Note: Descriptions are shown in the official language in which they were submitted.
216~381~
2-AMINO-1,3-THIAZEPINES AND THEIR USE
AS INHIBITORS OF NITRIC OXIDE SYNTHASE
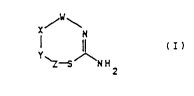
The present invention relates to thiazepines of the
general formula I
X~
S
~Z-~ ~ H2 (I)
which on account of their capacity to modulate endogenous
nitric oxide production are useful medicaments for the
prevention and control of conditions which are characterized
by an impaired nitric oxide level. Commercial packages
comprising pharmaceutically effective amounts of such compounds
together with instructions for use in prevention or treatment
of a disease caused by an increased nitric oxide level comprise
another aspect of the invention.
Nitric oxide (NO) plays an important part in all
sorts of physiological processes (see e.g. R. Henning, Nachr.
Chem. Tech. Lab. 41 (1993), 413; J. F. Kerwin, M. Heller, Med.
Res. Rev. 14 (1994), 23).
It has e.g. a relaxing effect on the smooth vessel
musculature and in this way is substantially involved in the
regulation of blood pressure, it controls blood clotting via
inhibition of platelet aggregation, and it is involved, for
example, in the brain as a neurotransmitter in the building up
of the long-term memory. In NANC nerves of the peripheral
nervous system, NO also functions as a messenger substance.
23233-310
216~386
- la -
The cytotoxic action of NO is used by macrophages for defence
against infection.
Endogenous NO is formed from arginine with the aid
of at least three different NO synthase isoenzymes.
All isoenzymes require NADPH, flavine adenine
dinucleotide, flavine mononucleotide and tetrahydrobiopterin
as cofactors. They differ with respect to their localization
in the body, their controllability by Ca /calmodulin and
their inducibility by endotoxins and cytokines. The
constitutive, calcium-dependent NO
23233-310
216~386
Ref. 3595 - 2 -
Dr.ER/L10956
synthases are found, for example, in the endothelium and
in the brain and are involved there in the -egulation of
blood pressure and clotting or in conduction processes.
The cyto~ine-inducible, calcium-independe~t isoform
occurs, for example, in macrophages, smooth muscle cells
and hepatocytes. It i8 able to produce relati~ely large
amounts of NO over a long period of time and is held
responsible for inflammatory processes and the cytotoxic
activity of the macrophages.
An impaired NO metabolism results in serious
disorders and damage. The excessive formation of NO in
septic or haemorrhagic shock thus leads to massive
pathological blood pressure decreases. Excessively raised
NO production is involved in the genesis of type 1
diabetes and atherosclerosis and also appears to be
-esponsible for the glutamate-induced neurotoxicity after
cerebral ischaemia. ~igh NO concent_ations can moreover
lead to DNA damage by d~min~tion of cytosine.
The attempt to use a modulat on of NO production
for the treatment of these syndromes was until now mainly
realized with the aid of arginine analogues (GB-A-2 240
041; WO-A-93/13055; WO-A-93/24126; WO-A-94/02453; WO-A-
94/07482). Further potential NO synthase inhibitors
discussed in the literature are N-iminoethylornithine
(McCall et al., Br.J.Pha-macol. 102 (1991), 234), ~m;no-
guanidine (T.P.Mis~o et al., Eu-.J.Pharmacol. 233 (1993),
119; EP-A-547588), methylguanidine (~S-A-5 246 971),
nitro- and cyanoaryls (WO-A-94/12163) and also pyri-
midines, pyridines, pteridinones and indazoles (WO-A-
94/14780). The inhibiting action of isothioureas on NO
synthase is disclosed in WO-A-94/12165. In add-tion to
the open-chain isothioureas mainly in~estigated there,
2-amino-1,3-thiazole deri~ati~es are also mentioned.
Surpris-ngly it has now been found that thiaze-
pine deri~ati~es of the general formula I are particu-
larly highly suitable inhibitors o~-inducible NO synthase
and inhibit NO formation substantially more strongly than
the thiazole deri~atives mentioned i~ WO-A-94/12165. They
. ~16538~
Ref. 3595 - 3 -
Dr.ER/L10956
are thus suitable for the modulatiou of endogenous NO
production and can be used as active sub6tances i~
medicaments for the treatment of diseases which are
characterized by an excessively high NO le~el. Only a few
representatives of thiazepi~es of the general formula I
ha~e previously been described in the literature, but the
ph~rm~cological properties or medicinal uses of compounds
of the ge~eral formula I were pre~iously usk~own.
The present in~ention therefore relates to
2-amino-1,3thiazepi3es of the general formula I,
X / N
Y`z-S ~ NH
n which W is CR R2 a~d
R1 and R2 independently of one another a-e hydrosen,
halogen, R3, R~, ORs, SR5, S(O)p-R6, where p is 1 or 2,
~R7R3, cyano, nitro, COOR9, CoNR7R~ or P(O)(O~) 2; or Rl and
R2 together are (C1-C6)-alkylidene or (C3-C,)-cycloalkyl-
idene, each of which can also ca-ry one or more Rl
radicals, or R1 and R2 together are =0, =S, =N~, =NR3 or
=NO~; or R1 and R2, together with the carbon atom to which
they are bonded, form a radical of the general formula II
(CH2)~\ ~II)
/\ / ,
( C H 2 ~ n
which can be completely or partly dehydrogenated, which
can also be benzo-fused, which can be substituted by one
or more identical or different R~ radicals a~d wherein m
and n independently of one another are the numbers 0, 1,
2, 3, 4 or 5, but the sum of m + n has o~e of the ~alues
1, 2, 3, 4 or 5; or R1 or R2 is a f~ee ~alency which, with
a similar free ~ale~cy on a~ atom which is bonded
directly to the carbon atom carrying R1 and R2, forms a
2165386
~ Ref. 3595 _ 4 _
Dr.ER/L1095
double bond;
_
R i 8 (Cl-Clo)-alkyl, (C2-C10)-alkenyl, (C2-C10)-alkynyl,
(C3-C,)- cycloalkyl, (Cg-Cl~)-be~zocycloalkyl or the
radical of a 4- to 10-membered heterocycle ha~ing one to
four ide~tical or different hete-oatoms from the series
~itrogen, oxygen and sulphur, which can be aromatic,
pa-tly saturated or completely satu-ated, it being
possible for the alkyl, alkenyl, alkynyl, cycloalkyl and
the heterocyclic radical to be substituted by o~e or more
identical or different Rl radicals;
R~ is phenyl or naphthyl, which can be substituted by one
to three identical or diffe-ent radicals from the series
(C~-C~)-alkyl, (C~-C,)-alkoxy, halogen, nitro, trifluorom-
ethyl, cyano, hydroxyl, (C~-C~)-alkoxycarbonyl, carboxyl,
carbamoyl, (Cl-C~)-alkyl-S(0)~, whe~e q is 0, 1 or 2,
~m;~o, (C~-C,)-alkylca-~o~yl~i~o, (C~-C4)-alkyl~m;no and
di((Cl-C~)-alkyl)am no;
R5 and R5 i3depende~tly of one another are hyd-ogen,
(Cl-C10)-alkyl in which the alkyl chain can also be
i~terrupted by one to three oxygen atoms,
(C2-C10)-alkenyl, (C3-C,) -cycloalkyl, R~, R~-(Cl-C6)-alkyl,
the radical of a 5- to 7-membe-ed heterocycle ha~ing one
to four ide~tical or diffe-e~t hete-oatoms from the
series ~it-oge~, cxysen a-d sul?hu_, which can be
aromatic, partly saturated or completely saturated,
(Cl-C10)-alkanoyl, R'-C0, R'-(Cl-C6)-alkyl-C0,
(Cl-C10)-alkoxycarbonyl, R~0-C0, R~-(Cl-Cc)-alkoxy-CO or a
radical of the general formula III
NRll
~~ (III~
NR12R13
R6 is (C1-C~0)-alkyl, (C2-C~0)-alkenyl, R~ or
R~-(Cl- C6 ) - alkyl;
- 2~6~386
- Ref. 3595 5
Dr.ER/~10956
R' and RJ independently of one another ha~e the me~n;ngs
specified for Rs O~ are (Cl-C6)-alkylsulphonyl or R~-S(O) 2
or R' and R8, together with the nitrogen atom to which
they are bonded, form a radical of the general formula rv
/(CH2~r\
-N A (~v)
( CH2 ) s
which can be substituted on the C~2 groups by one or more
idestical or different Rl radicals and wherei~ r and s
indepen-dently of one a~other are the numbers 1, 2, 3 or
4, but the sum of r + s is smaller than 6;
R' and R8 independently of R' and R8 ha~e the me~n;ngg
speci ied for R' and R8;
R9 has one of the me~nings of R5, but is not the radical
of the ge~eral formula II~;
RlD is (Cl-C5)-alkyl, (C3-C,)-cycloalkyl, the radical of a
5- to 7-m~mhered heterocycle ha~ing one to three ident-
ical or differe~t heteroatoms from the series nitrogen,oxygen and sulphur, which can be aromatic, partly satu-
rated or completely saturated, R4, halogen, oR5, SR5,
S(O)p-R6, whe-e p is 1 or 2, NR7R3, the hydroY m;no group,
the oxo s-ou?, cyano, ni~~o, COOR9, CONR' R8 or P(O) (~)2;
the radicals Rll, Rl2 and Rl3 independently of one anothe~
are hydrogen, (Cl-C6)-alkyl, ( C3 - C~ ) - CyC loalkyl, R~,
(Cl-C6)-~lk~noyl, (Cl-C6)-alkyl-S(O) 2 or R~-S(O) 2;
Rl~ is (Cl-C~)-alkyl, benzyl, phenyl, (Cl-C6)-~lk~nQyl,
(Cl-C6)-alkyl-S(O) 2 or R~-S(O) 2;
A is a methylene group which can b~-substituted by one or
two identical or different Rl radicals, or is oxygen,
sulphur or NRl~;
216~6
Ref. 3595 - 6 -
Dr.ER/L10956
X i8 CR20R2l and R20 and R2l independently of the m~nings
of the radicals RL-and R2 have the m~n;ngs specified for
Rl and R2;
Y i8 CR22R23 and R;2 and R23 independently of the m~n i ng8
of the radicals Rl and R2 have the mea~ings specified for
Rl and R2;
Z is CR2~R2s and R2~ and R25 independently of the me~n; ng8
of the radicals Rl and R2 have the me~n;ngs specified for
R and R2;
where radicals of the g-oups W, X, Y and Z ca~ also be
linked via a bond such that a bicyclic system o-
tricyclic system is formed;
and their tautomeric f 0nm8 and al60 their pharmaco-
losically tolerable salts as ph~rm~colosical active
subst~ces.
Examples of compounds of the general fon~ula I in
which a substituent i~ the groups W, X, Y and Z is a free
valency which forms an endocyclic double bond with a f-ee
valency on an atom bonded di~ectly to this group a-e,
inter alia, the compounds of the formula Va to Vf
~ alogen is fluorine, chlorine, bromine or iodine,
preferably fluor ne or chlo~ine.
Alkyl groups can be straight-chain or branched.
This also applies if they occur in other groups, for
example in alkoxy, alkylmercapto, alkylidene, alkoxy-
carbonyl or alkanoyl groups. Examples of alkyl groups
which can occur in the compounds of the general formula
I to be used according to the invention as such, i.e. as
( Cl - C~ ) -, ( Cl - C5 ) - or (C~-C10)-alkyl, or in other groups,
are methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl,
sec-butyl, tert-butyl, n-pentyl,~-3-methylbutyl, 2,2-
dimethylpropyl, n-hexyl, n-heptyl, n-octyl, n-nonyl or
n-decyl. Preferred alkyl groups are methyl, ethyl, n-
216~38~
Ref. 3595 - 7 -
Dr.ER/L10956
R I
2 ~~5 NN ~Z2
(Va) (Yb) ~Yc)
2 ~H2 XJ~
R 2 4
(Yd) (V~) (Vf)
propyl, i-propyl, n-butyl, i-butyl a d tert-butyl.
Alke~yl and alky~yl radicals can also ~e
6t-aight-chain or branched. Exam?les of alke~yl radicals
are ~inyl, l-propenyl, allyl, butenyl, 3-methyl-2-
bute~yl, a~d examples of alkynyl radicals are ethy~yl, 1-
propy~yl, proparsyl or 5-hexynyl.
The (C3-C,)-cycloalkyl radical is, for
example, a cyclopropyl radical, a cyclobutyl radical, a
cyclopentyl radical, a cyclohexyl radical or a cyclo-
heptyl radical. Preferred cycloalkyl radicals are thecyclopentyl radical and the cyclohexyl radical.
The heterocyclic radical ha~ing 1, 2, 3 or 4
heteroatoms from the series nitrogen, oxygen and sulphur
can be aromatic, partly saturated or completely saturated
and can be fused. Preferred ring sizes of the heterocycle
are 5-membered rinss, 6-m~hered rings and 7-membered
rings. Examples of heterocycles from which the radical i5
deri~ed are azetidine, pyrrolidine, pyrrole, indole,
pyrazole, imidazolidine, ;~;dazoline, ;~;dazole,
1,2,3-triazole, 1,2,4-triazole, tetrazole, tetrahydro-
furan, furan, l,3-dioxolane, tetrahydrothiophene,
thiophene, benzothiophene, l,3-dithiolane, 1,3-oxazoline,
216S~8~
Ref. 3595 - 8 -
Dr.E~/110956
1,3-oxazole, 1,3,4-ox~ ole, furazan, 1,3-thiazolidine,
1,3-thiazole, piperidine, 1,2,5,6-tetrahydropyridine,
1,4-dihydropyridine, pyridine, ~uinoline, isoquinoline,
pyridazine, pyrimidine, piperazine, 1,2,3-triazine,
1,3,5-triazine, perhydL~yLa~, 1,3-dioxane, 1,4-dioxane,
1,3-dithiane, dihydro-1,3-oxazine, morpholine, pe hydLo-
1,4-thiazine, 5,6-dihydro-4~-1,3-thiazine, perhydro-
azepine, pteridine and its deri~atives. The radicals can
be bonded in any desired positions, pyridyl radicals e.g.
in the 2-position, the 3-position or the 4-position.
Nitrogen heterocycles can also be bonded via a nitrogen
atom.
Examples of su~stituents which can be found on
alkyl racicals and other radicals are phenyl, pyridyl,
methoxy, hydroxyl, acetoxy, acetyl, 2-oxopropyl,
ca-boxyl, methoxycarbonyl, ethoxycarbonyl, methyl-
mercapto, methanesulphonyl, fluorine, ~;nocarbonyl,
diethyl~mino and suanidino.
Exam?les of substituted alkyl sroups a-e benzyl,
2-?henylethyl, hydroxymethyl, methoxycarbonylmethyl,
~;noca-bonylmethyl and diethyl~minnmethyl.
In monosubstituted phenyl radicals the
substituent can be located in the 2-position, the
3-position or the 4-position. If phenyl is disubstituted,
25 the substituents can be in the 2,3-, 2,4-, 2,5-, 2,6-,
3,4- or 3,5-position. Na?hthyl radicals can be present as
l-napht~yl and 2-naphthyl radicals and can also be
substituted in any desired positions. Examples of substi-
tuted deri~atives of the phenyl radical preferred as aryl
radical are 2-, 3- or 4-methylphenyl, 4-tert-butylphenyl,
2-, 3- or 4-methoxyphenyl, 3-ethoxyphenyl, 2,3-, 3,4- or
3,5-dimethoxyphenyl, 3,4,5-trimethoxyphenyl, 3- ~m; no-
phenyl, 3- or 4-dimethyl~nophenyl, 4-acetylaminophenyl,
2-, 3- or 4-fluorophenyl, 2,3- or 3,4-difluorophenyl, 2-,
35 3- or 4-chlorophenyl, 2,3-, 3,4-, 3,5- or 2,6-dichloro-
phenyl, 4-bromophenyl, 4-hydroxyphenyl, 3-hydroxy-4-
methoxyphenyl, 2-, 3- or 4-nitrophenyl, 3- or 4-tri-
fluoromethylphenyl, 2-, 3- or 4-cyanophenyl.
216~38G
- Ref. 3595 9
Dr.ER/L10956
- R5 radicals of alkyl ch~;nc~ which are inter-
rupted by oxygen atoms, are, for exa~ple~ 2-methoxyethyl,
2-ethoxyethyl, 2-(2-methoxyethoxy)ethyl or 2-(2-(2-
methoxyethoxy)ethoxy)ethyl.
Example6 of radicals of the general fo-mula rv
are 1-azetidi~yl, pyrrolidino, 2,5-dimethylpyrrolidino,
piperidino, 2,6-d~ethylpiperidino, 2,2-dimethyl-
piperidi~o, morpholino, 1,4-thiazin-4-yl, 3,3-dimethyl-
1,4-thiazin-4-yl, piperazino, 4-methylpiperazino,
4-acetylpiperazino, 4-methylsulphonylpipe-azi30, 4-
phenylpiperazino or azepino.
The compounds of the general formula I can be
present in various tautomeric for~s and in ~arious
stereoisomeric forms, thus i3 the tautomeric form of the
general formula VI.
X N H
~Z--S ~N H
The present invention includes not only the use
of all tautomeric forms, but also that of all stereoiso-
meric forms, that is e.g. that of pure enantiomers,
enantiomer mixtures and race-mates, pure diastereomers
and diastereomer mixtu-es or cis/tr~ns or E/Z isomers.
Suitable acids for the for~ation of pha-maco-
losically acceptable acid addition salts of the compou~s
of the general formula I are, for example: hydrochloric
acid, hydrobromic acid, naphthalenedisulphonic acids, in
particular naphthalene-1,5-disulphonic acid, phosphoric,
nit-ic, sulphuric, oxalic, lactic, tarta-ic, acetic,
salicylic, benzoic, formic, propionic, pivalic, diethyl-
acetic, malonic, succinic, pimelic, fuma-ic, maleic,
malic, sulph~;c, phenylpropionic, g'uconic, ascorbic,
isonicotinic, methanesulphonic, p-toluenesulphonic,
citric or adipic acid. The acid addition salts can be
prepared as is customary by combination of the compo-
nents, expediently in a suit2ble solvent or diluent.
216~8~
Ref. 3595 - 10 -
Dr.ER/LlOg56
Compounds of the general formula I which contain
an acidic group, ~or example a carboxylic acid group, ca~
form salts of this with inorganic or organic bases.
Suitable ph~rm~cologically acceptable 6alts are, for
example, sodium salts, potassium salts, magnesium salts,
calcium salts, ~mmon;um salts or salts with organic
~mines, for example ethanolamine or ~ino acids.
The thiazepine of the general formula I in which
W, X, Y and Z simultaneously are the C~2 group is pre-
ferred.
A few representatives of thiazepines of the
general formula I are known as such, see J. Les6el,
ph~-m-~ie 48 (19g3), 812; L.I. Rirkovskii, Metalloorg.
Rhim. 3 (1990), 1115; T. Rato et al., ~eterocycles 15
(1981), 399; J. B. Bream and J. Schmutz, ~elv. Chim. Acta
60 (1977), 2872; JP-B-46/021713; DE-A-1965309;
Y. Migalina et al., ~kr. Rhim. Zh. 35 (1969), 526 and
E. W. Schubert and 0. Behner, Arch. Pha-m. 301 (1968),
750.
The present invention also relates to the
previously undesc-ibed compou~ds of the general formula
I as such, i.e. 2-7mino-1,3-th~azepines of the general
formula I
I ll (I)
r~Z_~NH
in which W is CRlRl and
Rl and Rl independently of one another are hydrogen,
halogen, R3, R~, oR5, SR5, S(O)p-R6, where p is 1 or 2,
NR7R~, cyano, nitro, COOR9, CONR'R8 or P(O) (~)2; or Rl and
R2 together are (Cl-C6)-alkylidene or (C3-C7)-cycloalkyl-
idene, each of which can also carry one or more Rl
radicals, or Rl and R7 together are~~=O, =S, =N~, =NR3 or
=NO~; or Rl and R2, together with the carbon atom to which
they are bonded, form a radical of the general formula II
2165386
Ref. 3595 - 11 -
Dr.ER/~10956
tCH2)m
C A (II)
/\ /
(cH2)n
which can be completely or pa-tly dehydrogenated, whlch
can also be benzo-fused, which can be substltuted by one
or more ideutical or different Rl radicals and whereiu m
and n independently of one another are the numbers 0, 1,
2, 3, 4 or 5, but the sum of m + n has one of the ~alues
1, 2, 3, 4 or 5; or R~ or R2 is a f-ee ~alency which, with
a similar f-ee ~alency o~ an atom which is bonded
directly to the carbon atom ca-rying Rl and R', forms a
double bond;
R3 is (C1-C10)-alkyl, (C2-C10)-alkenyl, (C2-C10)-alkynyl,
(C3-C,)- cycloalkyl, (Cg-Cll)-benzocycloalkyl or the
radical of a 4- to 10-m~be-ed hete-ocycle ha~iug one to
four identical or different hete-oatoms f~om the series
~lt-ogen, oxygen and sulphur, which can be aromatic,
partly saturated or completely satu-ated, it being
possible for the alkyl, alkenyl, alkyuyl, cycloalkyl and
the heterocyclic radical to be substituted by one or more
identical or different Rl radicals;
R~ is phenyl or naphthyl, which can be substituted by one
to three identical or different radicals from the ~e-ies
(Cl-C~)-alkyl, (Cl-C~)-alkoxy, halogen, nitro, t-ifluoro-
methyl, cyano, hydroxyl, (Cl-C~)-alkoxycarbonyl, ca-boxyl,
carbamoyl, (Cl-C~)-alkyl-S(0)~, where q i8 0 ~ 1 or 2,
a~; no, (C~-C~)-alkylcarbonylamino, (Cl-C~)-alkyl a~; ~0 and
di((C~-C~)-alkyl)amino;
R5 and R5 independently of one another are hydrogen,
(Cl-C10)-alkyl in which the alkyl chain can also be
interrupted by cne to three o~yye~ atoms,
(C2-C~0)-alkenyl, (C3-C7) -cycloalkyl, R~, R~-(C1-Cc)-alkyl,
the radical of a 5- to 7-m~m~ered heterocycle ha~ing one
216~386
Ref. 3~95 - 12 -
Dr.Eg/L10956
to four identical or different heteroatoms from the
series nitroges, o~yy~ and sulphur, which can be aro-
matic, partly saturated or completely saturated, (C -C10)-
~1 k~noyl, R~-CO, R~- (Cl-C6) -alkyl-CO, (Cl-ClO)-alkoxy-
carbonyl, R~O-CO, R~- (Cl-C6) -alkoxy-CO or a radical of the
general formula III
~NRl 1
-C ~III)
\~R12R13
R6 i8 (C~-C1O)-alkyl, (C2-C1O)-alkenyl, R~ or
R~-(Cl-C6)-alkyl;
R7 and R~ independently of one another ha~e the me~ninss
specified for Rs or are (Cl-C6)-alkylsulphonyl or R~-S(O),
or R' and R3, together with the nitrogen atom to wkich
they are bonded, fo~ a radical of the general for~ula IV
/~ C H 2 ) r
-t~ A (~Y~
( CH2 ) S
which can be substituted on the C~2 groups by one or more
identical or different Rl radicals and wherein r and 8
l; independently of one another a-e the numbe-s 1, 2, 3 or
4, but the sum of r + 8 i5 smaller than 6;
R7 and RB independently of R7 and Ra ha~e the me~n;ngs
specified for R' and RB;
R9 has one of the m~nings of R~, but is not the radical
of the general formula III;
Rl i8 (Cl-C6)-alkyl, (C3-C~)-cycloalkyl, the radical of a
5- to 7-membered heterocycle ha~ing one to three
identical or different heteroatoms from the series
nitrogen, oxygen and sulphur, which can be aromatic,
21653~3~
Ref. 3595 - 13 -
Dr.ER/L10956
partly saturated or completely saturated, R~, haloge~,
oR5, SR5, S(O)p-R6, where p is 1 or 2, NR'R', the
hydroY;mino group, the oxo group, cyano, ~itro, CooR9,
CONR7RJ or P(0)(0~)2;
th adicals Rll Rl2 and Rl3 indepeude~tly Or one a~other
are hydrogen, (Cl-C6)-alkyl, (C3-C,)-cycloalkyl, R~,
(Cl-C6)-~lk~noyl, (Cl-C6)-alkyl-S(0)2 or R~-S(0)2;
Rl~ is (C~-C~)-alkyl, be~zyl, phe~yl ~ (Cl-C6) -A1 k~noyl,
(Cl-C6)-alkyl-S(0)2 or R~-S(0)2;
A is a methylene grou? which can be substituted by one or
two identical or differe~t Rl radicals, o is oxygen,
sulphur or NRl~;
X is CR20R2l a~d R20 a~d R2l i~depe~de~tly of the me~n;n5s
of the radicals Rl a~d R2 ha~e the me~nlngs specified for
Rl a~d R2;
22R23 ~d R22 a~d R23 indepe~de~tlY of _he e g
of the radicals Rl a~d R2 ha~e the me~nings specified for
R~ and R2;
Z is CR2~R25 and R2~ and R2s i~depe3de~tly of the me~n;ngs
of the radicals Rl a~d R2 ha~e the me~nlnss specified for
R~ and R2;
where radicals of the groups W, X, Y and Z can also be
li~ked ~ia a bo~d such that a bicyclic system or
tricyclic system is formed;
a~d their tautomeric forms a~d also their ph~r~co-
logically tolerable salts,
but where, ~~
a) if R20 and R2l are hydrogen,
216~386
Ref. 3595 - 14 -
Dr.E~/L10956
al) R1 R2 R22 R23, R2~ and R25 c~not be hydrogen
simultaneously;
a2) R22, R2~ and R25 cannot be hydrogen, R23 c~nnot be the
carboxyl group and Rl and R2 together c~nnot be =O simul-
taneously;a3) R22, R23 and R2~ cannot be hydrogen, R25 cannot be
bromomethyl and Rl and R2 together cannot be =O simul-
taneously;
a4) R1, R2, R2~ and R25 cannot be hydrogen and R22 and R23
together cannot be =O simultaneously;
a5) R1 c~nnot be hydrogen and R2 cannot be butyl and R22,
R23, R2~ and R2s, together with the two carbon atoms to
which they are bonded, cannot form a 2-oxo-1,2-dihydro-
qu noli~e-3,g-diyl rad cal simultaneously;
b) if R20, R21, R22 and R23, together with the two carbon
atoms to which they are bonded, form a 1,2-phenylene
radical or a 4-chloro-1,2-phenylene radical,
bl) Rl, R2 and R2~ cannot be hydrogen ~nd R2s c~nnot be
phenyl 8 imultaneously;
b2) Rl, R2~ a~d R25 c~nnot be hydrogen and R2 c~nnot be
phenyl simultaneously;
b3) R1 and R2 together cannot be =O and R2~ and R25
together c~nnot be =O simultaneously.
With respect to the substituents, such as haloge~
atoms, alkyl groups, etc. in the thiazepine derivatives
as 8uch, what has been said above applies correspon-
dingly.
The synthesis of the compounds of the general
formula I can be car-ied out, for example, according to
the following scheme:
Starting materials are ~=aminoalcohols of the
general formula VII, in which W, X, Y and Z ha~e the
me~n;n~s specified above. According to methods k~own per
216~386
~ef. 3595 - 15 -
Dr.ER/L10956
~2 ~I~NH2 ~-N=C=S
X X
r\ \ \z~
Z-~H z _
(YII~ ~VIII) (IX)
tSu-N=C=5 - t8u-NH2
~_~ ~ H-tBu
X S
~x)
z~
~Z--S N H 2 ~ ~1N H - t 8 U
(I) ~XI)
se, compounds of the general formula VIII are prepared
from these in which E- is a group which can be replaced
in a nucleophilic substitution reaction. E- is, for
example, Cl-, 8r-, I-, C~SO20-, C6~sSO20-, substituted
S benzenesulphonyloxy radicals, e.g. nitrobenzene-
sulphonyloxy or toluenesulphonyloxy, or another acti~ated
form of the hydroxyl group. ~he con~ersion of the
hydroxyl group in VII can be carried out under customary
conditions, for ex~ple, using thionyl chloride or
2165386
- ~ Ref. 3595 - 16 -
Dr.ER/L10956
bromide, using phosphorus halides, such as phosphorus
pentachloride, phospho_u3 t~ichloride or phosphorus
tribromide, using hydrogen bromide or hydrogen iodide or
using aliphatic or aromatic sulphouyl chlorides. The
compounds of the general formula VIII ca~ be cou~erted
directly to the thioureas of the general formula X usi~g
tert-butyl isothiocyanate or first converted, usins
r e a g e n t 8 8 u c h a 8 th i op h o 8 g e u e o r
N,N'-thiocarbo~yldiimidazole, to the isothiocya~ates of
the general formula IX, which with tert-butyl~m;2e yield
the thioureas of the general formula X, which cyclize to
the 2-tert-butylamino-1,3-thiazepi~es of the general
formula XI. The compouuds of the general formula I are
obt~;n~hle from these by removal of the tert-butyl s_oup,
for example by boiliug with dilute hydrochloric acid.
Sta_ting from the ~ no~lcohols of the general
formula VII, using tert-butyl isothiocyanate, the
thiourea3 of the general fo~mula XII
H H
H0~2~y~x~x ~ ~su ~XIi~
can also fi-st be prepared, which are then co~verted iuto
the compou~ds of the ge~eral fo~mula X by couversion of
the hydroxyl group to the g_oup E a3 specified above.
Sta-ti~g materials fo- anothe- synthetic route
are the compou~ds of the geueral formula X~II
G Xl N
X ~ ~N H 2 Y `z~ ~N H
2-~
(I)
~XIII)
2165386
Ref. 3595 - 17 -
Dr.E~/L10~56
in which G is a group which reacts with a~;no g_oups in
a substitution reaction or an addition reaction, for
example the carboxyl, alkoxycarbonyl, aryloxycarbonyl,
cyano or formyl group, the -CORl sroup or the -CRlR2-E'
group, Rl and Rl being defined as specified abo~e, and in
which E and also E' are identical or different s~oups
which - like E in the general formula VIII - can be
replaced in a nucleophilic substitution ~eaction, that is
e.g. by chlorine, bromine, iodine or aliphatic or aro-
matic 6ulphonyloxy radicals. The compounds of the generalformula XIII, w~ich ca~ be, for examrle, r-halocarbonyl
compounds or, if G- in the gene-al formula XIII is -W-E',
a,~-dibromides, ~,~-dichlorides or ~,~-disulphonates,
yield the thiazepines of the general formula I with
thlou-ea under customary conditions.
A further synthesis process is the reaction of
thiourea with compounds of the gene-al formula XIV
Z X /
(I~)
tXIV)
in which the double bond between the group Z and the
adjacent carbon atom is acti~ated by electron-withdrawing
substituents, such that addition of the sulphu~ atom in
the thiourea Z can take place, and i~ which G has the
~ean~ng specified for the formula XIII.
The details of the synthesis of compounds of the
general formula I in J.B. Bream and J. Schmutz, ~el~.
216~:~86
` Ref. 3595 - 18 -
Dr.ER/L10956
Chim. Acta 60 (1977), 2872; J8-B-46/021713 and ~.W.
Schubert and O. Be^hner, Arch. Pharm. 301 (1968), 750 are
completely part of the prese~t disclosure.
The inhibition of NO release according to the
invention by the compounds of the general formula I can
be determined by an acti~ity assay which is based 02
studies by ~redt and Snyder and also Sc~midt et al. (see
D.S. Bredt and S.S. Snyder, Isolation of nitric oxide
synthase, a c~lmo~ulin-requiriug enzyme, Proc. Natl.
Acad. Sci. ~SA 87 (1990), 682; E.~.~.W. Schmidt et al.,
Purification of a 601uble isoform of guanylyl cyclase-
acti~ating factor 6y3thase, P-oc. Natl. Acad. Sci. USA 88
(1991), 365). I3 this as6ay, the coproduct L-cit-ulline
for purified NO 6ynthase (NOS) obtained duri~g NO
formatio~ is deter_ined quantitatively. This is achieved
by the use of 3~-radiolabelled L-argi~ine as a substrate
of the e~zyme reactio~, which i8 reacted to gi~e
3~-L-citrulli~e and NO. Afte_ completion of ~e enzyme
i~cubation, L-cit-ulline formed is removed from the
reaction mixture f~om unused L-a~si~lne by mea~s o io~-
excha~ge chromatography; the 3~-acti~ity determined by
liquid scintillation measurement then co-responds to the
amount of L-citrulline.
The inhibition of NO release by the compounds of
the gene-al fo-mula I can also be determined i~
mac-ophages by NO measureme~t by means of oxyhaemoglobin:
Mice mac_ophages (M~) of the 6train RAW 264.7 a=e
cultured i~ Petri dishes (10 cm diamete-). The nutrient
medium employed is DMEM (Sigma No. D 5405), conditioned
w~th 4 _M l-glut~;ne, 3.5 g of D-glucose/l,
50 ~/50 ~g/ml of pen/strep and 10% FCS, employed under 5~
CO2, 95% atmospheric humidity, 37C. The subconfluent
cells a-e sc-aped off with the cell sc_ape-, taken up in
medium, the cell count is determ- n~ (counting i~ Trypan
81ue solution 0.2%) and inoculated i~to 24-well plates
(1 x 106 cells/ml, 1 ml /well). ~
About 18 hours after i~oculation, the well-
adhered mouse macrophages are ~t_mulated with LPS (lipo-
2165386
Ref. 3595 - 19 -
Dr.3~/L10956
polysaccharide from E. coli serotype 0111:B4, Sigma No.
2630) and y-;nterferon (yIFN, mouse recombinant,
Boehringer M~nnheim No. 1276905). To do this, the culture
medium i8 aspirated f_om the cells a~d 1 ml/well (24-well
plate) of i~cubation medium (~EM without Phenol Red
(Biochrom F0385)), conditioned with 4 mM glutami~e, 3.5 g
of glucose/l, 3.7 g of NaECO3/l, 110 mg of sodium
~yLuvGte/l, 50 ~/50 ~g/ml of peu/strep a~d 5% FCS, is
added. 1 ~1 of LPS and 1 ~1 of yIFN are pipetted per ml
of incubation medium.
The following concent-ations of the stimulators
result from this:
140 ng of LPS/~l + 5 U of yIFN/ml
During the stimulation for 4 hours, the de novo
synthesis of the NO-producing enzyme NO sy~thase (iNOS)
is induced. After this incubatio~ period, the incubation
medium is aspirated and each well is rinsed twice with
fresh incubation medium. Test medium (consisti~g of the
incubation medium with the addltion of SOD (30 ~/ml),
catalase (290 ~/ml), in~omethacin (3.5 ~g/ml), OxyEb
(about 8 ~m) and test substance (0.05-250 ~M)) is added
to the well (1 ml/well in the case of a 24-well plate).
As a control, the conditioned test medium is treated only
with the sol~ent for the test substance. The plates
charged with test medium a-e incubated in an incubator at
37C, 5% CO2 a~d 95% atmospheric humidity for 120
minutes. After the 120 minute incubation, the test medium
is pipetted into plastic semimicro cuvettes and the
extinction difference 576-592 nm is measured on a D~ 70.
Every 2 wells are charged with a test substance
(~ = 2), one control on each plate is u3stimulated, and
a stimulation control and a positive control (nitro-L-
arglni~e, 500 ~M) are in~estigated comparatively to the
test substa2ces. A6 a measurement of the test substances,
the extinction difference 576-5g2 nm is compared to the
s~im11l~tion control as a percentage. The extinction
differeuce 576-592 n~ at time 0 of the test medium
t_eated with the test substance is measured and the pE
216~81;
Ref. 3595 - 20 -
Dr.3R/L10956
and the osmolality i8 determined.
After pipétting off the test medium, the cyto-
toxicity of the test substance is tested (cell via-
bility).
Diseases which result due to an increased N0
level, and which can thus be treated according to the
in~ention with the compounds of the general formula I or
which can be prevented with these, are in particular
pathological blood pressure decreases, as occur in septic
or haemorrhagic shock, in tumou- or cancer therapy with
cytokines or in cirrhosis of the liver. In addition,
infl~mm~tory disorders, such as rheumatoid arth_itis and
in particular ulce-ative colitis, and also i3SUliD.-
dependent diabetes mellitus and transplant rejection
reactions.
~owever, the following disorders are also con-
nected with an inc-eased production of nitric oxide and
can be treated or prevented acco-ding to the invention.
In the ca-diovascular a-ea, these are arteriosclerosis,
post-ischaemic tissue damage and infarct damage, reper-
fusion d~ge, myoca-ditis based on a Coxsackie virus
infection and cardiomyopathy; in the nervous system/
central ne-vous system area neuritis of differing aetio-
genesis (forms of neuritis), encephalomyelitis, viral
neurodegenerative disorders, Alzheimer's disease,
hyperalgesia, epilepsy, migraine and opioid withdrawal
symptoms; in the renal area acute kidney failure and
nephritis of differing aetiogenesis, especially
glomerulonephritis.
Additionally, application areas for the compounds
of the general formula I are also treatments in the area
of the stomach and of the uterus/the placenta and also an
effect on sperm motility.
The compounds of the general formula I and their
ph~rm~cologically acceptable salts can be employed as
auxiliaries in biochemical and ph~rm~cological investiga-
tions in research and in ~;~gnostic processes, and they
can be ~min; stered to ~n;m:~l 8, preferably to m~ l 8,
. Ref. 3595 - 21 - 216 ~
Dr.ER/L10956
and in particular tQ h?.m~nR, as me~;cines by themsel~es,
in mixtu=es with one another or in the form of ph~rm~-
ceutical preparations which enable enteral or parenteral
~;n;stration and which as acti~e constituent contain an
effecti~e dose of at least one compound of the general
formula I or of a salt thereof, in addition to customa~y
ph~rmaceutically innocuous excipients and additives.
The medicines can be a~m;nistered orally, e.g. in
the form of pills, tablets~ lacquered tablets, coated
tablets, hard and soft gelatin capsules, solutions,
syrups, emulsions or suspensions or aerosol mixtures.
~m;n; stration, however, can also be ca-ried out rec-
tally, e.g. in the form of suppositories, o-
pa~enterally, e.g. in the form of injection solutio~s o_
inrusion solutio2s, or percutaneously, e.g. in the form
of oin~ments o- tinctu_es.
In addition to the acti~e substances and exci-
pients, the pharmaceutical p-epa_ations can additionally
contain adcit ves, such as e.g. fillers, extenders,
disintegrants, binders, glidants, wetting agents, stabi-
lizers, emulsifying agents, preser~ati~es, sweetene-s,
colourants, fla~ourings or a-omatizers, buffer sub-
stances, and also sol~ents or solubilizers or agents for
obt~;n;ng a depot effect, and also salts for altering the
osmotic pressure, coating agents or antioxidants. They
can also contain two o- more compounds of the gene-al
formula I or thei- phanmacologically acceptable salts and
additionally other therapeutically active substances.
Other therapeutically active substances of this
type are, for example: ~-receptor blockers, such as e.g.
propranolol, pindolol, metoprolol; ~asodilators, such as
e.g. carbocromen; t-anquillize-s, such as e.g. bar~ituric
acid deri~ati~es, 1,4-benzodiazepines and meprobamate;
diuretics, such as e.g. chlorothiazide; cardiotonic
agents, such as e.g. digitalis preparations; hypotensive
agents, such as e.g. hydralazine, dihydralazine,
ra~;pril, prazosin, clonidine, Rauwolfia alkaloids;
agents which dec~ease the fatty acid level in the blood,
2165386
Ref. 3595 - 22 -
Dr.Eg/LlOg56
such as e.g. bezafibrate, fenofibrate; agents for throm-
bosis prophylaxis~ such as e.g. phe~procoumon; anti-
i~flammatory substances, such as, for example, corti-
costeroids, salicylates or propionic acid deri~ati~es,
such as, for example, ibuprofen; antibiotics, such as
e.g. penicilli~s or cephalosporins; N0 donors, such as
e.g. organic nitrates or syd30ne imines or furoxanes.
The dose can ~ary within wide l;m;ts and i8 to be
suited to the indi~idual conditions in each indi~idual
case. In general, i3 the case of oral a~ministration a
daily dose of about 0.5 to 100 mg, preferably 1 to 20 mg,
per h~m~n indi~idual is appropriate. Also in the case of
other administ-ation forms the daily dose is in similar
ra~ges of amou3ts, i.e. in general also 0.5 to 100 mg/
person. The daily dose can be di~ided into two or more,
e.g. 2 to 4, pa-t ~m n;strations.
To prepare the ph~rm~ceutical preparations,
pk~ m~ceutically inert inorganic o- orsanic excipients
can be used. To prepare pills, tablets, coated tablets
and hard gelati~ capsules, e.g. lactose, maize starch or
deri~ati~es thereof, talc, stearic acid or its salts etc.
can be used. Excipients for soft gelatin capsules and
suppositories are e.g. fats, wzxes, semi-solid and liquid
polyols, natural or hardened oils etc. Suitable exci-
pients for the preparation of solutions and syrups aree.g. water, suc-ose, in~ert sugar, glucose, polyols etc.
Suitable ex^ipients for the preparation of injectio3
solutions are e.g. water, alcohols, glycerol, polyols or
~egetable oils.
ExamPle 1
2-Amino-4,5,6,7-tetrahydro-1,3-thiazepine hydrochloride
a) 2-Amino-4,5,6,7-tetrahydro-1,3-thiazepine
hydrochloride was prepared accordi~g to ~.W. Schubert and
0. Behner, Arch. Pharm. 301 (1968), 750. The physical
3~ data corresponded to the details there. l~-NMR (DMS0, 300
216~386
Ref. 359~ - 23 -
Dr.E~/L10956
M~z): 1.67 ppm (quintet, 2~, ~-6); 1.94 (quintet, 2~
5); 3.09-3.18 (m,~2~, 8-7); 3.24 (q, 2~, ~-4); 9.20 (s,
1~, N~); 9.53 (8, 1~, N~); 10.34 (8, 1~, N~).
b) The ph~-m~cological activity was determined on
macrophages by N0 measurement by means of oxyhaemoglobin
as described abo~e. The IC50 ~alue was 1.3xlO-' M.
ExamPle 2
3-Amino-1,5-dih~drobenzo[e~[1,3]thiaze~ine hvd~ochloride
a) 2-Am;n~m~thvlbenzyl alcohol hYdrochloride
20 g (0.13 mol) of 2-hydroxymethylbenzam;de in
450 ml of tet-ahydrofuran are added dropwise to a boiling
suspension of lithium aluminium hydride in 500 ml of
tet-ahydrofuran. The ~ixture is heated under reflnx for
5 h, stirred ove~night at room temperatu-e and treated
dropwise with 91 ml of saturated potassium ca_bonate
solution. The precipitate is filtered off with suction
and washed with tetrahydrofuran. The combined filtrates
are concentrated. The residue i8 taken up in 100 1 of
isopropanol, treated with ethereal hydrochloric acid, and
the precipitate formed is filtered off with suction and
rec-ystallized from isopropanol.
Yield: 19.2 g. M.p. 225C
b) 3-Amino-1,5-dihYdrobenzo~e] ~1,3]thiazePine
hydrochloride
7 g (40 mol) of 2-~m;n~methylbenzyl alcohol
hydrochloride are suspended in 80 ~l of ethanol, a
solution of potassium ethoxide in 10 ml of ethanol i8
added and the mixture is warmed to ~0C for 10 min. It is
then cooled to 5C and the precipitated potassium
chloride i8 filtered off with suction. 4.64 g (40 mol) of
Ref. 3595 - 24 - 216~386
Dr.E~/L10956
tert-butyl isothiocyanate are added dropwise to the
filtrate ~d the reaction mixture is heated under reflux
for 4 h. After stirring over3ight, it i8 concentrated 03
a rotary e~aporator, the residue i8 taken up in 30 ml of
aqueous hydrobromic acid (48% strength) and the mixture
is heated unde~ reflux for 2 h. The reaction mixtu-e
obt~;ne~ in this way is filtered, concentrated 03 a
rotary e~aporator and chromatographed on silica gel using
dichloromethane/methanol/water/acetic acid (85:15:2:2).
The main fraction is recrystallized f~om ethanol.
Yield: 0.88 g of white crystals. M.p. 246C (dec.)
~xam~le 3
2-Amino-5-~he~1-4,5,6,7-tetrah~d-o-1,3-thiazeDi~e
hydrochloride
a) 4-Amino-3-Dhe3Ylbutanol h~d-ochlo-ide
12.7 g (0.33 mol) of lithium aluminium hydride
a_e initially introduced into 250 ml of diethyl ether,
the mixture is cooled to 0C and 25 g (0.11 mol) of
methyl 4-n tro-3-phenylbutyrate (M. J. Leonard et al., J.
Am. Chem. Soc. 73 (lg51), 857), dissol~ed in 100 ml of
diethyl et~er, are added dropwise. The mixtu-e is heated
under reflux for 2 h, hydrolysed with saturated potassium
carbouate solution, treated with water and stir~ed at
room temperature for 1 h. The precipitate is filtered off
with suctio3 and boiled with dichloromethane. The com-
bined organic phases are concentrated. The residue i8
taken up in 1 N hydrochloric acid and extracted with
diethyl ether. The aqueous phase i8 rendered basic with
sodium hydroxide solution and extracted with dichloro-
methane. The residue which r~in~ after concen~ration is
taken up in tetrahydrofuran and ~he hydrochloride i8
precipitated by addition of ethereal hydrochloric acid.
10.4 g of the hydrochloride are obtained, which are
~ ef. 3595 - 25 - 216S~
Dr.ER/L10956
~loyed in the subsequent reaction without further
purification. --
b) 4-Chloro-2-phenylbutylamine hYdrochloride
A solution of 10.4 g (52 mmol) of 4-a~ino-3-
phenylbutanol hydrochloride in 50 ml of chloroform i8added dropwise at 0C to a suspension of 14.1 g (68 mmol)
of phosphorus pentachloride and 5.2 g (52 mmol) of
calcium carbonate in 100 ml of chloroform. The salt is
the~ filtered off, the filt_ate is concent-ated and the
residue which r~m~ n~ is employed in the subse~uent
reaction without further purification.
c) 4-Chloro-2-~he~YlbutYl isothiocYanate
11.4 g (52 _mol) of 4-chloro-2-phenylbutyl~;ne
hyd-ochloride a-e t-eated successi~ely with 50 1 of
water, 50 ml of ethylene chloride and 10.4 g (104 mmol)
of calcium carbonate. 6.9 g (60 mmol) of thiophosgene i~
10 ml of ethylene chloride are added dropwise at 10C
with cooling. The mixture is stirred at room temperature
for 16 h and finally the salt is filtered of~ with
suction. The phases of the filtrate are separated. After
drying the organic phase and stripping off the solvent,
it is distilled in a high vacuum.
Yield: 3.2 g of slightly yellow oil. B.p. (0.012 mbar)
130C.
d) 2-Amino-S-PhenYl-4,5,6,7-tetrahydro-1,3-thiazePine
hYdrochloride
3.1 g (14 mmol) of 4-chloro-2-phenylbutyl
isothiocyauate, dissol~ed in 5 ml of xylene, are
added dropwise to a solution of 2.0 g (28 mmol) of
tertbutyl7mine in 15 ml of xylene~ a~d the mixture is
heated under reflux for 2 h. The precipitated salt is
then filtered off and the filtrate is extracted three
211i538~
Ref. 3595 - 26 -
Dr.E~/L10956
times with 10 ml of 2 N hydrochloric acid each time. The
combi3ed aqueous -phases are heated under reflux until
reaction is complete. For working up, the mixture is
concentrated on a rotary e~aporator and, after chroma-
tography on silica gel using butanol/acetic acid/water(8:2:2) (org. phase), the residue i8 c-ystallized from
ethanol/ethyl acetate/hexane.
Yield: 0.5 g of white crystals. M.p. 234-235C.
ExamPle 4
2-Ami~o-6-~hen~1-4,5,6,7-tetrahYdro-1,3-thiaze~ine
hYdrochloride
a) E'hYl 4-nitro-2-~henYlbutvrate
147.7 g (0.34 mol) of Triton B (methanolic
solution) are added dropwise to 179.8 g (2.95 mol) of
~it_omethane in 200 ml of tert-butanol a~d the mixture is
sti~red at room temperature for 30 mi~. 86.5 g (0.49 mol)
of ethyl 2-phenylacrYlate (Chem. Ber. 119 (1986), 3694),
dissol~ed in 30 ml of tert-butanol, are added dropwise to
this mixture and it is stir~ed at 70C for 4 h. For
working up, it is concentrated on a rotary e~aporator,
taken up in dichloromethane, washed with water and
concentrated again. The residue is filtered through
silica gel using hexane/ethyl acetate (2:1) and finally
fractionally distilled in a high ~acuum. 26.7 g of
colourless oil are obtained. B.p. (0.5 =) 148C.
b) 4-Amino-2-PhenYlbutanol hYdrochloride
Correspon~ing to the preparation of 4-~ino-3-
phenylbutanol hydrochloride (Example 3a), 42 g of product
are obtained from 92.6 g (0.39 mol~ of ethyl 4-nitro-2-
phenylbutyrate and 47.2 g (1.24 mol) of lithium aluminium
hydride.
21S5386
Ref. 3595 - 27 -
Dr.ER/~10956
c) 4-Chloro-3-PhenYlbutYlamine hydro~hloride
. _
Correspon~ing to the preparation of 4-chloro-2-
phenybutyl~ine hydrochloride (Example 3b), 22.2 g of
crude product are obtained from 19.3 g (96 mmol) of
4-~m;no-2-phenylbutanol hydrochloride, which are employed
in the subsequent reaction without further purification.
d) 4-Chloro-3-PhenYlbut~l isothiocvanate
Corresponding to the preparation of 4-chloro-2-
phenylbutyl isothiocyanate (Example 3c), 3.~4 g of
product are obtained ~rom 22.2 g (0.1 mol) of 4-chloro-3-
phenylbutylamine hydrochloride. B.p. (0.04 mbar) 140C.
e) 2-Amino-6-Phenyl-4,5,6,7-tetrahydro-1,3-thiazepine
h~drochloride
3.7 g (16 ~mol) of 4-chloro-3-phenylbutyl iso-
thiocyanate are added d~opwise to a solution of 2.64 g(36 mmol) of tert-butylamine in 20 ml of xylene and the
mixture is heated under reflux for 8 h. After stirring at
room temperature for 16 h, the solid is filtered off with
suction, the filt-ate is extracted three times with 2 N
hydrochloric acid and the combined extracts are heated
under reflux for 2 h. The reaction mixture is concen-
trated on a rotary e~aporator and chromatographed on
silica gel using dichloromethane/methanol/acetic acid
(50:10:1). The main fraction is concentrated, taken up in
2 N hydrochloric acid and freeze-dried. The oil which
r~m~inR crystallizes on trituration with diethyl ether.
Yield: 0.33 g of beige crystals. M.p. 127C. (dec.)
ExamPle 5
_,
2-Amino-4,7-dihYdro-1,3-thiaze~ine hYdrochloride
216S ~8G
~ef. 3595 - 28 -
Dr.E~/L10956
a)- 4-Chlorobut-2-enYl isothiocYarate
._
Correspon~;ng to the preparation of 4-chloro-2-
phe~ylbutyl isothiocyanate (Example 3c), 22.1 g of
product are obtai~ed in the form of a colou-less oil from
30 g (0.21 mol) of 4-chlorobut-2-e~ylam;ne hydrochlo-ide
(Synthesis (1988), 347), 27.9 g (0.24 mol) of thiophos-
ge~e and 42.3 g (0.42 mol) of calcium carbonate. B.p.
(2 mm) 80C.
b) 2-Amino-4,7-dihYd-o-1,3-thiazePine hYd-ochloride
Cor-espon~in~ to the preparation of 2-~m;no-5-
phe~yl-4,5,6,7-tetrahydro-1,3-thiazepi3e hydrochloride
(Example 3d) 3.3 g of product a-e obtained from 20.7 g
(0.14 mol) of 4-c~lorobut-2-enyl isothiocyanate and
20.5 g (0.28 mol) of tert-butyl~m;~e after removal of the
lS tert-butyl residue with half-concentrated hyd~ochlo~ic
acid.
3eige crystals. M.p. 123-125C.
The following examples relate to ph~rm~ceutica
preparation forms.
kxample A
Soft gelatin capsules cont~;n;ng 100 mg of active ~ub-
stance per capsule:
per capsnle
Active substance 100 mg
25 Triglyceride mixture fractionated
from cocoa fat 400 mg
Capsule contents 500 mg
k~ample B
Injectio~ solution cont~; n i ng 2.0 mg of active substance30 per ml: ~
per ~1
216~386
Ref. 3595 - 29 -
Dr.E~/L10956
Acti~e substance 2.0 mg
Polyethylene glycol 400 5.0 mg
Sodium chloride 2.7 mg
Water for injection purposes to 1 ml
R~a~l e C
3mulsion containing 60 mg of acti~e substance per 5 ml:
per 100 ml of
~lsion
Active substance 1.2 g
10 Neut~al oil q.s.
Sodium carboxymethylcellulose 0.6 g
Polyoxyethylene stearate q.s.
Glycerol, pure 0.2 to 2.0 g
Fla~ouring q.s.
15 Water (demineralized o- distilled) to 100 ml
~xample D
Rectal pha~maceutical fo~m cont~ining 40 mg of active
substa~ce per suppository:
per suppository
20 Acti~e substance 40 mg
Suppository base to 2 g
13xamrle ~
Tablets contA i n i n5 40 mg of acti~e substance per tablet:
per tablet
25 Acti~e su~stance 40 mg
Lactose 600 mg
Maize sta-ch 300 mg
Solu~le starch 20 mg
Magnesium stearate 40 mg
1000 mg
F~amrle F
Coated tablets containing 50 mg of acti~e co~o~d per
coated tablet:
per coated
. 216~38B
Ref. 35g5 30
Dr.ER/L10956
tablet
Active substance ~ 50 mg
Maize starch 100 mg
Lactose 60 mg
5 sec calcium phosphate 30 mg
Soluble sta-ch 5 mg
Magnesium stearate 10 mg
Colloidal 8ilicic acid 5 mg
260 mg
~ample G
The following recipes are suitable for the preparatio~ o~
the co3te~ts of hard gelatln capsules:
a) Acti~e sub6tauce 100 mg
Maize starch 300 mg
400 mg
b) Acti~e substa~ce 140 mg
Mil~ sugar 180 mg
Maize sta-ch 180 mg
500 mg
~ample ~
Drops ca~ be prepared according to the followiug recipe
(100 mg of acti~e substance in 1 ml = 20 drops):
Acti~e substa3ce 10 g
Methyl be~zoate 0.07 g
Ethyl benzoate 0.03 g
Ethanol, 96% st~ength 5 ml
30 Demineralized water to 100 ml