Note: Descriptions are shown in the official language in which they were submitted.
CASE5580 2165633
..
METHOD OF REAGENT AND OXIDATION AIR DELIVERY
FIELD AND BACKGROUND OF THE INVENTION
The present invention relates in general to wet flue gas desulfurization (FGD)
processes and, in particular, to wet lim.os~on~ forced o~i-li7~ flue gas desulfurization
5 ~roc~es (LSFO-FGD) pr~sses and other cn.,-pAIihle procesæs. Wet LSFO-FGD systems
have s~ ox~ tion air and l;...~(o~e slurry streams. Theæ systems, in general, utilize
a wet ~ g circuit by ~ coarse 1;...~ f to prepare a limeston~ solids-liquid slurry
as the fresh reagent supplied to the absorber system. Typically, wet ball mills have been
used for ~is ~u.~ose. The wet CO~ m process has the advaD~age of ~ot~ ially lower
0 hOlY~ l~Ui~ than dry ~ ;. N1;. ~ ploce~xs, and the fact that the end product after
is a slurry also fits in well with the wet LSFO-FGD system design. This slurry is
commonly stored in a holding tank to provide surge c~a~i~y for :~ubse(lu.,.l~ controlled
supply to the absorber vessel based on l~m~n(3,
In wet FGD ~,.~ lls, the sulfur dioxide (SO2) absol~io~ ~locess l~u~s a nearly
lS contimloll~ supply of fresh l~e~ to ~ s~ti~f~-tory oper~tion and SO2 removal
CASE ~80 2 1 6 5 6 3 3
efficiencies. Often it is desirable to incorporate air addition to the wet FGD absorber to
provide in situ forced oxidation of the absorber reaction product.
Providing se~ e o~da~ll air and ;i....o~1...~ slurry streams increases the complexity
and expense of these- wet LSFO~G-l}systerns. -lt is thus apparent that an approach to
S providing these se~ala~ functions in a simple and cost-effective manner would be welcomed
by the industry.
SUMMARY OF THE INVENTION
The filn~m~n~l nature of the present invention is the use of the oxidation air system
air stream to not only provide oxidation air to an absorber vessel, but also to both
l0 pneumatically convey and inject dry reagent into the absorber vessel to satisfy reagent
addition requirements.
Accordingly, one aspect of the present invention is drawn to a method of forced
oxidation flue gas desulrul~lion wherein dry reagent and dry additive are supplied to an
absorber vessel cont~ining a liquid slurry through an oxidation air addition system. The
15 method colllylises the steps of: providing a rate controlled feed supply of ~r~ared dry
reagent and dry additive to a ~.~r.. AIic collv~ying pick-up point located within air supply
piping used to provide an oxidation air stream from the oxidation air addition system into
the absorber vessel; and using the oxidation air addition system air stream to provide
ox~ tion air into the abso~ vessel, and to ~ lly convey and inject the dry reagent
20 and dry addilive into the absoll~r vessel sufficient to partially desulfurize flue gas provided
to the al)so~ vessel.
The dry reagent is a ~ ~L ~1P~d from the group cO.~icli..g of solid alkali
colll~oullds used in flue gas desulruli~lion ~r(xesses inrl~lAing lillles~o~e, e~1cillm,
~o1~ c--.. ", ~1.. ;.. , so linm, and/or ~mmonillm colll~ullds. Other dry additives besides
25 reagent can be added sepz. ~ ly or added to the dry reagent so that the oxi-l~tion air stream
inje ts both the dry reagent and the dry additives into the absoll,cl vessel. These additives
could be added to promote oxi~ti~)n ~,vithin the absorber vessel; to ~ A.~-e cl~ ;r~l
al)sol~liol~ of the SO2 within the al)sollJcr vessel or to provide a desired degree of b~rL~ g
to the ~ lrl.. ;,,.~ion ~locess ~ ih; to inhibit scale growth vithin the al)soll~r vessel; or
CASE5580 - 2165633
to promote or specify the type of or degree of cryst~lli7~tion7 etc., in the desulfuri_ation
process occurring within the absorber vessel.
Acco,dil,~,ly, another aspect of the present invention is drawn to a method of forced
oxidation flue gas desulfurization, wherein.~dry~-r~litive i~ r.~ tir~lly supplied to an
absorber vessel cont~ining a liquid slurry through an oxidation air addition system. The
steps of this method conl~lise: providing a rate controlled feed supply of prepared dry
additive to a pn~nm~tic conveying pick-up point located within air supply piping used to
provide an oxidation air stream from the oxidation air addition system into the absorber
vessel; and using the oxidation air addition system air stream to provide oxidation air into
the abso,l,ci vessel, and to ~ ir~lly convey and inject the dry additive into the absorber
vessel.
The various feat~lrcs of novelty which characteri_e the invention are pointed out with
particularity in the claims annexed to and forming a part of this disclosure. For a better
underst~n-~in~ of the invention, its opela~ing advantages and specific results ~tt~in~A by its
uses, refc,c,lce is made to the ~ ol--p~.-yhlg drawings and descfl~live matter in which a
~ref~.,ed embodiment of the invention is illustrated.
BRIEF DESCRIPIION OF THE DRAWINGS
In the drawings:
Fig. 1 is a s~ P-~ ;c illllstr~tion of a system according to a first emb~im~nt of the
present invention; and
Fig. 2 is a sc-~ s~tion of a system acco~ing to a second embodiment of
the present invention.
DESCRIPTION OF THE PREFFRRFn EMBODIMENT
In the d~s, l~e mlm~o.r~lc ~ ". ~ the same or similar elem~o-nt~ throughout the
two drawings. ~.f~ to Fig. 1 in particular, one aspect of the present invention is
drawn to a system 10 using a m.o.th~1 of forced o~i~3ati~n flue gas ~e~llr~ lion~ wllc~ehl
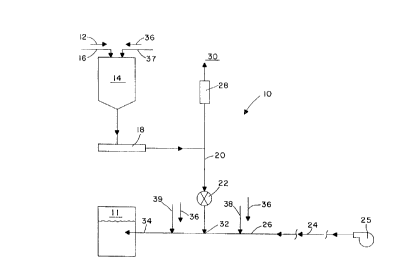
dry reagent is ~u~pli~d to an absollc~ vessel, s~ lly shovn at 11, through an
o~ tion air ~l-lition system. A p~ly p~ ,d reagent 12 in a dry form is IL~s~lL~d
CASE5580 2165633
to a reagent surge bin 14, either by m~h_nir~l conveyance, or by dilute or dense phase
conveyance, through a supply line 16. The dry reagent 12 is withdrawn from the surge bin
14 at a controlled rate by feeder means 18, such as a volumetric or gravimetric feeder. The
controlled feed rate of dry reagent 12 corresponds to an absorber.-reagent ~m~n(1 required
S by the absorber vessel 11. The feeder means 18 discl~ges to a gravity flow chute 20 which
directs the dry reagent 12 to an airlock 22. The airlock 22 provides the required level of
isolation between the gravity flow chute 20 and an oxidation air stream 24 provided from
oxidation air supply means 25, such as a blower or COlllpl~,SSOi station, at a higher pressure
via o~ tion air line supply piping 26. Oxidation air 24 which leaks across the airlock 22
can be vented through a vent filter 28 and into the atmosphere 30. Dry reagent 12 which
passes through the airlock 22 is introduced to the oxidation air line 26 at a solid pn~nm~tic
conveying pick-up point 32, incorporated within the air line supply piping 26 used to provide
the oxidation air stream 24 into the absorber vessel 11. The oxidation air stream 24
sl~bs~~ ntly provides oxidation air to the absorber vessel 11, and pn~llm~tir~lly conveys and
lS injects the dry reagent 12 to the absol~r vessel 11 through line 34 to partially or completely
satisfy the reagent addition requir~-m~nt~.
The dry reagent 12 is a m.omher selected from the group con~i~ting of solid alkali
col~ds used in flue gas le~llr~ ;on plocesses such as limestone, calcium, pot~inm,
~lnmimlm, sodiurn, and/or ammonium. Dry additives 36 can be added to the dry reagent
20 12 into the surge bin 14 by "~ a.,;~al con~ ~, or by dilute or dense phase collvey~ce~
through dry additive supply line 37, so that the oxidation air stream 24 injects both the dry
reagent 12 and the dry addilivcs 36 into absorber vessel 11. The dry additives 36 could also
be added s~ t~_ly into the o~ i~1al i~ air stream 24 at the same p ~ "~ COll~ illg pick-up
point 32, or at uy~ll~ or dow~ll~ dry addidves y..~ convt;ying pick-up points
38, 39 l~sye~ y. Various dry additives 36 could be added to promote oxidation within
the absoll,c~ vessel 11; to c~ A.~e cl~...i~al absorption of the SO2 within the absoll~. vessel
11 or to provide a desired degree of b..rr~ to the ~les~lr~ ;on process therein; to
inhibit scale growth within the absol1~ vessel 11; or to promote or specify the type of or
degree of cryst-lli7-tion, etc., in the ~ lr,.. ;,~ ,. process o~ , within the absorber
CASE 5580 2 ~ 6 5 6 3 3
vesæl 11. The dry additives can be added in this method even if the reagent is introduced
by other known methods.
F.Y~mples of dry additives 36 which could be added to promote oxidation within the
~ abso~ vessel 11 include compounds of iron, m~n~n~se; v~n~ lm an~other~metals that
5 promote or catalyze oxidation.
Examples of dry additives 36 which could be used to e~h~nre chemical absorption
of the S2 within the absorber vessel 11 include m~nesium oxide, ammonium hydroxide,
sodium carbonate, sodium sulfite, sodium hydroxide, m~n~sil-m sulfite, and other soluble
aLkali compounds of m~n~sillm~ ammonium, pot~ssillm~ calcium, and sodium, used to
10 enh~nr~ absorption in a calcium (limestone or lime) based FGD system.
Examples of dry additives 36 which could be used to provide a desired degree of
burr~.hlg to the desu!furization process occurring within the absorber vessel 11 include
sodium fo~ , dibasic acid (DBA), sodium citrate, adipic acid, succinic acid, and other
organic acids and/or their solid colL~poullds including formic, acetic, hydroxypropionic,
15 sulfosuGr-inic, adipic, phthalic, benzoic, fumaric, hydroxyacetic, succinic, and lactic.
F~ s of dry additives 36 which could be used to inhibit oxidation to reduce scale
growth within the absorber vessel 11 include coul~oullds of formate and thiosnlf~ and
el~m~nt~l sulfur.
An ~Y~.IIplr- of a dry additive 36 which could be used to promote or specify the type
20 of or degree of cryst~lli7~tion, etc. in the desulfuri_ation pl~cess occurring within the
absGll~r vessel 11 is polysulfonate.
As the examples Listed above in(~ir~t.q., some of the various dry additives 36 can
~)lOLU<~k: more than one kind of desired activity within the absorber vessel 11. For eY~n rle,
sod~m formate has been shown to inhibit oyi~tion and provide l UlLlldlS; ions to buffer and
25 ~l~LuO~ SO2 removal in r~lrillm based FGD ~ms. Also, additives such as iron EDTA
can be used to plOLuo~ removal of nitrous oxides in a flue gas desulrll. ;~ion system. It
wiU also be a~l~id~ that more than one dry additive 36 could be employed at the same
time, i.e., two or more dry additives 36 could be ~ lly injected into the absoll3el
vessel 11 via the q~ air ar1<liti~n system, so long as the two or more dry additives do
30 not ploduce an adverse .~ l reaction th~wæL~, or upon the ~esnl r~ ;on ~r~cess
` - 216S~33
CAS~ 5580
occurring within the absorber vessel 11. By way of example and not limitation, the dry
additives sodiurn formate and sodiurn thiosnlf~t~, or calcium carbonate and DBA, could be
pn~ lly injected simlllt~nfously without adverse effect. However, combinations of
u~.iron and thiosulfate, or sodium formate and DBA for example, would not be;desirable
S because the first combination ,e~resellls opposing goals of promoting and inhibiting oxidation
while the second is not desirable because the two additives are used to accomplish the same
goal of l~urr~ g.
While the solids pick-up point 32 can be located at virtually any point in the line 26
dc,w~l~ll of the oxidation air supply means 25, it is preferable to locate it as close to the
absorber vessel as practical. This will minimi7~ the length of line 34 and the associated
additional ~res~ulc: drop and abrasive wear due to the solids conveying function. Sirnilar
location considerations would apply to any sep~ate dry additives 36 pneumatic conveying
pick-up points 38, 39. l\~finimi7.ing the number of bends and line direction changes in line
34 will also serve this end.
Multiple system design configurations and arrangements are also possible. Through
the use of the present invention, each individual overall FGD system design may be adapted
for use with the present invention, even uniquely designf~l and e-nginf~ered arrangements,
equipment selections, and configurations. An exarnple of one such variation is illustrated
in Fig. 2.
Rf f~ g to Fig. 2, the system 40 l~ceivt;s dry reagent 12 through line 16, either by
~llfY`~ conveyallce, or by dilute or dense phase pl~f~..l.~ti- conveyance, in order to be
stored in surge bin 14. A rotary airlocl~/Ç~der 42 provides both the airlock and feed rate
control function to supply the dry reagent 12 through chute 44 to oxidation air line 26 at
pick-up point 32. I~e solids and air mix~re is then collv~d di~ly through a straight run
25 of pipe 46 to an injection point or points 48 located within an absorber reaction tank or
vessel 50. n~ f s or mllltiple alla~g~ can be configured in order to ~cr~ase theu~~ y of injection points 48 as l~uh~d by the FGD process. If desired, injection point
or points 48 can ~ e in a ~/i,i~ily of a pln,~ flow discllarge from a m~.l-
~
agitator 52, for lli~ .~;on of both o~iA~tinn air stream 24 and reagent 12 into the absofl~l30 reaction tank or vessel 50. To f~rilitslt~ other possible arrangements, t~he reagent flow
CASE5S80 216S633
control function could be provided remotely, wllel~y the dry reagent 12 is either dilute or
dense phase ~ lly conveyed, from a remote location, directly to the oxidation air
line 26 solids pick-up point 32 at the desired rate. This would allow the feed rate of the dry
reagent addition into the absorber vessel 50 and the feed rate of the oxidation air stream into !i.
5 the absorber vessel 50 to be independently controlled. In either case, the oxidation air line
26 solids pick-up point 32 should be provided at an elevation above a slurry liquid level 56
in absorber reaction tank 50, so that if the air supply means 25 fails, the slurry 54 will not
back-up into the e~ "~ piping 26, 44 located at or above the elevation corresponding to
the liquid level 56. Additionally, a lllillill""" feed rate of the oxidation air stream 24 would
10 usually be supplied whenever the feed rate of the dry reagent 12 is greater than zero, to
prevent solids dropout in the piping 26, 46.
Variations in the dis~ ing means 52 used to disperse the solids-air mixture into the
slurry 54, such as by the use of modified sparge headers, injection lances, jet
mixers/aerators, etc., can also be applied as deemed applicable to the overall process.
The process accoldillg to the present invention can reduce overall plant capital costs
by incorporating portions of the fresh reagent feed functions and the oxidation air addition
filnrfion~ into a single piping system. If a dry grinding system is required on site, it might
draw more power then a colllpalable wet grinding system. However, this could be off-set
by the lack of recycle water piping from a dewatering system to a reagent ~,~al~lion
system, reduced recycle water pump sizes, increased slurry solids den~itilo-s throughout the
plant and by the lo~ ing of hydraulic loadings throughout the plant.
The ~ invention allows increased FGD ~Irol~ce in the absorber reaction
tank 50 due to a higher slurry solids density. The present il~ ioll also eli.~ t~ the need
for fresh slurry feed pumps and associated slurry supply loops.
2S Additionally, the present invention provides for a r~llrtion in the size of the fresh
reagent surge storage vesæl; it also ~ tes the ~otelllial for reagent powder carryover
from the absoll,c. ~ ion tank 50 since the reagent is introduced below the liquid level 56
therein, rather than clæwll~, within the absorber reaction tank 50.
The ill~t;~i~ll can be used to ~rr~ively introduce a wide varie~ of reagents into the
absorber, such as the ~ lk~lin~ reagent, as well as various dry additives such as
- 2165633
CAS~: 5580
oxidation promoters, b~fr~ g agents, etc. Accordingly, while a specific embodiment of the
invention has been shown and d~-il,ed in detail to illustrate the application of the principles
of the invention, it will be understood that the invention may be embodied oth~L ~ise without
departing from such principles.- Such embodiments have been omitted herein for the sake
5 of conciselless and readability but properly fall within the scope of the following claims.