol. 28, 189
(1981)). In particular, substance P has been shown to be involved in the
tr~nsmission of pain in migraine (see B.E.B. Sandberg et al., Journal of
Medicinal Chemistry, 25, 1009 (1982)), and in arthritis (Levine et al.
Science, (1984) 226, 547-549).
In the airways, it has been indicated that NK1 receptors are
associated with microvascular leakage and mucus secretion, while NK2
receptors regulate smooth muscle contraction. Also, it has been shown
that both substance P and neurokinin A are effective in inducing airway
constriction and edema. Based on such findings, it is believed that
substance P and neurokinin A may be involved in the pathogenesis of
neurogenic infl~mm~tion, including allergic diseases such as asthma.
(Frossard et al., Life Sci., 49, 1941-1953 (1991); Advenier, et al.,
Biochem. Biophys. Res. Comm., 184(3), 1418-1424 (1992)).
In experimental studies, sensory neuropeptides, especially
tachykinins such as substance P and neurokinin A, can bring about many
of the pathophysiological features of asthma. Neurokinin A is a very
potent constrictor of human airways in vitro, and substance P causes
mucus secretion in the airways. (Barnes P.J., Lancet, pp 242-44 (1986);
WO 94/29309 PCT/US94/05545
2 1 63995
Rogers D.R., Aursudkij B., Barnes P.J., Euro. J. Pharmacol, 174, 283-
86 (1989)).
Inhalation of bradykinin causes bronchoconstriction in
asthmatic patients but not in normal subjects. (Fuller R.W., Dixon
C.M.S., Cuss F.M.C., Barnes P.J., Am Rev Respir Dis, 135, 176-80
(1987)). Since the bradykinin-induced bronchoconstriction is partly
opposed by anticholinergic agents and since bradykinin is only a weak
constrictor of human airways in vitro, it has been suggested that the
bronchoconstrictor response is partly mediated by a neural reflex.
Bradykinin stimulates vagal afferent C fibers and causes broncho-
constriction in dogs. (Kaufman M.P., Coleridge H.M., Coleridge
J.C.G., Baker D.G., J. Appl. Physio., 48, 511-17 (1980)). In guinea-pig
air~vays, bradykinin causes a bronchoconstrictor response by way of
cholinergic and sensory-nerve-mediated mech~ni~m.s. (Ichinoe M.,
Belvisi M.G., Barnes P.J., J. Pharmacol. Exp. Ther., 253, 594-99
(1990). Bradykinin-induced bronchoconstriction in human airways may
therefore be due partly to tachykinin released from sensory nerve
terminals via axon reflex mech~ni~m~. Clinical trials have shown that a
dual NK-1/NK-2 antagonist (such as FK-224) protects against
bradykinin induced bronchocontriction in asthmatic patients. (Ichinoe,
M. et al., Lancet,, vol. 340, pp 1248-1251 (1992)).
The tachykinins have also been implicated in gastro-
intestinal (GI) disorders and diseases of the GI tract, such as
infl~mm~tory bowel disease, ulcerative colitis and Crohn's disease, etc.
(see Mantyh et al., Neuroscience, 25 (3), 817-37 (1988) and D. Regoli
in "Trends in Cluster Headache" Ed. F. Sicuteri et al., Elsevier
Scientific Publishers, Amsterdam, 1987, pp. 85-95).
It is also hypothesized that there is a neurogenic mechanism
for arthritis in which substance P may play a role (Kidd et al., "A
Neurogenic Mech~nism for Symmetric Arthritis" in The Lancet, 11
November 1989 and Gronblad et al., "Neuropeptides in Synovium of
Patients with Rheumatoid Arthritis and Osteoarthritis" in J. Rheumatol.
(1988) 15(12) 1807-10). Therefore, substance P is believed to be
involved in the inflammatory response in diseases such as rheumatoid
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/0554~
arthritis and osteoarthritis (O'Byrne et al., in Arthritis and Rheumatism
(1990) 33, 1023-8). Other disease areas where tachykinin antagonists
are believed to be useful are allergic conditions (Hamelet et al., Can. J.
PhaImacol. Physiol. (1988) 66, 1361-7), immunoregulation (Lotz et al.,
Science (1988) 241, 1218-21, Kimball et al., J. Immunol. (1988) 141
(10) 3564-9 and A. Perianin, et al., Biochem. Biophys. Res. Commun.
161, 520 (1989)) vasodilation, bronchospasm, reflex or neuronal
control of the viscera (Mantyh et al., PNAS (1988) 85, 3235-9) and,
possibly by arresting or slowing ~-amyloid-mediated neurodegenerative
changes (Yankner etal., Science, (1990) 250, 279-82) in senile dementia
of the Alzheimer type, Alzheimer's disease and Downs Syndrome.
Substance P may also play a role in demyelinating diseases such as
multiple sclerosis and amyotrophic lateral sclerosis (J. Luber-Narod
et al., poster presented at C.I.N.P. XVIIIth Congress, 28th June-2nd
July, 1992). Antagonists selective for the substance P and/or the
neurokinin A receptor may be useful in the treatment of asthmatic
disease (Frossard et al., Life Sci., 49, 1941-1953 (1991); Advenier, et
al., Biochem. Biophys. Res. Comm., 184(3), 1418-1424 (1992)).
SUMMARY OF THE INVENTION
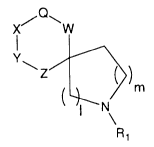
This invention is directed to compounds of forrnula I.
X' W
~) m
R1
The invention is also concerned with pharmaceutical
folmulations with these novel compounds as active ingredients and the
use of the novel compounds and their formulations in the treatment of
certain disorders.
WO 94/29309 PCT/US94/05545
21 6~995
The compounds of this invention are tachykinin receptor
antagonists and are useful in the treatment of infl~mm~tory diseases,
pain or migraine and asthma.
5 DETAILED DESCRIPTION OF THE INVENTION
This invention is directed to compounds of formula I.
X' `W
o ~Z~) m
I N
R1
or a pharmaceutically acceptable salt thereof,
wherein the nitrogen expressly shown above is optionally quaternized
with Cl 4alkyl or phenylCl 4alkyl or is optionally present as the N-
oxide (N+O-), and wherein:
20 l and m are each independently 0, 1, 2, 3, 4, or 5, with the proviso that
1 +misequalto 1,2,3,4,or5;
R1 is selected from a group consisting of:
( 1 ) hydrogen,
(2) linear or brarlched C1 8 alkyl, linear or branched C2 8
alkenyl, or linear or branched C2 8 alkynyl, wherein the
C1 8 alkyl, C2 g alkenyl or C2 g alkynyl is optionally
mono, di, tri or tetra substituted, the substitutents
independently selected from:
3 0 (a) hydroxy,
(b) oxo,
(c) cyano,
(d) halogen which is defined to include Br, Cl, I, and F,
(e) trifluoromethyl,
WO 94/29309 2 1 6 3 q ~ 5 PCT/US94/05545
(f) phenyl or mono, di or trisubstituted phenyl, the
substitutents independently selected from
( 1 ) phenyl,
(2) hydroxy,
(3) Cl 3alkyl,
(4) cyano,
(5) halogen,
(6) trifluoromethyl,
(7) -NR6COR7, wherein R6 and R7 are
o independently selected from:
(a) hydrogen,
(b) C1 6 alkyl, or mono or disubstituted C1 6
alkyl, the substitutents independently selected
from
(1) phenyl,unsubstituted or substituted
with hydroxy, C1 3alkyl, cyano,
halogen, trifluoromethyl or
C1 4alkoxy,
(2) hydroxy,
(3) oxo,
(4) cyano,
(S) halogen,
(6) trifluoromethyl,
(c) phenyl, pyridinyl or thiophene or mono, di or
trisubstituted phenyl, pyridinyl or thiophene,
the substitutents independently selected from
( 1 ) hydroxy,
(2) Cl 4alkyl,
(3) cyano,
3 (4) halogen,
(5) trifluoromethyl,
(d) C1 3alkyloxy,
or
WO 94/2930g PCTIUS94/05545
2 1 639~5
R6 and R7 are joined together to form a 5-, 6-,
or 7-membered monocyclic saturated ring containing
1 or 2 heteroatoms independently selected from
nitrogen, oxygen, and sulfur, and in which the ring is
unsubstituted or mono or disubstituted, the
substituents independently selected from
(a) hydroxy,
(b) oxo,
(c) cyano,
o (d) halogen,
(e) trifluoromethyl,
(8) -NR6C02R7,
(9) -NR6CONHR7,
(10) -NR6S(O)jR7, wherein j is 1 or 2,
(11) -CONR6R7,
(1 2) -COR6,
(1 3) -C02R6,
(14) -OR6,
(15) -S(O)kR6 wherein k is 0, 1 or 2,
(16) heteroaryl, wherein heteroaryl is selected from
the group consisting of:
( 1 ) benzimidazolyl,
(2) benzofuranyl,
(3) benzoxazolyl,
2 5 (4) furanyl,
(5) imidazolyl,
(6) indolyl,
(7) isoxazolyl,
(8) isothiazolyl,
3 (9) oxadiazolyl,
(10) oxazolyl,
( 1 1 ) pyrazinyl,
(12) pyrazolyl,
(1 3) pyridyl,
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
(14) pyrimidyl,
(1 S) pyrrolyl,
( 16) quinolyl,
(17) tetrazolyl,
(18) thiadiazolyl,
(1 9) thiazolyl,
(20) thienyl, and
(21 ) triazolyl,
wherein the heteroaryl is unsubstituted or mono, di
or trisubstituted, the substituents independently
selected from,
(a) hydroxy,
(b) oxo,
(c) cyano,
s (d) halogen,
(e) trifluoromethyl,
(g) -NR6R7,
(h) -NR6COR7,
(i) -NR6C02R7,
(j) -NR6CONHR7,
(k) -NR6S(O)jR7,
(I) -CONR6R7,
(m) -COR6,
(n) -C02R6,
2 5 (o) -OR6,
(p) -S(O)kR6,
(q) heteroaryl, wherein heteroaryl is selected from the
group consisting of:
( 1 ) benzimidazolyl,
3 (2) benzofuranyl,
(3) benzoxazolyl,
(4) furanyl,
(5) imidazolyl,
(6) indolyl,
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- (7) isoxazolyl,
(8) isothiazolyl,
(9) oxadiazolyl,
(10) oxazolyl,
(11) pyrazinyl,
(12) pyrazolyl,
(1 3) pyridyl,
( 14) pyrimidyl,
(15) pyrrolyl,
o (16) quinolyl,
(1 7) tetrazolyl,
(1 8) thiadiazolyl,
(19) thiazolyl,
(20) thienyl,
(21) triazolyl,
wherein the heteroaryl is unsubstituted or mono di or
trisubstituted, the substituents independently selected
from
( 1 ) phenyl,
2 o (2) hydroxy,
(3) oxo,
(4) cyano,
(5) halogen,
(6) trifluoromethyl,
wherein the nitrogen of definition R1 2(g) as defined above is optionally
quaternized with C1 4alkyl or phenylC1 4alkyl or is optionally present
as the N-oxide (N+O-);
W is selected from the group consisting of
3 ( 1 ) a covalent bond
(2) C1-3 alkyl, unsubstituted or substituted with
(a) oxo,
(b) hydroxy
(C) OR6,
WO 94/29309 PCT/US94/05545
21 63995
- 10 -
(d) halogen,
(e) trifluoromethyl,
(f) phenyl or mono, di or trisubstituted phenyl,
the substitutents independently selected from
( 1 ) hydroxy,
(2) cyano,
(3) halogen,
(4) trifluoromethyl,
3) S(O)k,
(4) (C1-3 alkyl)-S(O)k,
(5) S(O)k-(C1 -2 alkyl),
(6) S(O)k-NH,
(7) S(O)j-NH(C1 -2 alkyl),
(8) S(O)j-NR6,
(9) S(O)j-NR6-(C1-2 alkyl),
(10) CONH,
(11) CONH-(C1-2 alkyl),
(12) CONR6,
( 13) CONR6-(C 1-2 alkyl),
(14) CO2,
( 15) CO2-(C 1-2 alkyl);
Q = NR2, O, S, S(O), or SO2, with the proviso that when W is a
covalent bond and X is C1 3alkyl, then Q must be NR2;
R2 is selected from a group consisting of:
2 5 ( 1 ) hydrogen,
(2) C1-g linear or branched aLkyl, unsubstituted,
monosubstituted or multiply substituted with
(a) -OR6,
(b) = o,
3 0 (c) -NHCOR6,
(d) -NR6R7,
(e) -CN,
(f) -halogen,
(g) -CF3,
WO 94/29309 PCT/US94/05545
2 1 639~
(h) -phenyl, unsubstituted or substituted, wherein
the substitutents are selected from the group
consisting of
( 1 ) hydroxy,
(2) cyano,
(3) halogen,
(4) trifluoromethyl,
(3) S(O)Rg, wherein R8 is C1-6 linear or branched alkyl,
unsubstituted, mono di or trisubstituted with
o (a) hydroxy,
(b) oxo,
(c) cyano,
(d) -OR6,
(e) -NR6R7,
(f) -NR6COR7,
(g) -halogen,
(h) -CF3,
(i) -phenyl or mono, di or trisubstituted phenyl, the
substituents independently selected from
2 o ( 1 ) hydroxy,
(2) oxo,
(3) cyano,
(4) -NHR6,
(5) -NR6R7,
2 5 (6) -NR6COR7,
(7) -halogen,
(8) -CF3, and
(9) Cl 3 alkyl,
(4) S02R8,
3 0 (5) COR8,
(6) C02R8;
(7) CONR7R8;
X is selected from the group consisting of
WO 94129309 PCT/US94/05545
21 63'3~
( 1 ) a covalent bond
(2) C1 3 alkyl, unsubstituted or substituted with
(a) oxo,
(b) OR6,
(c) halogen,
(d) trifluoromethyl,
(e) phenyl or mono, di or trisubstituted phenyl,
the substitutents independently selected from
(1) OR6,
(2) halogen, and
(3) trifluoromethyl,
(3) S(O)k,
(4) (Cl -3 alkyl)S(O)k,
(5) S(O)k(C 1-2 alkyl),
(6) NHS(O)j,
(7) NH(C1-2 alkyl)S(O)j,
(8) S(O)jNR6,
(9) S(O)j-NR6-(C1-2 alkyl),
(10) NHCO,
(1 1 ) NHCO-(C1-2 alkyl),
(12) NR6CO,
(13) NR6-(Cl-2 alkyl)CO,
(14) O(CO), and
(15) (C1-2 alkyl)O(CO),
Y-Z considered together are 2 adjoining atoms of the ring
~Z
said ring being an phenyl, naphthyl or heteroaryl group, with the
heteroaryls selected *om the group consisting of:
( l ) benzimidazolyl,
(2) benzofuranyl,
(3) benzoxazolyl,
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
(4) furanyl,
(S) imidazolyl,
(6) indolyl,
(7) isoxazolyl,
(8) isothiazolyl,
(9) oxadiazolyl,
( 10) oxazolyl,
(11 ) pyrazinyl,
(12) pyrazolyl,
(13) pyridyl,
(14) pyrimidyl,
(15) pyrrolyl,
(16) quinolyl,
(1 7) tetrazolyl,
(18) thiadiazolyl,
(19) thiazolyl,
(20) thienyl,
(21 ) triazolyl,
and wherein the aryl or heteroaryl group is unsubstituted,
20 mono, di or tri substituted, the substitutents selected from:
(a) hydrogen,
(b) C1 6 alkyl, branched or unbranched, unsubstituted or
mono or disubstituted, the substituents being selected
from hydrogen and hydroxy,
2 5 (c) oxo
(d) OR6, wherein R6 is as defined immediately above,
(e) halogen,
(f) trifluoromethyl,
(g) nitro,
3 (h) cyano,
(i) NHR6,
(j) NR6R7~
(k) NHCOR6,
(1) NR6COR7,
WO 94/29309 PCT/US94/05545
21 6:~q5
- 14 -
(m) NHC02R6,
(n) NR6C02R7,
(o) NHS(0)jR6,
(p) NR6S(O)jR7,
(q) CONR6R7,
(r) C0R6,
(s) C02R6,
(t) S()jR6,
(u) heteroaryl, wherein heteroaryl is selected from the
group consisting of:
(a) benzimidazolyl,
(b) benzofuranyl,
(c) benzoxazolyl,
(d) furanyl,
(e) imidazolyl,
(f) indolyl,
(g) isoxazolyl,
(h) isothiazolyl,
(i) oxadiazolyl,
(j) oxazolyl,
(k) pyrazinyl,
(l) pyrazolyl,
(m) pyridyl,
(n) pyrimidyl,
2 5 (o) pyrrolyl,
(p) quinolyl,
(q) tetrazolyl,
(r) thiadiazolyl,
(s) thiazolyl,
3 (t) thienyl,
(u) triazolyl,
and wherein the heteroaryl is unsubstituted mono or
di substituted, the substitutents selected from
( 1 ) hydrogen,
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
(2) C1 6 alkyl, branched or unbranched, unsubstituted or
mono or disubstituted, the substituents being selected
from hydrogen and hydroxy,
(3) oxo,
(4) OR6,
(S) trifluoromethyl,
(6) nitro,
(7) cyano,
(8) NHR6,
o (9) NR6R7,
(10) NHCOR6,
( 1 1 ) NR6COR7,
( 12) NHC02R6,
(1 3) NR6C02R7,
(14) NHS(O)jR6,
(1 5) NR6S(O)jR7,
(16) CONR6R7,
(17) COR6,
(18) C02R6,
(19) S(O)jR6~ and
(20) phenyl.
One subclass of this invention consists of structures
tabulated below linked to R1 (as detailed immediately above) via the
25 broken bond, and optionally substituted at the positions indicated by
numbers 1-8 with
(a) hydroxy,
(b) oxo,
(c) cyano,
3 (d) -NR6R7,
(e) -NHCOR6R7,
(f) -halogen,
(g) -CF3,
WO 94/29309 PCT/US94/05545
21 639q5
- 16 -
(h) -phenyl or mono, di or trisubstituted phenyl, the
substituents independently selected from (a) through
(g) and Cl 3 alkyl;
the tabulated structures being
s
WO 94/29309 PCTIUS94/05545
21 63q95
~H ~,S~ Me ~)~H
4 ~?3 ~ ~
~ ~ ~r
0 0
~)~ Me ~ OMe~;
~e ~Me
~r ~
~N~Me ~N`Me ~,`Me
WO 94/29309 PCT/US94/05545
2 1 63~q5
- 18 -
Me Me
~N Me ~ ~N
~r ~
~r O
Me Me " Me
~ ~ N
1 5
Me 3~ ~
~ 5 ~ ~ ~S=O
4 ~3, 4 ~3' [~ [~
WO 94/29309 PCT/US94/05545
2 1 639~
- 19 -
~H ~' Me ~ H
~
/ N N / N
~Me ~)~OMe
~,r ~ ~/Me
N N N
~N~ Me ~ ` Me ~` Me
PCT/US94/05545
WO 94/29309
21 63q~5
- 20 -
Me Me 7
Me ~~O
Me Me
~ ~ ~N
~ Me
2s ~~=O
/N /N /N /N
WO 94/29309 PCT/US94/0554;
2 1 639q5
~ ~0~ /,0
5 Ç~
O O
~ Me ~ OMe g~
1 1 1
~ O
~r
~ ~N~ Me ~N` Me ~ Me
WO 94/29309 PCT/US94/05545
21 63q95
Me O Me
~N~ Me
~r ~
Me Me 1l Me
~ N
Me
~ ~r
~r
N ~ ~ ~
WO 94/29309 PCT/US94/0554~
2 1 ~39~5
`"S"
o o
~ Me ~J~OMe ~_'
~ ~ ~
~e ~e ~e
~N~ Me ~N` Me ~ Me
WO 94/29309 PCT/US94/05545
21 639q5
- 24 -
~ ~N
~ ~r
Me Me R Me
N~ [ ~N N~ N~N
1 5 0
~ Me
~ ~r
25 ~ ~o
WO 94/29309 PCT/US94/05545
2 1 63995
- 25 -
O~. ~, ~
/~J~Me/~)~OMe I~N/
~ ~
MeMe Me
N~ ~ N~ N~
2 5 N~N ~ MeN~ ~N~ Me ~ ` Me
WO 94129309 21 6 39 q 5 PCT/US94/05545
- 26 -
Me Me
~, ~ N~l N/
~r ~Ar
~r
Me Me ,, Me
/ ~N ~s~N/
N~5 N~ N~,~,
~r ~r ~
O
~r ~r~r
~r
N~ N~ N/~ N~
~ ~r ~Ar
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- 27 -
O~ // )~
~N
~)~ Me ~ OMe
~Me ~ ~
~N~Me ~`Me g~ Me
~r ~r
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 28 -
Me Me
~~Me
~r
~r
Me Me 1l Me
~ 0 ~S
~r
25 ~ ~=O
WO 94129309 2 1 6 3 9 9 5 PCT/US94/05545
- 29 -
~S~ Me
~N ~ [~
~ N;~ ~;
Me Me Me
~0 ~ ~ O
~ ~r
~N~Me ~`Me $~ ~ `Me
WO 94/29309 PCT/US94/0554~
2 ~ 6;~9~5
- 30 -
N` Me ~ ~ $
~r ~
~r
3 Me Me R Me
1 1 1
// Me a O 0
~N ~ lNH
~r
25 ~ ~=O
WO 94/29309 PCT/US94/05545
2 1 63995
- 31 -
S~H ~N'S`Me ~N)~H
~N ~ ~
O O Me
~ ~
Me ~M
~r ~Ar
S~ S S S~
\~X~ Me ~N` M ;~N~ Me
WO 94/29309 21 6 3 9 9 5 PCT/US94/05545
- 32 -
Me b' Me
S~ ~ ~N
Me Me 1I Me
S~- O S~lS S~
~;~ Me
~r ~r
5~ S~ 5~ 5~=
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
N~ N~ 'S`Me N~N~H
~N ~ ~
O O
~N~ Me ~ OMe
~N N N
1 5
b~
~r ~nAr
~N5~N. ~ ~\ S
~ I I
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- 34 -
Me 3 Me
c~N`Me ~ ~N~
Me Me 1l Me
N~ N~ N~N
/
~r ~
~' N~
wo 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
As is clear from the examples and schemes, the designation:
~) l or ~) m
in formula I is interchangeable with (CH2)l or (CH2)m respectively. As
appreciated by those of skill in the art, halo as used herein are intended
to include chloro, fluoro, bromo and iodo.
Exemplifying the invention are the compounds of the examples
including the group consisting of
(a) 1'-(3-(S)-(3,4-dichlorophenyl)-4-(t-butoxycarbonyl
(methylamino)) butyl)-1-methanesulfonyl-spiro(indoline-3,4'-
piperidine);
(b) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine);
(c) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-
(benzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
2 o piperidine);
(d) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
bistrifluoromethylbenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3,4'-piperidine);
(e) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-
2 5 methylbenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine);
(f) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-
chlorobenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine);
3 o (g) 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-
trifluoromethyl-benzoyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3,4' piperidine);
(h) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dichloro-
benzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine);
WO 94/29309 PCT/US94/05545
21 63qq5
- 36 -
(i) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-
trifluoromethyl-phenylacetyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3 ,4 '-piperidine);
(j) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-
5 isopropyloxy-phenylacetyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3 ,4'-piperidine);
(k) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-
(benzenesulfonyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3 ,4'-piperidine);
(l) 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -benzyoxycarbonyl-spiro(indoline-3,4'-
piperidine);
(m) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,S-dimethyl-
benzoyl)(methylamino))butyl)-spiro(indoline-3 ,4'-piperidine);
(n) 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -propionyl-spiro(indoline-3 ,4'-
piperidine);
(o) 1'(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino)) butyl)-1-formyl-spiro(indoline-3,4'-
20 piperidine);
(p) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -t-butylcarbonyl-spiro(indoline-3 ,4'-
piperidine);
(q) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
2 5 benzoyl)(methylamino))butyl)- 1 -methylaminocarbonyl-spiro(indoline-
3,4'-piperidine);
(r) 1'(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -ethoxycarbonyl-spiro(indoline-3 ,4'-
piperidine);
3 (s) 1 '(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3 ,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -ethanesulfonyl-spiro(indoline-3,4'-
piperidine);
WO 94/29309 2 1 6 3 9 q ~ PCT/US94/05545
- 37 -
(t) 1'(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -i-propanesulfonyl-spiro(indoline-3,4'-
piperidine);
(u) 1'(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
5 benzoyl)(methylarnino))butyl)-1'-methyl-1-methanesulfonyl-spiro-
indoline-3,4'-piperidinium iodide;
(v) 1'-(3-(S)-(3,4-dichlorophenyl)-4-(N-(R or S)-(3-
methylbenzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-
spiro(indoline-3 ,4'-piperidine);
(w) 1'-(3-(S)-(3,4-dichlorophenyl)-4-(N-(R or S)-(3,5-
bis(trifluoromethyl)benzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-
spiro(indoline-3 ,4'-piperidine);
(x) 1'-(3-(S)-(3,4-dichlorophenyl)-4-(N-(R or S)-(3,5-
dimethylbenzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-spiro-
5 (indoline-3 ,4'-piperidine);
(y) 1'-(3-(S)-(3,4-dichlorophenyl)-4-(N-(R or S)-(3,5-
dichlorobenzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-spiro-
(indoline-3 ,4'-piperidine);
2 o (aa) 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3 ,5-
difluorobenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro-
(indoline-3 ,4 '-piperidine);
(ab) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-fluoro-5-
25 (trifluoromethyl)benzoyl)(methylamino))butyl)-1-methanesulfonyl-
spiro(indoline-3 ,4'-piperidine);
(ac) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(1-naphthoyl)-
(methylamino))butyl)- l -methanesulfonyl-spiro(indoline-3,4'-
piperidine);
3 (ad) 1 '-(2-((S)-(3 ,4-dichlorophenyl))- 1 -(N-(2-chloro-
phenylsulfonyl)-(methylamino))-4-butyl)- 1 -methylsulfonyl-spiro-
(indoline-3 ,4'-piperidine);
WO 94/29309 21 6 39 ~ 5 PCT/US94/05545
- 38 -
(ae) 1 '-(2-((S)-(3,4-dichlorophenyl))- 1 -(N-(3-chloro-
phenylsulfonyl)-(methylamino))-4-butyl)- 1 -methylsulfonyl-spiro-
(indoline-3 ,4'-piperidine);
(af) 1 '-(2-((S)-(3 ,4-dichlorophenyl))- 1 -(N-(4-chloro-
5 phenylsulfonyl)-(methylamino))-4-butyl)- 1 -methylsulfonyl-spiro-
(indoline-3,4'-piperidine);
(ag) 1 '-(2-((S)-(3,4-dichlorophenyl))- 1 -(N-(3,5-dichloro-
phenylsulfonyl)-(methylamino))-4-butyl)- 1 -methylsulfonyl-spiro-
(indoline-3 ,4'-piperidine);
(ah) 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3-fluoro-5-
(trifluoromethyl)benzoyl)(methylamino))butyl)- 1 -acetyl-spiro(indoline-
3,4'-piperidine);
(ai) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -acetyl-spiro(indoline-3 ,4'-piperidine).
(aj) 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-bromo-5-
methylbenzoyl)(methylarnino))butyl)- 1 -methanesulfonyl-spiro(indoline-
3 ,4' -piperidine);
(ak) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
dimethylbenzoyl)(methylamino))butyl)- 1 -(2-aminoacetyl)-spiro-
20 (indoline-3,4'-piperidine);
(al) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
dimethylbenzoyl)(methylamino))butyl)- 1 -methyl-spiro(indol-2-one-
3,4'-piperidine);
(am) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
2 5 dichlorobenzoyl)(methylamino))butyl)- 1 -methyl-spiro(isoindol- 1 -one-
3,4'-piperidine);
(an) 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)-spiro(2-oxo-tetrahydroquinoline-4,4'-
piperidine); and
3 (ao) 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3 ,5-dichloro-
benzoyl)(methylamino))butyl)- 1 -methyl-spiro(2-oxo-tetrahydro-
quinoline-4,4'-piperidine).
WO 94/29309 PCT/US94/05545
21 639~5
- 39 -
In an alternative embodiment the above compounds may be
co-administered with a ~2-agonist such as Bambuterol, US 4,419,364
issued to Draco on 12/6/83; Bitolterol mesylate, US 4,138,581 issued to
Sterling 2/6/79; Carbuterol, US 3,763,232 issued to SmithKline 10/2/73;
Clenbuterol, US 3,536,712 issued to Boehringer Ingelheim 10/27/70;
Dopexamine, US 4,645,768 issued to Fisons 2/24/87; Formoterol, US
3,994,974 issued to Yamanouchi 11/30/76; Mabuterol, US 4,119,710
issued to Boehringer Ingelheim 10/10/78; Pirbuterol hydrochloride US
3,700,681 issued to Pfizer 10/24/72; Procaterol hydrochloride US
4,026,897 issued to Otsuka 5/31/77; Ritodrine hydrochloride US
3,410,944 issued to North American Philips 11/12/68; Brosaterol, US
4,276,299 issued to Zambon 6/30/81 and US 4,520,200 issued to
Zambon 5/28/85; Cimaterol, US 4,407,819 issued to American
Cyanamid 10/4/83; Docarpamine, US 4,228,183 issued to Tanabe
10/14/80; Salmeterol, US 4,992,474 i~sued to Glaxo 2/21/91 and US
5,091,422 issued to Glaxo 2/25/92.
The compounds of formula I are particularly useful in the
treatment of diseases or conditions that are advantageously treated by
concomitant antagonism of both NK1 and NK2 receptors or NK1, NK2
and NK3 receptors. These diseases include neuropathy, such as diabetic
or peripheral neuropathy and chemotherapy-induced neuropathy;
asthma; osteoarthritis; rheumatoid arthritis; and migraine.
In a second alternative embodiment the compounds of
formula I may be co-~lmini~tered with another NKl or NK2 antagonist
such as those described in
Appln No. DO-139125, filed 08-Jun-78, Pub. 12-Dec-79; Appln No.
EP-82568, filed 22-Dec-81, Pub. 29-Jun-83; Appln No. EP-490379,
filed 13-Dec-90, Pub. 17-Jun-92; Appln No. EP-353732, filed 05-Aug-
88, Pub. 07-Feb-90; Appln No. EP- 161007, filed 13 -Jan-84, Pub. 13-
Nov-85; Appln No. EP-385-43, filed 28-Feb-89, Pub. 05-Sep-90; Appln
No. WO8301251, filed 09-Oct-81, Pub. 14-Apr-83; Appln No. BE-
894602, filed 09-Oct-81, Pub. 31-Jan-83; Appln No. DE3205991, filed
19-Feb-82, Pub. 01-Sep-83; Appln No. EP-327009, filed 02-Feb-88,
Pub. 09-Aug-89; Appln No. EP-336230, filed 05-Apr-88, Pub. 11 -Oct-
WO 94/29309 PCT/US94/05545
q q 5
- 40 -
89; Appln N o. 394989, filed 28-Apr-89, Pub. 31-Oct-90; Appln No.
AU9068010, filed 22-Dec-89, Pub. 27-Jun-91; Appln No. EP-482539,
filed 24-Oct-90, Pub. 29-Apr-92; Appln No. EP-443132, filed 10-Dec-
90, Pub. 28-Aug-91; Appln No. EP-498069, filed 21-Dec-90, Pub. 12-
Aug-92; Appln No. W09222569, filed 19-Jun-91, Pub. 23-Dec-92;
Appln No. JO4297492, filed 24-Oct-91, Pub. 21-Oct-92; Appln No.
US4997853, filed 02-Dec-88, Pub. 05-Mar-91; Appln No. EP-272929,
filed 24-Dec-86, Pub. 29-Jun-88; Appln No. EP-360390, filed 25-Jul-
88, Pub. 28-Mar-90; Appln No. US3862114, filed 22-Nov-71, Pub. 21-
Jan-75; Appln N o. EP-219258, filed 30-Sep-85, Pub. 22-Apr-87, Appln
N o. US4742156, filed 30-Sep-85, Pub. 03-May-88; Appln No. EP-
401177, filed 29-May-89, Pub. 05-Dec-90; Appln N o. W09202546,
filed 03-Aug-90, Pub. 20-Feb-92; Appln No. EP-176436, filed 26-Sep-
84, Pub. 02-Apr-86; Appln No. US4680283, filed 26-Sep-84, Pub. 14-
lS Ju1-87; Appln No. WO9220661, filed 22-May-91, Pub. 26-Nov-92;
Appln No. EP-520555, filed 24-Jun-91, Pub. 30-Dec-92; Appln No.
EP-347802, filed 20-Jun-88, Pub. 27-Dec-89; Appln No. EP-412542,
filed 10-Aug-89, Pub. 13-Feb-91; Appln No. W09005729, filed 23-
Nov-88, Pub. 31-May-90; Appln No. W09005525, filed 23-Nov-88,
Pub. 31-May-90; Appln No. EP-436334, filed 04-Jan-90, Pub. 10-Jul-
91; Appln No. WO9118878, filed 31-May-90, Pub. 12-Dec-91; Appln
N o. W 0 9118899, filed 01-Jun-90, Pub. 12-Dec-91; Appln No.
WO9201688, filed 23-Jul-90, Pub. 06-Feb-92; Appln No. W09206079,
filed 28-Sep-90, Pub. 16-Apr-92; Appln N o. WO9212152, filed 03-Jan-
91, Pub. 23-Ju1-92; Appln No. WO9212151, filed 10-Jan-91, Pub. 23-
Ju1-92; WO9215585, filed 01-Mar-91, Pub. 29-Apr-92; Appln No.
W0022-676, filed 22-May-91, Pub. 26-Nov-92; Appln No.
WO9221677, filed 31 -May-91, Pub. 10-Dec-92; Appln No.
WO9300331, filed 20-Jun-91, Pub. 07-Jun-93; Appln No. W09300330,
filed 21-Jun-91, Pub. 07-Jan-93; Appln No. WO9109844, filed l 1-Jul-
91, Pub. 11-Jul-91; Appln N o. EP-429366, filed 23-Nov-89, Pub. 29-
May-91; Appln No. EP-430771, filed 23-Nov-89, Pub. 05-Jun-91;
Appln No. EP-514274, filed 17-May-91, Pub. 19-Nov-92; Appln No.
EP-514276, filed 17-May-91, Pub. 19-Nov-92; Appln No. EP-514275,
WO 94/29309 2 1 6 3 ~ q 5 PCT/US94/0554~
- 41 -
filed 17-May-91, Pub. 19-Nov-92; Appln No. EP-514273, filed 17-
May-91, Pub. 19-Nov-92; Appln No. EP-428434, filed 06-Nov-89, Pub.
22-May-91; Appln No. EP-474561, filed 09-May-90, Pub. 11-Mar-92;
Appln No. EP-512901, filed 03-May-91, Pub. 11-Nov-92; Appln No.
EP-512902, filed 03-May-91, Pub. 11 -Nov-92; Appln No. EP-515240,
filed 03-May-91, Pub. 25-Nov-92; Appln No. US4472305, filed 17-
May-83, Pub. 18-Sep-84; Appln No. US4839465, filed 20-Jan-87, Pub.
13-Jun-89; Appln No. EP-101929, filed 28-Ju1-82, Pub. 07-Mar-84;
Appln No. WO9102745, filed 16-Aug-89, Pub. 07-Mar-91; Appln No.
US3912711, filed 03-Ju1-72, Pub. 14-Oct-75; Appln No. US4059693,
filed 11 -Jun-76, Pub. 22-Nov-77; Appln No. US4481139, filed 13-Apr-
83, Pub. 06-Nov-84; Appln No. US7358073, filed 24-Oct-88, Pub. 19-
Dec-89; Appln No. US7261627, filed 24-Oct-88, Pub. 07-Mar-89,
which are hereby incorporated by reference.
The compounds of formula I are useful in the
prevention and treatment of a wide variety of clinical conditions (as
detailed in this specification) which are characterized by overstimulation
of the tachykinin receptors, in particular NK1, NK2 and NK3.
These conditions may include disorders of the central
nervous system such as anxiety, depression, psychosis and
schizophrenia; neurodegenerative disorders such as AIDS related
dementia, senile dementia of the Alzheimer type, Alzheimer's disease
and Down's syndrome; demyelin~ting diseases such as multiple sclerosis
and amyotrophic lateral sclerosis and other neuropathological disorders
such as diabetic or peripheral neuropathy, AIDS related neuropathy,
chemotherapy-induced neuropathy, and neuralgia; respiratory diseases
such as chronic obstructive airways disease, bronchopneumonia,
bronchospasm and asthma; infl~mm~tory diseases such as inflammatory
bowel disease, psoriasis, fibrositis, osteoarthritis and rheumatoid
arthritis; allergies such as eczema and rhinitis; hypersensitivity
disorders such as poison ivy; ophthalmic diseases such as conjunctivitis,
vernal conjunctivitis, and the like; cutaneous diseases such as contact
dermatitis, atopic dermatitis, urticaria, and other eczematoid dermatitis;
WO 94/29309 ~ ~ 6 ~ ~ ~ 5 PCT/US94/05545
- 42 -
addiction disorders such as alcholism; stress related somatic disorders;
reflex sympathetic dystrophy such as shoulder/hand syndrome;
dysthymic disorders; adverse immunological reactions such as rejection
of transplanted tissues and disorders related to immune enhancement or
5 suppression such as systemic lupus erythematosis; gastrointestinal (GI)
disorders and diseases of the GI tract such as disorders associated with
the neuronal control of viscera such as ulcerative colitis, Crohn's disease
and incontinence; disorders of bladder function; fibrosing and collagen
diseases such as scleroderma and eosinophilic fascioliasis; disorders of
blood flow caused by vasodilation and vasospastic diseases such as
angina, migraine and Reynaud's disease; and pain or nociception, for
example, that is attributable to or associated with any of the foregoing
conditions especially the tr~nsmission of pain in migraine. Hence, these
compounds are readily adapted to therapeutic use for the treatment of
5 physiological disorders associated with the overstimulation of the
tachykinin receptors, in particular NK1, NK2 and NK3.
The compounds of the present invention are particularly
useful in the treatment of pain or nociception and/or inflammation and
disorders associated therewith such as, for exarnple: neuropathy, such
20 as diabetic or peripheral neuropathy and chemotherapy-induced
neuropathy; asthma; osteoarthritis; rheumatoid arthritis; and migraine.
For the treatment of any of these diseases compounds of
Formula I may be a~lministered orally, topically, parenterally, ICV, by
inhala~ion spray or rectally in dosage unit formulations containing
25 conventional non-toxic pharmaceutically acceptable carriers, adjuvants
and vehicles. The term parenteral as used herein includes subcutaneous
injections, intravenous, intramuscular, intracisternal injection or
infusion techniques. In addition to the treatment of warm-blooded
~nim~ls such as mice, rats, horses, cattle, sheep, dogs, cats, etc., the
30 compounds of the invention are effective in the treatment of humans.
The pharmaceutical compositions cont~ining the active
ingredient may be in a form suitable for oral use, for example, as
tablets, troches, lozenges, aqueous or oily suspensions, dispersible
powders or granules, emulsions, hard or soft capsules, or syrups or
WO 94129309 PCT/US94/05545
2 1 63~95
- 43 -
elixirs. Compositions intended for oral use may be prepared according
to any method known to the art for the manufacture of pharmaceutical
compositions and such compositions may contain one or more agents
selected from the group consisting of sweetening agents, flavoring
5 agents, coloring agents and preserving agents in order to provide
pharmaceutically elegant and palatable preparations. Tablets contain the
active ingredient in admixture with non-toxic pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets.
These excipients may be for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; granulating and disintegrating agents, for example, corn
starch, or alginic acid; binding agents, for example starch, gelatin or
acacia, and lubricating agents, for example magnesium stearate, stearic
acid or talc. The tablets may be uncoated or they may be coated by
S known techniques to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a
longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed. They may also
be coated by the techniques described in the U.S. Patents 4,256,108;
20 4,166,452; and 4,265,874 to form osmotic therapeutic tablets for
control release.
Formulations for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert
solid diluent, for example, calcium carbonate, calcium phosphate or
25 kaolin, or as soft gelatin capsules wherein the active ingredient is mixed
with water or an oil medium, for example peanut oil, liquid paraffin, or
olive oil.
Aqueous suspensions contain the active materials in
admixture with excipients suitable for the manufacture of aqueous
3 suspensions. Such excipients are suspending agents, for example sodium
carboxymethylcellulose, methylcellulose, hydroxy-propylmethyl-
cellulose, sodium alginate, polyvinyl- pyrrolidone, gum tragacanth and
gum acacia; dispersing or wetting agents may be a naturally-occurring
phosphatide, for example lecithin, or condensation products of an
WO 94/29309 2 1 ~ 3 ~ 9 5 PCT/US94/05545
- 44 -
alkylene oxide with fatty acids, for example polyoxyethylene stearate,
or condensation products of ethylene oxide with long chain aliphatic
alcohols, for example heptadecaethyleneoxycetanol, or condensation
products of ethylene oxide with partial esters derived from fatty acids
and a hexitol such as polyoxyethylene sorbitol monooleate, or
condensation products of ethylene oxide with partial esters derived from
fatty acids and hexitol anhydrides, for example polyethylene sorbitan
monooleate. The aqueous suspensions may also contain one or more
preservatives, for example ethyl, or n-propyl, p-hydroxybenzoate, one
or more coloring agents, one or more flavoring agents, and one or
more sweetening agents, such as sucrose or saccharin.
Oily suspensions may be formulated by suspending the
active ingredient in a vegetable oil, for example arachis oil, olive oil,
sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
The oily suspensions may contain a thickening agent, for example
beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as
those set forth above, and flavoring agents may be added to provide a
palatable oral preparation. These compositions may be preserved by the
addition of an anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation
of an aqueous suspension by the addition of water provide the active
ingredient in admixture with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending agents are exemplified by those already
2s mentioned above. Additional excipients, for example sweetening,
flavoring and coloring agents, may also be present.
The pharmaceutical compositions of the invention may also
be in the form of oil-in-water emulsions. The oily phase may be a
vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally-occurring gums, for example gum acacia or
gum tragacanth, naturally-occurring phosphatides, for example soy
bean, lecithin, and esters or partial esters derived from fatty acids and
hexitol anhydrides, for example sorbitan monooleate, and condensation
WO 94/29309 PCT/US94/05545
2 1 63~95
- 45 -
products of the said partial esters with ethylene oxide, for example
polyoxyethylene sorbitan monooleate. The emulsions may also contain
sweetening and flavoring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example glycerol, propylene glycol, sorbitol or sucrose.
Such formulations may also contain a demulcent, a preservative and
flavoring and coloring agents. The pharmaceutical compositions may
be in the form of a sterile injectable aqueous or oleagenous suspension.
This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents which
have been mentioned above. The sterile injectable preparation may also
be a sterile injectable solution or suspension in a non-toxic parenterally-
acceptable diluent or solvent, for example as a solution in 1,3-butane
diol. Among the acceptable vehicles and solvents that may be employed
are water, Ringer's solution and isotonic sodium chloride solution. In
addition, sterile, fixed oils are conventionally employed as a solvent or
suspending medium. For this purpose any bland fixed oil may be
employed including synthetic mono- or diglycerides. In addition, fatty
acids such as oleic acid find use in the preparation of injectables.
The compounds of formula I may also be ~clministered in
the form of suppositories for rectal a-l-ninistration of the drug. These
compositions can be prepared by mixing the drug with a suitable non-
irritating excipient which is solid at ordinary temperatures but liquid at
the rectal temperature and will therefore melt in the rectum to release
the drug. Such materials are cocoa butter and polyethylene glycols.
For topical use, creams, ointments, jellies, solutions or
suspensions, etc., containing the compounds of Formula I are employed.
(For purposes of this application, topical application shall include mouth
washes and gargles.)
3 In the treatment of a condition associated with an excess oftachykinins, an appropriate dosage level will generally be about 0.001 to
50 mg per kg patient body weight per day which can be a~lministered in
single or multiple doses. Preferably, the dosage level will be about 0.01
to about 25 mg/kg per day; more preferably about 0.05 to about 10
WO 94/29309 PCT/US94/0~545
2 ~ 6~3~ 95
- 46 -
mg/kg per day. A suitable dosage level may be about 0.001 to 25 mg/kg
per day, about 0.005 to 10 mg/kg per day, or about 0.005 to 5 mg/kg
per day. Within this range the dosage may be 0.005 to 0.05, 0.05 to 0.5
or 0.5 to 5.0 mg/kg per day. The compounds may be a~lmini~tered on a
5 regimen of 1 to 4 times per day, preferably once or twice per day.
TACHYK~IN ANTAGONISM ASSAY
The compounds of this invention are useful for
antagonizing tachykinins, in particular substance P and neurokinin A in
the treatment of gastrointestinal disorders, central nervous system
disorders, infl~mm~tory diseases, pain or migraine and asthma in a
m~mmal in need of such treatment. This activity can be demonstrated
by the following assay.
A. Receptor Expression in COS
To express the cloned human neurokinin-1 receptor
(NKlR) transiently in COS, the cDNA for the human NKlR was cloned
into the expression vector pCDM9 which was derived from pCDM8
20 (INVITROGEN) by inserting the ampicillin resistance gene (nucleotide
1973 to 2964 from BLUESCRIPT SK+) into the Sac II site.
Transfection of 20 ~g of the plasmid DNA into 10 million COS cells
was achieved by electroporation in 800 ~l of transfection buffer (135
mM NaCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 2.4 mM K2HPO4, 0.6 mM
25 KH2PO4, 10 mM glucose, 10 mM HEPES pH 7.4) at 260 V and 950 uF
using the IBI GENEZAPPER (IBI, New Haven, CT). The cells were
incubated in 10% fetal calf serum, 2 mM glllt~mine, 100U/ml penicillin-
streptomycin, and 90% DMEM media (GIBCO, Grand Island, NY) in
5% C2 at 37C for three days before the binding assay.
30 Similar methods were used to express the NK2 receptor.
WO 94/29309 PCT/US94/05545
2 1 63995
- 47 -
B. Stable Expression in CHO
To establish a stable cell line expressing the cloned human
NKlR, the cDNA was subcloned into the vector pRcCMV
(INVITROGEN). Transfection of 20 ,ug of the plasmid DNA into CHO
5 cells was achieved by electroporation in 800 ~l of transfection buffer
suplemented with 0.625 mg/ml Herring sperm DNA at 300 V and 950
uF using the IBI GENEZAPPER (IBI). The transfected cells were
incubated in CHO media (10% fetal calf serum, 100 U/ml pennicilin-
streptomycin, 2 mM glutamine, 1/500 hypoxanthine-thymidine (ATCC),
90% IMDM media (JRH BIOSCIENCES, Lenexa, KS), 0.7 mg/ml G418
(GIBCO)) in 5% CO2 at 37C until colonies were visible. Each colony
was separated and propagated. The cell clone with the highest number
of human NKlR was selected for subsequent applications such as drug
screening.
15 Similar methods were used to express the human NK2 receptor.
C. Assay Protocol usin~ COS or CHO
The binding assay of human NKlR expressed in either COS
or CHO cells is based on the use of 125I-substance P (125I-SP, from
20 DU PONT, Boston, MA) as a radioactively labeled ligand which
competes with unlabeled substance P or any other ligand for binding to
the human NKlR. Monolayer cell cultures of COS or CHO were
dissociated by the non-enzymatic solution (SPECIALTY MEDIA,
Lavallette, NJ) and resuspended in appropriate volume of the binding
25 buffer (50 mM Tris pH 7.5, 5 mM MnCl2~ 150 mM NaCl, 0.04 mg/ml
bacitracin, 0.004 mg/ml leupeptin, 0.02 mg/ml BSA, 0.01 mM
phosphoramidon) such that 200 ~11 of the cell suspension would give rise
to about 10,000 epm of speeifie 125I-SP binding (approximately 50,000
to 200,000 cells). In the binding assay, 500 ul of cells were added to a
30 tube containing 20 ~l of 1.5 to 0.25 nM of 125I-SP and 5 ~l of
unlabeled substance P or any other test compound in DMSO. The tubes
were incubated at 4C or at room temperature for 1 hour with gentle
shaking. The bound radioactivity was separated from unbound
radioactivity by GF/C filter (BRANDEL, Gaithersburg, MD) which was
WO 94/29309 PCT/US94/0~545
2 1 63995
- 48 -
pre-wetted with 0.1% polyethylenimine. The filter was washed with 3
ml of wash buffer (50 mM Tris pH 7.5, 5 mM MnCl2, 150 mM NaCl)
three times and its radioactivity was determined by gamma counter. A
similar assay was used for NK2 except 125I-NKA was used as the
ligand.
The activation of phospholipase C by NKlR may also be
measured in CHO cells expressing the human NKlR by determining the
accumulation of inositol monophosphate which is a degradation product
of IP3. CHO cells are seeded in 12-well plate at 250,000 cells per well.
After incubating in CHO media for 4 days, cells are loaded with 0.025
uCi/ml of 3H-myoinositol by overnight incubation. The extracellular
radioactivity is removed by washing with phosphate buffered saline.
LiCl is added to the well at final concentration of 0.1 mM with or
without the test compound, and incubation is continued at 37C for 15
min. Substance P is added to the well at final concentration of 0.3 nM
to activate the human NKlR. After 30 min of incubation at 37C, the
media is removed and 0.1 N HCl is added. Each well is sonicated at 4C
and extracted with CHCl3/methanol (1:1). The aqueous phase is applied
to a 1 ml Dowex AG lX8 ion exchange column. The column is washed
with 0.1 N formic acid followed by 0.025 M ammonium formate-0.1 N
formic acid. The inositol monophosphate is eluted with 0.2 M
ammonium formate-0.1 N formic acid and quantitated by beta counter.
Similar methods were used to assess antagonism at the NK2 receptor,
except NKA was used as the stimulating agonist.
The compounds of of Formula I as Exemplified in the
EXAMPLES below have been found to displace radioactive ligand for
the NK-1 receptor at a concentration range of 0.01 nM to 1.0 ,uM, for
the NK-2 receptor, 0.01 nM to S ~M, and for the NK-3 receptor, 1.0
nM to 10 ~lM.
Several methods for preparing the compounds of this
invention are illustrated in the following Schemes and Examples.
The compounds of the present invention are prepared by
alkylating azacycle 1, in which R1 = H, under appropriate conditions
(Scheme 1). The required azacycle starting materials are prepared
WO 94/29309 PCT/US94/0554~
2 1 6~ 9 ~
- 49 -
using methods described in the literature; such as described in Ong, H.
H. et al, Journal of Medicmal Chemistry, 1983,26, 981-986, and
Nargund, R. e~ al, USSN 08/147,226 (November 3, 1993), EP
93309867.5 hereby encorporated by reference. None of the compounds
in these references are claimed to be neurokinin antagonists.
Thus, azacycle 1 (R1=H) is combined with the appropriate
aldehyde and the intermediate imine is reduced to the tertiary amine
chemically (e.g. using sodium cyanoborohydride) or catalytically (e.g.
using hydrogen and palladium on carbon or Raney nickel catalyst)
(Scheme 1). The aldehyde needed for this reaction can be prepared by
methods generally known in the chemical literature; for the purposes of
the present invention the preparation of a representative aldehyde is
described in Hale, J.J.; Finke, P.E.; MacCoss, M. Bioorganic &
Medicinal Chemistry Letters 1993,3, 319-322.
In an alternative embodiment of the present
invention, azacycle 1 (Rl=H) can be alkylated with an alkyl halide or
alkyl sulfonate ester (with or without an added base to neutralize the
mineral acid or sulfonic acid by-product) to give the desired compound
(Scheme 1). The alkyl halide or alkyl sulfonate needed for this
reaction can be prepared by methods generally known in the chemical
literature; for the purposes of the present invention an aldehyde,
prepared as described above, can be reduced to an alcohol with sodium
borohydride, diisobutylaluminum hydride or lithium aluminum
hydride, and the product alcohol converted to either the aL~yl halide
using methods described in March J. "Advanced Organic Chemistry",
25 3rd ed., John Wiley & Sons, New York, pp. 382-384 (1985), or alkyl
sulfonate ester using methods described in March J. "Advanced Organic
Chemistry", 3rd ed., John Wiley & Sons, New York, p. 444 (1985).
In an alternative embodiment of the present invention, 1
(R1 = H) can be acylated to give the tertiary amide and subsequent
30 reduction with a strong reducing agent (e.g. diborane including borane
dimethylsulfide; and, lithium alllminllm hydride) will give the desired
compound (Scheme 1). The acylating agent needed for this reaction can
be prepared by methods generally known in the chemical literature; for
WO 94/29309 PCT/US94/05545
~l 63q95
- 50 -
the purposes of the present invention an aldehyde, prepared as described
above, can be oxidized using such commonly used reagents as
permanganate in acid or silver oxide, and the resulting acid activated as
an acid chloride or mixed anhydride which can be used to acylate I (Rl
5 = H). The product amide can in and of itself be a neurokinin antagonist
or can be reduced with a strong reducing agent, such as diborane or
lithium aluminum hydride, to give the tertiary amine.
Optionally, compound 1 formed in the alkylation step may
be further modified in subsequent reactions. In one illustration of such
an approach,the aldehyde fragment contained a t-butoxycarbonylamino
group (Example 2). After reductive ~min~tion, the t-butoxycarbonyl
protecting group is removed by treatment with a strong acid such as
trifluoroacetic acid or formic acid and the resulting amine is acylated to
furnish the desired compounds (Example 3). Alternatively, the
5 protecting group may also be present in the azacycle portion as
illustrated with a benzyloxycarbonyl group in Example 6. Thus an
azacycle cont~ining a benzyloxycarbonylindoline (prepared in Example
4) is alkylated with an aldehyde in the presence of a reducing agent.
Next, the protecting group is removed to liberate a free amine
20 (Example 7) and the amine is further reacted to provide additional
analogs (Example 8).
WO 94/29309 PCT/US94/05545
2 1 63~95
SCHEM:E 1
RCHO, [H]
X W R 1 X X' W
~;;z~)m ~
H 1 R1
RCO~\ ~trong [H]
~X-Q`W
~ )m
O R
WO 94/29309 PCT/US94/05545
~1 63995
In an alternative embodiment of the present invention, the
allyl acid 2 (described in Hale et al; see above) can be converted into the
N-methyl methoxy amide 3, which is then treated with an alkyl or aryl
metal reagent, for example methyllithium or butyllithium, to provide
5 the ketone 4 (Scheme 2). The ketone can be converted into an imine
which can then be reduced to secondary amine 5 chemically, (e.g using
sodium cyanoborohydride or sodium borohydride), or catalytically (e.g.
using hydrogen and palladium on carbon or Raney nickel catalyst).
Acylation under standard conditions, for example with an acid chloride,
provides amide 6. The allyl group in 6 can be oxidatively cleaved to
aldehyde 7 with osmium tetroxide followed by sodium periodate or
with ozone at low temperature. Reductive ~min~tion of aldehyde 7 with
azacycle 1 can then be carried out under the conditions described above.
Substituted spiro(indoline-3,4'-piperidine) derivatives can
5 be prepared as shown in Scheme 3 starting from the appropriately
substituted phenylhydrazines. Following the Fischer indole reaction and
reduction of the intermediate imine with a mild reducing agent such as
sodium borohydride, the indoline nitrogen can be reacted with an
electrophile such as an acyl chloride or a sulfonyl chloride. The
20 protecting group on the piperidine nitrogen, for example a
benzyloxycarbonyl group, can be removed by treatment with hydrogen
in the presence of palladium on carbon or by exposure to trimethylsilyl
iodide, to give the deprotected substituted spiro(indoline-3,4'-
piperidine).
WO 94/29309 PCT/US94/05545
2 1 63qq5
SCHEME 2
E DAC O
HOBT-H20 ~N,OMe
OH HN(Me)(OMe) HCI - ~e RLi, -78C or
Cl' ~ Cl/~3 RMgBr, rt
`\ R R6NH3CI ~N,H (BOC)2o
R6 TEA
Cl/~ C~
Cl 4 Cl 5
1 ) OSO4
R NMO R6
N,BOC acetone/ tBuOH/H20 H N,BOC
- R 3:1 CIJ~3
Cl 6 Cl 7
CF3CO2H Amine HCI C ~ ~
anisole, CH2C12 NaBH3CN - R 7
Cl/~
Cl 8
WO 94/29309 PCT/US94/05545
2 1 63~5
- 54 -
SCHEME 3
O OBn 1) pyridine, toluene ~OBn
+ ~ 2) CF3CO~H, 60C ~)
NH3CI CHO 3) NaBH4, MeOH H
R2-X
~ OBn
N HCI Me3Sil; HCI N
~ ~ H2, Pd/C; HCI
R ~ R
R2 R2
Preparation of spiro(2,3-dihydrobenzothiophene-3,4'-
piperidine) derivatives is shown in Scheme 4. Reaction of N-Boc
protected 4-piperidone with the lithium salt of methyl phenyl sulfoxide
followed by base-mediated elimin~tion-rearrangement and basic
cleavage provides the indicated allylic alcohol. The alcohol can be
30 converted to the rearranged allylic chloride with thionyl chloride in
toluene in the presence of 2,6-lutidine as a proton scavenger.
Displacement of the chloride with functionalized 2-bromothiophenol
provides the allylic sulfide, which can be cyclized under radical
conditions to give the illustrated spiro(2,3-dihydrobenzothiophene-3,4'-
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
piperidine). Cleavage of the t-butoxycarbonyl group under standard
conditions, such as trifluoroacetic acid, then provides the desired
spirocycle.
S SCHEME 4
Boc
Boc methyl phenyl N
~N~ sulfoxide ~ KOtBu, tBuOH
LDA, THF HO ~ 50C
OC ~S~O
R ~
Boc Boc ~ SH
N N Br
~ ~ SOCI2, PhMe ~ K2CO3, 40C
OH 2,6-lutidine Cl
Boc
N~ Boc
I~J Br Bu3SnH, AIBN ~N~ 1 ) TFA
S~¦~ PhH, 80C ~ 2) HCI, MeOH
H HCI R
N
30 ~
R
WO 94/29309 PCT/US94/05545
2 1 6~9~5
- 56 -
Spiro(2,3-dihydrobenzofuran-3,4'-piperidine) derivatives
can be prepared as illustrated in Scheme 5. Treatment of an
appropiately substituted ester of 2-fluorophenylacetate with
mechloret~mine hydrochloride under basic conditions provides the
5 piperidine product, which on treatment with a strong reducing agent
such as lithium aluminum hydride produces the corresponding 4-
(hydroxymethyl) compound. Cyclization with base provides the
benzofuran derivative, and cleavage of the N-methyl group can then be
carried out using l-chloroethyl chloroformate or other suitable N-
o demethylating agents.
SCHEME 5
WO 94/29309 PCT/US94/05545
21 639~5
- 57 -
N NaH
MeO Cl Cl f ~ F
O N
Me
~I LiAlH4, THF
DMF HO ~
R ~ F
Cl ~ Me
Me O Cl
1 ,2-dichloroethane,
reflux
~NH-HCI
Compounds with alternate arrangements of the amide bond
30 can be prepared as shown in Scheme 6. The illustrated acid can be
homologated under Arndt-EisteIt conditions to give the chain-extended
acid, which can be derivatized under standard acylating conditions with,
for example, an aniline derivative, to give the corresponding amide.
Oxidative cleavage of the olefin with osmium tetroxide or ozone then
WO 94129309 PCT/US94/05545
21 639~5
- 58 -
provides the aldehyde intermediate suitable for coupling as described
earller.
WO 94/29309 2 1 6 3 9 9 5 PCTIUS94/05545
- 59 -
SCHEME 6
R R
~1 1) (COCI)2
~OH 2) CH2N2, Et20 ~N2
R
AgN03 ~s~H
H20 o
1) (COCI)2
e` ,~
R
1 ) OSO4, H20, tBuOH
N-Me-morpholine-N-oxide ~\'q
2) NalO4, H20, THF ~ Me
O ~5~ N
Me
WO 94/29309 PCT/US94/05545
2 ~ 63~95
- 60 -
In addition, ketone derivatives can be prepared by an
extension of the chemistry given above, as shown in Scheme 7. A
second Arndt-Eistert chain extension provides the illustrated heptenoic
acid derivative, which after conversion into its N-methoxy-N-methyl
5 amide, can be reacted with an aryl organometallic reagent, such as an
aryl magnesium bromide, to provide the ketone. Routine oxidative
cleavage then gives the desired aldehyde, which can be coupled with a
spiro-piperidine derivative as described above.
SCHEME 7
WO 94/29309 PCT/US94/05545
21 63995
- 61 -
R
1) (C )2
~ 2) CH2N2 ~J~ OH
1) (COCI)2 ~q~
- Me
2) HN(Me)OMe ~ ~N~OMe
~ R'
BrMg
THF
1 ) OsO4, H2O, tBuOH
N-Me-morpholine-N-oxide R
2) NalO4, H2O, THF ~q
'1~ 1~ R'
R ~ J
2 ~ O
WO 94/29309 PCT/US94/05545
2 1 6399~
- 62 -
D~ 1) (COC~
s2) C H2N2 ~ ,I~,OH
101) (COc1)2 , ~q
~ Me
2) HN(Me)OMe l I
~N~ OM
BrMgJ3
THF '''
1 ) OSO4, H20, tBuOH
N-Me-morpholine-N-oxide R
2) NalO4, H20, THF ~\~
,. ~ ~ R'
R ~ ~
~ O
~ 1~ R'
~/
O
Alcohol containing antagonists can be prepared according
to procedures given in Scheme 8. Formation of the N-methyl-N-
methoxy amide of the indicated acid followed by oxidative cleavage of
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- 63 -
the olefin provides the intermediate aldehyde. Coupling with a
spiro(indoline-3,4'-piperidine) derivative followed by addition of an
organometallic reagent to the amide provides the illustrated ketone.
Treatment with a hydride reducing agent, such as sodium borohydride,
5 then yields the desired alcohol derivatives.
WO 94/29309 2 1 6 3 ~ ') 5
- 64 -
SCHE~'IE 8
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- 65 -
O O
~OH ~N~ Me
- 1) (coC1)2 . ,~, OMe
l~;~ 2) HN(Me)OMe ~J
1 ) 0sO4,H20, tBuOH ~ N~ Me SO2CH3
N-Me-morpholine-N-oxlde ~ OMe ~< ~
2) NalO4, H20,THF R' R/~ NH HCI
7O2CH3
MeOH, NaBH3CN R\,=< O R"MgCI
mol. sieves ~--NI--~`N'Me
OMe
Sl 02C H3
20 R~N ~--~` R~ NaBH4
R' SO2CH3
so2CH3
N N
R N~ R~ R~--
~+ ~1
R' R'
WO 94/29309 PCT/US94/05545
21 63995
- 66 -
Formation of heterocycle substituted antagonists can be
carried out according to the procedure given in Scheme 9 for
substituted imidazoles. Reduction of the allyl acid with a strong
reducing agent such as lithium aluminum hydride followed by in situ
5 formation of the trifluoromethanesulfonate of the formed alcohol allows
for displacement of the triflate with a nucleophile such as 2-
phenylimidazole. Oxidative cleavage under standard conditions
provides the indicated aldehyde which can then be coupled under the
conditions described above to the appropriate spiro derivative.
SCHEME 9
WO 94/Z9309 2 1 6 3 9 9 5 PCT/US94105~4~
- 67 -
LiAlH4 ~--OH (CF~SO2)0
T~F R' 2,6-lutidine
R'
1 ) OSO4, H2O, tBuOH
N-Me-morpholine-N-oxide
/_\ ~J 2) NalO4, tl2O, THF
HN~f N ~ N)~N
R'
~--N~N
2 0 \=~
R'
Spiro(2-oxo- 1,2,3 ,4-tetrahydroquinoline-4,4'-piperidine)
and spiro(1-oxo-1,2,3,4-tetrahydroisoquinoline-4,4'-piperidine) can be
prepared as shown in Scheme 10. Starting from the indicated spiro(2-
oxoindane-3,4'-piperidine) (described in Claremon, D.A. et al,
30 European Patent 0 431 943 943 A2, Ev~ns, B.E. et ~1, U.s. Patent
5~091.387, Davis, L. et al, U,s. Patent 4~420~485, all of which are
incorporated by reference, and Parham et al, Journal of Or~anic
Chemi~try, 41, 2628 (1976)), deprotection of the piperidine nitrogen is
carried out by treatment with acid, for example trifluoroacetic acid,
followed by protection as the trifluoroacetamide, and the product is
WO 94/29309 ~ 1 ~ 3 q q 5 PCT/US94/05545
- 68 -
exposed to hydrazoic acid in the presence of sulfuric acid. Heating of
this mixture effects a Schmidt rearrangement, to provide both the
tetrahydroquinoline and the tetrahydroisoquinoline derivatives. These
spiro compounds can then be separated and coupled to functionalized
5 aldehydes by the methodology given above.
SCHEME 10
0 --NH-CF3CO2H
--N Ot-Bu CF3C02H o~J
HN3, H2S04
rt to 45 deg C
--NH --NH
~~J + HN J
HN`~
Neurokinin antagonists with ether substituents can also be prepared by
the route shown in Scheme 11. Thus, the allyl acid discussed earlier can
be reduced to the corresponding alcohol with, for example, lithium
aluminum hydride. This alcohol can be alkylated by a Williamson ether
30 synthesis, by deprotonation with a strong base such as sodium hydride
or sodium hexamethyldisilazide followed by reaction with a benzyl
halide such as benzyl bromide. The product can be processed through
the oxidative cleavage steps described earlier to provide the aldehyde,
which can then be coupled with a spirocycle kunder reductive amination
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
- 69 -
conditions or else by reduction to the corresponding alcohol and
conversion to the bromide. the bromide can then be used to alkylate a
spirocycle under the conditions detailed above.
SCHEME 11
OH 4 ~ - OH
R~ R
1 ) OsO4, H20, tBuOH
,, n ~ N-Me-morpholine-N-oxide
~1 2) NalO4, H20, THF
~- ~oJ
2 0 R R'
-
,~
R
Accordingly in another aspect the invention encompasses a
process of making compounds of formula I
WO 94/29309 PCT/US94/05545
21 639q5
- 70 -
X' `W
~Z ~) m
R1
as defined above, comprising the steps of:
(a) reacting a compound of formula A
~Q~
X W
~Z~>) m
I N
H
A
in a suitable solvent such as acetonitrile or dimethylacetamide with a
compound of formula Rl-xl wherein X1 is a leaving group such as
bromo, chloro, tosyl or mesyl optionally in the presence of a suitable
2 5 base such as trialkylamine; or
(b) reacting a compound of formula A in a second suitable solvent such
as methanol or ethanol with a compound of the formula R-CH(O),
wherein R-CH2 is R1 as defined above, in the presence of a reducing
agent such as sodium cyanoborohydride or hydrogen and a palladium on
carbon catalyst; or
(c) reacting a compound of formula A in a halocarbon solvent such as
methylene chloride with a compound of the formula R-C(O)X2 wherein
WO 94/29309 PCT/US94/05545
21 63995
X2 is a suitable leaving group such as bromo or chloro in the presence
of a suitable base such as trialkylamine,
to yield a compound of formula I.
EXAMPLE 1
3 -(S)-(3 ,4-Dichlorophenvl)-4-(N-(t-butoxycarbonyl)methylamino)
butanal
A solution of 10 g (41 mmol) of 3-(S)-(3,4-dichloro-
phenyl)-4-methylamino-1-pentene in 100 mL of CH2C12 was cooled in
an ice bath and treated with 5.8 mL (41 mmol) of triethylamine (Et3N)
and 9 g (41 mmol) of di-t-butyl dicarbonate. The cold bath was
removed after 5 min and the stirring was continued for 1 h. The
reaction mixture was diluted with CH2Cl2 and washed with water, 1.2
15 N HCl, saturated NaHCO3 and brine. The solution was dried over
Na2SO4 and concentrated to give 14.58 g of residual oil.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) o 1.36 (s, 9H), 2.33 (m, 2H), 2.60 & 2.70 (2s, 3H),
2.8-3.6 (m, 3H), 4.94 (m, 2H), 5.59 (m, lH), 6.9-7.4 (m, 3H).
The residue was dissolved in 80 mL of acetone, 40 mL of t-
butanol and 40 mL of water. To this solution 1 mL of osmium
tetroxide (4% solution in water) and 5.15 g (44 mmol) of 4-
methylmorpholine N-oxide were added. After stirring for 26 h, the
reaction was quenched with approximately 5 g of Na2SO3 and
25 concentrated to 25% of the original volume. The residue was
partitioned between water and 1:1 ether (Et2o)~ ethyl acetate (EtOAc),
the layers were separated and the aqueous layer was extracted with
Et2O:EtOAc. Each organic layer was washed with water, brine and
dried by filtering through Na2so4. The filtrate was concentrated to
30 afford the crude diol.
A solution of the diol in 120 mL of tetrahydrofuran (THF)
and 40 mL of water was treated with 9.42 g (44 mmol) of sodium
periodate. After stirring for 2 h, the reaction was diluted with
Et2O:EtOAc and washed with water and brine. The organic layer was
wo 94/29309 2 1 6 3 9 9 5 PCT/US94/0~545
- 72 -
dried (Na2SO4) and the filtrate was concentrated. The residue was
purified by prepLC using 30% EtOAc/hexane to furnish 11.74 g (83%
yield for three steps) of the title compound as a thick oil.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) o 1.38 (s, 9H), 2.69 & 2.75 (2s, 3H), 2.6-3.65 (m, SH),
6.95-7.4 (m, 3H), 9.67 (s, lH).
EXAMPLE 2
0 1 '-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(t-
butoxycarbonyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3 ~4 '-piperidine)
To a solution of 0.76 g (2.2 mmol) of 3-(S)-(3,4-
dichlorophenyl)-4-(N-(t-butoxycarbonyl)methylamino)butanal ( from
Example 1) in 4 mL of methanol were added 0.608 g (2 mmol) of 1-
methane-sulfonyl-spiro(indoline-3,4'-piperidine) hydrochloride and 0.6
g of powdered 4 A molecular sieves. After 15 min a solution of 0.554
g (8.8 mmol) of NaCNBH3 in 8 mL of THF was dropwise added. Some
gas evolution was obser~ed. After 2 h, when the reaction was complete
by TLC, the mixture was filtered through a pad of celite, the reaction
flask and the pad were rinsed with methanol. The filtrate was
concentrated to approximately 5 ml and the residue was partitioned
between saturated NaHCO3 and Et2o:EtoAc. The organic layer was
washed with water, brine and dried over Na2so4. The filtrate was
concentrated and the residue was chromatographed on a flash column
using a gradient of 49:49:2 to 74:24:2 EtOAc:hexane: triethylamine to
furnish 0.94 g (72%) of the title compound as a foam.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.37 (s, 9H), 1.6-3.6 (m, 15H), 2.61 & 2.72 (2s, 3H),
2.86 (s, 3H), 3.74 (s, 2H), 6.95-7.4 (m, 7H).
EXAMPLE 3
WO 94/29309 PCT/US94/05545
2163~95
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5-
dimethylbenzoyl)(methylamino)) butyl)-1-methanesulfonyl-
spiro(indoline-3 .4'-piperidine)
Step A: 1'-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-1-
methanesulfonyl-spiro(indoline-3 4'-piperidine)
Cold trifluoroacetic acid (TFA, 4 mL) and 0.2 mL of
anisole were added to 0.94 g (1.57 mmol) of 1'-(3-(S)-(3,4-
dichlorophenyl)-4-(N-(t-butoxycarbonyl)(methylamino))butyl)- 1 -
methanesulfonyl-spiro(indoline-3,4'-piperidine) and the mixture was
stirred in an ice bath until all the foam dissolved. After stirring the
resulting solution at room temperature for 30 min, it was concentrated
in vacuo. The residue was partitioned between 0.5 N NaOH and
CH2C12 and the layers were separated. The organic layer was washed
with brine, dried over Na2S04 and concentrated to give 0.7 g of foam
which was used in the next step without purification.
lH NMR (CDC13, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.7-2.7 (m, 10H), 2.64 (s, 3H), 2.88 (s, 3H), 2.9-3.4
(m, SH), 3.70 (s, 2H), 6.8-7.4 (m, 7H).
Step B: 1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-
dimethylbenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3 4'-piperidine)
A solution of 0.12 g (0.52 mmol) of 3,5-dimethylbenzoic
acid in 2 mL of CH2C12 containing 1 drop of DMF was treated with 85
ul of oxalyl chloride. (Caution-gas evolution!) After 20 min the
solution was concentrated in vacuo and the residue was mixed with 0.2 g
(0.4 mmol) of 1'-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-
1-methane-sulfonyl-spiro(indoline-3,4'-piperidine) obtained from Step
A, and 0.14 mL (1 mmol) of Et3N in 2 mL of CH2C12. After 1 h the
reaction mixture was diluted with CH2C12 and washed with saturated
NaHCO3, water, and brine. The CH2C12 solution was dried over
Na2SO4, filtered and concentrated. Purification of the residue by prep
WO 94/29309 PCT/US94/05545
~1 639q5
- 74 -
TLC using 2% Et3N/EtOAc afforded 0.238 g (93% yield) of the title
compound as a foam.
1H NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.6-2.4 (m, 10H), 2.27 (s, 6 H), 2.6-3.9 (m, 10H),
2.86 (s, 3H), 6.6-7.5 (m, 10H).
The following compounds were prepared by substituting
the required acid chloride for 3,5-dimethylbenzoyl chloride in Step B.
1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(benzoyl)(methylamino)) butyl)-
1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
Mass Spectrum (FAB) 602 (37Cl + 35Cl isotope), 600 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5-
bistrifluoromethylbenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-
5 spiro(indoline-3 ,4'-piperidine)
Mass Spectrum (FAB) 738 (37Cl + 35Cl isotope), 736 (35Cl + 35Cl
isotope).
1 '-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 -methylbenzoyl)
2 (methylamino))-butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine)
1H NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.6-2.4 (m, 10H), 2.32 (s, 3H), 2.6-3.9 (m, 10H),
2.86 (s, 3H), 6.75-7.5 (m, 1 lH).
25 Mass Spectrum (FAB) 616 (37Cl + 35Cl isotope), 614 (35Cl + 35Cl
isotope).
1 '-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 -chlorobenzoyl)
(methylamino))-butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
3 piperidine)
1H NMR (CDC13, ppm ranges are given because of amide rotomers and
line broadening) o 1.6-2.4 (m, 10H), 2.6-3.9 (m, 10H), 2.86 (s, 3H),
6.75-7.5 (m, 1 lH).
WO 94/29309 2 1 6 3 q 9 5 PCT/US94/05545
Mass Spectrum (FAB) 635 (37Cl + 35Cl isotope), 633 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3 -trifluoromethylbenzoyl)
5 (methyl-amino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Mass Spectrum (FAB) 669 (37Cl + 35Cl isotope), 667 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl)
(methylamino))-butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.6-2.4 (m, lOH), 2.6-3.9 (m, lOH), 2.86 (s, 3H),
5 6.75-7.5 (m, 1 OH).
Mass Spectrum (FAB) 671 (37Cl + 35Cl isotope), 669 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-trifluoromethylphenylacetyl)
20 (methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum (FAB) 684 (37Cl + 35Cl isotope), 682 (35Cl + 35CI
isotope).
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3-isopropyloxyphenylacetyl)
25 (methyl-amino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(benzenesulfonyl)
(methylamino))-butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
30 piperidine)lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.65 (m, 3H), 1.8-2.3 (m, 7H), 2.62 (s, 3H), 2.7-3.05
(m, 4H), 2.86 (s, 3H), 3.3 (m, 2H), 3.74 (s, 2H), 7.0-7.7 (m, 12H).
WO 94/29309 2 l 6 3 9 ~ 5 PCT/US94/05545
- 76 -
Mass Spectrum (FAB) 637 (37Cl + 35Cl isotope), 635 (35Cl + 35Cl
isotope).
The following compounds were also prepared by using the
5 appropriate acid chloride under the conditions given in Step B above:
1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(N-(3,5-difluorobenzoyl)(methyl-
amino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
Mass Spectrum (FAB) 638(37Cl + 35Cl isotope), 636(35Cl + 35Cl
isotope).
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 -fluoro-5 -(trifluoromethyl)-
benzoyl)(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine)
Mass Spectrum (CI) 688 (37Cl + 35Cl isotope), 686 (35Cl + 35Cl
5 isotope).
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-( 1 -naphthoyl)(methylamino))-
butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
1 H NMR (CDCl3, ppm ranges are given because of amide rotomers and
20 line broadening) d
Mass Spectrum (FAB) (37Cl + 35Cl isotope), (35Cl + 35Cl isotope).
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(N-(2-chlorophenylsulfonyl)-
(methylamino))butyl)- 1 -methylsulfonyl-spiro(indoline-3 ,4'-piperidine)
25 Mass Spectrum: 200, 202, 228, 230, 279, 308, 310, 494, 496, 670, 672
(cluster).
1 '-(3-((S)-(3,4-Dichlorophenyl))- 1 -(N-(3-chlorophenylsulfonyl)-
(methylamino))-4-butyl)- 1 -methylsulfonyl-spiro(indoline-3 ,4'-
3 piperidiIle)Mass Spectrum: 200, 202, 228, 230, 279, 308, 310, 494, 496, 670, 672
(cluster).
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-chlorophenylsulfonyl)-
(methylamino))butyl)- 1 -methylsulfonyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum: 200, 228, 230, 279, 494, 496, 669 (cluster).
1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorophenylsulfonyl)-
(methylamino))butyl)- 1 -methylsulfonyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum: 228, 230, 279, 494, 496, 703, 705 (cluster).
EXAMPLE 4
l-Benzvloxycarbonyl-spiro(indoline-3.4'-piperidinium) hydrochloride
A solution of 99 g (489 mmol) of 1'-methylspiro(indoline-
3,4'-piperidine) (prepared according to Ong, H. H. et al, J. Med. Chem.,
1983, 26, 981-986) in 1 L of CH2Cl2 and 82 mL (539 mmol) of Et3N
l5 was cooled to 0-5C with an ice bath and 77 mL (539 mmol) of benzyl
chloroformate was added over 30 min keeping the reaction temperature
below 10C. After stirring for 2 h 19 mL (136 mmol) of Et3N and 15
mL (105 mmol) of benzyl chloroformate were added since the reaction
was incomplete and stirred for 2 h. At this time, additional 19 mL (136
20 mmol) of Et3N and 15 mL (105 mmol) of benzyl chloroformate were
added. After 1 h, when a TLC indicated a complete reaction, the
solution was concentrated in vacuo and the residue was partitioned
between ether and saturated NaHCO3. The layers were separated, the
organic layer was washed with saturated NaHCO3 and brine, and dried
25 over MgSO4. The filtrate was concentrated and the residue was
chromatographed on 2 kg of silica gel using 1-5% MeOH/CH2Cl2 to
obtain 117 g (71 %) of 1 -benzyloxycarbonyl- 1 '-methylspiro(indoline-
3,4'-piperidine) as a yellow oil.
The yellow oil was dissolved in 800 mL of 1,2-
30 dichloroethane and cooled in ice bath as 50 mL (463 mmol) of 1-
chloroethyl chloroformate keeping the tempera~ure below 10C. The
resulting solution was heated to reflux. Gas evolution was noticed when
the reaction temperature reached 70-75C. After 1 h the solution was
cooled, concentrated to ca. 250 mL in vacuo and 700 mL of methanol
WO 94/29309 2 1 6 3 ~ 9 5 PCT/US94/05545
- 78 -
was added. The mixture was refluxed for 1.5 h and gas evolution was
observed. The reaction was cooled to room temperature and
concentrated in vacuo to a wet solid. The solid was slurried with cold
methanol, the solid was filtered, washed with cold methanol and dried.
5 The filtrates and the washing were combined and concentrated to a
brown foam. The brown foam and the filtered solid were suspended in
CH2Cl2, washed with 2.5 N NaOH and the CH2Cl2 solution was dried.
The residue was chromatographed on 2 kg of silica gel using a gradient
of 94:5:1 to 89:10:1 CH2Cl2, methanol, NH40H to isolate 91.3 g of free
base as a brown oil. The oil was dissolved in 1 L of EtOAc by adding
methanol (ca. 10 mL) and HCl gas was passed through the solution.
After stirring the acidic solution for 10 min, it was concentrated to a
foam. The foam was triturated with ether and the solid was filtered,
washed with more ether and dried to furnish 91.5 g (73%) of title
5 compound as a light yellow solid.
EXAMPLE S
3 -((S)-(3 ,4-Dichlorophenyl))-4-((3 ,5 -dimethylbenzoyl)methylamino)-
20 butanal
The title compound was prepared using the proceduresdescribed in Example 1 by substituting 3,5-dimethylbenzoyl chloride
for di-t-butyl dicarbonate.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
25 line broadening) ~ 2.27 (s, 6H), 2.6-3.9 (m, 8H), 6.5-7.5 (m, 6H), 9.73
(s, lH).
EXAMPLE 6
1 '-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5 -dimethylbenzoyl)(methyl-
3 amino))butyl)- 1 -benzyoxycarbonyl-spiro(indoline-3 ~4'-piperidine)
The title compound was prepared from 3-((S)-(3,4-
dichlorophenyl))-4-((3,5-dimethylbenzoyljmethylamino)butanal
(Example S) and 1-benzyloxycarbonyl-spiro(indoline-3,4'-
WO 94/29309 PCT/US94/05545
21 63q~5
- 79 -
piperidinium) hydrochloride (Example 4) following the procedure of
Example 2.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.6-2.35 (m, 10H), 2.27 (s, 6H), 2.6-3.9 (m, 10H),
5.23 & 5.3 (2 s, 2H), 6.6-7.6 (m, 15H).
Mass Spectrum (FAB) 686 (37Cl + 35Cl isotope), 684 (35Cl + 35Cl
isotope).
EXAMPLE 7
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)
(methylamino))butyl )-spiro(indoline-3,4'-piperidine)
To a solution of 1.23 g (1.8 mmol) of 1'-(3-((S)-(3,4-
dichlorophenyl))-4-(3,5-dimethylbenzoyl(methylamino))butyl)- 1 -
benzyoxycarbonyl-spiro(indoline-3,4'-piperidine) (Example 6) in 10
mL of ethanol and 0.8 mL of acetic acid (HOAc) was added 0.15 g of
10% Pd/C. The resulting mixture was hydrogenated on a Parr
apparatus for 20 h. The catalyst was filtered and washed with EtOH.
The combined filtrate was concentrated in vacuo and the residue was
dissolved in CH2Cl2. The CH2Cl2 solution was washed with dilute (ca
0.5 N) NaOH and brine, and dried by filtering through Na2SO4. The
filtrate was concentrated to furnish 1.03 g (quantitative) of the title
compound as a foam which was used in the next reaction without
purification.
1H NMR (CDCl3, ppm ranges are given because of amide rotomers and
2s line broadening) ~ 1.6-2.45 (m, 10H), 2.27 (s, 6H), 2.6-3.9 (m, 10H),
6.5-7.5 (m, 10H).
Mass Spectrum (FAB) 552 (37Cl + 35Cl isotope), 550 (35Cl + 35Cl
isotope).
EXAMPLE 8
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)
(methylamino))butyl)- 1 -acetvl-spiro(indoline-3.4'-piperidine)
WO 94/29309 PCT/US94/05545
2~ 63~5
- 80 -
Acetyl chloride (16 uL) was added to a solution of 0.1 g
(0.18 mmol) of 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)-spiro(indoline-3,4'-piperidine) (Example
7) in 4 mL of CH2Cl2 containing 30 mL of pyridine. After stirring for
5 2 h, the reaction mixture was diluted with CH2Cl2 and washed with
saturated NaHCO3, water, brine and dried. ~he residue after
concentration of the filtrate was purified by prep TLC using 5%
Et3N/EtOAc as an eluent to afford 90 mg (84%) of the title compound.
lH NMR (CDCl3, ppm ranges are given because of amide rotomers and
line broadening) ~ 1.55-2.5 (m, 10H), 2.22 (s, 3H), 2.27 (s, 6H), 2.6-3.9
(m, 10H), 6.6-7.5 (m, 9H), 8.17 (d, lH, J = 12Hz).
Mass Spectrum (FAB) 594 (37Cl + 35Cl isotope), 592 (35Cl + 35Cl
isotope).
The following analogs were prepared by substituting the
appropriate acylation reagent for acetyl chloride in the above
procedure.
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)(methyl-
amino))butyl)- 1 -propionyl-spiro(indoline-3,4'-piperidine)
20 Mass Spectrum (FAB) 608 (37Cl + 35Cl isotope), 606 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)
(methylamino)) butyl)-1-formyl-spiro(indoline-3,4'-piperidine)
25 Mass Spectrum (FAB) 580 (37Cl + 35Cl isotope), 578 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)
(methylamino)) butyl)-1-t-butylcarbonyl-spiro(indoline-3,4'-piperidine)
30 Mass Spectrum (FAB) 636 (37Cl + 35Cl isotope), 634 (35Cl + 35Cl
isotope).
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 81 -
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)
(methylamino)) butyl)-1-methylaminocarbonyl-spiro(indoline-3,4'-
piperidine)
Mass Spectrum (FAB) 609 (M+H, 37Cl + 35Cl isotope), 607 (M+H,
5 35Cl + 35CI isotope).
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)
(methylamino)) butyl)-1-ethoxycarbonyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum (FAB) 624 (37CI + 35Cl isotope), 622 (35Cl + 35Cl
isotope).
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)
(methylamino)) butyl)-1-ethanesulfonyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum (FAB) 643 (37Cl + 35Cl isotope), 641 (35Cl + 35Cl
l 5 isotope).
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)
(methylamino)) butyl)-l-i-propanesulfonyl-spiro(indoline-3,4'-
piperidine)
20 Mass Spectrum (FAB) 657 (37Cl + 35Cl isotope), 655 (35Cl + 35Cl
isotope).
The following compound can also be prepared under the
conditions given above:
25 1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-fluoro-5-(trifluoromethyl)-
benzoyl)(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass Spectrum (FAB) (CI) 652 (37Cl + 35Cl isotope), 650 (35Cl +35Cl
isotope).
An alternative method (method B) is given below:
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)(methyl-
amino))butyl)- l -acetyl-spiro(indoline-3,4'-piperidine)
l -Acetyl-spiro(indoline-3,4'-piperidine)
WO 94/29309 PCT/US94/05545
2 T ~3~5
- 82 -
Acetyl chloride (1.4 mL, 19.9 mmol) was added to a
solution of 5.35 g (16.6 mmol) of 1'-benzyloxycarbonyl-spiro(indoline-
3,4'-piperidine) in 33 mL of CH2Cl2 and 3.2 mL (23.2 mmol) of Et3N
5 keeping the temperature between 0-5C by cooling in ice bath. After 10
min the cold bath was removed and reaction was stirred for 30 min at
which time a TLC indicated complete reaction. The solution was
diluted with CH2Cl2 and washed with water, brine and dried over
Na2SO4. The filtrate was concentrated to a thick oil and the oil was
dissolved in 40 mL of EtOH. Acetic acid (3 mL) and 0.8 g of 10%
Pd/C were added to the solution and the resulting mixture was
hydrogenated on a Parr apparatus for 3 h. The catalyst was filtered and
washed with EtOAc and the combined filtrate was concentrated. The
residue was partitioned between CH2Cl2 and water and 2N NaOH was
5 added to this mixture until the aqueous layer was basic. The layers
were separated and the aqueous layer was extracted with CH2Cl2. The
combined organic layer was washed with brine, dried over Na2SO4 and
the filtrate was concentrated to give 2.93 g (77%) of the title compound
sufficiently pure for use in the next reaction.
1 '-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5 -dimethylbenzoyl)(methyl-
amino)) butyl)-1-acetyl-spiro(indoline-3.4'-piperidine)
To a solution of 0.284 g (0.75 mmol)of 3-((S)-(3,4-
dichlorophenyl))-4-(N-(3 ,S -dimethylbenzoyl)(methylamino))butanal
25 (Example 5) in 2 mL of MeOH were added 0.166 g (0.72 mmol) of 1-
acetyl-spiro(indoline-3,4'-piperidine), 0.5 g of powdered 4 A molecular
sieves and 10 drops (ca. 0.1 mL) of acetic acid. After stirring the
mixture for 1.5 h a solution of 0.189 g (3 mmol) of NaCNBH3 in 3 mL
of THF was added. Some gas evolution was observed. After 30 min
30 when the reaction was complete by TLC the mixture was filtered
through a pad of celite, the reaction flask and the pad were rinsed with
MeOH. The filtrate was concentrated to approximately 3 mL and the
residue was diluted with EtOAc. The EtOAc solution was washed with
water, brine and dried over Na2so4. The filtrate was concentrated and
WO 94/29309 PCT/US94/05545
21 639~5
- 83 -
the residue was chromatographed on a flash column using 50% EtOAc-
hexane followed by 2% Et3N-EtOAc and finally 93:5:2 EtOAc: MeOH:
Et3N to isolate 0.317 g (74%) of the title compound as a white foam.
EXAMPLE 9
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(3,5-
dimethylbenzoyl(methylamino)) butyl)- 1 '-methyl- 1 -methanesulfonyl-
spiro(indoline-3,4'-piperidinium) iodide
A solution of 53 mg (0.084 mmol) of 1'-(3-((S)-(3,4-
dichlorophenyl))-4-(3,5-dimethylbenzoyl(methylamino))butyl)- 1 -
methanesulfonyl-spiro(indoline-3,4'-piperidine) in 5 drops of MeOH
was diluted with 1 mL of ether and 0.5 mL of methyl iodide was added.
The reaction mixture was stirred overnight while a solid was formed.
5 The yellowish solid was allowed to settle and the supernatent was
removed. The solid was washed with ether and dried to furnish 51 mg
(78%) of the title compound.
EXAMPLE 10
1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(R or S)-(3-methylbenzoyl)
(methyl-amino))pentyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
2s Step 1: N-Methoxy-N-methyl-2-(S)-(3,4-dichlorophenyl)-4-
pentenamide
A mixture of 306 mg (1.25 mmol) of (2S)-(3,4-
dichlorophenyl)-4-pentenoic acid (prepared according to the procedure
of Hale, J.J.; Finke, P.E.; MacCoss, M. Bioorganic & Medicinal
30 Chemistry Letters 1993,3, 319-322) and 202 mg (1.50 mmol) of 1-
hydroxybenzotriazole hydrate in 10 mL of methylene chloride was
cooled to 0C and treated with 287 mg (1.50 mmol) of 1-(3-dimethyl-
aminopropyl)-3-ethylcarbodiimide. The cooling bath was removed and
after 45 min. a solution of 365 mg (3.75 mmol) of N,O-dimethyl-
WO 94/29309 PCT/US94/05545
~163~5
- 84 -
hydroxylamine hydrochloride and 522 ~1 (3.75 mmol) of triethylamine
in 10 mL of methylene chloride was added via cannula. The mixture
was then stirred at 22C for 4 hours and then quenched with 10 mL of
water and diluted with 8 mL of methylene chloride. The layers were
5 separated and the aqueous layer was extracted with methylene chloride
(2 x 10 mL). The combined organic layers were washed with 10 mL of
brine, dried over anhydrous sodium sulfate, filtered, and concentrated
in vacuo. Flash chromatography on 75 g of silica gel using 1:9 v/v
ethyl acetate/ hexane as the eluant afforded 319 mg (89%) of the title
o compound as a clear oil.
lH NMR (400 MHz, CDCl3) ~ 2.40 (pentet, lH), 2.75 (pentet, lH), 3.13
(s, 3H), 3.52 (s, 3H), 3.99-4.01 (m, lH), 4.96-5.05 (m, 2H), 5.63-5.70
(m, lH), 7.15 (dd, lH), 7.35 (d, lH), 7.41 (d, lH).
Mass Spectrum (FAB): m/z 290 (M+H, 37Cl + 35Cl isotope, 50%), 288
5 (M+H, 37Cl + 37Cl isotope, 100%).
Step 2: 3-(S)-(3.4-dichlorophenyl)-5-hexen-2-one
A solution of 319 mg (1.11 mmoL) of N-methoxy-N-
methyl-2-(S)-(3,4-dichlorophenyl)-4-pentenamide (from Step 1 above)
20 in 10 mL of dry tetrahydrofuran was cooled to -70C and treated with
1.0 mL (1.40 mmol) of methyllithium and stirred between -70C to
-40C. After 3 hours, the reaction was quenched with 5 mL of water,
and diluted with 10 mL of ethyl acetate. The layers were separated and
the organic layer was washed with water (3 x 10 mL). The aqueous
25 layers were extracted with 10 mL of ethyl acetate. The combined
organic layers were washed with 10 mL of saturated aqueous sodium
chloride solution, dried over anhydrous sodium sulfate, filtered, and
concentrated in vacuo. Flash chromatography on 44 g of silica gel
using 1:3 v/v ethyl acetate/hexane as the eluant afforded 250 mg (93%)
3 of the title compound as a clear oil.
lH NMR (400 MHz, CDCl3) o 2.07 (s, 3H), 2.36 (pentet, lH), 2.72
(pentet, lH), 3.64 (t, lH), 4.95-5.01 (m, 2H), 5.55-5.65 (m, lH), 7.03
(dd, lH), 7.30 (d, lH), 7.39 (d, lH).
WO 94/29309 PCT/US94/05545
2 1 639q5
- 85 -
Mass Spectrum (FAB): m/z 245 (M+H, 37Cl + 35Cl isotope, 30%), 243
(M+H, 37Cl + 37Cl isotope, 50%), 155 (60%), 119 (100%).
Step 3: N-Methyl 3-(S)-(3,4-dichlorophenyl)-5-hexen-2-(RS)-
amine
A mixture of 102 mg (0.42 mmol) of 3-(S)-(3,4-
dichlorophenyl)-5-hexen-2-one (from Step 2 above), 170 mg (2.52
mmol) of methylamine hydrochloride, and 234 ,ul (1.68 mmol) of
triethylamine in 4.0 mL of methanol was treated with 16 mg (0.25
mmol) of sodium cyanoborohydride and stirred at 22C for 20 hours.
Saturated aqueous sodium bicarbonate solution (1.0 mL) was added and
the resulting milky mixture was diluted with 5.0 mL of ethyl acetate and
5.0 mL of water. The layers were separated and the organic layer was
washed with water (3 x 5 mL). The aqueous layers were extracted with
10 mL of ethyl acetate. The combined organic layers were washed with
10 mL of saturated aqueous sodium chloride solution, dried over
anhydrous sodium sulfate, filtered, and concentrated in vacuo. Flash
chromatography on 42 g of silica gel using 10:1 v/v ether/ hexane as the
eluant afforded 64 mg of the higher Rf isomer (Isomer A) and 22 mg of
a lower Rf isomer (Isomer B) both as yellow oils.
lH NMR (400 MHz, CDCl3); Isomer A: o 1.04 (d, 3 H), 2.29-2.35 (m,
4 H), 2.50-2.68 (m, 3H), 4.86-4.95 (m, 2H), 5.48-5.56 (m, lH), 7.01
(dd, lH), 7.26 (d, lH), 7.34 (d, lH); Isomer B: ~ 0.86 (d, 3H), 2.32-
2.50 (m, 4H), 2.51-2.53 (m, lH), 2.68-2.73 (m, 2H), 4.88-4.98 (m, 2H),
5.54-5.61 (m, lH), 6.97 (dd, lH), 7.22 (d, lH), 7.33 (d, lH).
Mass Spectrum (Isomer A) (FAB): m/z 260 (M+H, 37Cl + 35Cl
isotope, 70%), 258 (M+H, 35Cl + 35Cl isotope, 100%).
Step 4: N-Methyl-N-t-butoxycarbonyl-3-(S)-(3,4-dichlorophenyl)-
5 -hexen-2-(RS )-amine
A solution of 1.1 g (4.1 mmol) of N-methyl-(3-(S)-(3,4-
dichlorophenyl)-5-hexen-2-(R or S)-amine (Isomer B *om Step 3
above) in lO mL of dry methylene chloride was cooled to 0C and
treated with 690 ~l (5.0 mmol) of triethylamine and 1.2 g (5.3 mmol)
WO 94/29309 2 1 6 3 ~ 9 5 PCT/US94/05545
- 86 -
of di-tert-butyl dicarbonate. The cooling bath was removed and the
reaction was stirred at 22C for 20 hours. The reaction was quenched
with 10 mL of water and diluted with 25 mL of methylene chloride.
The layers were separated and the aqueous layer was extracted with
5 methylene chloride (2 x 10 mL). The combined organic layers were
washed with 15 mL of brine, dried over anhydrous sodium sulfate,
filtered, and concentrated in vacuo. Flash chromatography on 72 g of
silica gel using 1 :3 v/v ethyl acetate/ hexane as the eluant afforded 1.4 g
(95%) of the title compound as a yellow oil.
o l H NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) ~ 1.24-5.70 (22H), 6.88-7.40 (3H), 1.50 (s, 3H, N-
CH3).
Mass Spectrum (FAB): m/z 358 (M+H, 37CI + 35Cl isotope, 30%), 302
(100%).
Step 5: N-Methyl-N-t-butoxycarbonyl-3-(S)-(3,4-dichlorophenyl)-
4- (RS ) -amino-pentanal
A solution of 1.4 g (3.9 mmol) of N-methyl-N-t-butoxy-
carbonyl-3-(S)-(3,4-dichlorophenyl)-5-hexen-2-(RS)-amine (from Step
4 above) in 20 mL of 2:1:1 v/v/v acetone/t-butanol/water was treated
with 30 mg (0.12 mmol) of osmium tetroxide. After 5 min., 691 mg
(5.90 mmol) of N-methylmorpholine N-oxide was added and the
resulting mixture was stirred at 22C for 4 hours. The reaction was
quenched with 491 mg of sodium bisulfite and concentrated in vacuo to
25% of the original volume. The residue was partitioned between 20
mL of methylene chloride and 10 mL of water and the layers were
separated. The aqueous layer was extracted with methylene chloride (2
x 10 mL). The combined organic layers were dried over anhydrous
sodium sulfate, filtered, and concentrated in vacuo.
A solution of the crude diol in 24 mL of 3:1 v/v
tetrahydrofuran/water was treated with 1.1 g (5.1 mmol) of sodium
periodate and stirred at 22C for 20 hours. The reaction mixture was
partitioned between 20 mL of ethyl ether and 10 mL of water and the
layers were separated. The organic layer was washed with water (2 x
WO 94/29309 PCT/US94/05545
2 1 639~5
- 87 -
15 mL), dried over anhydrous sodium sulfate, filtered, and concentrated
in vacuo. Flash chromatography on 68 g of silica gel using 4:1 v/v
ethyl ether/hexane as the eluant afforded 372 mg of the higher Rf
isomer (Isomer A) and 879 mg of a lower Rf isomer (Isomer B) both as
5 yellow oils.
1H NMR (400 MHz, CDCl3) Isomer B: o 1.19-1.34 (m, 13H), 2.45 (s,
3H, N-CH3), 2.68-2.81 (m, 2H), 3.28-3.34 (m, lH), 4.20-4.50 (m, lH),
6.98-7.32 (m, 3H), 9.60 (s, lH, -CHO).
Mass Spectrum (Isomer B) (FAB): m/z 360 (M+H, 37Cl + 35Cl
isotope, 20%), 242 (100%).
Step 6: 1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(R or S)-(t-butoxy-
carbonyl)(methylamino))pentyl)- 1 -methanesulfonyl-
spiro(indoline-3 ~4'-piperidine)
A mixture of 217 mg (0.60 mmol) of N-methyl-N-t-
butoxycarbonyl-3-(S)-(3,4-dichlorophenyl)-4-(RS)-amino-pentanal
(from Step 5 above) and 262 mg (0.86 mmol) of 1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) hydrochloride in 13 mL of methanol was
treated with 115 mg (1.83 mmol) of sodium cyanoborohydride and
20 stirred at 22C for 20 hours. Saturated sodium bicarbonate solution
(1.0 ml) was added and the resulting milky mixture was concentrated to
50% of its original volume. The residue was partitioned between 25
mL of ethyl acetate and 15 mL of water and the layers were separated.
The organic layer was washed with water (3 x 10 mL). The aqueous
25 layers were extracted with 20 mL of ethyl acetate. The combined
organic layers were washed with 15 mL of brine, dried over anhydrous
sodium sulfate, filtered, and concentrated in vacuo. Flash chromato-
graphy on 42 g of silica gel using 5:95 v/v methanol/methylene chloride
as the eluant afforded 329 mg (89%) of the title compound as a white
30 foam.
1H NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) ~ 1.20-2.90 (31H), 3.74 (s, 3H, N-SO2CH3), 7.05-
7.41 (m, 8H).
WO 94/29309 PCT/US94/05545
21 63',q5
- 88 -
Mass Spectrum (FAB): m/z 612 (M+H, 37Cl + 35Cl isotope, 70%), 610
(M+H, 37Cl + 37Cl isotope, 100%).
Step 7: 1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(R or S)-
(methylamino))pentyl)- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine)
To a solution of 329 mg (0.54 mmol) of 1'-(3-(S)-(3,4-
dichlorophenyl)-4-N((R or S)-(t-butoxycarbonyl)
(methylamino))pentyl) 1-methanesulfonyl-spiro(indoline-3,4'-
piperidine) (from Step 6 above) in 8.0 mL of dry methylene chloride at
0C was added 117 ~l (1.1 mmol) of anisole and 2.0 mL of
trifluoroacetic acid. The cooling bath was removed and the reaction
was stirred at 22C for 20 minutes. The reaction was concentrated in
vacuo. The residue was partitioned between 10 mL of methylene
5 chloride and 5.0 mL of water. The organic layer was washed with 2N
NaOH (3 x 5 mL), dried over anhydrous sodium sulfate, filtered, and
concentrated in vacuo. Flash chromatography on 42 g of silica gel
using 5:95:0.5 v/v/v methanol/methylene chloride/ammonium hydroxide
as the eluant afforded 221 mg (80%) of the title compound as a clear
20 oil.
1H NMR (400 MHz, CDCl3) ~ 1.05 (d, 3H, J = 6.2Hz), 1.62-2.85 (m,
17H), 2.30 (s, 3H, N-CH3), (7.03-7.37 (m, 7H).
Mass Spectrum (FAB): m/z 512 (M+H, 37Cl + 35Cl isotope, 70%), 510
(M+H, 37Cl + 37Cl isotope, 100%).
Step 8: 1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(R or S)-(3-methyl-
benzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-
spiro(indoline -3.4' -piperidine)
The title compound was prepared from 1'-(3-(S)-(3,4-
30 dichlorophenyl)-4-(R or S)-(methylamino)pentyl)-1 -methanesulfonyl-
spiro(indoline-3,4'-piperidine) (from Step 7 above) using a procedure
identical to Example 3, Step (b), substituting m-toluoyl chloride for
3,5-dimethylbenzoyl chloride.
WO 94/29309 ~ 1 6 3 9 9 5 PCT/US94/05545
- 89 -
lH NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) ~ 1.42 (â, 3H, J = 6.7Hz), 1.60-2.30 (16H), 2.54
(s, 3H, Ph-CH3), 2.87 (s,3H, N-CH3),3.74 (s, 3H, N-SO2CH3),7.05-
7.79 (m, 1 lH).
Mass Spectrum (FAB): m/z 630 (M+H,37Cl + 35Cl isotope,70%), 628
(M+H, 37Cl + 37Cl isotope, 100%).
EXAMPLE 11
1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(R or S)-(3,5-bis(trifluoromethyl)-
benzoyl)(methylamino))pentyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
The title compound was prepared from 1'-(3-(S)-(3,4-
dichlorophenyl)-4-(R or S)-(methylamino)pentyl)-1-methanesulfonyl-
15 spiro(indoline-3,4'-piperidine) (from Example 1, Step 7 above) using a
procedure identical to Example 3 Step (b), substituting 3,5-
bis(trifluoromethyl)benzoyl chloride for 3,5-dimethylbenzoyl chloride.
lH NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) ~ 1.38-3.00 (22H), 3.74 (s, 3H, N-SO2CH3), 6.40-
7.41 (m, lOH).
Mass Spectrum (FAB): m/z 752 (M+H,37Cl + 35Cl isotope, 40%),750
(M+H, 37Cl + 37Cl isotope, 60%), 241 (100%).
EXAMPLE 12
1'-(3-(S)-(3,4-Dichlorophenyl)-4-(R or S)-(3,5-
dimethylbenzoyl(methyl-amino))pentyl)- 1 -methanesulfonyl-
spiro(indoline-3 ~4'-piperidine)
The title compound was prepared from 1'-(3-(S)-(3,4-
dichlorophenyl)-4-(R or S)-(methylamino)pentyl)-1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) (from Example 1, Step 7 above) using a
procedure identical to Example 3, Step (b).
WO 94/29309 PCTIUS94/05545
21 63~5
- 90
lH NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) â 1.37-2.86 (28H), 3.74 (s, 3H, N-S02CH3), 6.24-
7.41 (m, lOH).
Mass Spectrum (FAB): m/z 642 (M+H,37Cl + 35Cl isotope, 70%), 644
(M+H, 37Cl + 37Cl isotope, 100%).
EXAMPLE 13
(1'-(3-(S)-(3,4-Dichlorophenyl)-4-(R or S)-(3,5-dichlorobenzoyl-
(methylamino))pentyl)-1-methanesulfonyl-spiro(indoline-3,4'-
piperidine)
The title compound was prepared from 1'-(3-(S)-(3,4-
dichlorophenyl)-4-(R or S)-(methylamino)pentyl)-1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) (from Example 1, Step 7 above) using a
procedure identical to Example 3, Step (b), substituting 3,5-dichloro-
benzoyl chloride for 3,5-dimethylbenzoyl chloride.
lH NMR (400 MHz, CDCl3, ranges are given due to amide rotamers
and line broadening) ~ 1.38-2.93 (22H), 3.73 (s, 3H, N-SO2CH3), 6.53-
7.42 (m, lOH).
Mass Spectrum (FAB): m/z 684 (M+H,37Cl + 35Cl isotope,70%), 686
(M+H,37Cl + 37Cl isotope, 100%).
EXAMPLE 14
1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-bromo-5-methylbenzoyl)-
2 5 (methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3.4'-piperidine)
Step A: 3-Bromo-5-methylbenzoic acid
To a solution of 0.38 g (1.44 mmol) of 3-bromo-5-methyl-
benzyl bromide (prepared by NBS bromination of 3,5-dimethyl-
3 bromobenzene) in 22 mL of MeCN and 50 mL of water was added 7.8
mL (28.8 mmol) of agueous sodium hypochlorite (13% active Cl). The
mixture was allowed to stand in an ultrasonic cleaning bath for 14 h.
The reaction was acidified with HCl to pH 3 and extracted with
CH2Cl2. The organic layer was washed with water, brine and dried
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 91 -
with Na2SO4. The filtrate was concentrated and the residue which was
a mixture of the desired acid and the aldehyde was dissolved in 3 mL of
acetone. The solution was treated with 6 N Jones reagent until the
orange color persisted. After stirring for 20 min the excess reagent was
5 destroyed by adding few drops of i-PrOH. The solution was diluted
with water and extracted with CH2Cl2. The CH2Cl2 layer was washed
with brine, dried and the filtrate was concentrated. The residue was
purified by prep TLC using 0.5:30:69.5 of HOAc:EtOAc:hexane to
isolate 0.14 g (45 %) of 3-bromo-5-methylbenzoic acid.
Step B: 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-bromo-5-
methylbenzoyl)(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3.4'-piperidine)
3-Bromo-5-methylbenzoic acid was used in the acylation
5 reaction according to the procedure of Example 3, Step B to obtain the
title compound.
Mass Spectrum (CI) 696 (37Cl + 35Cl isotope), 694 (35Cl + 35Cl
isotope).
EXAMPLE 15
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)-
(methvlamino))butyl)- 1 -(2-aminoacetyl)-spiro(indoline-3.4'-piperidine)
A solution of 65 mg (0.31 mmol) of carbobenzyl-
2s oxyglycine in 3 mL of CH2Cl2 was treated with 82 mg (0.41 mmol) of1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 56 mg (0.41
mmol) of 1-hydroxybenzotriazole and 42 mg (0.41 mmol) of N-
methylmorpholine. After 10 min 123 mg (0.21 mmol) of 1'-(3-((S)-
(3,4-dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)(methylamino))-
30 butyl)-spiro(indoline-3,4'-piperidine) (Example 7) was added and the
reaction was stirred.for 2 h. The mixture was diluted with CH2Cl2 and
washed with water, brine, dried and concentrated to give 0.184 g of
residue. The residue in 10 drops of HOAc was dissolved in 3 mL of
EtOH and the solution was hydrogenated on a Parr apparatus for 16 h.
WO 94/29309 PCT/US94/05545
2 1 639q5
- 92 -
The catalyst was filtered and washed with EtOAc. The filtrate was
washed with 10% Na2co3~ brine and concentrated. The residue was
purified by prep TLC using 30% MeOH-EtOAc to give 80 mg (59%) of
the title compound.
Mass Spectrum (CI) 651 (37Cl + 35Cl isotope), 649 (35Cl + 35Cl
isotope).
EXAMPLE 16
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)-
(methylamino))butyl)- 1 -methyl-spiro(indol-2-one-3,4'-piperidine)
Step A: 1.1'-Dimethvl-spiro(indol-2-one-3.4'-piperidine)
A solution of 0.1 g (0.68 mmol) of N-methyl-2-oxo-indole
in 2 mL of THF was added to a well stirred suspension of 0.14 g (3.4
mmol) of NaH in 2 mL of THF with cooling in ice bath. After the gas
evolution had stopped the cold bath was removed and the mixture was
heated in a 50C bath for another 15 min. The reaction was allowed to
cool to room temperature and 0.68 mL of DMSO was added and more
gas evolution was observed. After stirring for 10 min, the reaction
mixture was cooled in ice bath and 0.144 g of mechloreth~mine hydro-
chloride was added. The mixture was warmed to room temperature and
stirred overnight. Next morning, the reaction was quenched with water
and extracted with EtOAc. The EtOAc extact was washed with brine,
dried with Na2so4 and filtered. The filtrate was concentrated and the
residue was purified by prep TLC using 89:10:1 EtOAc:MeOH:Et3N to
furnish 25 mg (15%) of the title compound.
Step B: l-Methyl-spiro(indol-2-one-3.4'-piperidine)
A solution of 25 mg (0.11 mmol) of 1,1'-dimethyl-
spiro(indol-2-one-3,4'-piperidine) (from Step A above) in 1 mL of dry
dichloroethane was treated with 0.023 mL (0.22 mmol) of 1-chloroethyl
chloroformate (ACECl) under a dry N2 atmosphere. After 30 min at
room temperature, the solution was kept in a 50C bath for 30 min.
The reaction mixture was cooled to room temperature, 2 mL of MeOH
was added and reheated to 60C. After 30 min the solution was cooled
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 93 -
and concentrated in vacuo. The residue was partitioned between water
and EtOAc and the aqueous phase was adjusted to pH 9 by adding lN
NaOH. The layers were separated and the combined EtOAc solution
was washed with brine and dried. The filtrate upon concentration gave
5 34 mg of a residue which was a mixture of the desired compound and
the starting material, but was sufficiently pure to be used in the next
reaction.
Step C: 1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)- 1 -methyl-spiro(indol-2-one-
3 4'-piperidine)
A reaction of 49 mg (0.13 mmol) of 3-((S)-(3,4-dichloro-
phenyl))-4-(N-(3,5-dimethylbenzoyl)methylamino)butanal (Example 5)
with 34 mg of impure 1-methyl-spiro(indol-2-one-3,4'-piperidine)
5 (from Step B) according to the procedure of Example 8, method B
furnished 32 mg of the title compound after purification by prep TLC.
Mass Spectrum (CI) 580 (37Cl + 35Cl isotope), 578 (35Cl + 35Cl
isotope).
EXAMPLE 17
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl)-
(methylamino))butyl)- 1 -methyl-spiro(isoindol- 1 -one-3.4'-piperidine)
Mass Spectrum (CI) 622 (37Cl + 35Cl isotope), 620 (35Cl + 35Cl
25 isotope).
EXAMPLE 18
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)-
3 (methylamino))butyl)-spiro(2-oxo-tetrahydroquinoline-4.4'-piperidine)
Step A: l'-Trifluoroacetyl-spiro(1-indanone-3.4'-piperidine)
Cold trifluoroacetic acid (15 mL) and 0.6 mL of anisole
were added to 2 g (6.6 mmol) of 1'-t-butoxycarbonyl-spiro(1-indanone-
WO 94/29309 PCT/US94/05545
21 63~95
- 94 -
3,4'-piperidine) and the resulting solution was stirred in ice bath for lh.
The reaction mixture was concentrated in vacuo and the residue was
partitioned between CH2cl2 and 0.5 N NaOH. The organic layer was
washed with brine, dried with Na2so4 and concentrated. The residual
orange oil was dissolved in 10 mL of CH2cl2 and 1.92 mL (13.7 mmol)
of Et3N, 1 mL (7.1 mmol) of trifluoroacetic anhydride and 3 crystals
of DMAP were added. After stirring for 4 h, the reaction mixture was
diluted with CH2Cl2 and washed with water, brine and dried. The
solution was filtered and the filtrate was concentrated to yield 2.0 g
(quantitative) of the desired product as a solid.
lH NMR (CDCl3) ~ 1.65 (d, 2H, J=14Hz), 2.05 (m, 2H), 2.67 (ABq,
2H), 2.89 (m, lH), 3.28 (m, lH), 4.1 l(d, lH, J=14Hz), 4.67 (dt, lH,
J=14 and 2Hz), 7.5-7.8 (m, 4H).
Step B: 1'-Trifluoroactyl-spiro-(2-oxo-1,2,3,4-tetrahydro-
quinoline-4,4'-piperidine) and 1'-trifluoroactyl-spiro-(1-
oxo- 1~2~3 .4-tetrahvdroisoquinoline-4.4'-piperidine)
To a mixture of 1.09 g (16.8 mmol) of Sodium azide in 1.2
mL of water and 6.6 mL of CHCl3 was added 0.46 mL of concentrated
H2SO4 (36 N) keeping the temperature between 0-5C. (Caution!)
After 10 min the cold bath was removed and the reaction was stirred
for 3 h, at which time the CHCl3 layer was separated from the aqueous
layer. The CHCl3 layer containing HN3 was dried and the filtrate was
added to a solution of 2 g (6.7 mmol) of 1'-trifluoroacetyl-spiro(1-
2s indanone-3,4'-piperidine) (from Step A) in 7 mL of CHCl3.
Concentrated H2SO4 (1.8 mL) was added to this solution and the
reaction was allowed to age for 30 min. The mixture was heated in a
45C bath for 45 min and then stirred at room temperature for 16 h.
Next morning, the reaction mixture was poured into ice and the layers
were separated. The aqueous layer was neutralized with aq. NaOH and
extracted with EtOAc. The combined organic phases were washed with
brine, dried and concentrated. The residue was chromatographed using
50-80% EtOAc-CH2Cl2 to isolate 0.34 g (16%) of 1'-trifluoroactyl-
spiro(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-piperidine) and 0.13 g of
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 95 -
1 '-trifluoroactyl-spiro(1 -oxo- 1,23,4-tetrahydroisoquinoline-4,4'-
piperidine). In addition, 0.72 g (36%) of the starting indanone was
recovered.
lH NMR (CDCl3) Isomer A: ~ 1.82 (m, 2H), 1.96 (m, 2H), 2.75 (ABq,
2 H, J=14Hz), 3.16 (t, lH), 3.46 (t, lH), 3.9 (d, lH), 4.42 (d, lH), 6.8-
7.3 (m, 4H), 8.49 (br s, lH); Isomer B: ~ 1.9-2.1 (m, 4H), 3.09 (t, lH),
3.42 (m, lH), 3.61 (ABq, 2H), 3.94 (d, lH), 4.45 (d, lH), 6.72 (br s,
lH), 7.3-7.6 (m, 3H), 8.11 (d, lH).
Step C: Spiro-(2-oxo-1.2.3.4-tetrahydroquinoline-4.4'-piperidine)
To a solution of 0.3 g (0.97 mmol) of 1'-trifluoroacetyl-
spiro-(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-piperidine) (from Step B)
in 4 mL of MeOH was added 0.16 g (2.9 mmol) of KOH in 1 mL of
water. After stirring the reaction for 16 H the solution was
15 concentrated and the residue was partitioned between EtOAc and water.
The EtOAc layer was washed with brine, dried with Na2SO4 and
concentrated to give 0.16 g (76%) of the title compound as a white
solid.
lH NMR (CDCl3) ~ 1.6-2.0 (m, 4H), 2.72 (s, 2H), 2.95 (m, 4H), 6.7-
20 7.4 (m, 4H), 8.4 (br s, lH).
Step D: 1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethyl-
benzoyl)(methylamino))butyl)-spiro(2-oxo-tetrahydro-
quinoline-4.4'-piperidine)
The title compound was obtained by reductive ~min~tion of
3 -((S)-(3,4-dichlorophenyl))-4-((3,5-dimethylbenzoyl)methylamino)-
butanal (Example 5) by spiro-(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-
piperidine) obtained in Step C according to the procedure of Example 8,
method B.
30 Mass Spectrum (CI) 580 (37Cl + 35Cl isotope), 578(35Cl + 35Cl
isotope).
WO 94/29309 21 6 3~ q 5 PCT/US94/05545
- 96 -
EXAMPLE 19
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5-dichlorobenzoyl)(methyl-
amino))butyl)- 1 -methyl-spiro(2-oxo-tetrahydroquinoline-4,4'-
5 piperidine)
Step A: 1-Methyl-spiro-(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-
piperidine)
To a solution of 0.15 g (0.48 mmol) of 1'-trifluoroacetyl-
spiro-(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-piperidine) (from
Example 18, Step B) in 1.7 mL of DMF was added 19 mg (0.77 mmol)
of 95% NaH at 0C. After 15 min 0.063 mL (1 mmol) of methyl iodide
was added and the reaction was allowed to warm to room temperature.
After stirring for 16 H the reaction was not complete, so an additional
5 0.015 mL of methyl iodide was added and the solution was heated in a
45C bath. After 2 H the reaction was cooled to room temperature and
partitioned between EtOAc and water. The EtOAc layer was washed
with brine, dried and the filtrate was concentrated. The residue was
purified by prep TLC using 33% EtOAc-hexane to provide 1-methyl-
2 1 '-trifluoroacetyl-spiro-(2-oxo- 1 ,2,3 ,4-tetrahydroquinoline-4,4'-
piperidine). Hydrolysis of this trifluoroacetamide as described in
Example 55, Step C furnished 71 mg (64%) of the title compound.
lH NMR (CDCl3) â 1.61 (d, 2H), 1.92 (m, 2H), 2.74 (s, 2H), 2.98 (m,
4H), 3.36 (s, 3H), 7.0-7.4 (m, 4H).
2s
Step B: 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dichloro-
benzoyl)(methylamino))butyl)- 1 -methyl-spiro(2-oxo-
tetrahydroquinoline-4 ~4'-piperidine
The title compound was prepared by reaction of the amine
30 from Step A and 3-((S)-(3,4-dichlorophenyl))-4-((3,5-dichlorobenzoyl)-
methylamino)butanal as described in Example 18, Step D.
Mass Spectrum (CI) 636 (37Cl + 35Cl isotope), 634 (35Cl + 35Cl
isotope).
EXAMPLE 20
WO 94/29309 PCT/US94/05~45
21 63995
- 97 -
4-Bromo-2-(S)-(4-fluorophenyl)- 1 -(N-(3,5-
bistrifluoromethylbenzoyl)methylamino)butane .
Step A: 3-(S)-(4-Fluorophenyl)-4-(N-(3,5-
5 bistrifluoromethylbenzoyl)methylamino)butanol.
A solution of 1.67 g (3.84 mmol) of 3-((S)-(4-fluorophenyl)-4-(N-(3,5-
(bistrifluoromethyl)benzoyl)(methylamino))butanal (prepared from 4-
fluorophenylacetic acid as described by J. Hale et. al., Bioorganic &
10 Medicinal Chemistry Letters 1993,3, 319-322) in 16 mL of absolute
ethanol at 0 C was treated with 172 mg (4.55 mmol) of sodium
borohydride. After stirring for 1 h at room temperature, the reaction
was quenched with saturated NH4Cl and extracted twice with ethyl
acetate. The combined organic layers were washed with brine, dried
15 (MgSO4) and evaporated to give 1.59 of residual oil. Purification on a
silica gel flash column (30:70 then 50:50 ethyl acetate:hexanes) provided
1.21 g (72%) of the title compound as a viscous oil.
Mass Spectrum (CI/NH3) M+H= 438.
Step B: 4-Bromo-2-(S)-(4-fluorophenyl)- 1 -(N-(3,5-
bistrifluoromethylbenzoyl)methylamino)butane.
To a solution of 1.19 g (2.72 mmol) of 3-(S)-(4-fluorophenyl)-4-(N-
25 (3,5-bistrifluoromethylbenzoyl)methylamino)butanol in 20 mL of
acetonitrile was added 1.49 g (3.53 mmol) of triphenylphosphine
dibromide. After 1.5 h the reaction was partitioned between ethyl
acetate and water. The organic layer was washed with brine, dried
(MgSO4) and concentrated to give 2.33 g of crude white solid.
30 Purification on a silica gel flash column (30:70 ethyl acetate:hexanes)
gave 944 mg (69%) of the desired bromide as a viscous oil.
Mass Spectrum (CIJNH3) M+H=S00, 502 (79,81Br isotope).
EXAMPLE 21
WO 94/29309 PCT/US94/05~45
~1 63~95
- 98 -
1 '-(3-(S)-(4-Fluorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine).
To 40 mg (0.08 mmol) of the bromide prepared in Example 20, Step B
and 0.21 ul (0.12 mmol) of N,N-diisopropylethylamine in 0.5 mL of
acetonitrile was added 37 mg (0.16 mmol) of 1-acetyl-spiro(indoline-
3,4'-piperidine). The resultant mixture was heated in a tightly capped
vial at 50 C for four days. The solvent was evaporated and the
residue was purified on a 1000 micron silica gel prep plate (93:5:2 ethyl
acetate:methanol:triethylamine) to furnish 46.6 mg (90%) of the title
compound as a white foam.
Mass Spectrum (CIINH3) M+H=650.
The compounds in Examples 22-26 were prepared as in Example 21
from the requisite bromide, prepared from the corresponding
20 phenylacetic acid as described in Example 20, and the required 1-
substituted-spiro(indoline-3,4'-piperidine) .
EXAMPLE 22
1 '-(3 -(S )-(3 -Chlorophenyl)-4-(N-(3,5 -bistrifluoromethylbenzoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine).
Mass Spectrum (CI/NH3) M+H= 666, 668 (35,37Cl-isotope).
EXAMPLE 23
WO 94/29309 PCT/US94/05545
2 1 63995
99
1 '-(3-(S)-(4-Chlorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine).
Mass Spectrum (CI/NH3) M+H= 666, 668 (35,37Cl-isotope).
EXAMPLE 24
1 '-(3-(S)-(3,4-Difluorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine).
Mass Spectrum (CI/NH3) M+H= 668.
EXAMPLE 25
l '-(3-(S)-(3,4-Methylenedioxyphenyl)-4-(N-(3,5-
20 bistrifluoromethylbenzoyl)-(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3,4'-piperidine) .
Mass Spectrum (CI/NH3) M+H=712.
2s
EXAMPLE 26
l '-(3-(RS)-(3,5-Dichlorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
30 (methylamino))butyl)- l -methanesulfonyl-spiro(indoline-3,4'-
piperidine).
Mass Spectrum (CI/NH3) M+H=736, 738 (35,37Cl-isotope).
WO 94/29309 PCT/US94/0~54~
2 1~ 6~995
- 100-
EXAMPLE 27
1 '-(3 -(S)-(4-Chlorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
5 (methylamino))butyl)-spiro(2,3-dihydrobenzothiophene-3,4'-
piperidine).
The title compound was prepared as in Example 21 from 4-bromo-2-
(S)-(4-chlorophenyl)- 1 -(N-(3,5-
bistrifluoromethylbenzoyl)methylamino)butane and spiro(2,3-
dihydrobenzothiophene-3,4'-piperidine) hydrochloride except that 3 eq.
of diisopropylethylamine were used.
Mass Spectrum (CI/NH3) M+H=641,643 (35,37Cl-isotope).
EXAMPLE 28
1 '-(3 -(RS)-(4-Pyridyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
2 o (methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine).
The title compound was prepared from 3-(S)-(4-pyridyl)-4-(N-(3,5-
bistrifluoromethyl-benzoyl)methylamino)butanal (prepared from 4-
pyridylacetic acid as described by J. Hale et. al., Bioorganic &
25 Medicinal Chemistry Letters 1993,3, 319-322) by reductive amination
as described in Example 2.
Mass Spectrum (CVNH3) M+H=633.
EXAMPLE 29
1 '-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(3,5-dimethylbenzoyl)-
(ethylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine).
WO 94/29309 PCT/US94/05545
2 1 639~5
- 101 -
Step A: 4-Bromo-2-(S)-(3,4-dichlorophenyl)-1-(N-(3,5-
dimethylbenzoyl)methylamino)butane.
The title compound was prepared as in Example 20, Steps A and B,
from 3-(S)-(3,4-dichlorophenyl)-4-(N-(3,5-dimethyl-
benzoyl)ethylamino)butanal (prepared from 3,4-dichlorophenylacetic
acid as described by J. Hale et. al., (Bioorganic & Medicinal Chemistry
Letters 1993,3, 319-322) using ethylamine rather than methylamine to
form the intermediate amide).
Mass Spectrum (CI/NH3) M = 454, 456 (79,81Br isotope).
Step B: 1'-(3-(S)-(3,4-Dichlorophenyl)-4-(N-(3,5-
dimethylbenzoyl)-(ethylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3,4'-piperidine).
The title compound was prepared from the bromide prepared in Step A
and 1-acetyl-spiro(indoline-3,4'-piperidine) as described in Example 21.
Mass Spectrum (CVNH3) M+H = 641, 643 (35,37Cl-isotope).
EXAMPLE 30
2s
5-Fluoro-spiro(2 3-dihydrobenzofuran-3.4'-piperidine) hydrochloride
salt
Step 1) 4-(2,5-Difluorophenyl)-4-methoxycarbonyl- 1 -methylpiperidine
Methyl 2,5-difluorophenylacetate (4.8 g, 26 mmol) and
mechloreth~mine hydrochloride (5.0 g, 26 mmol) in DMSO (15 mL)
and THF (50 mL) at 0C was carefully treated with NaH (2.5 g, 104
mmol). The reaction was gradually warmed to reflux over 1 h and
WO 94129309 2` 1 6 3 9 9 5 PCT/US94/05545
- 102-
refluxed further for 1 h. The reaction was cooled to 0C, and 6N HCl
(25 mL) was slowly added. The reaction was diluted with lN HCl (200
mL) and washed with hexane (200 mL). The aqueous layer was cooled
to 0C and adjusted to pH 12 with solid K2co3. The product was
extracted with ethyl acetate (200 mL), washed with brine (100 mL),
dried (MgSO4), and concentrated to 4.1 g (59%) of the title compound
as an oil.
H NMR (400 MHz, CDCl3) ~ 7.34 (dq, lH), 6.88 (m, lH) 6.78 (ddd,
lH) 6.69 (minor NMe invertomer, dm), 6.59 (minor NMe invertomer,
dd), 3.69 (s, 3H), 3.80 (minor invertomer, s), 2.71 (d, 2H), 2.48 (d,
2H), 2.38 (t, 2H), 2.25 (s, 3H), 2.10 (t, 2H) ppm.
Step 2) 4-(2,5-Difluorophenyl)-4-hydroxymethyl-1-methylpiperidine
EtOH (5.1 mL, 86 mmol) was added to 0.5 M LiAlH4 in glyme (82 mL,
41 mmol) at 0C. 4-(2,5-difluorophenyl)-4-methoxycarbonyl- 1 -
methylpiperidine (3.45 g, 12.8 mmol) in glyme (4 mL) was added.
Saturated aqueous sodium potassium tartrate (50 mL) was added along
with Celite (10 g), and the mixture was mechanically stirred 1 h at
room temp. The slurry was filtered, and the organic layer was
extracted with lN HCl. The HCl was washed with EtOAc and then
basified with 3N NaOH. The product was extracted with CH2Cl2,
washed with 20% brine, dried (MgSO4), and concentrated to a crude
solid, which was recrystallized (EtOAc) to yield 1.46 g (52%) of the
title compound as colorless crystals.
IH NMR (400 MHz, CDC13) ~ 7.28 (dt, lH, J =7,9 Hz), 6.88 (ddd, lH,
J=3,9,9 Hz), 6.81 (ddd, J=3,9,13 Hz), 3.76 (s, 2H), 2.59 (m, 2H), 2.32-
2.20 (m, 4H), 2.23 (s, 3H), 1.96 (t, 2H, J=5 Hz) ppm.
WO 94129309 2 1 6 3 9 ~ 5 PCT/US94/0554~
- 103 -
Step 3) 5-Fluoro-1'-methyl-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
NaH (158 mg, 6.56 mmol) was added to 4-(2,5-difluorophenyl)-4-
hydroxymethyl-1-methylpiperidine (1.45 g, 6.56 mmol) in DMF (20
mL). The slurry was heated to 90C for 6 h. The reaction was diluted
with hexane (200 mL), washed with water (200 mL), brine (50 mL),
dried (MgSO4), and concentrated to yield 1.21 g (92%) of the title
compound as a white crystalline solid;
1H NMR (400 MHz, CDC13) ~ 6.98 (dd, lH), 6.54 (dt, lH), 6.48 (dd,
lH), 4.37 (s, 2H), 2.84 (m, 2H), 2.31 (s, 3H), 1.97 (4H, pentuplet), 1.71
(m, 2H) ppm.
Step 4) 5-Fluoro-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
hydrochloride salt
5 -fluoro- 1 '-methyl-spiro(2,3 -dihydrobenzofuran-3,4'-piperidine) (1.21
g, 5.48 mmol) in 1,2-dichloroethane (12 mL) at room temp was treated
with 2-chloroethyl chloroformate (1 mL, 9 mmol). A white precipitate
formed, and the reaction was refluxed 2 h. MeOH (12 mL) was added
and refluxing was continued for 2 h. The reaction was concentrated to
a crude solid, which was triturated with EtOAc (5 mL) and filtered to
yield 1.27 g (95%) of the title compound as a white crystalline solid.
1H NMR (400 MHz, d6-DMSO) ~ 9.12 (s, lH), 9.04 (s, lH), 7.11 (dd,
lH), 7.74-7.66 (m, 2H), 4.53 (s, 2H), 3.26 (d, 2H), 2.95 (t, 2H), 2.08 (t,
2H), 1. 79 (d, 2H) ppm.
Reaction of 5-fluoro-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
hydrochloride with 3-((S)-(3,4-dichlorophenyl))-4-(N-(t-
butoxycarbonyl)-(methylamino))butanal according to the procedure
WO 94/29309 2 1 6 3 q q 5 PCT/US94/05545
- 104-
given in Example 8, Method B gave 1'-(3-((S)-(3,4-dichlorophenyl))-4-
(N-(t-butoxycarbonyl)-(methylamino))butyl)-5 -fluoro-spiro(2,3 -
dihydrobenzofuran-3,4'-piperidine). Removal of the BOC group and
benzamide formation according to the procedure given in Example 3,
5 Steps A and B afforded the compounds listed in Examples 31-36:
EXAMPLE 31
l '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl) -
(methylamino))butyl)-5-fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
15 Mass spectrum (CI): m/z = 611.2 (35Cl + 35Cl isotope + H+), 613.2
(37Cl + 35Cl isotope + H+).
EXAMPLE 32
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-5 -fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
Mass spectrum (CI): m/z = 609.3 (35Cl + 35Cl isotope + H+), 611.3
(37Cl + 35Cl isotope + H+).
EXAMPLE 33
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-chlorobenzoyl)-
(methylamino))butyl)-5-fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
WO 94/29309 PCT/US94/0554~
21 639q5
- 105-
Mass spectrum (CI): m/z = 575.2 (35Cl + 35Cl isotope + H+), 577.2
(37Cl + 35Cl isotope + H+), 579.2 (37Cl + 37Cl isotope + H+).
EXAMPLE 34
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-S-fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
Mass spectrum (CI): m/z = 569.3 (35Cl + 35Cl isotope + H+), 571.3
5 (37Cl + 35Cl isotope + H+).
EXAMPLE 35
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-methylbenzoyl)-
(methylamino))butyl)-5 -fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
25 Mass spectrum (CI): m/z = 555.3 (35Cl + 35Cl isotope + H+), 557.3
(37Cl + 35Cl isotope + H+).
EXAMPLE 36
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(benzoyl)-(methylamino))butyl)-
S-fluoro-spiro(2,3 -dihydrobenzofuran-3,4'-piperidine)
WO 94/29309 2 1 6 3 ~ 9 5 PCT/US94/05545
- 106-
Mass spectrum (CI): m/z = 541.3 (35Cl + 35Cl isotope + H+), 543.3
(37Cl + 35Cl isotope + H+).
Preparation of spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
hydrochloride was carried out by analogy to the procedure given in
Example 30, starting with methyl 2-fluorophenylacetate. Reaction of
spiro(2,3-dihydrobenzofuran-3,4'-piperidine) hydrochloride with 3-(S)-
(3,4-dichlorophenyl)-4-(t-butoxycarbonyl-methylamino)butanal
according to the procedure given in Example 8, Step B gave 1'-(3-((S)-
(3,4-dichlorophenyl))-4-(N-(t-butoxycarbonyl)-(methylamino))butyl)-
spiro(2,3-dihydrobenzofuran-3,4'-piperidine). Removal of the BOC
group and benzamide formation according to the procedure given in
5 Example 3, Steps A and B afforded the compounds listed in Exarnples
37-43:
EXAMPLE 37
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(benzoyl)-(methylamino))butyl)-
spiro(2,3 -dihydrobenzofuran-3,4'-piperidine)
Mass spectrum (CI): m/z = 523.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 38
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-methylbenzoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
WO 94/29309 PCT/US94/05545
2 1 ~3995
- 107-
Mass spectrum (CI): m/z = 537.2 (35Cl + 35CI isotope + H+), 539.2
(37Cl + 35Cl isotope + H+).
EXAMPLE 39
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
Mass spectrum (CI): m/z = 551.2 (35Cl + 35Cl isotope + H+), 553.2
(37Cl + 35Cl isotope + H+).
EXAMPLE 40
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-chlorobenzoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
20 Mass spectrum (CI): m/z = 557.0 (35Cl + 35Cl isotope + H+).
EXAMPLE 41
25 1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
Mass spectrum (CI): m/z = 591.0 (35Cl + 35Cl isotope + H+), 593.1
(37Cl + 35Cl isotope + H+).
EXAMPLE 42
WO 94/29309 PCT/US94/05~45
216~5`
- 108-
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzofuran-3 ,4 '-piperidine)
Mass spectrum (CI): m/z = 591.3 (35Cl + 35Cl isotope + H+), 593.2
5 (37Cl + 35Cl isotope + H+).
0 EXAMPLE 44
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzothiophene-3 ,4'-piperidine)
15 Step 1) 1-t-Butoxycarbonyl-3-hydroxy-4-methylenepiperidine
n-Butyl lithium (9.57 mL, 2.45M in hexane, 23.7 mmol) was added to a
-78C solution of diisopropylamine (3.32 mL, 23.7 mmol) in THF (15
mL). After 30 min at -78C, methyl phenyl sulfoxide (3.32 g, 23.7
20 mmol) in THF (4 mL) was added. The solution was warmed to 0C and
cooled back down to -78C. 1-t-butoxycarbonyl-4-piperidinone (4.69 g,
23.7 mmol) in TH~ (20 mL) was added. The reaction was warmed to
room temp, quenched by addition of solid NH4Cl, concentrated in
vacuo, and partitioned between H2O (100 mL) and EtOAc (100 mL).
25 The organic layer was washed with H2O (50 mL) brine (50 mL), dried
(MgSO4), and concentrated in vacuo. The resultant oil was heated at
80C in t-butanol (50 mL) with potassium t-butoxide (3.4g, 30 mmol)
for 2 h. Solid NH4Cl was added, and the reaction was concentrated in
vacuo and partitioned between H2O (100 mL) and EtOAc (100 mL).
3 The EtOAc was washed with brine (50 mL), dried (MgSO4 ),
concentrated in vacuo and purified by column chromatography (silica
gel 60, 0-50% EtOAc/hexane) to yield 4.47 g (79%) of the title
compound as a crystalline solid.
WO 94/29309 PCT/US94/0~45
2~ 6~995
- 109-
lH NMR (400 MHz, DMSO-d6) â 5.21 (d,lH), 4.96 (s, lH), 4.77 (s,
lH), 3.82 (t, 2H), 3.67 (dt, lH), 2.83 (dt, lH), 2.77-2.50 (brd d, lH),
2.26 (dt, lH), 2.01 (ddd, lH), 1.38 (s, 9H) ppm.
Step 2) 1-t-Butoxycarbonyl-3,4-didehydro-4-(chloromethyl)piperidine
To 1-t-butoxycarbonyl-3-hydroxy-4-methylenepiperidine (5.329 g, 25.1
mmol) in toluene (120 mL) and 2,6-lutidine (3.1 mL, 26 mmol) at 0C
o was added SOCl2 (2.0 mL, 26 mmol). The reaction was heated at 40C
for 30 min, cooled to 0C, washed with 0C lN HCl (100 mL), 0.1 N
HCl (100 mL), H2O (100 mL), brine (50 mL), dried (MgSO4), and
concentrated in vacuo to afford 5.18 g (89%) of allylic chloride as a
yellow oil.
1H NMR (400 MHz, CDCl3) ~ 5.78 (s, lH), 4.04 (s, 2H), 3.95 (s, 2H),
3.55 (t, 2H, J=6 Hz), 2.24 (s, 2H), 1.45 (s, 9H) ppm.
Step 3) l -t-Butoxycarbonyl-4-((2-bromophenyl)thio)methyl- 1,2,5,6-
tetrahydropyridine
The allylic chloride (330 mg, 1.43 mmole) was dissolved in acetone (10
mL) and 2-bromothiophenol (172 ml, 1.43 mmole) and K2CO3 (390
mg, 2.86 mmole) were added. The reaction mixture was heated to 60C
for 1 h and then filtered though silica gel ( ether). The organic layer
was concentrated in vacuo and purified by column chromatography
(silica gel 60, hexanes/ethyl acetate 10/1) to give the title compound in
84% yield (460 mg).
Step 4) l'-t-Butoxycarbonyl-spiro(2,3-dihydrobenzothiophene-3,4'-
piperidine).
WO 94/29309 PCT/US94/0554~
21; ~ 39~5
- 110-
The intermediate adduct from step 3 above (450 mg, 1.17 mmole) was
dissolved in benzene (60 mL) and AIBN (10 mg) and tributyltin hydride
(644 mL, 2.39 mmole) were added. This mixture was refluxed for 2 h
and concentrated. The residue was dissolved in Et2O and Br2 was
5 added until the reaction solution turned to a brownish color. To this
brownish solution at room temp was added DBU (650 mL) dropwise.
The resulting cloudy solution was filtered though silica gel and washed
with Et2O. The Et2O solution was concentrated and the residue was
purified by radial chromatography (silic gel 60, 10/1 hexanes/EtOAc)
to give the title compound (157 mg) in 43% yield.
1H NMR (400 MHz, CDCl3) ~ 7.18 (d, 7 Hz, 1 H), 7.12 (t, 7 Hz, 1 H),
7.06 (m, 2 H), 4.11(m, 2 H), 3.30 (s, 3 H), 2.89 (m, 2 H), 1.79 (m, 4
H), 1.47 (s, 9 H) ppm.
Removal of the BOC group according to the procedure given in
Example 3, Step A followed by reaction with 3-((S)-(3,4-
dichlorophenyl))-4-(N-(t-butoxycarbonyl)-(methylamino))butanal
20 according to the procedure given in Example 8, Method B gave 1'-(3-
((S)-(3,4-dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine). Removal of the BOC group and benzamide formation
according to the procedures described in Example 3, Steps A and B
25 gave the compounds listed in Examples 45-46:
EXAMPLE 45
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5 -dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
WO 94/29309 2 1 6 3 9 9 5 PCT/US94/05545
Mass spectrum (CI): m/z = 567.2 (35Cl + 35Cl isotope + H+), 569.2
(37Cl + 35Cl isotope + H+).
EXAMPLE 46
1 '-(3-((S)-(4-Chlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
Mass spectrum (CI): m/z = 533 (35Cl + 35Cl isotope + H+), 535 (37Cl
+ 35Cl isotope + H+).
EXAMPLE 47
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine)- 1 -oxide.
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
(222 mg, 415 ~mol) in CH2Cl2 (500 ,uL) at -78C was treated with a
solution of m-chloroperbenzoic acid (86 mg, 498 ,umol) in CH2C12 (1
25 mL). The reaction was poured into 0C saturated aqueous NaHSO3.
The organic layer was washed with saturated aqueous NaHCO3 (1 mL),
brine (1 mL), dried (MgSO4), concentrated in vacuo and purified by
column chromatography (silica gel 60, 0-100% acetone/CH2Cl2) to
yield 54.3 mg (24%) of the title compound as a white foam.
1H NMR (500 MHz, CDC13) ~ 7.84 (d, lH, J=7.5 Hz), 7.60 (t, lH,
J=7.5 Hz), 7.48 (t, lH, J=7.5 Hz), 7.44 (m, lH), 7.39 (dd, lH, J=2.0,
8.5 Hz), 7.32 (m, lH), 7.10-7.04 (rotamer multiplets, lH), 3.6-3.2 (m,
2H), 3.34, 3.32 (two doublets of one diastereomer, lH), 3.16, 3.14 (two
WO 94/29309 PCT/US94/05~45
- 112-
doublets of other diastereomer, lH), 3.1-2.8 (m, 3H), 2.75-2.65
(rotamer singlets, 3H), 2.3-1.7 (m, 10 H), 1.42 (s, 9H) ppm.
EXAMPLE 48
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthylmethyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzothiophene-3,4~-
piperidine)- 1 -oxide.
The title compound was prepared by oxidizing 1'-(3-((S)-(3,4-
dichlorophenyl))-4-(N-(t-butoxycarbonyl)-(methylamino))butyl)-
5 spiro(2,3-dihydrobenzothiophene-3,4'-piperidine) as described in
Example 47 above, and then removing the BOC group and N-
benzoylating according to the procedures given in Example 3, Steps A
and B.
20 Mass spectrum (CI): m~z = 623.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 49
2 5 1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3 ,4'-
piperidine)- 1,1 -dioxide.
To 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(t-butoxycarbonyl)-
3 (methylamino))butyl)-spiro(2,3-dihydrobenzothiophene-3,4'-piperidine)
(102 mg, 191 ~lmol) in MeOH (0.8 mL) at 0C was added Oxone (176
mg, 287 ~) in water (0.4 mL). After 30 min at room temp, the reaction
was filtered through a plug of silica gel and concentrated to yield 39.5
mg (36%) of the title compound as a white foam.
WO 94/29309 2 1 6 3 9 ~ ~ PCT/US94/0~54~
- 113-
1H NMR (500 MHz, CDCl3) o 7.72 (d, lH, J=7.5 Hz), 7.66 (t, lH,
J=7.5 Hz), 7.51 (t, lH, J=7.3 Hz), 7.39 (t, lH, J=8.3 Hz), 3.65-3.25 (m,
2H), 3.38 (s, 2H), 3.15-2.85 (m, 3H), 2.76,2.66 (rotamer singlets, 3H),
2.25 (m, 2H), 2.15-1.95 (m, 3H), 1.95-1.65 (m, 5H), 1.40 (s, 9H) ppm.
EXAMPLE 50
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzothiophene-3,4'-
piperidine)- 1,1 -dioxide.
lS The title compound was prepared by removing the BOC group and N-
benzoylating (according to the procedures given in Example 3, Steps A
and B) the product from Example 49.
Mass spectrum (CI): m/z = 639.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 51
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine)- 1,1 -dioxide.
30 To 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
(10 mg, 20 ~mol) in MeOH (0.1 mL) at 0C was added 0.4 M aqueous
Oxone (75 IlL, 30 !lmol). The reaction was warmed to room temp and
stirred overnight. The reaction was concentrated in vacuo, partitioned
WO 94/29309 ~ 3 9 9 5 PCT/US94/05545
- 114-
between lN NaOH (1 mL) and CH2cl2 (1 mL). The organic layer was
concentrated and purified by column chromatography (silica gel 60, 0-
100% acetone/CH2Cl2) to yield 9.0 mg (90%) of the title compound as
a clear filrn.
Mass spectrum (CI): m/z = 599.1 (35Cl + 35Cl isotope + H+), 601.1
(37Cl + 35Cl isotope + H+).
EXAMPLE 52
1 '-(3-((S)-(4-Chlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
15 piperidine)- 1,1 -dioxide.
This compound was prepared according to the procedure given in
Example 51 above.
20 Mass spectrum (CI): m/z = 567 (35Cl + 35Cl isotope + H+), 565 (37Cl
+ 35Cl isotope + H+).
EXAMPLE 53
1 '-(3 -((S)-(4-Chlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine)- 1 -oxide.
To 1'-(3-((S)-(4-chlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
(25 mg, 47 ,umol) in MeOH (1.0 mL) at 0C was added a solution of
Oxone (38 mg, 61 ~mol) in H2O (1.0 mL). The reaction was stirred 2
WO 94/29309 2 1 6 3 ~ ~ 5 PCT/US94/05545
- 115 -
min and quenched with 10% aqueous sodium bisulfite. The reaction
mixture was diluted with H2O (10 mL), neutralized with sat. aqueous
NaHCO3 (15 mL), extracted with CH2cl2 (3 x 25 mL), washed with
brine (10 mL), dried (Na2so4)~ concentrated in vacuo, and purified by
column chromatography (silica gel 60, 5-8% MeOH/CH2Cl2) to yield
25 mg (99%) of a colorless solid; Mass spectrum (CI): m/z = 549 (35Cl
+ 35Cl isotope + H+), 551 (37Cl + 35Cl isotope + H+).
EXAMPLE 54
l '-(3 -(S)-(4-Chlorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine), l-oxide.
The title compound was prepared by the oxone oxidation method
15 described in Example 53.
Mass Spectrum (CVNH3) M+H=657, 659 (35,37Cl-isotope).
EXAMPLE 55
1 '-(3-(S)-(4-Chlorophenyl)-4-(N-(3,5-bistrifluoromethylbenzoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-
piperidine), l, l-dioxide.
The title compound was prepared by the oxone oxidation method
25 described in Example 51.
Mass Spectrum (CVNH3) M+H=673, 675 (35,37Cl-isotope).
30 Substituted indoline spiropiperidine derivatives were obtained by
employing substituted phenyl hydrazines and 1-
benzyloxycarbonylpiperidine-4-carboxyaldehyde in the Fisher indole
synthesis. Wlhen regioisomers were formed, they were separated as the
1 '-benzyloxycarbonyl- l -methanesulfonyl-spiro(indoline-3,4'-piperidine)
derivative by chromatography (silica gel 60, THF/hexane). Preparation
WO 94/29309 PCT/US94/05~45
~1 63~5
- 116-
of a representative substituted spiro(indoline-3,4'-piperidinium)
hydrochloride is described below:
s EXAMPLE 56
1 '-Benzyloxycarbonyl-5 -fluoro-spiro(indoline-3,4'-piperidine)
A slurry of 4-fluorophenylhydrazine hydrochloride (6.504 g, 40
mmol), pyridine (6.56 ml, 80 mmol), toluene (360 mL), acetonitrile (40
mL), and N-benzylcarboxy-4-piperidine carboxyaldehyde (9.88g, 40
mmol) was maintained at 0C for 1 h. Trifluoroacetic acid (18.5 mL,
240 mmol) was added, and the reaction was heated 20 h at 60C. The
reaction was cooled to 0C, and methanol (40 mL) was added followed
5 by NaBH4 (1.51 g, 40 mmol). The cooling bath was removed and 30%
aqueous NH40H (100 mL) was added. The organic layer was
separated, washed with 5% aqueous NH40H (100 mL) brine (50 mL),
dried (MgSO4), and concentrated to a crude oil which was purified by
column chromatography (SG 60 silica, 0-5% acetone/CH2Cl2) to yield
20 6.48 g (48%) of the title compound as a clear oil.
1H NMR (400 MHz, CDCl3): ~ 7.23-7.36 (m, SH), 6.76-6.71 (m, 2H),
6.58 (dd, lH, J=4.4, 8.0 Hz), 5.14 (s, 2H), 4.12 (br s, 2H), 3.49 (s, 2H),
2.95 (br s, 2H) 1.73 (br s, 4H) ppm.
2s
EXAMPLE 57
30 Step 1) l'-Benzyloxycarbonyl-S-fluoro-1-methanesulfonyl-
spiro(indoline-3,4'-piperidine)
To a solution of 1'-benzyloxycarbonyl-5-fluoro-spiro(indoline-3,4'-
piperidine) (6.48 g, 19.0 mmol) in CH2cl2 (19 mL) and pyridine (38
WO 94/29309 PCT/US94/05545
21 639~5
- 117-
mmol, 3.1 mL) at 0C was added methanesulfonyl chloride (19 mmol,
1.52 mL). The reaction was warmed to room temp., diluted with ethyl
acetate (200 mL), washed with lN aqueous HCl (100 mL) saturated
aqeuous NaHCO3 (100 mL) brine (50 mL), dried (MgSO4), and
5 concentrated to 7.81 g (98%) of the title compound as a red foam.
lH NMR (400MHz, CDCl3) â 7.35 (m, 5H), 7.32 (dd, lH, J=4.2, 9.0
Hz), 6.90 (dt, lH, J=2.7, 8.8 Hz), 6.81 (lH, dd, J=2.6, 8.2 Hz), 5.14 (s,
2H), 4.22 (br s, 2H), 3.84 (s, 2H), 2.92 (br s, 2H), 2.88 (s, 3H), 1.79 (br
s, 2H), 1.69 (d, 2H, 13 Hz) ppm.
Step 2) 5-Fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
hydrochloride salt
To 1 '-benzyloxycarbonyl-5-fluoro- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine) (7.81 g, 18.7 mmol) in CHCl3 (18 mL) at room temp.
was added trimethylsilyl iodide (20.5 mmol, 2.93 ml). After 5 min, the
rxn was cooled to 0C, and a 5M solution of HCl in methanol/methyl
20 acetate is added with vigorous stirring. The HCl solution was prepared
by adding acetyl chloride (190 mmol, 14 ml) to methanol (20 mL) at
0C. 40 ml of EtOAc was added, and the slurry was vigorously stirred
at 0C for 4 h. The solid was filtered off under dry nitrogen, washed
with 0C ethyl acetate (10 mL) and then with hexane (10 mL), and dried
25 under vacuum to yield 4.77 g (80%) of the title compound as a light
pink solid.
1H NMR (400 MHz, DMSO-d6) ~ 8.85 (br s, lH), 8.77 (br s, lH), 7.26
(dd, lH, J=4.4, 8.8 Hz), 7.11 (dt, lH, J=2.8, 8.8 Hz), 7.02 (dd, lH,
30 J=2.8, 8.4 Hz), 3.97 (s, 3H), 3.30 (m, 2H), 3.06 (m, 2H), 3.06 (s, 3H),
2.04 (m, 2H), 1.83 (d 2H, J=14 Hz) ppm.
WO 94/29309 PCT/US94/05545
2 T 63995
- 118-
The substituted 1-methanesulfonyl-spiro(indoline-3,4'-piperidinium)
hydrochlorides could be reductively ~min~ted with 3-(S)-(3,4-
dichlorophenyl)-4-(t-butoxycarbonyl-methylarnino)butanal according to
the procedure described in Example 8, Method B. Removal of the BOC
5 group by the procedure given in Example 3, Step A provided
intermediate secondary amine compounds described below which could
then be benzoylated under conditions given in Example 3, Step B to give
the indicated benzamide derivatives.
EXAMPLE 58
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(methylamino)butyl)- 1-
methanesulfonyl-5 -methoxy-spiro(indoline-3 ,4'-piperidine)
1H NMR (CDC13, 400 MHz) ~ 7.37 (d, lH, J=8.2 Hz), 7.29 (d, lH),
7.25 (d, lH), 7.04 (dd, lH, J=2.1, 8.3 Hz), 6.72 (m, 2H), 3.76 (s, 3H),
3.73 (s, 2H), 2.87 (m, 2H), 2.82 (s, 3H), 2.78 (d, 2H, J=7.1 Hz), 2.41 (s,
3H), 2.32-2.18 (m, 2H), 2.05-1.85 (m, 5H), 1.7 (m, 3H) ppm.
EXAMPLE 59
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(methylamino)butyl)- 1-
2 5 methanesulfonyl-5-methyl-spiro(indoline-3 ,4'-piperidine)
1H NMR (CDCl3, 400 MHz) o 7.37 (d, lH, J=6.2 Hz), 7.30 (d, lH,
J=2.0 Hz), 7.24 (d, lH, J=10 Hz), 7.05 (dd, lH, J=2.0, 8.2 Hz), 7.00 (d,
lH, J= 8.8 Hz), 6.95 (s, lH), 3.71 (dd, 2H, J=16, 5.4 Hz), 2.9 (m, 3H),
30 2.84 (s, 3H), 2.79 (d, 2H, J=7.4 Hz), 2.43 (s, 3H), 2.30 (s, 3H), 2.24 (m,
lH), 2.05-1.85 (m, 5H),.1.75-1.60 (m, 3H) ppm.
EXAMPLE 60
WO 94/29309 PCT/US94/05545
2 1 63~5
- 119-
5-Chloro- 1 '-(3-((S)-(3 ,4-dichlorophenyl))-4-(methylamino)butyl)- 1-
methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
lH NMR (CDCl3, 400 MHz) ~ 7.39 (d, lH, J=8.2 Hz), 7.29 (d, lH,
J=2.1), 7.24 (s, lH), 7.17 (dd, lH, J=2.2, 8.5 Hz), 7.11 (d, lH, J=2.1
Hz), 7.05 (dd, lH, J=2.0, 8.3 Hz), 3.76 (dd, 2H, J=4.5, 25 Hz), 3.18 (p,
lH), 2.10-2.85 (m, 4H), 2.87 (s, 3H), 2.61 (s, 3H), 2.47 (m, lH), 2.34
(m, lH), 2.15 (t, lH), 2.04 (m, 2H), 1.95-1.70 (m, SH) ppm.
EXAMPLE 61
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(methylamino)butyl)-S-fluoro- 1-
methanesulfonyl-spiro(indoline-3,4'-piperidine)
lH NMR (CDCl3, 400 MHz) ~ 7.38 (d, lH), 7.3 (m, 2H), 7.05 (dd, lH),
7.91-7.85 (m, 2H), 3.75 (dd, 2H), 3.0-2.8 (m, 3H), 2.81 (d, 2H), 2.43
(s, 3H), 2.42 (m, lH), 2.34 (m, lH), 2.1-1.8 (m, SH), 1.7 (m, 3H) ppm.
EXAMPLE 62
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(methylamino)butyl)-7-fluoro- 1 -
2 ~ methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
lH NMR (CDCl3, 400 MHz) ~ 7.38 (d, lH), 7.29 (d, lH), 7.05 (m, 2H),
6.95 (m, 2H), 3.99 (dd, 2H), 3.25 (s, 3H), 2.9 (m, 2H), 2.81 (t, lH),
2.45 (s, 3H), 2.38 (m, lH), 2.28 (m, lH), 2.1-1.8 (m, 5H), 1.75 (m, 3H)
ppm.
EXAMPLE 63
WO 94/29309 PCT/US94/05545
~ 1~ b3~95
- 120-
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3 ,5-dimethylbenzoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-5-methyl-spiro(indoline-3 ,4'-
piperidine)
Mass spectrum (FAB): m/z = 64~.0 (35Cl + 35Cl isotope + H+).
EXAMPLE 64
5-Chloro-1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-methylbenzoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
15 Mass spectrum (FAB): m/z = 648.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 65
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 ,5-dimethylbenzoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-5-methoxy-spiro(indoline-
3,4'-piperidine)
25 Mass spectrum (FAB): m/z = 658 (35Cl + 35Cl isotope + H+).
EXAMPLE 66
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(3 -methylbenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine)
WO 94/29309 PCT/US94/05~45
~1 63995
- 121 -
Mass spectrum (CI): m/z = 632.2 (35Cl + 35Cl isotope + H+), 634.2
(37Cl + 35Cl isotope + H+).
EXAMPLE 67
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Mass spectrum (FAB): m/z = 688.0 (37Cl + 35Cl isotope + 35Cl +
35Cl isotope+ H+).
EXAMPLE 68
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
20 (methylamino))butyl)-5-fluoro-1-methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Mass spectrum (CI): m/z = 646.1 (35Cl + 35Cl isotope + H+), 648.1
(37Cl + 35Cl isotope + H+).
2s
EXAMPLE 69
30 l '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-chlorobenzoyl)-
(methylamino))butyl)-5-fluoro- l -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
WO 94/29309 PCT/US94/0554~
21 63'tq5
- 122-
Mass spectrum (CI): m/z = 652.2 (35Cl + 35Cl isotope + 35Cl + 35Cl
isotope + H+), 656.2 (37Cl + 35Cl isotope + 37Cl + 35Cl isotope+ H+),
657.2 (37Cl + 37Cl isotope + 37Cl + 35Cl isotope+ H+),. 658.2 (37Cl +
37Cl isotope + 37Cl + 37Cl isotope+ H+).
EXAMPLE 70
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-
o bis(trifluoromethyl)benzoyl)-(methylamino))butyl)-5-fluoro- l -
methanesulfonyl-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 754.1 (35Cl + 35Cl isotope + H+), 756.1
(37Cl + 35Cl isotope + H+).
EXAMPLE 71
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
20 (methylamino))butyl)-7-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Mass spectrum (CI): m/z = 646.1 (35Cl + 35Cl isotope + H+), 648.1
(37Cl + 35Cl isotope + H+).
EXAMPLE 72
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-t-butoxycarbonyl)-
30 (methylamino))butyl)-5-fluoro-spiro(indoline-3,4'-piperidine)
To 1'-(3-((S)-(3,4-dichlorophenyl))-4-(N-t-butoxycarbonyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine) (1.32 g 2.15 mmol) in toluene (5 mL) at 0C was added
WO 94/29309 2 1 6 3 ~ ~ 5 PCT/US94/05545
- 123 -
3.4M Red-Al/toluene (5.1 mL, 17.2 mmol). After 4 h at room temp,
the reaction was cooled to 0C and quenched by cautious addition of lN
aqueous NaOH (2 mL). Cold saturated aqueous sodium potassium
tartrate (30 mL) was added, and the biphasic mixture was mechanically
5 stirred at 0C for 1 h. The product was extracted with toluene (3 x 10
mL), washed with 50~ saturated aqueous sodium potassium tartrate (10
mL), H2O (10 mL), brine (10 mL), dried (MgSO4), and concentrated
to roughly 5 mL volume, and cooled to 0C. Pyridine (705 !lL, 8.6
mmol) and acetic anhydride (410 ,uL, 4.3 mmol) were added. After 16
hours at room temp, the reaction was concentrated and purified by
column chromatography (silica gel 60, 0-50% acetone/CH2Cl2) to yield
830 mg (72%) of the title compound as a white foam.
1H NMR (400 MHz, CDCl3) ~ 8.14 (dd, lH), 7.37 (d, lH), 7.28 (m,
15 lH), 7.1-7.0 (m, lH), 6.87 (m, 2H), 3.95, 3.81 (rotamer singlets, 2H),
3.53 (m, lH), 3.36 (m, 2H), 3.22 (m, lH), 3.01 (m, lH), 2.90 (m, lH),
2.82 (m, lH), 2.74, 2.63 (rotamer singlets, 3H), 2.39, 2.20 (rotamer
singlets, 3H), 1.89 (m, 4H), 1.65 (m, 4H) ppm.
20 The corresponding 1 -acetyl-spiro(indoline-3,4'-piperidine) compounds
were obtained by selectively removing the methanesulfonyl group with
Red-Al and then treating with acetic anhydride/pyridine at the stage
where the methylamino group is protected with BOC; a representative
procedure is given in Example 72 above. The BOC group could be
25 removed using the procedure given in Example 3, step A to give
intermediate methylamino compounds which were benzoylated
according to Example 3, step B to give the compounds in Examples 73-
90:
EXAMPLE 73
1 -Acetyl-5-chloro- l '-(3-((S)-(3,4-dichlorophenyl))-4-
(methylamino)butyl)-spiro(indoline-3,4'-piperidine)
WO 94/29309 PCTIUS94/0~545
2t 639qF~
- 124-
lH NMR (CDCl3, 400 MHz) ~ 7.83 (d, lH, J=6.6 Hz), 7.12 (d, lH,
J=5.2 Hz), 7.09 (d, lH, J=2.0 Hz), 6.87 (dd, 2H, J=2.0, 10.0 Hz), 6.84
(s, lH), 2.81 (p, lH0, 2.75-2.55 (m, 4H), 2.27 (s, 3H), 2.12 (m, lH),
2.04 (m, lH), 1.95 (s, 3H), 1.9-1.7 (m, 3H), 1.6 (t, 2H), 1.5-1.4 (m, 3H)
ppm.
EXAMPLE 74
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-5-
methyl-spiro(indoline-3,4' -piperidine)
H NMR (CDCl3, 400 MHz) ~ 8.05 (d, lH)~ 7.38 (d, lH), 7.30 (d, lH),
5 7.05 (dd, lH), 7.00 (d, lH), 6.92 (s, lH), 3.79 (s, 2H), 3.01 (p, 2H), 2.9
(m, 3H), 2.52 (s, 3H), 2.5-2.1 (m, 2H), 2.29 (s, 3H), 2.20 (s, 3H), 2.1-
1.7 (m, 6H),. 1.65 (m, 2H) ppm.
EXAMPLE 75
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-5-
fluoro-spiro(indoline-3,4'-piperidine)
25 lH NMR (CDCl3, 400 MHz) ~ 8.14 (dd, lH), 7.38 (d, lH), 7.24 (s, lH),
7.05 (dd, lH), 6.88 (dt, lH), 6.82 (dd, lH). 3.96, 3.83 (rotamer
singlets, 2H), 3.13 (p, lH), 3.04 (dd, 2H), 2.92 (dd, 2H), 2.69, 2.66
(rotamer singlets, 3H), 2.50 (p, lH), 2.33 (p, lH), 2.38, 2.20 (rotamer
singlets, 3H), 2.13 (t, lH), 2.05 (m, lH), 1.7 (m, 4H), 1.73 (dd, 2H)
30 ppm-
EXAMPLE 76
wo 94/29309 2 1 6 :~ 9 9 5 PCT/US94/05545
- 125 -
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-6-
fluoro-spiro(indoline-3,4'-piperidine)
lH NMR (DMSO-d6, 500 MHz) â 7.76 (dd, lH), 7.58-7.53 (m, 2H),
7.26 (dd, lH), 7.21 (dd, lH), 6.80 (dt, lH), 3.93 (s, 2H), 2.98-2.86 (m,
3H), 2.82 (d, lH), 2.65 (d, lH), 2.38 (s, 3H), 2.19 (m, lH), 2.16 (s,
3H), 2.09 (m, lH), 2.05 (t, lH), 1.90 (t, 2H), 1.78-1.6 (m, 3H), 1.6-1.5
(m, 2H) ppm.
EXAMPLE 77
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(methylamino)butyl)-4-
fluoro-spiro(indoline-3,4'-piperidine)
lH NMR (DMSO-d6, 500 MHz) ~ 7.88 (d, lH), 7.63-7.58 (m, 2H), 7.29
(dd, lH), 7.19 (q, lH), 6.79 (t, lH), 3.86 (s, 2H), 3.23-3.13 (m, 3H),
2.97 (m, lH), 2.72 (m, lH), 2.52 (s, 3H), 2.26 (m, lH), 2.16 (s, 3H),
2.09 (t, 4H), 1.97 (p, 2H), 1.76-1.62 (m, 3H) ppm.
EXAMPLE 78
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(benzoyl)-
(methylamino))butyl)-4-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 588.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 79
WO 94/29309 PCT/US94/05545
? 5
- 126-
1 -Acetyl- 1 '-(3 -((S)-(3,4-dichlorophenyl))-4-(N-(3,5,-dimethylbenzoyl)-
(methylamino))butyl)-6-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 610.2 (35Cl + 35Cl isotope + H+), 612.2
5 (37Cl + 35Cl isotope + H+).
EXAMPLE 80
.
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(benzoyl)-
(methylamino))butyl)-6-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 582.3 (35Cl + 35Cl isotope + H+).
EXAMPLE 81
20 1 -Acetyl- l '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5,-dimethylbenzoyl)-
(methylamino))butyl)-4-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 610.3 (35Cl + 35Cl isotope + H+)
EXAMPLE 82
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(benzoyl)-
30 (methylamino))butyl)-5-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 582.2 (35Cl + 35Cl isotope + H+).
WO 94/29309 PCTIUS94/05545
2 1 63995
- 127 -
EXAMPLE 83
1 -Acetyl- l '-5-chloro-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
5 dimethylbenzoyl)-(methylamino))butyl)-spiro(indoline-3,4'-piperidine)
Mass spectrum (FAB): m/z = 626.0 (35Cl + 35Cl isotope + H+), 628.1
(37Cl + 35Cl isotope + H+).
EXAMPLE 84
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-chlorobenzoyl)-
15 (methylamino))butyl)-5-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 616.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 85
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dichlorobenzoyl)-
(methylamino))butyl)-5 -fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 650.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 86
l -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3-methylbenzoyl)-
(methylamino))butyl)-5 -fluoro-spiro(indoline-3,4'-piperidine)
WO 94/29309 2 1 6 3 9 q 5 PCT/US94/05545
- 128-
Mass spectrum (CI): m/z = 596.2 (35Cl + 35Cl isotope + H+), 598.3
(37Cl + 35Cl isotope + H+).
EXAMPLE 87
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
(methylamino))butyl)-5-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 610.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 88
1 -Acetyl- 1 '-(3 -((S)-(3,4-dichlorophenyl))-4-(N-(3-isopropoxybenzoyl)-
(methylamino))butyl)-5 -fluoro-spiro(indoline-3,4'-piperidine)
20 Mass spectrum (CI): m/z = 640.3 (35Cl + 35Cl isotope + H+), 642.3
(37Cl + 35Cl isotope + H+).
EXAMPLE 89
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-
bis(trifluoromethyl)-(methylamino))butyl)-5-fluoro-spiro(indoline-3,4' -
piperidine)
Mass spectrum (CI): m/z = 718.2 (35Cl + 35Cl isotope + H+), 720.2
(37Cl + 35Cl isotope + H+).
WO 94/29309 PCT/US94/05545
2 ~ 6 3 ~ ~ 5!
- 129-
EXAMPLE 90
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
5 (methylamino))butyl)-S-methyl-spiro(indoline-3,4'-piperidine)
Mass spectrum (FAB): m/z = 606.1 (35Cl + 35Cl isotope + H+), 608.2
(37Cl + 35Cl isotope + H+).
N-Napthoyl- methylamino derivatives (Examples 91 - 101) were
prepared by analogy to the benzoyl derivatives, employing
commercially available 1-napthoyl chlorides in place of benzoyl
15 chlorides:
EXAMPLE 91
1 -Acetyl- 1 '-(3 -((S)-(3,4-dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-S -fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 650.3 (35Cl + 35Cl isotope + H+).
EXAMPLE 92
30 1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(1 -napthoyl)-
(methylamino))butyl). S-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 632.2 (35Cl + 35Cl isotope + H+), 634.2
(37Cl + 35Cl isotope + H+).
WO 94/29309 PCT/US94/05545
21 ~qq5
- 130-
EXAMPLE 93
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(1 -napthoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Mass spectrum (CI): m/z = 668.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 94
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
20 Mass spectrum (CI): m/z = 668.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 95
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
Mass spectrum (CI): m/z = 607.2 (35Cl + 35Cl isotope + H+).
EXAMPLE 96
WO 94/29309 PCT/US94/0~45
21 63i~
- 131 -
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- l -napthoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene-3,4'-piperidine)
sulfone
Mass spectrum (CI): m/z = 639.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 97
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- l -napthoyl)-
(methylamino))butyl)-spiro(2,3 -dihydrobenzothiophene -3,4'-piperidine)
Mass spectrum (CI): m~z = 623.1 (35Cl + 35Cl isotope + H+).
EXAMPLE 98
2 1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-5 -fluoro-spiro(2,3-dihydrobenzofuran-3,4'-
piperidine)
Mass spectrum (CI): m/z = 609.3 (35Cl + 35Cl isotope + H+), 611.3
25 (37Cl + 35Cl isotope + H+).
EXAMPLE 99
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- l -napthoyl)-
(methylamino))butyl)-spiro(2,3-dihydrobenzofuran-3,4'-piperidine)
WO 94/29309 PCT/US94/05545
2 1 63~
- 132-
Mass spectrum (CI): m/z = 591.3 (35Cl + 35Cl isotope + H+), 593.3
(37Cl + 35Cl isotope + H+).
EXAMPLE 100
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-6-fluoro-spiro(indoline-3,4'-piperidine)
o Mass spectrum (CI): m/z = 650.3 (35Cl + 35Cl isotope + H+).
EXAMPLE 101
1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-4-fluoro-spiro(indoline-3,4'-piperidine)
Mass spectrum (CI): m/z = 650.3 (35Cl + 35Cl isotope + H+).
Benzylamine derivatives could be synthesized by reducing the
benzamide of the 1-methanesulfonyl-spiro(indoline-3,4'-piperidine)
25 derivatives described in some of the Examples. The methanesulfonyl
group could be removed by heating with HBr/acetic acid/phenol and
then be replaced with an acetyl group by treating with acetic
anhydride/pyridine. Representative procedures and compounds are
given in Examples 102 and 103 below:
EXAMPLE 102
WO 94/29309 PCT/US94/05545
21 63995
- 133-
l '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthylmethyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine) (96 mg) was dissolved in lM Dibal-H in toluene (160 uL).
After 1/2 h, saturated aqueous sodium potassium tartrate (5 mL) and
EtOAc (5 mL) were added and stirred vigorously for 2 h. The organic
layer was washed with H2O (5 mL), brine (5 mL), dried (MgSO4), and
concentrated to a crude oil, which was purified by column
chromatography (silica gel 60, 0-10% acetone/CH2Cl2) to yield 55 mg
(59%) of the title compound as a white foam; 1H NMR (400 MHz,
CDCl3) ~ 8.09 (d, lH, J=8.5 Hz), 7.91 (d, lH, J=8.5 Hz), 7.53 (t, lH,
5 J=7.5 Hz), 7.38 (t, lH, J=7.5 Hz), 7.33 (dd, lH, J=4.3, 8,8 Hz), 7.22
(dd, lH, J=5.8, 7.8 Hz), 7.18 (d, lH, J=8.5Hz), 7.09 (d, J=2.0 Hz), 7.04
(dd, lH, J=7.5, 10. 0 Hz), 6.92 (dt, lH, J=2.5, 8.5 Hz), 6.88 (d, lH),
6.77 (dd, lH, J=1.8, 8.3 Hz), 3.85 (dd, lH, J=8.0 Hz), 3.76 (s, 2H), 3.75
(dd, lH, J=8.0 Hz), 2.88 (s, 3H), 2.80-2.66 (m, 3H), 2.62 (dd, lH,
20 J=8.8, 12.3 Hz), 2.51 (dd, lH, J=6.5, 12.5 Hz), 2.28 (s, 3H), 2.18-2.06
(m, 2H), 1.88-1.80 (m,4H), 1.65 (d, 2H, J=10.5 Hz) ppm;
Mass spectrum (CI): m/z = 672.4 (35Cl + 35Cl isotope + H+).
EXAMPLE 103
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthylmethyl)-
30 (methylamino))butyl)-5-fluoro-spiro(indoline-3,4'-piperidine)
1 '-(3 -((S)-(3,4-dichlorophenyl))-4-(N-(4-fluoro- 1 -napthylmethyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine) (45.6 mg) and phenol (19 mg) in 30% HBr/HOAc (270 ~L)
WO 94129309 2 1 6 3 ~ q ~ PCT/US94/05~4~
- 134-
were heated to 70C for 6 h in a sealed vessel. The reaction was
concentrated and partitioned between CH2Cl2 (1 ml) and lN NaOH (2
mL). The organic layer was eluted through a 3x3 cm silica gel plug
with 0-100% acetonelcH2cl2 to yield 30 mg (74%) of the title
5 compound as a yellow oil.
1H NMR (400 MHz, CDCl3) ~ 8.09 (d, lH, J=8.0 Hz), 7.93 (d, lH,
J=8.5 Hz), 7.52 (t, lH, J=7.5 Hz), 7.43 (t, lH, J=7.3 Hz), 7.22 (dd, lH,
J=5.5, 7.5 Hz), 7.17 (d, lH, J=8.5 Hz), 7.06 (dd, lH, J=8.8, 10.3 Hz),
7.02 (d, lH, J=1.5 Hz), 6.87 (d, lH, J=3.5 Hz) 6.78 (dd, lH, J=2.3, 8.3
Hz), 6.75 (dd, lH, J=2.3, 7.8 Hz), 6.56 (dd, lH, J=4.0, 8.5 Hz) ppm;
Mass spectrum (CI): m/z = 594.3 (35Cl + 35Cl isotope + H+), 596.3
(37Cl + 35Cl isotope + H+)
EXAMPLE 104
2 1 -Acetyl- 1 '-(3-((S)-(3,4-dichlorophenyl))-4-(N-(4-fluoro- 1-
napthylmethyl)-(methylamino))butyl)-5 -fluoro-spiro(indoline-3 ,4'-
piperidine)
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -napthylmethyl)-
2 5 (methylamino))butyl)-5-fluoro-spiro(indoline-3 ,4'-piperidine) ( 10 mg)
in CH2C12 (100 !lL) was treated with one drop acetic anhydride and 1
drop pyridine. After 30 min, the reaction was eluted through a lx2 cm
silica gel column using 0-100% acetone/CH2Cl2 plus 1% NH40H to
yield 10 mg (93%) of the title compound as a clear film.
1H NMR (CDC13) ~ 8.17 (dd, lH, J=4.3, 8.8 Hz), 8.09 (d, lH, J=8.5
Hz), 7.91 (d, lH, J=8.0 Hz), 7.52 (t, lH, 7.3 Hz), 7.38 (t, lH, J=7.3 Hz),
7.22 (dd lH, 6.8, 7.0 Hz), 7.18 (d, lH, J=8.8 Hz), 7.07 (d, lH, J=2.0
Hz), 7.04 (dd, lH, J=8.0, 10.5 Hz), 6.91 (dt, lH, J=2.0, 9.0 Hz), 6.86
WO 94t29309 ~1 63 ~ ~ ~ PCT/US94/0554~
- 135 -
(dd, lH, J=2.0, 7.5 Hz), 6.76 (dd, lH, J=2.0, 8.5 Hz), 3.96, 3.81
(rotamer singlets, 3H), 3.85 (d, 1 H, J= 13 Hz), 3.75 (d, 1 H, J= 13 Hz),
2.84 (m, 2H), 2.74 (m, lH), 2.61 (dd, lH, J=8.5, 13Hz), 2.51 (dd, lH,
J=7.0, 13 Hz), 2.43, 2.35 (rotamer singlets, 3H), 2.24 (s, 3H), 2.3-2.2
(m, 3H), 2.0-1.85 (m, 4H), 1.65 (m, 2H), 1.50 (m, lH) ppm;
Mass spectrum (CI): m/z = 636.4 (35Cl + 35Cl isotope + H+), 638.4
(37Cl + 35Cl isotope + H+).
EXAMPLE 105
1 '-(5-Fluoroindolyl-3-(2-ethanoyl))- 1 -methanesulfonyl-spiro(indoline-
5 3 4 -piperidine)
To a solution of l-methanesulfonyl-spiro(indoline-3,4'-piperidine)
hydrochloride (373 mg, 1.23 mmol), 5-fluoroindole-3-acetic acid (500
mg, 2.59 mmol), in DMF (15 mL) at room temp. was added N-methyl
20 morpholine (261 mg, 2.59 mmol), hyroxybenzotriazole (381 mg, 2.82
mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (473 mg,
2.47 mmol). The reaction was stirred 48 h, diluted with H2O (250
mL), extracted with EtOAc (3 x 100 mL), washed with H2O (2 x 150
mL), brine (150 mL), dried (Na2SO4), concentrated in vacuo and
25 purified by column chromatography (SG 60 silica, 5% MeOH/CH2Cl2)
to afford 486 mg (89%) of the title compound as a colorless oil.
lH NMR (500 MHz, CDCl3) o 8.39 (br s, lH), 7.37 (d, lH, J = 8.2 Hz),
7.34 (dd, lH, J = 9.6, 2.3 Hz), 7.29 (dd, lH, J = 8.9, 4.4 Hz), 7.23 (dt,
30 lH, J = 7.8, 1.2 Hz), 7.14 (d, lH, J = 2.3 Hz), 7.03 (t, lH, J = 7.3 Hz),
6.98 (dt, lH, J = 8.9, 2.5 Hz), 6.87 (d, lH, J = 7.5 Hz), 4.73 (d, lH, J =
13.7 Hz), 3.96 (d, lH, J = 14.0 Hz), 3.82-3.92 (m, 2H), 3.72-3.78 (m,
lH), 3.13 (t, lH, J = 13.4 Hz), 2.91 (s, 3H), 2.73 (t, lH, J = 13.5 Hz),
WO 94/29309 PCT/US94/05545
;2 1 ~3995
- 136-
1.83 (dt, lH, J = 13.5, 4.4 Hz), 1.65-1.75 (m, 2H), 1.52-1.58 (m, lH),
1.40 (dt, lH, J = 13.0, 4.3 Hz) ppm; Mass spec (CI) m/z 441 (M+H).
EXAMPLE 106
1 '-(2-(3-(5-Fluoroindolyl))ethyl))- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine).
To a solution of 1'-(5-fluoroindolyl-3-(2-ethanoyl))-1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) (100 mg, .226 mmol) in CH2C12 (8 mL)
at -70C was added Dibal-H (lM in THF, 0.91 mL, .906 mmol). After
2.5 h the mixture was quenched by addition of 1 M NaOH (20 mL),
15 diluted with CH2cl2 and stirred vigorously for 15 min. The mixture
was extracted with CH2Cl2 (3 x 50 mL), washed with brine (50 mL),
dried (Na2SO4), concentrated in vacuo and purified by column
chromatography (SG60 silica, 5% MeOH/CH2Cl2) to afford 66 mg
(68%) of the title compound as a colorless solid.
lH NMR (500 MHz, CDCl3) â 8.20 (br s, lH), 7.42 (d, lH, J = 8.0 Hz),
7.20-7.30 (m, 4H), 7.06-7.14 (m, 2H), 6.93-6.97 (m, lH), 3.84 (s, 2H),
3.08 (d, 2H, J = 11.7 Hz), 2.94-3.00 (m, 2H), 2.93 (s, 3H), 2.71-2.77
(m, 2H), 2.19 (t, 2H, J = 12.3 Hz), 2.07 (dt, 2H, J = 13.2, 3.9 Hz), 1.75
25 (d, 2H, J = 13.0 Hz) ppm; Mass spec (CI) m/z 428 (M+H).
EXAMPLE 107
30 4-Fluoro-3,5-dimethylbenzoic acid
Step 1) 1-Bromo-4-fluoro-3,5-dimethylbenzene
wo 94/29309 21 6 3 q 9 5 PCT/US94/05545
- 137-
- To a mixture of 4-Bromo-2,6-dimethylaniline (8.3 g, 42 mmol) at 5C
and H2O (50 mL) was added conc H2SO4 (6.25 mL). NaNO2 (4.1 g)
was added in portions until an excess was indicated by starch iodide
paper. Water (30 mL) was added to make the mixture homogeneous.
After transferring to a plastic container, HBF4 (50%, 13.7 g) was added
dropwise with stirring. The resultant white precipitate was collected by
vacuum filtration, washed with H2O (30 mL), MeOH (30 mL), and
Et2O (60 mL), and dried over P2O5 under vacuum for 16 h. The solid
was then heated in a glass flask with an open flame until all the solid had
decomposed. The rem~ining liquid was diluted with Et2O (50 mL) and
0.5 M NaOH (30 mL). The organic layer was separated, washed with
0.5 M NaOH (25 mL), H2O (25 mL), brine (25 mL), dried (MgSO4),
and concentrated in vacuo yielding 6.06 g (72%) of 1-bromo-4-fluoro-
3,5-dimethylbenzene as a pale yellow liquid.
1HNMR(500MHZ,CDCl3)~7.17(d,2H,J=6.2Hz),2.21 (s,6H)
ppm.
Step B) 4-Fluoro-3,5-dimethylbenzoic acid
To a mixture of magnesium shavings (120 mg, 4.92 mmol) in THF (2
mL) was added a crystal of iodine followed by slow addition of a
solution of the bromide (1.0 g, 4.92 mmol) in THF (3 mL). The
2s reaction mixture was heated to reflux for 1 h followed by cooling to
room temp. and addition of CO2(s) (excess), stirred 1 h and quenched
by addition of lM HCl (10 mL). The mixture was extracted with Et2O
(3 x 25 mL), washed with brine (25 mL), dried (MgSO4), and
concentrated in vacuo to afford 0.82 g (99%) of the title compound as a
pale yellow solid.
1H NMR (500 MHz, CDC13) ~ 7.81 (d, 2H, J = 6.7 Hz), 2.36 (s, 6H)
ppm;
WO 94/29309 PCT/US94/05545
2~3~Y5
- 138-
Mass spec (CI) m/z 168 (M-H).
The compounds of Examples 108-120 were prepared as per Example 3
5 Step B utilizing the previously prepared amines and the appropriate
benzoic or naphthoic acids:
EXAMPLE 108
l '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(3-chloro-4-fluorobenzoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
15 Mass spec (CI) 656 (37Cl + 35Cl isotope + H+), 654 (35CI + 35CI
isotope + H+).
EXAMPLE 109
1 '-(3-((S)-(3 ,4-Dichlorophenyl))-4-(N-(3-chloro-4-fluorobenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine)
Mass spec (CI) 674 (37Cl + 35Cl isotope + H+), 672 (35Cl + 35Cl
isotope + H+).
EXAMPLE 110
1'-(3 -((S)-(3 ,4-Dichlorophenyl))-4-(N-(4-fluorobenzoyl)-
(methylamino))butyl)- l -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
WO 94/29309 2 1 6 3 5 9 5 PCT/US94/05545
- 139-
Mass spec (CI) 620 (37Cl + 35Cl isotope + H+), 618 (35Cl + 35Cl
isotope + H+).
EXAMPLE 111
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluorobenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 618 (37Cl + 35Cl isotope + H+), 616 (35CI + 35Cl
isotope + H+).
EXAMPLE 112
1 '-(3 -((S) -(3,4-Dichlorophenyl))-4-(N-(3 -chloro-4-fluorobenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 636 (37Cl + 35Cl isotope + H+), 634 (35CI + 35Cl
isotope + H+).
2 s EXAMPLE 113
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro-3,5-dimethylbenzoyl)-
(methylamino))butyl)-5-fluoro- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 630 (37Cl + 35Cl isotope + H+), 628 (35Cl + 35Cl
isotope + H+).
wo 94/29309 21 S 3 9 q 5 PCT/US94/05545
- 140-
EXAMPLE 114
1'-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro-3,5-dimethylbenzoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 648 (37Cl + 35Cl isotope + H+), 646 (35Cl + 35Cl
isotope + H+).
EXAMPLE 115
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro-3 -
trifluoromethylbenzoyl)-(methylamino))butyl)- 1 -methanesulfonyl-
spiro(indoline-3,4'-piperidine)
Mass spec (CI) 688 (37Cl + 35Cl isotope + H+), 686 (35Cl + 35Cl
isotope + H+).
EXAMPLE 116
l '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro-3,5 -dimethylbenzoyl)-
2 5 (methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 612 (37Cl + 35Cl isotope + H+), 610 (35Cl + 35Cl
isotope + H+).
EXAMPLE 117
WO 94/29309 PCT/US94/05545
21 ~3995
- 141 -
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro-3-
trifluoromethylbenzoyl)-(methylamino))butyl)- 1 -acetyl-spiro(indoline-
3,4'-piperidine)
Mass spec (CI) 652 (37Cl + 35Cl isotope + H+), 650 (35Cl + 35Cl
isotope + H+).
EXAMPLE 118
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -naphthoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 634 (37Cl + 35Cl isotope + H+), 632 (35Cl + 35Cl
isotope + H+).
EXAMPLE 119
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(4-fluoro- 1 -naphthoyl)-
(methylamino))butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
Mass spec (CI) 670 (37Cl + 35Cl isotope + H+), 668 (35Cl + 35Cl
isotope + H+).
EXAMPLE 120
1 '-(3-((S)-(3,4-Dichlorophenyl))-4-(N-(1 -naphthoyl)-
(methylamino))butyl)- 1 -acetyl-spiro(indolme-3,4'-piperidine)
Mass spec (CI) 616 (37Cl + 35Cl isotope + H+), 614 (35Cl + 35Cl
isotope + H+).
WO 94/29309 PCT/US94/05545
21 63q95
- 142-
EXAMPLE 121
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(N-(3,5-dimethylbenzoyl)-
5(methylamino))-4-phenyl-butyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
The title compound was prepared in 6 steps from 2-(S)-
(3,4-dichlorophenyl)-4-pentenoic acid using procedures identical to
othose in Example 10, substituting phenyllithium for methyllithium in
Example 10, Step 2.
Mass Spectrum (FAB): m/z 704 (M+H, 37Cl + 35Cl isotope, 100%),
706 (M+H, 37Cl + 37Cl isotope, 80%).
EXAMPLE 122
1 '-(4-(N-(3,5-Dimethylbenzoyl)-(methylamino))-4-(phenyl)butyl)- 1 -
20acetyl-spiro(indoline-3,4'-piperidine)
The title compound was prepared in 6 steps from 4-
pentenoic acid using procedures identical to those in Example 10,
substituting phenyllithium for methyllithium in Example 10, Step 2.
Mass Spectrum (FAB): m/z 524 (M+H, 37Cl + 35Cl isotope, 100%),
526 (M+H, 37CI + 37Cl isotope, 50%).
30EXAMPLE 123
1 '-(3 -((S)-(3,4-Dichlorophenyl))-4-(1 -(2-phenylimidazolo))butyl)- 1 -
methanesulfonyl-spiro(indoline-3,4'-piperidine)
WO 94/29309 PCT/~lS94/0554~
21 b3~9G3
- 143 -
Step 1) 1-(2-Phenylimidazolo)-2-((S)-(3,4-dichlorophenyl))-4-pentene
To a solution of 0.178 g (0.77 mmole) of 2-((S)-(3,4-dichlorophenyl))-
4-penten-1-ol (prepared in Example 136, Step A) and 0.099 mL (0.85
mmole) of 2,6-lutidine in 1.5 mL of methylene chloride at -53 deg C
under nitrogen was added 0.136 mL (0.81 mmole) of
trifluoromethanesulfonic anhydride. The solution was stirred between
-30 deg C and -40 deg C for 15 min at which point 0.333 g (2.31
mmole) of 2-phenylimidazole was added. The temperature was allowed
o to warm to -20 deg C briefly, and the mixture was then cooled to -60
deg C, stirred at that temperature for 1 hr, stirred at -20 deg Cfor 2 hr,
and then held at 4 deg C for 16 hr. After stirring at room temperature
for 8 hr, the mixture was treated with 10 mL of saturated sodium
carbonate solution and 10 mL of ethyl acetate and the layers were
separated. The aqueous phase was extracted with 2x15 mL of ethyl
acetate and the combined aqueous layers were dried over sodium
sulfate, filtered and concentrated in vacuo. The residue was partly
purified by flash chromatography on 36 g of silica eluting with 500 mL
of 3:100 methanol:methylene chloride then 300 mL of 5:100:0.1
methanol:methylene chloride: ammonia water. The partly purified
product fractions were flash chromatographed on 66 g of silica eluting
with 1.2 L of 83:17 methylene chloride:ethyl acetate to give 85 mg
(31%) of an oil.
1H-NMR (400 MHz, CDC13) ~ 2.26 (app. t, 2H), 2.85 (pentet, lH), 4.08
(dd, lH), 4.27 (dd, lH), 4.9-5.0 (m, 2H), 5.45-5.55 (m, lH), 6.59 (dd,
lH), 6.79 (s, lH), 6.85 (d, lH), 7.18 (d, lH), 7.23-7.30 (m, 2H), 7.35-
7.4 (m, 3H).
Mass Spectrum (FAB): m/z 359 (M+H, 65%), 357 (M+H, 100%), 145
(7%).
WO 94/29309 PCT/IJS94/05545
2 1 63995
- 144-
Step 2) 1 '-(2-((S)-(3,4-Dichlorophenyl))- 1 -(1 -(2-phenylimidazolo))-4-
butyl)- l -methanesulfonyl-spiro(indoline-3,4'-piperidine)
The title compound was prepared by employing the chemistry outlined
in Examples 1 and 2, using 1-(2-phenylimidazolo)-2-((S)-(3,4-
dichlorophenyl))-3-butene in place of 3-(S)-(3,4-dichlorophenyl)-4-
methylamino-1-pentene, and beginning with the osmium tetroxide step.
H-NMR (400 MHz, CDCl3) ~ 1.55-2 (m, 8H), 2.08 (t, J = 7.3, 2H),
o 2.63 (br d, J = 11, lH), 2.70 (br d, J = 8.3, lH), 2.86 (s, 3H), 2.9-3.0(m, lH), 3.71 (s, 2H), 4.13 (dd, J = 14, 8.8, lH), 4.25 (dd, J = 14, 6.2,
lH), 6.66 (dd, J = 6.2, 2.1, lH), 6.79 (d, J = 1.3, lH), 6.94 (d, J = 2.1,
lH), 7.03 (d, J = 1.3, lH), 7.05 (d, J = 6.4, lH), 7.15 (d, J = 6.5, lH),
7.15-7.25 (m, 2H), 7.35-7.45 (m, 6H)
Mass Spectrum (FAB): m/z 609 (M+H, 25%), 279 (100%), 267 (50%),
212 (30%), 187 (35%).
EXAMPLE 124
1'-(3-((S)-(3,4-Dichlorophenyl))-4-((N-(R or S)-(3,5-dimethylbenzoyl)-
(methylamino))pentyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine)
The title compound was prepared in 6 steps from (2S)-(3,4-
dichlorophenyl)-4-pentenoic acid using procedures identical to those in
Example lO, substituting l-acetyl-spiro(indoline-3,4'-piperidine) for 1-
methanesulfonyl-spiro(indoline-3,4'-piperidine) in Example 10, Step 6.
Mass Spectrum (FAB): m/z 606 (M+H, 37Cl + 35Cl isotope, 100%),
608 (M+H, 37CI + 37Cl isotope, 80%).
WO 94129309 2 1 6 3 9 9 5 PCT/US94/05545
- 145-
EXAMPLE 125
1'-(3-((S)-(3,4-Dichlorophenyl))-4-((N-(R or S)-(4-fluoro-1-napthyl)-
(methylamino))pentyl)- 1 -acetyl-spiro(indoline-3,4'-piperidine)
The title compound was prepared in 6 steps from (2S)-(3,4-
dichlorophenyl)-4-pentenoic acid using procedures identical to those in
Example 10, substituting 1-acetyl-spiro(indoline-3,4'-piperidine) for 1-
methanesulfonyl-spiro(indoline-3,4'-piperidine) in Example 10, Step 6,
and substituting 4-fluoro-1-napthoyl chloride for benzoyl chloride.
Mass Spectrum (FAB): m/z 646 (M+H, 37Cl + 35Cl isotope, 30%), 204
(1 00%).
The following compounds described in Examples 126-129
were prepared by the method described in Scheme II and in
Example 10, except that in step 2 ethylmagnesium chloride or
- propylmagnesium chloride was used at room temperature instead
20 of methyllithium at -78C.
EXAMPLE 126
25 1'-(3-((S)-(3,4-Dichlorophenyl))-4-(R or S)-(N-(3,5-
dimethylbenzoyl)-(methylamino))hexyl)- 1 -acetyl-spiro(indoline-
3,4'-piperidine)
1H-NMR (400 MHz, CDCl3) ~ 0.97 (t, 3H), 2.20 (s, 6H), 2.21 (s,
30 3H), 2.42-2.46 (s+m, 4H), 6.23 (s, 2H), 6.89 (s, lH), 7.04 (t, lH),
7.15-7.21 (m, 3H), 7.39 (t, 2H), 8.18 (d, lH).
Mass Spectrum (FAB) m/z 620 (m+).
WO 94/29309 PCT/US94/05~45
21 639~5
- 146-
EXAMPLE 127
1'-(3-((S)-(3,4-Dichlorophenyl))-4-(R or S)-(N-(3,5-
dimethylbenzoyl)-(methylamino))hexyl)- 1 -acetyl-5-fluoro-
5 spiro(indoline-3,4'-piperidine)
1H-NMR (400 MHz, CDCl3) ~ 0.96 (t, 3H), 2.20 (s, 9H), 2.45 (s,
3H), 3.81 (s, 2H), 6.24 (s, 2H), 6.84-6.89 (m, 3H), 7.19 (dd, lH),
7.39 (t, 2H), 8.13 (dd, lH).
Mass Spectrum (FAB) m/z 638 (m+).
EXAMPLE 128
1'-(3-(S)-(3,4-Dichlorophenyl)-4-(R or S)-(N-(3,5-
dimethylbenzoyl)-(methylamino))heptyl)- 1 -acetyl-spiro(indoline-
3,4'-piperidine)
20 lH NMR (400 MHz, CDC13) ~ 0.96 (t, 3H), 2.20 (s, 6H), 2.21 (s,
3H), 2.41-2.45 (s+m, 4H), 3.78 (s, 2H), 6.22 (s, 2H). 6.89 (s, lH),
7.03 (t, lH), 7.15-7.21 (m, 3H), 7.39 (t, 2H), 8.18 (d, lH).
Mass Spectrum (FAB): m/z 634 (m+).
EXAMPLE 129
1'-(3-(S)-(3,4-Dichlorophenyl)-4-(R or S)-(N-(3,5-
3 0 dimethylbenzoyl)-(methylamino))heptyl)- 1 -acetyl-5-fluoro-
spiro(indoline-3,4'-piperidine)
1H-NMR (400 MHz, CDCl3) ~ 0.97 (t, 3H), 2.20 (s, 9H), 2.44 (s,
3H), 3.81 (s, 2H), 6.22 (s, 2H), 6.83-6.88 (m, 3H), 7.18 (dd, lH),
7.38 (t, 2H), 8.13 (dd, lH).
WO 94/29309 2 1 6 3 q q 5 PCT/US94/05545
- 147 -
Mass Spectrum (FAB) m/z 652 (m~).
EXAMPLE 130
1'-(3-((S)-(3,4-Dichlorophenyl))-4-(R or S)-hydroxy-5-(3,5-
dimethylphenyl)pentyl)- 1 -methane-sulfonyl-spiro(indoline-3,4'-
piperidine)
To a THF (3 mL) solution of 3,5-
dimethylbenzylmagnesium chloride (generated from 290 mg (1.9
mmol) of 3,5-dimethylbenzyl chloride and 53 mg (2.2 mmol) of
magnesium in THF) was added slowly 1'-(3-((S)-(3,4-
dichlorophenyl))-3 -(N-methoxy-N-
15 methylaminocarbonyl)propyl)- 1 -methanesulfonyl-spiro(indoline-
3,4'-piperidine) (100 mg, 0.19 mmol, prepared by reacting the
product obtained in Example 10, Step 1 under the oxidative
cleavage conditions given in Example 1 followed by the coupling
procedure given in Example 2) in 1 mL of THF. The reaction
20 mixture was stirred at 60C for 40 min and poured into 20 mL of
lN HCl. The solution was extracted with 3 x 10 mL of EtOAc.
The organic extracts were combined, dried, and concentrated.
The product was purified by preparative TLC (30% EtOAc in
CH2Cl2) to afford 20 mg of ketone.
25 To a MeOH (3 mL) solution of ketone (19.4 mg) was added
sodium borohydride (7 mg). The mixture was stirred at 55C for
lh and concentrated. The residue was purified by preparative
TLC (4% MeOH in CH2Cl2) to give 15 mg of the higher Rf
isomer (Isomer A) and 4 mg of a lower Rf isomer (Isomer B).
1H-NMR (400 MHz, CDCl3), Isomer A: d 1.71 (d, 2H), 1.92-
2.12 (m, 6H), 2.23-2.29 (s+m, 9H), 2.50-2.60 (m, 2H), 2.72-2.76
(m, lH), 2.88 (s, 3H). 2.95 (d, 2H), 3.76 (s, 2H), 4.00-4.06 (m,
WO 94/29309 PCT/US94/05545
21 63995
- 148-
lH), 6.69 (s, 2H), 6.83 (s, lH), 7.05 (d, lH), 7.19-7.24 (m, 3H),
7.37 (t, 2H), 7.44 (s, lH).
Mass spectrum (FAB) Isomer A, m/z 601 (m+), 603 (m++ 2).
lH-NMR (400 MHz, CDCl3), Isomer B: d 1.69 (d, 2H), 1.74-
1.79 (m, lH), 1.83-1.90 (m, lH), 1.93-2.05 (m, 2H), 2.07-2.20
(m, 2H), 2.24-2.36 (s+m, 8H), 2.42-2.47 (m, lH), 2.55-2.58 (dd,
lH), 2.66-2.72 (d+dd, 2H), 2.87 (s, 3H), 2.86-3.00 (m, 2H), 3.76
(s, 2H), 3.91-3.95 (m, lH), 6.72 (s, 2H), 6.82 (s, 2H), 7.13-7.19
(m, 2H), 7.18-7.21 (m, 2H), 7.36 (t, 2H).
Mass spectrum (FAB) Isomer B, m/z 601 (m+) 603 (m+ + 2).
EXAMPLE 131
1 '-(3 -(R)-(3,4-Dichlorophenyl)-5 -(N-3,5 -dimethylphenyl-
2 0 methylamino)-S-oxo-pentyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-
piperidine)
Step 1) Diazomethyl-(2-(S)-(3,4-dichlorophenyl)-pent-4-en-yl)-ketone.
To a solution of 2-(S)-(3,4-dichlorophenyl)-pent-4-enoic acid
(5.04g, 20.6mmol) in 60mL of dichloromethane was added oxalyl
chloride 2.15mL (24.6mmol) and dimethylformamide (O.lmL) upon
cooling in an ice-water bath. The cooling bath was then removed and
30 the reaction mixture was stirred at rt overnight. The solvent was
removed under reduced pressure. The resulting material was diluted in
ethyl acetate and concentrated in vacuo in order to remove residual HCl.
The residual crude acid chloride was dissolved in 70mL of ether and
was slowly added to a lOOmL ether solution of diazomethane (77mmol).
After stirring for 2hr at rt, the solvent was removed under vacuum.
WO 94/29309 2 1 6 3 q ? ~i PCT/US94/0~545
- 149 -
The resulting yellow oil was chromatographed on silica gel column
eluting with a gradient of hexane: ethyl acetate = 20: 1 to 3: 1 to give
4.66g (84%) of diazomethyl-(2-(S)-(3,4-dichlorophenyl)-pent-4-en-yl)-
ketone.
H-NMR (CDCl3 400MHz): o 2.44(app. quint. lH), 2.82(app. qunit.
lH), 3.43(br s. lH), 4.98 & 5.02 (d of AB quart., 2H), 5.16 (br s, lH),
5.63(m, lH), 7.09 (dd, J=2.2Hz, 8.3Hz, lH), 7.34(d, J=2.2Hz, lH),7.38
(d J=8.3Hz).
Step 2) 3-(R)-(3,4-Dichlorophenyl)-hex-4-en-oic acid
To a solution of the above diazoketone 4.56g (17.0mmol) in
15 340mL of tetrahydrofuran was added 170mL aquous solution of silver
nitrate 3.02g (17.8mmol). After stirring at rt overnight,
tetrahydrofuran was removed under reduced pressure. The remaining
aqueous layer was extracted with two lOOmL portions of
dichloromethane. The combined organic phases were washed with
20 brine, dried over anhydrous magnesium sulfate, filtered, and
concentrated. The resulting material was purified by silica gel column
chromatography. Elution with dichloromethane: methanol = 10: 1
gave 3.94g (90%) of 3-(R)-(3,4-dichlorophenyl)-hex-4-en-oic acid.
25 Step 3) (N-(3,5-Dimethylphenyl)-N-methyl)-((3-(R)-(3,4-
dichlorophenyl)-hex-5-en-yl)-amide
The carboxylic acid from Step 2 (300mg, 1.16mmol) was
dissolved in 5mL of dichloromethane. To it was added 0.131mL
30 (1.50mmol) of oxalyl chloride followed by the addition of a drop of
dimethylformamide upon cooling in an ice-water bath. The cooling
bath was then removed and the reaction mixture was stirred at rt for
2hr. The solvent and residual HCl was removed as described above.
The resulting crude acid chloride was then dissolved in 5mL of
WO 94/29309 PCT/US94/05545
21 63qq~
- 150-
dichloromethane. To it was added N-methyl-3,5-dimethylaniline 313mg
(3.32mmol) (Prepared from 3,5-Dimethylaniline following the
procedure of Barluenga J., Bayon A.M., and Asensio G. J. Chem. Soc.
Chem. Comm. 1984 1334.) followed by the addition of triethylamine
5 0.5mL (3.6mmol) upon cooling in an ice-water bath. Then the cooling
bath was removed and the reaction mixture was stirred at rt overnight.
The solvent was removed under reduced pressure. The residual solid
material was dissolved in 15mL of ethyl acetate and 5mL of water. The
organic phase was separated and aqueous phase was extracted with two
o 7mL portions of ethyl acetate. The combined organic phases were
washed with brine, dried over anhydrous magnesium sulfate, filtered,
and concentrated. This crude material was chromatographed on silica
gel eluting with a gradient of 10: 1 to 3: 1 hexane-ethyl acetate to give
386mg of (N-(3,5-dimethylphenyl)-N-methyl)-((3-(R)-(3,4-
15 dichlorophenyl)-hex-5-en-yl)-amide (88%).
lH-NMR(CDCl3 400MHz): ~ 2.15-2.35 (m., 4H), 2.29 (s, 6H), 3.09 (s,
3H), 3.26 (quint, J=7.2Hz, lH), 4.88 (d, J=7.6Hz, lH), 4.92 (s, lH), 5.5
(m, lH), 6.45 (s, 2H), 6.91 (dd, J=2Hz, 7Hz, lH), 6.93 (s, lH), 7.30 (d,
20 J=g.3Hz, 1 H)-
Step 4) 3-(R)-(3,4-Dichlorophenyl)-5-(N-(3,5-dimethylphenyl)-
methylamino)-5-oxo-pentanal
To 386mg (1.03mmol) of the product from the previous step was
oxidized by osmium tetroxide to corresponding diol as described in
Example 1 to give 413mg of crude diol. 381mg of this material was
then dissolved in lOmL of benzene. To it was added lead tetraacetate
30 452mg (1.02mmol). After stirring for lhr at rt, 5mL of water was
added to quench the reaction. The reaction mixture was extracted with
two lOmL portions of ethyl acetate. The combined organic phases were
dried over anhydrous magnesium sulfate, filtered, and concentrated.
The crude material was chromatographed on silica gel eluting with
WO 94/29309 PCTIUS94/05~45
~1 63995
- 151 -
hexane: ethyl acetate = 2: 1 to give 329mg of 3-(R)-(3,4-
dichlorophenyl)-5 -(N-(3 ,5 -dimethylphenyl)-methylamino)-5 -oxo-
pentanal (94% overtwo steps).
Step 5) 1'-(3-(R)-(3,4-Dichlorophenyl)-5-(N-3,5-dimethylphenyl-
methylamino)-5-oxo-pentyl)- 1 -methanesulfonyl-spiro(indoline-3 ,4'-
piperidine)
Following the procedure described in Example 2, 107mg
(0.287mmol) of this aldehyde was treated with 1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) hydrochloride to give 1 03mg (58 %
yield) of the title compound .
15 1H-NMR (CDCl3 400MHz): â 2.23 (s, 6H), 2.86 (s, 3H), 3.09 (s, 3H),
3.72 (s, 2H), 6.49 (s, 2H), 6.9-7.2 (s, 8H).
MS(CI): 628 (M++1: 35Clx2), 630 (M++1: 35Cl & 37Cl)
EXAMPLE 132
1 '-(3 -(R)-(3 ,4-Dichlorophenyl))-5 -(3 ,5 -dimethylphenyl)-5 -oxo-pentyl)-
2 5 1 -methanesulfonyl-spiro(indoline-3 ,4'-piperidine)
Step 1) (N-Methoxy-N-methyl)-(3-(R)-(3,4-dichlorophenyl)-4-
hexenyl)-amide
To a solution of 3-(R)-(3,4-dichlorophenyl)-5-hexenoic acid
(Example 132, Step 1) 744mg (2.87mmol) was added 1-
hydroxybenzotriazole hydrate 465mg (3.44mmol), and 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloroide 660mg
(3.44mmol) with cooling in an ice-water bath. The cooling bath was
WO 94/29309 PCT/US94/05545
21 63~5
- 152-
then removed. After stirring at rt for lhr, to it was added 5mL
dichloromethane suspension of N, O-dimethylhydroxyl amine
hydrochloride 840mg (8.61mmol) and triethylamine 1.2mL (8.6mmol).
After stirring overnight, the solvent was removed under vacuum,
5 diluted with ethyl acetate and water. The organic phase was separated.
Aqueous phase was extracted twice with ethyl acetate. Combined
organic phases were washed with brine, dried over anhydrous
magnesium sulfate, filtered, concentrated, chromatographed on silica
gel eluting on a gradient of hexane: ethyl acetate = 5: 1 to 2: 1 to give
o 762mg (88%) of (N-methoxy-N-methyl)-(3-(R)-(3,4,-dichlorophenyl)-
4-hexenyl)-amide.
1H-NMR (CDCI3 400MHz): ~ 2.34(m, lH), 2.69 (App. d, 2H), 3.09 (s,
3H), 3.23 (quint. J=7.3Hz, lH), 3.56 (s, 3H), 4.95 (s, lH), 4.98 (app. d,
15 lH), 5.6 (m, lH),7.0 (dd, J=2.1Hz, 8.4Hz, lH),7.28 (d, J=2.1Hz, lH),
7.32 (d, J=8.3Hz, lH).
Step 2) 3-(R)-(3,4-Dichlorophenyl)-(N-methoxy-methylamino)-S-oxo-
2 pentaIlal
This above material was subjected to the osmium tetroxide
oxidation to the corresponding diol as described in Example 1. The
crude product was then treated with 1.23g (2.77mmol) of lead
25 tetraacetate as described in example 131, Step 4. Chromatographic
purification on silica gel (eluant; dichloromethane: ethyl acetate = S: 1)
afforded 618mg (81% two steps)of 3-(R)-(3,4-dichlorophenyl)-(N-
methoxy-methylamino)-S -oxo-pentanal .
Step 3) 1'-(3-(R)-(3,4-Dichlorophenyl)-5-(N-methoxy-methylamino)-5-
oxo-pentyl)- l -methanesulfonyl-spiro(indoline-3,4'-piperidine)
WO 94/29309 PCT/US94/05545
~ 1 6:~q95
- 153-
- A sample of 332mg (1.09mmol) of the aldehyde from Step 2
above was subjected to reductive ~min~tion with 1-methanesulfonyl-
spiro(indoline-3,4'-piperidine) hydrochloride as described in Example 2
to give 369mg (61%) of 1'-(3-(R)-(3,4-dichlorophenyl)-5-(N-methoxy)-
5 N-(methyl)amino)-5-oxo-pentyl)-1-methanesulfonyl-spiro(indoline-3,4'-
piperidine).
1H-NMR (CDCl3 400MHz): ~ 2.87 (s 3H), 3.10 (s, 3H), 3.60 (s, 3H),
7.0-7.4 (m, 7H).
Step 4) 1 '-(3-(R)-(3,4-Dichlorophenyl))-5-(3,5-dimethylphenyl)-5-oxo-
pentyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
To a 1.2mL THF solution of the amide from Step 3 above (73mg,
0.13mmol) was added l.lmL of 0.7M 3,5-dimethylphenylmagnesium
bromide solution in THF (prepared from 5-bromo-m-xylene and
magnesium turnings in THF). Then the reaction mixture was heated to
500C. After stirring for 1.5hr, the reaction mixture was allowed to cool
20 down to rt and the reaction was quenched by sat NH4Cl aq solution.
THF was removed under reduced pressure, diluted with ethyl acetate.
The organic phase was separated and the aqueous phase was extracted
twice with ethyl acetate. Combined organic phases were dried over
anhydrous magnesium sulfate, filtered, concentrated, chromatographed
25 on silica gel eluting with a gradient of dichloromethane: ethyl acetate =
10: 1 to 1: 1 to give 55mg (70%) of the title compound.
lH-NMR (CDCl3 400MHz): â 2.34 (s, 6H), 2.86 (s, 3H), 3.23 (m, 2H),
3.74 (s, 2H), 7.0-7.5 (m, lOH).
MS (CI): 599 (M++1;35Clx2), 601 (M++1: 35Cl& 37Cl).
EXAMPLE 133
WO 94/29309 PCT/US94/05545
2l 63~5
- 154 -
1 '-(3-(R)-(3,4-Dichlorophenyl)-6-(3,5-dimethylphenyl)-5-oxo-hexyl)- 1 -
methanesulfonyl-spiro(indoline-3,4'-piperidine)
70mg (0.126mmol) of 1'-(3-(R)-(3,4-dichlorophenyl)-5-(N-
methoxy)-N-(methyl)amino)-5-oxo-pentyl)- 1 -methanesulfonyl-
spiro(indoline-3,4r-piperidine) (Example 132, Step 3) was treated with
0.8M THF solution of 3,5-dimethyl benzylmagnesium chloride as in the
case of Example 132. The crude material was chromatographed on
silica gel in the same solvent system to afford 33mg of the title
compound (43%).
1H-NMR (CDCl3 400MHz): ~ 2.24 (s, 6H), 2.86 (s, 3H), 3.47 (s, 2H),
3.72 (s, 2H), 6.64 (s, 2H), 6.8-7.4 (m, 8H).
MS (CI): 613 (M++1: 35Clx2), 615 (M++1: 35CI& 37CI).
EXAMPLE 134
1 '-(3-(S)-(3,4-Dichlorophenyl)-6-(3,5-dimethylphenyl)-6-oxo-hexyl)- 1 -
methanesulfonyl-spiro(indoline-3,4'-piperidine)
3-(R)-(3,4-Dichlorophenyl)-4-hexenoic acid (Example 131, Step
2) was converted into 4-(S)-(3,4-Dichlorophenyl)-5-heptenoic acid as in
Example 131, Steps 1 and 2. 4-(S)-(3,4-dichlorophenyl)-4-heptenoic
acid was converted to (N-methoxyl-N-methyl)-(4-(S)-(3,4-
dichlorophenyl)-6-heptenyl)-amide followed by treatment with 3,5-
dimethylphenylmagnesium bromide as described in Example 132, Step 4
to give the title compound.
1H-NMR (CDCl3 400MHz): ~ 2.32 (s, 6H), 2.80 (s, 3H), 3.74 (s, 3H),
7.0-7.4 (m, lOH).
WO 94/29309 2 1 6 3 q ~ j PCT/US94/05545
- 155 -
MS (CI): 613 (M++1: 35Clx2), 615 (M++1: 3~Cl& 37Cl).
EXAMPLE 135
1 '-(3-(S)-(3,4-Dichlorophenyl)-6-(3,5-dimethylphenyl)-5-(RS)-methyl-
6-oxo-hexyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
Step 1) 4-(S)-(3,4-Dichlorophenyl)- 1 -(3,5-dimethylphenyl)-hept-6-
ene-1 -one
1.42g (4.50mmol) of (N-Methoxy-N-methyl)-(4-(S)-(3,4-
dichlorophenyl)-6-heptenyl)-amide (prepared in Example 134) was
dissolved in 20mL of dry THF. To it added 10mL THF solution of 3,5-
5 dimethylphenylmagnesium bromide prepared from 1.8g (9.6mmol) of
5-bromo-m-xylene and 463mg of magnesium turnings. After stirring
for 2hr at rt, the reaction was quenched with saturated aqueous
ammonium chloride solution. THF was removed under reduced
pressure. The residual material was diluted with ethyl acetate. The
20 organic phase was separated, aqueous phase was extracted twice with
ethyl acetate. Combined organic phases were washed with brine, dried
over anhydrous magnesium sulfate, filtered, concentrated,
chromatogrpraphed on silica gel eluting with a gradient of hexane:
ethyl acetate = 10: 1 to 5: 1 to give 1.57g of 4-(S)-(3,4-
25 dichlorophenyl)-1 -(3,5-dimethylphenyl)-hept-6-ene-1 -one (97%).
Step 2) 4-(R)-(3,4-Dichlorophenyl)-1-(3,5-dimethylphenyl)-2-(RS)-
methyl-hept-6-ene- 1 -one
Hexamethyldisilazane (0.108mL, 0.512mmol), and 0.089mL of
hexamethylphosphoramide were dissolved in 2mL of dry THF. To it
was added 0.306mL (0.49mmol) of n-butyllithium (1.6M hexane
solution) after cooling in an ice-water bath. After stirring for 20min,
WO 94129309 2 1 6 3 ~ ~ 5 PCT/US94/05545
- 156-
the ice-water bath was replaced by a dry ice-acetone bath and 2mL of a
dry THF solution of 4-(S)-(3,4-dichlorophenyl)-1-(3,5-
dimethylphenyl)-hept-6-ene-1-one (154mg, 0.426mmol) was added via
syringe. After stirring for lhr, 0.066mL (1.06mmol) of iodomethane
5 was added . The cooling bath was removed and the mixture stirred at rt
overnight. The solvent was then removed under reduced pressure and
the residual material was diluted in ethyl acetate and water. The
organic phase was separated. The a~ueous phase was extracted twice
with ethyl acetate. The combined organic phases were washed with
o brine, dried over anhydrous magnesium sulfate, filtered, concentrated,
and chromatographed on silica gel eluting with a gradient of hexane:
ethyl acetate = 10: 1 to 7: l to give l50mg of 4-(R)-(3,4-
dichlorophenyl)- l -(3,5-dimethylphenyl)-2-(R &S)-methyl-hept-6-ene- 1 -
one (94%). This was a 1 to 1 mixture of two diastereomers as revealed
5 by proton NMR.
H-NMR (CDCI3 400MHz): ~ 1.06 ( d, J=7Hz, l.5H), 1.14 ( d, J=6.7Hz,
l.5H), 2.30, 2.31 (s, 6H), 2.5 (m, O.5H), 2.6 (m, O.5H), 3.1-3.2 (m,
lH), 4.9 (m, 2H), 5.5 (m, lH), 6.8-7.4 (m, 6H).
Step 3) 3-(S)-(3,4-Dichlorophenyl)-5-(RS)-methyl-6-(3,5-
dimethylphenyl)-6-oxo-hexanal
The product from Step 2 above was subjected to osmium
tetroxide oxidation followed by the treatment with sodium periodate as
described in Example 1 to give 3-(S)-(3,4-dichlorophenyl)-5-(RS)-
methyl-6-(3,5 -dimethylphenyl)-6-oxo-hexanal.
Step 4) l '-(3-(S)-(3,4-Dichlorophenyl)-6-(3,5-dimethylphenyl)-5-(RS)-
methyl-6-oxo-hexyl)- 1 -methanesulfonyl-spiro(indoline-3,4'-piperidine)
WO 94/29309 PCT/US94/05545
21 6~qq5
- 157-
This product from Step 3 above was subjected to reductive amination
with 1-methanesulonyl-spiro(indoline-3,4'-piperidine) as described in
Example 2 to give the title compound.
1H-NMR (CDCl3 400MHz): ~ 1.05 (d, J=7Hz),1.08 (d, J=6.7Hz), 2.30
& 2.32 (s, 6H), 2.89 (s, 3H), 3.72 (S, 2H), 6.8-7.0 (m, 10H).
MS (CI): 627 (M++1: 35Clx2), 629 (M++1: 35Cl& 37Cl).
EXAMPLE 136
1 '-(3 -(S )-(3,4-Dichlorophenyl)-4-(3,5 -(bistrifluoromethyl)benzyloxy)-
l -acetyl -spiro(indoline-3,4 ' -piperidine)
Step A: 2-(S)-(3,4-Dichlorophenyl)-4-penten-1-ol
To a solution of 2-(S)-(3,4-dichlorophenyl)-4-pentenoic acid (7.0 gm)
(prepared as described by J. Hale et. al., Bioorganic & Medicinal
Chemistry Letters 1993,3, 319-322.) in ether (50 mL) at r.t. was added
portionwise over 5 min solid lithium aluminum hydride (700 mg). The
reaction was heated to 40 C for 3 hr and then stirred at r.t. for 16 hr.
The reaction was poured into water cont~ining 25 mL of 2N NaOH and
extracted twice with ether. The ether layers were washed with brine,
combined and dried over Na2SO4. Flash chromatograghy afforded the
title compound (4.5 gm) as an oil. [a]D = +14 (EtOH, c = 1.5).
Step B: 2-(S)-(3,4-Dichlorophenyl)-1-(3,5-
(bistrifluoromethyl)benzyloxy)-4-pentene
To a solution of 2-(S)-(3,4-dichlorophenyl)-4-penten-1-ol (1.0 gm) in
DMF (25 mL) was added sodium hydride (175 mg) while cooled in an
ice bath. After 1 min, 3,5-(bistrifluoromethyl)benzyl bromide (2.0 gm)
was added followed by a second portion of sodium hydride (175 mg).
After 1 hr, the reaction was poured into water and extracted twice with
ether. The ether layers were washed with brine, combined and dried
over Na2SO4. Flash chromatograghy (hexanes, then 2 and 5% ethyl
acetate/hexanes) afforded the title compound (2.0 gm) as an oil.
WO 94/29309 PCT/US94/05545
2 1 6399~
- 158-
NMR (CDCl3): ~ 2.30-2.40 and 2.50-2.60 (2 m, 2 H), 2.90-3.00 (m, l
H), 3.55-3.65 (d of AB q, 2 H, J = 6 and 9 Hz), 4.54 (AB q, 2 H, J = 13
Hz), 4.90-5.00 (m, 2 H), 5.55-5.70 (m, 1 H), 7.04 (dd, I H, J = 2 and
Hz), 7.30 (d, 1 h, J = 2 Hz), 7.36 (d, 1 h, J = 8 Hz), 7.64 (s, 2 h), 7.76
(s, 1 H).
Step C: 3-(S)-(3,4-Dichlorophenyl)-4-(3,5-
(bistrifluoromethyl)benzyloxy)butan- 1 -ol
o A solution of 2-(S)-(3,4-dichlorophenyl)-1-(3,5-
(bistrifluoromethyl)benzyloxy)-4-pentene (1.5 gm) in methanol (50
mL) was cooled to -70 C in a dry ice/acetone bath and ozone bubbled
thru for 15 min until a blue coloration was seen. The solution was
purged with N2 for 10 min and sodium borohydride was added. The
reaction was allowed to warm to r.t. and was stirred for 2 hr. The
volatiles were removed in vacuo and the residue was flash
chromatograghed (30 then 50% ethyl acetate/hexanes) to give the title
compound as a clear oil.
NMR (CDCl3): ~ 1.78-1.88 and 2.00-2.10 (2 m, 2 H), 3.05-3.15 (m, 1
H), 3.45-3.55 (m, 1 H), 3.55-3.68 (2 m, 3 H), 4.55 (AB q, 2 H, J = 13
Hz), 7.04 (dd, 1 H, J = 2 and 8 Hz), 7.32 (d, 1 h, J = 2 Hz), 7.36 (d, 1 h,
J = 8 Hz), 7.65 (s, 2 h), 7.76 (s, 1 H).
Step D: 4-Bromo-2-(S)-(3,4-dichlorophenyl)-1-(3,5-
(bistrifluoromethyl)benzyloxy)butane
3-(S)-(3,4-Dichlorophenyl)-4 (3,5
(bistrifluoromethyl)benzyloxy)butan-1-ol from Step C (500 mg) was
converted to the title compound (530 mg) with Ph3P-Br2 as described
in Example 20, Step B.
Step E: 1'-(3-(S)-(3,4-Dichlorophenyl)-4-(3,5-
(bistrifluoromethyl)benzyloxy)- 1 -acetyl-spiro(indoline-
3,4r-piperidine).
4-Bromo-2-(S)-(3,4-dichlorophenyl)- 1 -(3,5 -
(bistrifluoromethyl)benzyloxy)butane (30 mg) from Step D was
converted to the title compound (42 mg) as described in Example 20,
Step C.
WO 94/29309 PCT/US94/05545
2 1 ~39~5
- 159-
NMR (CDCl3): ~ 1.48-2.05 (m,10 H),2.14 and 2.34 (2 s,3 H),2.10-
2.25 (m,2 H),2.70-2.85 (m,2 H),2.90 (m, l H),3.48-3.58 (m,2 H),
3.70 and 3.84 (2 s,2 H),4.55 (AB q,2 H, J = 13 Hz),6.90-7.15 (m,4
h),7.33 (d, l h, J = 2 Hz),7.37 (d, l h, J = 8 Hz),7.66 (s,2 h),7.76
(s,1 H),8.18(d,1 h,8Hz).