Note: Descriptions are shown in the official language in which they were submitted.
2165141
.
-- 1 --
TITT,~ OF TH~ INV~NTION
USE OF SULEPAROIDE IN THE PREVENTION OF ENDOTHELITIS AND
OTHER ENDOTHELIAL REACTIONS INDUCED BY THERAPEUTIC
INTRAVENOUS INFUSIONS.
D~ cRrpTIoN
F;el~ of the Inv~nt1on
The present invention refers to a glycosaminoglycan
known as "suleparoide" (HHS.5), prepared in a
pharmaceutical form for parenteral use, endowed with
protective activity towards endothelitis, endothelial
damages and subendothelial sclerotic reactions, caused by
intravenous infusions with therapeutic purposes (e.g.
cancer chemotherapy, parenteral nutrition, etc.).
~ Descript1on of the pr1Or art
Cancer is the most important cause of death in the
industrialized countries after the cardiovascular
diseases. Its prevalence and incidence are continuously
growing because of the increase in life expectation
determined by the reduced mortality due to infective and
cardiovascular diseases.
In the last years, interesting results have been
achieved in cancer chemotherapy, which can dispose of
powerfull drugs with high selectivity of action towards
tumoral cells. The anticancer chemotherapy allows to
achieve good results in the treatment of certain cancers,
for which high percentages of success can be obtained,
such as cancer of lymphocytic system, urogenital tract,
gastrointestinal apparatus, m~mm~ry glands, etc..
However, the use of cytostatic drugs is hampered by
their poor tolerability because of the side effects on
rapidly growing normal tissues, such as gastrointestinal
mucosa, hematopoietic system, germinal cells, etc..
Another problem which fre~uently limits the use of
cytostatic drugs, is the damage of the endothelial cells
2165141
- 2
of the superficial vein (usually, of the elbow) through
which the drug has been infused. The damage can reveal
itself as a simple endothelial irritation (with skin
erythema and spontaneous or induced pain along the vessel)
or as a clinically evident phlebitis (with perivasal
phlogosis, eventually complicated by a thrombophlebitis).
The chemical damage of the venous endothelial cells
is always complicated by a perivascular fibrotic reaction,
even in absence of phlebitic symptomatology. The clinical
result is the disappearance of the vein, which can be
hardly found for a new venous infusion. This obliges the
physician or the nurse to look for another vein in other
less comfortable regions (hand, leg, foot, etc.) and
increases the (already heavy) sufferings of the patient.
Moreover, in many cases, the impossibility to find other
venous accesses obliges the physician to install a central
venous catheter, which cause further sufferings to the
patients and brings the risk of severe septicaemia.
Sl~mm~ry of the ;nventlon
It is therefore of great scientific interest the
surprising invention that the heparinoid "suleparoide'~
(HHS-5) is able to protect the vascular endothelium toward
the chemical damage induced by anticancer drugs.
In addition, suleparoide has been found
therapeutically effective in preventing the infusion
flebitis provoked by parenteral nutrition.
The invention can be considered surprising, because
suleparoide is known for its antithrombotic and
profibrinolytic activity, and its ability to protect the
endothelium against chemical damage by anticancer
chemotherapeutics could not be foreseen because it is
based on biochemical and physiopathogenetic mechanism
which are completely different from those responsible for
the antithrombotic activity.
~lG5141`
.
-- 3
The discovery has a relevant therapeutic interest,
because suleparoide is the first compund pharmacologically
active and therapeutically effective in the prevention of
endothelitis and subendothelial sclerotic reactions
provoked by venous infusion. At present, there is no
convalidated pharmacological therapy for this problem and
the auxiliary aids are usually palliative, such as
dilution of the drugs, washout of the vessel with saline
after the infusion, use of a central venous catheter,
etc.. It is noteworthy to mention that these aids do not
guarantee an effective protection or cause other side
effects (e.g. septicaemia).
Recently, a clinical trial has shown that heparin
can reduce the incidence of phlebitis induced by cancer
chemotherapy (Thurlimann B., Bachmann I., Effective
prevention of chemotherapy-induced phlebitis by low-dose
heparin: a prospective randomised trial. Ann. Oncol.
1992;3:311-3)
However, heparin can be handled with difficulty in
patients with cancer, because of its side effects on long-
term treatment (haemorrhagies on the site of infusion,
osteoporosis, hypersensibility, alopecia, thrombo-
cytopenia, etc.)
Best mode of carrying out the ;nvention
Suleparoide is a glycosaminoglycan, chemically
described as polyhexuronyl-l-4-~-D-N-acetyl-(N-sulphonyl-)
glucosamine 6-O-sulfate, sodium salt. It is devoid of the
heparin-like anticoagulant, but it is endowed with in vivo
antithrombotic and profibrinolytic activity.
The hexuronyl residues are represented by: D-
glucoronic, L-iduronic, L-iduronyl-2-sulfate.
Suleparoide (HHS-5), which can be generally defined
as an heparan sulfate, according to the definition
proposed by Lindhal and Lane (heparin-like structure with
216514i
a content of N-acetyl higher than 20%), is a white powder,
slightly hygroscopic. The chemical structure is:
co ~ c~ as~3~_
~ O--
where
R = H o -SO3Na
R' = -SO3Na o CH3-CO-
(about 50~ each)
n (average) = about 20
The molecular structure is:
for R' = CH3CO,R = H: (C14HlgNO14SNa2)n, MW - 503 D
for R' = CH3CO,R = SO3Na: (C14H18NO17SNa3)n, MW = 605 D
The mean molecular weight is: MW = 10.250 + 1.350 D (MW =
weighed mean molecular weight).
Suleparoide is chemically obtained as a N-acetylated
derivative of heparin, through a reaction of a N-
desulfated heparin (heparamine) with acetic anidride in
alkaline environment. The final product has the following
chemical characteristics:
Anticoagulant activity < 10 IU/mg
Content of N-acetyl (as CH3CO-) 3-4,7~ W/W
(corresponding to a degree of N-acetylation between
40 and 60~)
Content of O-acetyl absent
Uronic acid (after hydrolysis) 31 i 1
Hexosamines (after hydrolysis) 32 i 1
Organic sulfate (as S) 8 + 1~
Molar rate uronic/hexosamines/S/acetyl 1:1:1.5-
1.7:0.4-0.6
Specific optic rotation ~ + 35
21651~
-
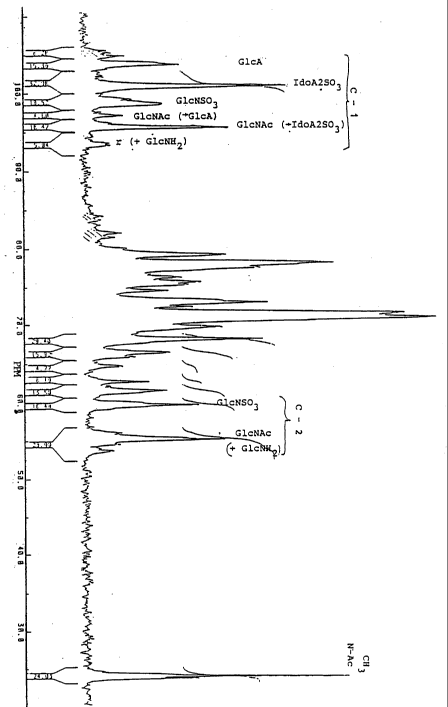
Figure 1 shows the N.M.R. (Nuclear Magnetic
Resonance) spectra of suleparoide (HHS-5), with the
identification of the relative chemical groups. In the
l3C-NMR spectra obtained in D20, operating at 75 Mhz, the
peak relative to the N-acetyl is clearly evident (main
difference vs héparin), as well as the peaks of
glucosamine N-sulfate (GlcNSO3) and N-acetylglucosamine
(GlcNac) in the zone identified as C-2, and the peaks of
glucoronate (GlcA) and iduronyl-C -sulfate (IdoA2SO3) in
the zone C-1.
Suleparoide (HHS-5) and its method of production are
disclosed in EP-A-0413248.
Suleparoide is a glycosaminoglycan, generically
called as "heparan sulfate", according to the statement of
Gallagher et al (1985) that: "...the term "heparin" should
be restricted to a polysaccharide in which more than 80
percent of the glucosamine residues are N-sulfated and the
concentration of O-sulfate groups exceeds that of the N-
sulfate groups. It was recommended that all other related
polysaccharides should be referred to as heparan sulfate"
(Biochemical Journal 230, 665-674, 1985)
Suleparoide exerts antithombotic activity, as shown
in different experimental models of arterial and venous
thrombosis. This activity is supported by a dose-dependent
activation of fibrinolysis, that has been demonstrated
both in animals and in patients with cardiovascular
diseases (chronic obstructive arteriopathy, chronic
cerebrovascular disease, atheroschlerotic coronaropathy,
etc.). The profibrinolytic activity of suleparoide is
supported by an increase in the plasma levels of the
tissue-type plasminogen activator (tPA) and a reduction in
its inhibitor (PAI). Because of its profibrinolytic
activity, suleparoide is indicated in the diseases caused
by impaired fibrinolysis and, particularly, in the
.,
21~5141
-
-- 6
treatment and prevention of thrombotic complications of
cardiovascular diseases.
Since Suleparoide has been studied and developed for
its antithrombotic activity and effects on coagulation and
fibrinolysis, it was surprising to discover that
suleparoide is endowed with other than the antithrombotic
activity, e.g. the protective effects towards the
endothelitis and endothelial damage provoked by venous
infusion of anticancer chemotherapeutics and by parenteral
nutrition.
~ ffects of Sl~leparo;~e on the ~ndothellt;s ~n~
flebosclerosis in~uced hy ~nticancer chemotherapy.
The Suleparoide's protective effects on the venous
endothelium has been experimentally studied in a model oon
endothelitis induced by intravenous administration of
anticancer drugs.
Male Wistar rats (180-220 gr.b.w.) have been treated
with the anticancer drugs that more frequently induce in
patients phlogistic reactions of the endothelium and
reactive perivasal sclerosis. The endothelial damage has
been measured on the basis of the number of detached
endothelial cells (EC), found in plasma 2 hours after the
treatment with the anticancer drugs. The protective
activity of suleparoide has been evaluated, giving the
coumpound 30 minutes before the cytostatic drugs. The
plasma has been obtained by centrifugation at 2,100 rpm x
10 min from blood collected with siliconized material and
added with trisodic citrate 3.8~ 1:10. The precipitate
obtained after removal of platelets with ADP and
centrifugation at 3,000 rpm x 15 min., was resuspended in
3 ml of a 0.5 procaine solution, and the Ecs counted with
a Burker chamber. The number of EC has been evaluated as a
mean of 4 different determinations and expressed as n
CE/9 ~l.
216~141
,
-- 7
Groups of 18-20 rats have been treated with the
following anticancer drugs: vinorelbine (0.3 mg/kg),
vindesine (0.04 mg/kg), dacarbazine (2 mg/kg), carmustine
(BCNU) (2 mg/kg), doxorubicin (1 mg/kg), cyclophosphamide
(0.1 mg/kg).
All the drugs provoked an endothelial damage as
shown by a statistically significant increase in plasma
ECs; these data confirm the irritant effects of anticancer
chemotherapeutics. The administration of suleparoide dose-
dependently reduced the number of ECs at doses rangingbetween 0.01 and 10 mg/kg: the activity of the
glycosaminoglycan towards the different drugs is reported
in Table 1. In the same Table, the effects of other
glycosaminoglycans are reported; it is noteworthy to
underline that no other compound was active (dermatan
sulfate, condroitin sulfate, hyaluronic acid).
2165141
C ;, ~
~ 7
~} o 2
y v 3
~ ~ * c~l O O O O CJ~
2 8 ~ ~ ~ ~ -- ~ ~ o x o~ Y
~ V { o o o o o ~-
~ 3 ^ X _ ~ o _ ~ o r~ x
~, U ~ ~o ~ ~ u 'H
~ _ O O O ~ 'u
._ ,................ . .
~, -
o - ~ o~ o o o o o ~ *
z o +~ +~ 0 ~ o~
~ o o o o o o o o o .5
U~ o ~ , O ~ ~ ~ ~ ~ +l +l H +I V .u
c~ Z .,,~ o ~1 o. o~ o o -- o o ~ D
~ o
~ 3 0 ~; ~
~ 3 ~ x
o t~ _ o o o _ _ _ o y
u ~ 3 ~ o 2 ~ ~ ~ ,-? ~
. ..
216514~
.
The therapeutic efficacy of suleparoide has been
confirmed in a double-blind placebo-controlled randomized
multicenter clinical trial, which enrolled 182 patients
receiving multiple-day chemotherapy for cancer. The
cytostatics most often used were vinorelbine, vindesine,
dacarbazine, mechloretamine, antrhacyclines, bleomycin.
Patients were randomly assigned to Group (A) or Group (B),
and treated with 2 vials of placebo or suleparoide,
respectively. One vial was administered before starting of
chemotherapy infusion and another one at the end of
infusion. The treatment was repeated before any other
infusion. The therapeutic efficacy of suleparoide has been
evaluated with the following parameters:
a) Phlebitis: the total number of phlebitis observed
in each group;
b) Phleb./pat (%): the incidence of phlebitis for
100 patients (or, the probability of each patient to
develop at least one phlebitis during a therapy lasting on
average 4 infusions);
c) Phleb./Infus. (%): the incidence of phlebitis for
100 infusions (or the probability of each infusion to be
complicated by a phlebitis);
d) Vein lost: the total number in each group of the
veins lost for perivascular reactive fibrosis;
e) Vein lost/pat (%): the incidence of vein lost for
100 patients (or, the probability of each patient to loss
at least one vein during a therapy lasting on average 4
infusions);
f) Vein lost/infus.(%): the incidence of vein lost
for 100 infusions (or the probability of each infusion to
be complicated by the loss of one vein).
The results of the clinical trial are summarized in
Table 2, and they show that:
~ 1 6 ~
-
- 10
- s2.69% of patients given with anticancer
chemotherapy and placebo develop a phlebitis;
- 87.10% of patients loss one vein at least during
the therapy lasting 4 infusions.
Howe~er, when the infusions of chemotherapeutic
agents are preceded by suleparoide intravenous
administration:
- only 4.49~ of patients develop a phlebitis;
- only 23.60% of patients loss a vein during the
therapy.
~ahl~
N~ml~cr ~,r in~si~n~inpati~nt~undcr~rcatmcntwithcancerchemorhcrapyandtrealcd
withpla~eboorsul~paloid~i.v.andirlci~ccofphlebilisandveinslo~
Pl~cebo Sufeparoicle
N p~ticn~ 93 R9
Infus~on.~ 3~t) 346
Inf./pat. 4.0g :~: 0.13Q ~.89 ~ O.IR3
Plllcbiti~
I'hleb.Jpat. (%) 52.69 4 49
Phlcb.llnfus. (%) ~.89 1.16
Vein l()st ~1 21~
Vci~ st~p~t. (%) 87.1Q 23.60~*
Vein lostJin~us. (~) 21.~2 6.07~
Tl~e p~raluetric data were sr~ictica~ly analy~ed with theonewayanalysi~of variancc and
Slud~nt's tn te.s~. l he non-parametric data werc analyzcd with chi2-two-tailed tcs~ corrcct~d
by Y~s and tl1c on~-way Kruskall-Wallis test. Statistically si~nificant diff~rence~ c().
and *~p ~c O.(I I vs placcb~.
216514 ~
-
~ ffects of SuleD~ro;~e ln the en~othel;t;s ~n~
phl~hosclerosls ;n~uced hy p~renter~l nl]trition ther~py
Parenteral nutrition provides life-sustaining
therapy for patients who cannot take adequate nutrition by
mouth and who consequently are at risk for the
debilitating complications of malnutrition. Partial and
short-term parenteral nutrition can be provided via a
peripheral vein, but the repeated use of a venous access
can be complicated by an infusion phlebitis. In another
double-blind clinical multicenter trial, 146 patients who
needed parenteral nutrition (massive small bowel
resection, moderate to severe acute pancreatitis,
necrotizing enterocolitis, GI major surgery, extensive
burns, etc.) were ruled into the study. Patients have been
randomly assigned to Group (A) or Group (B), and treated
with 2 vials of placebo or suleparoide, respectively. One
vial was administered before starting of parenteral
infusion, and another one immediately after. The treatment
was repeated before any other infusion. The therapeutic
efficacy of suleparoide has been evaluated with the same
above-mentioned parameters:
a) Phlebitis: the total number of phelibitis
observed in each group;
b) Phleb./pat (%): the incidence of phlebitis for
100 patients (or, the probability of each patient to
develop at least one phlebitis during a therapy lasting on
average 8 infusions);
c) Phleb./Infus.(%): the incidence of phlebitis for
100 infusions (or the probability of each infusion to be
complicated by a phlebitis);
d) Vein lost: the total number of veins lost in each
group;
e) Vein lost/pat.(%): the incidence of vein lost for
100 patients (or, the probability of each patient to loss
2165141
- 12
at least one vein during a therapy lasting on average 8
infusions);
f) Vein lost/infus.(%): the incidence of vein lost
for 100 infusions (or the probability or each infusion to
be complicated by the loss of one vein).
The results of the clinical trial are summarized in
Table 3, and they show that:
- 47.22~ of patients given with parenteral nutrition
and placebo develop a phlebitis;
lo - 70.83~ of patients loss one vein at least during
the therapy lasting on average 8 infusions.
However, when the infusion of the nutritive
preparation was preceded by suleparoide intravenous
administration:
- only 4.05~ of patients develop a phlebitis;
- only 16.22~ of patients loss a vein during the
therapy.
l'~blc~
Incidcnce ~ phlcbitis and veinsloslin patient.~ under ~arentcral nutri~i~n via
pcripheral vein, treatcd with placebo or sllleparo~de i.v. berorc ca~h infimsion.
Pk~eh~ SulepQroidc
-
N patienls 72 74
Il~ru~ ns 61 8 623
Illf.fp~t. 8.5~ ~: 0.756 ~.42 ~ O fi71
Phlehitis 34
Pl~lcb.lpat. (%) 47.22 4.05
Pllle~.llnfus. (9rv) 5 50 0 4
Vcitl lost 5 1 1 Z~
Vci~ lp~t. (~) 70.~3 ~6 22
Vein lo!;t/infiJs. ~%) 8.25 1.93~
Tl-e parametric d~la w~re statistically analyzed with the one-way analy~;is uf variancc antl
Studcnt's "t" test. lhe non-para.~et~ic dala were analy7~d with chi2 ~wo-t~ilcd t~stcorr~te~
by Yates alld the one-way Kruskall-Wallis test. St~tistic~lly .~igni~lcantdiffcrcnce *pcO.()~
and ~p~0.01 v~ l lacebo.
21 G51 4~
-
- 13
A typical pharmaceutical preparation of suleparoide
is in the form of an ampoule containing 100 mg of this
drug in a sterile physiological solution. The preparation
is used to prevent endothelitis and other endothelial
S reactions induced by therapeutic intravenous infusion and
is administered intravenously at a dose of 50-200 mg
before and after the therapeutic infusion.