Note: Descriptions are shown in the official language in which they were submitted.
2i 65587
-
Backqround of the Invention
Polyvinylchloride (PVC) is the accepted material for use
as tubing in various medical applications, such as peritoneal
dialysis, blood processing, chemotherapy and other uses. In such
uses, a consumable is conveyed through the tubing from one location
to another. For peritoneal dialysis (CAPD), for example, it is
also the practice to replace a used dialysate bag with a new bag.
This is accomplished by cutting through the PVC tubing leading from
the used bag and then welding tubing from a new bag to the cut
portion of the tubing so that one bag may replace another. PVC is
also the generally accepted material for forming bags and other
medical containers and is commonly used as tubing in food process-
ing particularly for fluids and semi-solids. In addition, PVC is
the material generally used for forming sheets and films for
bacterial and virus exclusion. Despite its acceptance by the art,
PVC has a number of disadvantages which would be desirable to
overcome in such uses. For example, conventional PVC includes a
plasticizer (DOP) which might leach into the solutions in the bag.
Further, after PVC has leached its DOP, large volumes of PVC
particulates are released. Other disadvantages will be later
referred to.
Summary of the Invention
This invention provides a material which will act as an
improved replacement for PVC in the conventional uses of PVC.
~1 655~7
This invention can also provide such a material which can
be used as tubing for medical applications, as bags and other
medical containers, for tubing food processing and as sheets and
films for bacterial and virus exclusion. --
In accordance with this invention the material whichmeets the above requirements is an ionomeric modified poly-ether-
ester. The ionomeric modifier is added in a range of 1~ to 20
depending upon the end use requirement.
The Drawinqs:
Figure 1 is a perspective view of a tube made from
ionomeric modified poly-ether-ester in accordance with this
invention;
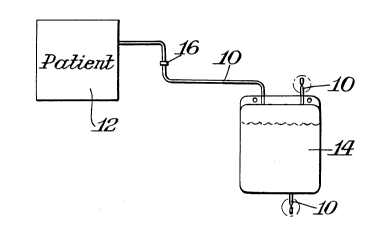
Figure 2 is a schematic view of a urinary drainage system
utilizing the tubing of Figure 1;
Figure 2A is an enlarged cross-sectional view of a top
air seal shown in the corresponding circled portion of Figure 2;
Figure 2B is an enlarged cross-sectional view of a
discharge line in the corresponding circled portion of Figure 2;
Figures 3-4 are graphs comparing the elongation and
strength characteristics of PVC welded to the material of this
invention (Figure 3) and of the ionomeric modified poly-ether-ester
of this invention alone (Figure 4);
Figure 5 is a schematic view of a prior art system for
steam sterilizing when using PVC tubing in peritoneal dialysis;
21 65587
~ Figure 6 is a schematic view showing a sterile solution
fed to sterile bags using the ionomeric modified poly-ether-ester
of this invention;
Figure 7 is a schematic view showing a primary blood bag
and four satellite bags connected by the tubing of this invention
as used in blood processing;
Figure 8 is a schematic view showing prior art chemother-
apy treatment using PVC tubing;
Figure 9 is a view similar to Figure 8 showing use of the
tubing of this invention; and
Figure 10 is a schematic view showing parenteral feeding
of the body using the tubing of this invention.
Detailed Description
The present invention is directed to providing à material
which can be used as a substitute for PVC in the conventional uses
of PVC while having advantages over such PVC material. The
material of this invention is an ionomeric modified poly-ether-
ester. Poly-ether-ester is a well known material generally used to
take advantage of its high strength characteristics. Such material
is used, for example, in clothing generally referred to as
polyester clothing. Ordinarily, the strength of such material
would make it unsuitable for use as a substitute for PVC in the
uses of PVC with which this invention deals. It has been discov-
ered, however, that the poly-ether-ester material can be modified
by incorporation of a small amount of an ionomer which would modify
2~l 655a 1
` -
the characteristics of the poly-ether-ester by giving it sufficient
frigility to, for example, permit the material when used as a
sealed tube to pop open. The specific amount of ionomer would
depend upon the end use.
In general, the ionomeric modifier comprises from 0.5-50~
and preferably from 1~ to 25~ of the combination depending upon the
end use requirement. For example, in tubing such as for CAPD use
where the material is to be sealed, welded and the lumen reopened
the broad ionomer composition range would be 2~ to 15~, a more
preferred range of 3~ - 10~. A range of 7-10~ is ideal. The
result is a tube which is strong, sufficiently rubber like, and a
degree of fracture to make reopening of the sealed tubes by finger
pressure. For bags, films and other thin structures the ionomer
would be present in a range of from 1~ to 50~ of the combination
depending on the end use requirements. For food processing tubes
and aseptic surgical draping procedures a range of 10~ to 20~ is
desirable. For implanted catheters and sutures the range of 0.5 to
1~ is preferred.
The material of this invention is thus an ionomeric
modified poly-ether-ester wherein the material has from 1~ to 25
ionomer and from 99~ to 75% poly-ether-ester with mixtures of 50-
50~ being feasible. The basic material components are as follows:
Poly-ether-ester
POLY-ETHER-ESTERBLOCKCOPOLYMER (Blockpoly-ether-ester)
(Co-poly-ether-ester) (Polyester elastomer) (Thermoplastic poly-
21 655~7
-
ether-ester) Trademarks: Hytrel, Lomod, Pelprene and others. A
block copolymer containing both polyether and ester blocks. The
best known example is poly-(tetramethyleneterephthalate)-b-poly-
oxytetramethyleneterephthalate).
Ionomer
IONOMER Trade mark Surlyn, among others. A copolymer of
ethylene with 1-10~ methacrylic acid, which has been converted to
methacrylate salt, often the sodium, magnesium or zinc salt, by
neutralization with the appropriate base. The resultant ionic
groups tend to aggregate to form domains which act as physical
cross-links for the polyethylene. However, the domains break down
on heating, so the material may be melt processed as other
thermoplastics. The copolymers are produced by the high pressure
ethylene polymerization process and so are similar to low density
polyethylene. The comonomer decreases crystallinity but consequent
loss of stiffness is restored by the physical cross-links. The
material is more transparent than LDPE and shows better adhesion,
which makes it useful as a layer in laminated coextruded packaging
films and in, therefore, homogenous mixing.
2l S~587
Material Properties
Poly-ether-ester
Property ASTM Test Units Typical
Hardness, Durometer D D 2240 points 30
Hardness, Durometer A D 2240 points 85
Processing
Melt Flow Rate at 190C(374F)/2.16 g load D 1238 g/10 min 5
Melting Point .. . ............... D 3418b
Peak of Endotherm . C(F) 170 (338)
Extrapolated End Point C(F) 200 (392)
Stress/Strain D 638C
Tensile Strength MPa(psi) 26.2 (3800)
Elongation at Break ............. % 700
Stress at 5% Strain . MPa(psi) 1.3 (190)
Stress at 10% Strain MPa(psi) 2.1 (300)
Stress at 15% Strain .. MPa(psi) 2.6 (380)
Stiffness
Flexural Modulus: D 790d
at-40C(-40F) . MPa(psi) 145 (21,000)
at 23C (73F) ...................... MPa(psi) 28 ( 4,060)
at 100C(212F) ..................... MPa(psi) 14 ( 2,030)
Brittleness Temperature, Solenoid D 746 C(F) <-105 (<-157)
Toughness
Initial Tear Resistance, Die C D 1004e ~ kN/m (Ib ~/in) 77 (440)
Resistance to Flex Cut Growth, Ross(pierced) D 1052 cycles to 5x cut >1 x 106
growth
Izod Impact (Notched) D 256~
at -40C(-40F) . J/m (ft Ib f/in) No Break
at 23C(73F) . J/m (ft lb f/in) No Break
Abrasion Resistance
Taber, CS-17 wheel, 1 kg load D 1044 mg/1,000 cycles 2
Taber, H-18 wheel, lkg load D 1044mg/1,000 cycles 90
Miscellaneous
Compression set, after 22h at 70C (158F)
2.8MPa (400psi) load D 395g % 16
Specific Gravity .. . D 792 - 1.07
Water Absorption (24 h) . . D 570 % 3
Softening Point, Vicat . D 1525hC(F) 83 (181)
Heat Deflection Temperature . D 648
0.5 MPa (66 psi) C(F) 46 (115)
21 65581
Ionomer
Film Property
(2 mil blown, 3:1 BUR) Typical Value Test Method
Ultimate Tensile Strength 4900 (33.8)/ ASTM D-882
MD/TD, psi (MPa)5900 (40.7)
Ultimate Elongation, MD/TD, % 350/400 ASTM D-882
Secant Modulus,35,000 (241)/ASTM D-882
MD/TD, psi (MPa)38,000 (262)
Spencer Impact Strength 7.0 (31) ASTM D-3420
in Ib/mil (J/mm)
Dart Drop Strength300 (11.8)ASTM D-1709
g/mil (g/llm) Method B
Elmendorf Tear Strength 18 (6.9)/ ASTM D-1922
MD/TD, g/mil (mN/Ilm)19 (7.3)
Gloss 20 75 ASTM D-2457
% Haze 3.0 ASTM D-1003
Resin PropertyTypical ValueTest Method
Melt flow index 1.3 ASTM D-1238
dg/min condition 190/2.16
Melt Point, C(F)98 (208) ASTM D-3418
(DSC)
Freeze Point, C(F)68 (154) ASTM D-3418
(DSC)
Vicat softening point, C(F) 74 (165) ASTM D-1525
Ion type Sodium
2~ ~55~-~
Poly-ether-esters are valuable materials because they
have excellent low temperature properties (freezing) and are
impervious to chemicals, oils and tissue. They have one serious
negative, however, for many end-uses: They stretch 7x their length
under low stress. For example, a tube one foot long will stretch
to seven feet before breaking. Industrial goods such as bags,
films, tubes, etc. readily warp out of shape and become unaccept-
able as end-use products. The present invention adds an ionomer to
the composition to make useful, thin products.
Ionomers are somewhat like polyethylene in that they are
useful as films because they are excellent for food wrapping,
medical and pharmaceutical packing and are impervious to most oils
and chemicals. Like polyethylene, they puncture readily and only
stretch 3x before fracture.
A common use is a coating over stronger materials and are
frequently used as a co-extrusions on nylon and other films to
provide heat sealing.
The invention ma~es use of the fact that both poly-ester-
ethers and ionomers melt at the same temperature. (191C) Instead
of a coating, the invention mixes them together. In this way, the
negatives of the two materials could be adjusted in a variety of
ways to make new materials that are stronger than the ionomers, and
less stretchy than the poly-ether-esters.
Combining the two materials as described also reduces
another major limitation of the poly-ester-ethers (P.E.E.). They
2l 655&1
can absorb excessive moisture depending upon temperature and
humidity. In the case of P.E.E. use in autoclaved (steam
sterilized) medical tubing for example, the moisture pick-up makes
the tubing unacceptable for further processing. Adding 5% to 7~
ionomer to the composition reduces the moisture absorbance to less
than 1% by weight. A level comparable to medical grade PVC and
well within the limits re~uired for TCD0 to welding.
For forming the material of this invention the following
process may be used. The two materials are fed separately in
pellet form into a single screw extruder. A twin screw extruder
could be used satisfactorily but a single screw is sufficient and
simpler to control. The two pellet streams are mixed to the
required ratios and fed into the extruder. Extrusion rates of 10
to 2~0 lbs per hour are practical at melt temperatures of 180C to
200C, with 191C being ideal. The melt is fed through a sizing
die and liquid quenched as it exists the die for accuracy and
handling purposes.
A typical extruder heating and mixing profile would
involve feeding the ionomeric and P.E.E. pellets from individual
feed hoppers into the extruder where the feed zone of the extruder
is at 300F. The material would then pass to a compression zone at
350F. The materials would then pass into a melting zone at 375F.
Finally, the material would be extruded into the desired shapes.
Techniques such as conventionally used for PVC could then be used
for forming the end products.
21 655~7
-
Figure 1 illustrates a tube lo made from an ionomeric
modified poly-ether-ester in accordance with this invention. Tube
has characteristics which make it ideally suited for its
intended uses as an improved replacement for PVC. For example,
using a modification ratio of 3% to 10%, the material will provide
seal strengths in excess of 60 psi internal pressure as indicated
in Table 1. The material will weld to itself with tensile
strengths of 10 kgs for tubes of 5.4 mm outside diameter and a wall
thickness of 0.7 mm as indicated in Figure 4. Such tensile
strengths can even exceed 10 kgs.
The welds for tube lO can be reopened with the same ease
as PVC by external finger pressure such as by squeezing.
There is no degradation such as the smoke or particulates
characteristic of the PVC with the material of this invention being
generated at welding temperatures of 320~C and exposure times of
less than 1 second. The ionomeric modified poly-ether-ester of
this invention can be steam or ET0 sterilized without geometry
changes. In particular the material will permit steam steriliza-
tion of itself and the tube contents without changing the sealing
or welding conditions because of extremely low water (steam)
adsorption or retention.
Figures 2, 2A and 2B illustrate use of this invention for
urinary drainage application. As shown therein tubing 10 is 5~
ionomer modified and is utilized as the tubing which leads from an
operative site, such as the patient 12 to a urinary bag 14.
11 ,
_ ~l 65587
Urinary bag 14 may also be made of the ionomeric modified poly-
ether-ester of this invention. In use for urinary drainage
application the invention has advantages over medical grade (Class
VI) di-octyl-phlate (DOP) plasticized PVC. For example, since
there is no plasticizer with the material of this invention there
is no leaching of plasticizer into the bag solutions as is common
with PVC. Additionally, after PVC has leached its DOP, large
volumes of PVC particulates are released. This does not happen
with the material of this invention.
For urinary drainage application the full bag 14 is to be
separated from the patient discharge line so that it can be
replaced by a fresh bag. A preferred practice of accomplishing
such substitution would be to use the total containment sterile
connection device as described in U.S. Patent Nos. 5,141,592;
5,156,701; 5,158,630; 5,209,800; 5,244,522 and 5,279,685.
Such sterile connection device is referred to as TCD~. sy using
such techniques the discharge line is disconnected at any suitable
location such as the location 16. This leaves a portion of the
tubing extending from the operative site or patient 12. A new bag
14 is attached by welding a tubing 10 leading from the new bag so
that the tube sections become integral at the weld connection at
location 16. The full or used bag is completely sealed and set
aside to be weighed for total volume and volume per unit time for
determining the discharge rate. The sealing is advantageously
12
21 65~7
accomplished because of the sealing characteristics of the
ionomeric modified poly-ether-ester. Figure 2A, for example, shows
a seal 18 formed at the top of bag 14 and Figure 2B shows a seal 20
formed at the discharge line of bag 14 by use of TCD~.
The full bag 14 and its contents can be disposed of as a
sealed biological waste. Alternatively, the bag 14 can be
suspended on a standard bag holding pole over a commode and the
bottom seal 20 can be opened by use of the fingers and thumb as is
possible from seals formed by the total containment sterile
discharge connection use for PVC. The bag contents will not drain
at this point because of lack of air displacement. When the top
seal 18 is opened again by finger pressure if desired the bag
contents will immediately begin draining. The use of these two
seals 18,20 created by the total containment device have the
advantage of draining the bag contents without tools and in the
manner that the bag contents do not contact the nurse's hands.
This feature is of extreme importance to protect the nurses from
contamination and also prevents cross-contamination with a hospital
ICU facility. Cross-contamination in ICUs is currently a major
problem.
The new or empty and sterile bag 14 is welded to the
patient line at welding site 16 immediately after removing the full
bag to provide uninterrupted flow from the patient.
2 1 6~5~7
The following represents an experiment to compare the
ionomeric modified poly-ether-ester designated in the experiment as
E77-200-7-3 as a substitute for PVC tubing. The E77-200-7-3 was
made from a blend of 90% Hytrel poly-ether-ester and 10% Surlyn
ionomer. The poly-ether-ester had a hardness of Durometer 30 on
the D scale and a melt flow rate of 5 grams/10 minutes. The
ionomer had a hardness of Durometer 60 on the D scale and a melt
flow rate of 1.3 grams/10 minutes.
Experimental plan:
PART A: (Disconnect Seal Test)
1) Cut the tubing (E77-200-7-3) into 25 samples 6
inches long.
2) Place each sample in the TCD~ and perform a discon-
nect. This will cut the tube sample in half and
seal both ends producing 50 sealed pieces of tubing
3) Place each piece of tubing onto the pressure tester
and pump up to 60 psi. If the tubing maintains
that pressure for 1 minute then it passes.
4) Place the seal of each piece of tubing in the Arbor
Press and squeeze the seal 5 times.
5) Remove the tubing from the press and see if the
seal has broken open.
6) Repeat steps 4 & 5, 20 times or until the seal
breaks, which ever occurs first.
7) Repeat step 3.
- 14
5~7
P~RT B: (PVC Disconnect Seal Test)
1) Repeat Part A using 5 samples of PVC tubing.
PART C: (Connect Test)
1) Place two of the disconnect pieces of tubing from
PART A into the TCD~ and perform a connect.
2) Record whether the tubing can be popped open or
not.
3) Repeat steps 1 & 2 using all 50 pieces, thus pro-
ducing 25 samples 6 inches long.
PART D: (Welded Flow Test)
1) Using a metal can that has a tubing connector at
its base, attach one of the welded 6 inch samples
to the can.
2) Fill the can with 2 cups of water and record the
amount of time required for the can to drain
through the tubing. Make sure that the draining
water is caught in another can so that the same
amount of water can be used in all the tests.
3) Repeat step 2 with eight more welded tube samples
and one non-welded tube sample.
PART E: (Weld Tensile Strength Test)
1) Load and run the Tensile Instrument program
"INSTFIXE.BAS~. This program wills save to disk the-
tensile strength curve results for each of the
Zl 65587
~ samples, ~o they can be regenerated using the
J~1
program "EXCELn.
2) Calibrate the Tensile Instrument.
3) Place one of the welded samples into the clamps of
the Tensile instruments and start the test.
4) Stop the test when the lower clamp of the Tensile
Instrument reaches the automatic stop switch.
5) Save the results to disk.
6) Repeat the steps 3, 4 & S with the remaining 24
samples and one non-welded tubing.
PART F: (Weld Tubing to PVC Tubing)
1) Set the TCD~ to the CONNECT mode.
2) Place one 3 inch piece of Tubing (E77-200-7-3) into
one of the TCD~ clamps.
3) Place one 3 inch piece of PVC Tubing into the other
TCD~ clamp.
4) Perform the connect operation so that the E77-200-
7-3 tubing and the PVC tubing are welded together.
5) Remove the tubing from the TCD~ and record whether
the weld can be popped open,
6) Place the E77-200-7-3-PVC tubing in the Tensile
instrument and repeat steps 1-5 of PART E.
~1 65587
Observations and Data: Table 1
PART A: (Disconnect Test)
E-77-200- Disconnect Seal Strength of each End Seal Squeeze Test using Arbor Press
7-3 Tubing
Sample # 1" Half of Tubing 2nd Half of Tubing 1" Half of Tubing 2nd Half of Tubing
61 psi * 60 psi *100 squeezes * 100 squeeæs *
2 60 psi * 60 psi *100 squeezes * 100 squeezes *
3 60 psi * 60 psi *100 squeezes * 100 squeezes *
4 60 psi * 60 psi *100 squeezes * 100 squeezes *
60 psi * 60 psi *100 squeezes * 100 squeezes *
6 60 psi * 60 psi *100 squeezes * 100 squeeæs *
7 60 psi * 60 psi *100 squeezes * 100 squeezes *
8 62 psi * 60 psi *100 squeezes * 100 squeezes *
9 62 psi * 61 psi *100 squeezes * 100 squeezes *
60 psi * 60 psi *100 squeeæs * 100 squeezes *
Il 60 psi * 60 psi *100 squeezes * 100 squeezes *
12 60 psi * 60 psi *100 squeezes * 100 squeezes *
13 60 psi * 60 psi *100 squeezes * 100 squeezes *
14 60 psi * 60 psi *100 squeezes * 100 squeeæs *
60 psi * 64 psi *15 squeezes * 100 squeezes *
16 60 psi * 60 psi *100 squeeæs * 100 squeeæs *
17 60 psi * 60 psi *100 squeezes * 100 squeezes *
18 60 psi * 60 psi *100 squeezes * 25 squeezes *
19 60 psi * 61 psi *100 squeezes * 100 squeeæs *
60 psi * 60 psi *100 squeezes * 100 squeeæs *
21 62 psi * 60 psi *100 squeezes * 100 squeezes *
22 60 psi * 60 psi *100 squeezes * 100 squeezes *
23 60 psi * 63 psi *100 squeezes * 100 squeeæs *
24 60 psi * 60 psi *100 squeezes * 100 squeezes *
61 psi * 60 psi *100 squeezes * 100 squeezes *
~ote: * indicates the seal did not break or leak.
17
21 65~7
Table 2
PART B: (PVC Disconnect Test)
PVC Tubing Disconnect Seal Strength of each End Seal Squeeze Test using Arbor Press
Sample # lSt Half of Tubing 2nd Half of Tubing 1S~ Half of Tubing 2nd Half of Tubing
62 psi * 60 psi *10 squeeæs 10 squeeæs
2 60 psi * 60 psi *15 squeezes 10 squeezes
3 60 psi * 60 psi *10 squeezes 15 squeeæs
4 62 psi * 63 psi *10 squeezes 10 squeezes
60 psi * 60 psi *20 squeezes 10 squeezes
6 60 psi * 60 psi *10 squeezes 10 squeezes
7 60 psi * 60 psi *10 squeezes 15 squeezes
8 62 psi * 60 psi *5 squeezes 10 squeeæs
9 60 psi * 63 psi *10 squeeæs 10 squeezes
60 psi * 60 psi *10 squeezes 10 squeezes
Part C: (Ability of weld to pop open.)
All 25 connected (welded) Samples (E77-200-7-3) popped open easily
Note: * indicates the seal did not break or leak.
18
21 6~587
Table 3
Part D: (Effect of Weld on Water Flow through E77-200-7-3 Tubing)
Sample #Flow TimeFlow TimeFlow TimeAvg. Flow TimeFlowChange
(sec) (sec) (sec) (sec) (%)
No Weld 90.74 90.41 90.3 90.48 100%
97.97 98.29 98.4 98.22 91.40%
2 101.27 101.6 100.9 101.25 88.10%
3 95.88 95.21 95.28 95.45 94.50%
4 93.51 92.89 93.45 93.28 96.90%
104.45 103.52 103.44 103.81 85.20%
6 101.24 100.96 100.26 100.82 88.60%
7 101.62 100.34 100.12 100.69 88.70%
8 98.67 97.81 97.27 97.91 91.80%
9 99.64 99.65 99.48 99.59 89.90%
18a
2l 655~7
-
An advantage of the ionomeric modified poly-ether-ester
is its ability to be effectively welded to conventional PVC. This
is important because it permits the replacement of some of the PVC
components with the new material. Thus, for example, in CAPD usage
it is necessary to remove a used filled bag for replacement by a
new empty bag. The conventional PVC tubing could be cut and tubing
from the new material could be welded to the remaining PVC tube
segment. The new material components could thus gradually replace
their PVC counterparts. Figure 3 demonstrates the weld strength of
PVC tubing to ionomeric modified poly-ether-ester tubing.
Figures 3 and 4 are graphs illustrating the ability of
the weld to pop open. As shown therein with use of the ionomeric
modified poly-ether-ester of this invention (Figure 4) the
unsealing characteristic is accomplished with approximately the
same ease as in PVC tubing welded to the modified poly-ether-ester
tubing (Figure 3).
Figures 5-6 illustrate use of the invention with respect
to peritoneal dialysis (CAPD). Figure 5 represents the prior art
practices wherein a tube 22 is utilized made from PVC. In such
applications PVC is the material of choice of the health care
industry for both the tubing and the solution bags 24. The reasons
for favoring PVC are that its long standing use started early in
the 1960s. Additionally, there is film clarity for visual
clarification and PvC provides low cost and abundant supplies. The
PVC used for peritoneal dialysis, however, must undergo bulk
19
21 655~,7
-
sterilization after packaging to insure sterility to the entire
product offering prior to patient use. Steam sterilization (250F
for thirty minutes) of PVC in contact with dialysis solutions
causes several serious problems. For example, the PVC plasticizer
(DI-OCTYL-PHLATE), DOP, leaches out of the PVC composition during
steam sterilization and into the dialysis solution. DOP is under
suspicion of being a low level carcinogen. A further product that
is after DOP leaching the residual PVC composition is vulnerable to
particulates being formed and entering the dialysis solution which
in turn enters the patient's body. Further, water adsorption (from
the steam sterilization) into the patient tubing and bags reduces
the plastic strength by approximately 50% causing bag leakage and
sterility failures. Figure 5 illustrates the conventional practice
of using PVC as a material for the tubing 22 and bag 24 wherein the
solution 26 in the bag 24 is caused to flow through tubing 22 to
the patient's site 28. Steam sterilization takes place in chamber
30. Figure 5 also illustrates the separation and connection area
32 used for replacing solution bags by separating and then
rejoining the PVC transfusion line 22.
Figure 6 illustrates use of the ionomeric modified poly-
ether-ester for the tube 10 and bag 14. This material overcomes
the PVC problems in several ways without sacrificing the positive
attributes of PVC. With the material of this invention there is no
plasticizer, thus leaching is not a pro~lem. The material also
does not form particulates and is impervious to steam sterilization
21 65587
to retain 100% of its strength. The ionomeric modified poly-ether-
ester has another positive feature that is unique to sterile
connection devices that use a heated blade to achieve sterility.
The material does not make smoke. Conversely, when PVC is heated
using the TCD~ instrument of the aforenoted patent the plasticizer
DOP evolves as fine aerosol droplets that qives the appearance of
smoke. Means must be provided therefore to contain and dispose of
the smoke harmlessly. Since the poly-ether-ester of this invention
has no plasticizer, no smoke is formed using the TCD~ process.
The use of the total containment device TCD~ has a more
significant potential for peritoneal dialysis, however, in that
steam sterilization and its problems can be avoided entirely. For
example, the ionomeric modified poly-ether-ester consumable bags,
tubes, etc. can be bulk sterilized by ethylene oxide at the point
of manufacture. The solutions can also be sterilized in bulk
storage tanks and made ready to fill the previously sterilized
bags. Using the TCD~ the sterile solutions can be fed to the
sterile consumable thus avoiding steam sterilization. Figure 6
illustrates by use of the connect/disconnect areas 16 how the
ionomeric modified poly-ether-ester of this invention for tubing 10
can be used for replacing bags thereby resulting in a plurality of
filled bags 34 with the unfilled bags 36 ready to be connected by
weld 16 which would join its tubing 10 to the tubing leading from
the sterile solution container 14.
~l 65~7
Figure 7 illustrates use of the invention for blood
processing. In this application the use of ionomeric modified
poly-ether-ester materials has several end-use advantages. Since
the material has no plasticizers the problems of plasticizer
leaching into the blood are eliminated. The problems of mixing and
matching of various sources of blood bags and tubing are also
eliminated. Mixing and matching is viewed by the FDA as a health
threat when bags and tubin~ of unknown source and composition are
introduced into blood centers because of cost or availability.
Eventually the material can be frozen and retain its flexibility,
thus avoiding brittle fracture.
Mix and match is considered by the FDA as a serious
guality control and safety problem in the blood processing
industry. Mix and match is an expression used to describe the
chaotic situation in blood banks brought on by economics. Blood
banks buy blood bags and tubing strictly on price without too much
regard to source. Global dumping practices, questionable polymer
compositions and tube size variation all contribute to the problem.
In order to exercise a measure of control the FDA would prefer to
be able to mandate a set of regulations regarding tube quality and
be able somehow to exclude tubing that does not meet the FDA
criteria.
The ionomeric modified poly-ether-ester material meets
this requirement again because the material does not contain
plasticizers. Commonly used medical grade PVC contains up to 40%
2~ 655~7
-
DOP as a plasticizer that vaporizes to a smoke when the welding
temperature exceeds 200C. By using what amounts to a small smoke
detector in the TCD~ the instrument can detect the smoke generation
and refuse to complete the welding cycle. Specifically, prior to
making a weld the tube ends are made to touch the wafer for one
second. If no smoke is generated the process will proceed to make
a weld. If smoke is detected the instrument will abort. In this
way, the mixture will mix and match problem so important to the FDA
and safety of the nation's blood supply can be avoided.
Use of the ionomeric modified poly-ether-esters have a
positive effect when used in conjunction with TCD~ in that tube
sealing (separating) can be accomplished in the TCD as well as
connections. Blood banks have the capability of sealing off tubing
by radio frequencies (RF) sealers. Because of the radio frequency,
however, the equipment is normally located away from the blood
processing areas and shielded for safety (booths). The inconve-
nience of going to a sealer encourages people to make poor seals
via hot plates and bunsen flames rather than taking the bags to the
RF sealer. Use of the TCD~ avoids this problem. The TCD~ is
located in the work place. The use of the TCD~ in blood banking
has another important advantage. It is called "un-bundling" and
means complete flexibility to disconnect and connect blood bags at-
will. Without the sterile connection technology afforded by TCD~
this concept is not possible. For example, when blood is collected
at the blood banks it is not known at that time what blood
23
~ ~ 65587
fractions will be required to meet end use needs. To overcome this
unknown all blood is collected in bag sets. That is, a primary
blood bag and four satellite bags are sent as a sterilized set.
This is illustrated in Figure 7 wherein the blood supply needle 38
has its tubing 10 leading to primary blood bag 40 with tubing 10
also leading to the four satellite bags 42 each of which has its
tubing 10 connected to the main tubing by the TCD~ connect or
disconnect location 16.
After the blood is centrifuged the fractions are
expressed over into various component bags and sealed off. Once
sealed off the bags can not be re-entered without compromising
sterility. In the practice of this invention all of the tubing 10
and the various bags 40,42 would be made of the ionomeric modified
poly-ether-ester material.
If, for example, only whole blood is needed, the four
unused satellite bags 42 are separated and discarded. At approxi-
mately $2.00 per bag this loss is keenly felt by the blood bank.
Moreover, if the bags are used and sealed it is not possible to
enter the bags to enhance the cell life by the addition of
stabilizers, nutrients or enhancers without compromising sterility.
The use of the TCD~ has made "un-bundling" possible. The
TCD~ is designed to sterilely disconnect and connect sterile
containers at will thereby providing the medical profession a
desired level of flexibility and at lower consumable costs.
~ 655~7
-
The use of the ionomeric modified poly-ether-ester
materials has proven to be non-pyrogenic to human tissue through
wide spread use of permanently placed interval sutures. For this
reason, the poly-ether-ester material is approved by the FDA for
internal human use and is ideal for blood bags.
Figures 8-9 relate to use of the invention for chemother-
apy. Figure 8 illustrates the conventional practice to administer
chemo-therapeutic drugs for cancer patients by introducing the
drugs via syringe 44 or a pump into the intra-venous transfer
tubing 22. Solution would also flow into PV~ tubing 22 from PVC
bag 24. The syringe port is a convenient entry site for bacteria.
For patients whose immune system is depressed from the therapy
bacteria entry is life threatening. This problem can be eliminated
by the use of the TCD~ instrument.
Use of the TCD~ instrument in co~bination with the
ionomeric modified poly-ether-ester material for chemotherapy
application has several end use advantages. Since there is no
plasticizers involved, the danger of plasticizer leaching into the
patient is eliminated. Since there are no particulates this form
of contamination into the patient's blood stream is also eliminat-
ed. The poly-ether-esters are already used widely within humans
for sutures and arterial repair. They present no pyro-genic
defects therefore for human use.
Figure 9 illustrates use of ionomeric modified poly-
ether-ester material for bag 14 and tubing lO. As shown therein at
~i 655~1
the normal entry port location 46 a ionomeric modified poly-ether
ester tube 48 is spliced into the line 50 also made of ionomeric
modified poly-ether-ester and is terminated with a closed (sealed)
distal end 52. The syringe 44 or pump is sterilely enclosed in a
sheath 54 and terminated with a closed sealed distal end.
Making a sterile connection via the TCD~ the connection
is totally contained. No bio-burden can enter the patient and no
chemotherapy agents can escape outside the system. The syringe can
also be sterilely disconnected by the TCD~ when the procedure is
complete.
Figure 10 illustrates use of the invention for parenteral
feeding. For cancer patients who have suffered alimentary tract
removal because of the cancer, feeding is accomplished by liquid
nutrients fed into the patient's blood stream by way of a tube
inserted into a sub-clavian vein and then into the heart.
Long term implantation of PVC tubing is also a problem.
PVC plasticizer extraction directly into a patient's blood stream
is particularly troublesome.
As pointed out in Biocompatible Polymers, Metals and
Composites by M. Szycher, Sponsored by Society of Plastics
Engineers Inc., Techmonic Publishing Co. 1983, flexible PVC
compounds can contain up to 40~ plasticizer. While the chosen-
material is very carefully selected for its high purity and low
toxicity, it can be extracted from PVC by contact with some fatty
substances, such as by blood or the digestive system. When contact
26
~ 65~a7
times are short there is no difficulty, but for a number of
applications in which PVC may be in contact with the patient's
system for longer periods extraction of plasticizer is much less
acceptable. Stiffening of PVC due to plasticizer being extracted
may cause patient's considerable discomfort when a feeding or wound
drainage tube is removed after prolonged use. Blood stored in PVC
bags or flowing through PVC tubes for long periods will also
extract plasticizer. While that most commonly used, DEHP, has been
shown to be of low toxicity it is naturally desirable to avoid any
extra burden on a patient's system. This is obviously more
important for those needing regular hemodialysis for blood
transfusions.
The use of ionomeric modified poly-ether-ester materials
for tubing is beneficial in two ways. There is no leaching of
plasticizers into the patient's system from the implanted tube.
Additionally, the poly-ether-ester material is compatible with the
TCD~ for making sterile connects and disconnects from the implant.
Figure 10, for example, illustrates the use of a poly-ether-ester
bag 14 supplying the liquid nutrients through poly-ether-ester
tubing 10 into the patient's heart 56. By use of the TCD~ a
sterile connect/disconnect procedure 16 can be effectively used to
replace the bag 14 with a new bag 14.
The invention may also be used for cell culturing and
particularly has applications in connection with the TCD~ in
~ 65587
biotechnology. In such applications the ionomeric modified poly-
ether-ester would be used as tubing from the reactor.
Biotechnology is a rapidly growing business area with R&D
expenditures of $2BLN in 1994 (U.S.). TCD~ technology can fill
unmet needs for improved connections in several segments of this
market. The value-in-use is a ~2-10/connection with a total market
opportunity of $28MM (1990) and estimated to grow to $78MM (1995).
Mammalian cell fermentation is targeted as the market entry segment
for the TCD~ technology.
The biotechnology business utilizes many techniques
developed by molecular and cellular biologists in the 1980's for
increasing the production of a specific protein in a cell by
redesigning, or engineering, its DNA. This business area produced
high value proteins which are used in diagnosis or therapy of
diseases as well as cost reducing processes in energy conversion
and agriculture processes. The common factors in all biotechnology
segments are the creation and isolation of a unique cell type and
then the growth of this cell to isolate product(s).
Cell growth occurs in a sterilely isolated system which
provides nutrients and removes waste products, a process called
fermentation. To maximize product yield, this fermentation system
must also prevent the entry of other "non-unique" cells which would-
grow and contaminate the system.
The technology to produce and isolate unique cells is
well developed, although it requires highly skilled personnel.
28
~ ~ ~,5587
Unique cells may be created from either bacteria or cells isolated
from more complex organisms, including humans. The technology to
grow large quantities of bacterial cells is well established.
Cells from complex organisms, called mammalian cells, grow more
slowly than bacterial cells and require more complex nutrients.
the development of new ways to grow these unique mammalian cells in
large quantities is one of the major problems in biotechnology
today.
Preventing non-unique cells from entering the fermenta-
tion system is important for all types of cells. In bacterial
systems contamination is not as significant because of the design
of fermentors and the length of the fermentation process, a few
days. A typical mammalian cell fermentation cycle is three weeks
~g but the nutrients and wastes are exchanged several times a
day. Accidentally introducing bacterial contamination when
exchanging the fluids is a real possibility. Such contamination
destroys the mammalian cells in several days.
Presently about 90% of the products produced by biotech-
nology are grown in bacterial cells lines. However, research is
focusing more on producing unique cells from mammalian cells
because some proteins can be produced more effectively in these
cells. By 1995, it is expected that at least 50%, and perhaps ase
much as 90%, of the genetically engineered products for health care
will be produced from mammalian cells.
29
`- 2t 655~7
The biotechnology market may be segmented in several
different ways, the preferred methods are by product source and by
stage of the product development cycle. Product source defines the
type of cell the protein is isolated from and product development
cycle defines whether the product is in the stage of research,
development, or manufacturing. The following charts give more
information on segmentation and estimates of value-in-use (VIU) and
market opportunity for the TCD~ technology. To date, market
analysis has been focused on the high value products for health
care applications. The segmentation and rest of this discussion
focuses on this area.
The early phase of product development, research, is done
by a large number of highly skilled scientists whose primary
objective is the construction and isolation of unique cells with
specific properties. Cell growth in research is done on a small
scale and lose due to contaminations of a fermentation, although a
nuisance, has small economic consequences. However, in the
development and manufacturing phases, where the objective is the
growth of large quantities of these unique cells, contamination
results in a significant economic loss.
The following tables compare several important aspects of
the termination process and the market opportunity by cell type.
2~ 6~87
Average Length Number ofRelative Value Average
Cell Type of Connectionsof Materials VIU
Fermentation Per Cycle per
Cycle Connection
Bacterial 4 Days 8 lX $3
M~mm~ n 21 Days 60 10X $7
Whole Organ21 Days 42 8X $4
Number of Connections Market Opportunity
Cell Type 1994 2000 1994 2000
Bacterial 5.2MM 7.9MM $1lMM $17MM
M~mm~ n 3.0MM 8.5MM $15MM $58MM
W~ole Organ0.5MM 0.8MM $2MM $3MM
8.7MM 17.2MM $28MM $78MM
TOTAL
The increase in the number of products in the development
and manufacturing phases as well as more products derived from
mammalian cells, both with higher VIU's, account for much of
the growth in the market opportunity.
The initial targets for the TCD~ are the development and
manufacturing phases of mammalian cell culture. The reasons
for this choice are:
significant product loss due to contamination
high VIU per connection
very high income potential per TCD~ placement
rapidly growing segments
technology still evolving.
31
21 65587
Our initial TCD~ placements will be with small companies
which produce monoclonal antibodies by mammalian cell culture on
contract to other companies. These antibodies are used in
diagnostic tests and a~re in clinical studies for disease treat-
ments.
Six companies worldwide produce monoclonals on contract
which sell for ~2M to $5M per gram. Their 19~0 sales were $3MM and
are forecast to grow to $8MM (l~g5) a~d as high as $60MM (2000).
The demand for monoclonal pr~ducts exceedS the present capacity of
these companies. In addjtion, at least 20 companies produce
monoclonals for t~eir own use. At contract companies, the product
loss due to fermentation system contamination is approximately
15%/week. Discussions with these companies indicate that most of
this contamination appears to occur when entering the system to add
fresh nutrients and remove wastes and product (the monoclonals
produced by the cells). Presently, there is no simply means to
sterilely access these fermentation systems.
The strategies for market development in the U.S. are:
establish the TCD~ as the state-of-the-art connec-
tion technology;
utilize scientific meetings and seminars to expose
TCD~ technology to marketplace;
initially place the TCD~ in a high value-in-use
segment.
~1 6~5~7
._
Establishing the TCD~ as the standard connection
technology for biotechnology depends on demonstrating its effec-
tiveness in reducing contamination. Its convenience and simplicity
will also be important. Effectively demonstrating these attributes
requires information collected under normal use conditions.
Developing and publishing the experience of TCD~ in cell
culture will create the exposure to develop a demand for this
technology in the other biotechnology applications and help
establish the TCD~ as the state-of-the-art connection technology
for this market.
In addition to the above described uses of the invention
the ionomeric modified poly-ether-ester may also be used in food
processing particularly as tubing for fluids and semi-solids and as
the material for making sheets and films for bacterial and virus
extrusion. When used as sheets or films, the sheet could be draped
over the patient and the surgeon would cut through the sheet into
the patient. The ionomeric modified poly-ether-ester may in effect
be used as a suitable replacement for PVC in virtually all uses of
PVC .