Note: Descriptions are shown in the official language in which they were submitted.
NSC-B857/PCT
- 1 -
DESCRIPTION
Process for Producing Steel by Converter
FIELD OF INVENTION
The present invention relates to a refining process,
using a converter having a bottom-blowing function, in
steel production. The present invention relates, in more
detail, to a converter refining process wherein molten iron
is refined by desiliconization and dephosphorization in the
same converter, intermediate slag discharge is conducted,
and the molten iron is successively refined by
decarbonization, and to the operation conditions of the
dephosphorization refining.
PRIOR ART
Demand for quality of steel materials has become more
strict as the utilization technologies become advanced and
diversified, and the need for the production of a high
purity steel has further increased. In order to meet such
a requirement for the production of such a high purity
steel, molten iron pretreatment installations or secondary
refining installations have been enlarged and arranged in a
steel production process. Since dephosphorization is
particularly efficient in the molten iron stage where the
temperature level is low, precedent dephosphorization is
generally carried out in the molten iron pretreatment step.
In precedent dephosphorization, there are refining vessel
systems such as a torpedo car system, a ladle system and a
two converter system where decarbonization is conducted in
a separate furnace. Any of the systems can be carried out
by charging flux such as Ca0 and iron oxide either through
top addition or injection, and agitating through nitrogen
bubbling or nitrogen bubbling and oxygen top blowing in
combination. For example, Japanese Patent Publication
Kokai No. 58-16007 discloses a Process for Dephosphorizing
and Desulfurizing Molten Iron wherein a Ca0 flux is blown
into a molten iron, together with a carrier gas, while
oxygen is being top blown, the molten iron is subsequently
dephosphorized so that the slag basicity and the iron oxide
- 2 - 2.1~6~9'~
content subsequent to the treatment become at least 2.0 and
up to 15~, respectively, top blowing oxygen is then
stopped, and the molten iron is desulfurized by blowing a
desulfurizing agent without forcibly removing the slag.
Moreover, Japanese Patent Publication Kokai No. 62-109908
discloses a Process for Desiliconizing, Dephosphorizing and
Desulfurizing Moten Iron wherein a dephosphorizing flux
containing Ca0 as its main component is added to a molten
iron surface from the initial stage of pretreating the
molten iron, oxygen or an oxygen source in a solid state is
added to the molten iron surface while iron oxide flux
powder is being blown into the molten iron with a carrier
gas, and the flux is changed to an alkali type flux after
the desiliconization stage to conduct dephosphorization and
desulfurization simultaneously. In addition to the
Japanese Patent Publications mentioned above, Japanese
Patent Publication Kokai No. 63-195209 discloses a Process
for Producing Steel wherein two converters, a top-blowing
converter and a bottom-blowing converter, are used, one is
employed as a dephosphorizing furnace and the other is
employed as a decarbonizing furnace, the converter slag
produced in the decarbonizing furnace is recycled to the
dephosphorizing furnace, and the dephosphorized molten iron
obtained by dephosphorization is charged into the
decarbonizing furnace.
As described above, in order to make the
decarbonization step in a converter efficient and improve
the productivity therein by carrying out the
desiliconization step and the dephosphorization step as a
primary refining process in the molten iron stage, steel
companies have directed, their attention to separate
refining and have conducted studies and realized
installations of this type.
In view of only the capacity of the dephosphorization
step according the process as mentioned above, a relatively
low phosphorus content level can be achieved. However, the
step has the following drawbacks: the treating time is long
and the heat loss at the time of treating is large; it
takes much time to supply the molten iron to a converter;
~I~~~~7
- 3 -
and, even when two converters are utilized, a decrease in
the molten iron temperature is unavoidable due to the
discharge of the molten iron subsequent to the treatment
from a first converter and the recharge thereof into the
other converter. Accordingly, the process is by no means a
satisfactory one in view of a heat margin. Moreover,
dephosphorization of the total amount of the molten iron in
recent years has further lowered the heat margin in the
converter process. As a result, freedom to select the raw
materials to be used is lost, and there will arise a
serious problem, from the standpoint of positively
recycling scrap in converters, in the future.
In contrast to the process as mentioned above, there
is a refining process termed a double slag process wherein
predephosphorization and decarbonization refining are
practiced in one converter, as disclosed in the Collection
of Papers in Commemoration of 10th Anniversary of LD
Committee by Japan BOT Group, LD Committee, 235, (1969).
The process is directed to conduct dephosphorization
refining by soft blowing refining in the first blowing
within a converter, and comprises discharging
dephosphorizing slag in such a manner that the molten iron
does not flow out from the furnace mouth subsequently to
dephosphorization, and then conducting decarbonization
refining continuously. However, there can be found no
techniques in the process which improve the refining
process and the slag dischargeability.
Although the double slag process has a high heat
margin, the cost of the process is high and refractory
materials consumed therein is large as described below: (1)
since refining by soft blowing (the agitation force of the
molten iron within the converter is lowered, and the
material transfer of [C] in the molten iron is made in a
rate-determining state) is intentionally conducted and the
(~ T.Fe) concentration in the slag is maintained at least
at about 15~ to make the slag liable to foam, the iron loss
increases, (2) in order to maintain the flowability of the
slag, the refining temperature is increased so that the
blowing-off temperature during dephosphorization refining
CA 02166097 1999-07-06
- 4 -
becomes at least 1,400°C, and consequently the wear and
melt loss of refractory materials at converter-inclined
portions increase, and (3) since the dephosphorization
efficiency is lowered due to a high blowing-out
temperature, the slag basicity, Ca0/SiOz, is maintained at
least at 3.0, and the flux cost increases: Accordingly,
the technique has not been applicable to practical
operations.
In the process as mentioned above, recycling
decarbonizing slag as a dephosphorizing agent by leaving
the decarbonizing slag having a high Ca0 concentration in
the furnace anal charging a molten iron of the next charge
thereinto is effective in reducing flux costs. However,
the decarboniz;ing slag in the converter generally has a
high oxygen activity. As a result, when a molten iron is
charged into t:he converter while the converter
decarbonizing slag in a molten state is left therein, C in
the molten iron explosively reacts with oxygen in the
converter decarbonizing slag. There may, therefore, arise
a problem that. the converter operation is hindered by
bumping or sl<ig foaming.
DISCLOSURE OF THE IN~fION
The present invention has been achieved under such
circumstances. Although separate refining is directed in
order to desi:liconizing and dephosp orizing a molten iron
in the conventional process, the pr sent invention makes it
possible to combine the pretreatmen steps in a converter
process. An object of the present nvention is to provide
a refining process effective in gre tly improving a heat
margin and greatly reducing steel r fining costs.
The subject matter of the pres nt invention is as
described below.
CA 02166097 1999-07-06
5
(1) A converter refining process for obtaining a
dephosphorized molten iron comprising the steps of
charging a molten iron into a converter having a top- and
bottom-blowing function, dephosphorizing the molten iron
by controlling the amounts of charged flux and charged
coolants so that the Ca0/Si02 ratio in slag becomes at
least 0.7 and up to 2.5 and the molten iron temperature
becomes at least 1,200°C and up to 1,450°C after the
treatment, wherein the flow rate of bottom-blown gas is
being controlled so that an agitation energy ~ of the
formula
0.0285 x Q x 103 x T x {log(1+L°/1.48)}/W
wherein a is the agitation energy per ton of molten iron,
Q is the flow rate of the bottom-blown gas (Nm3/min)
which is measured in a normal state, T is a bath
temperature (K), L° is a bath depth (m), W is the weight
of the molten iron (ton), becomes at leat 0.5 kW/ton,
interrupting refining once, discharging at least 600 of
the slag within the converter by tilting the converter,
making the furnace stand vertically, and conducting
decarbonizat:ion refining.
(2) The converter refining process according to (1),
wherein the process further comprises the step of top
blowing oxygen so that the sum of a T.Fe concentration
being sum of the iron concentrations of Fe0 and Fe203 and
a Mn0 concenl_ration becomes from 10 to 35o by weight in
the slag.
(3) The converter refining process according to (1) or
( 2 ) , wherein oxygen is top blown while a L/L° ratio of
the formula
L/L° = L,, ~10-3~exp (-0.78h/L,,) /L°
wherein L° i:~ a bath depth (m), h is a height of a top-
blowing lance of oxygen, L is a recess depth, L,, is
represented x>y the formula 63.0 x (k~Qo2/n/d)2/3 (wherein
CA 02166097 1999-07-06
6
Qo2 is a flow rake of oxygen (Nm3/h) , n is a number of
nozzles, d is a diameter of each of the nozzles (mm), and
k is a constant determined by the ejecting angled of the
nozzles, is being maintained at 0.1 to 0.3.
(4) The converter refining process according to (1),
(2) or (3), wherein the decarbonizing slag formed during
decarbonization refining is left in the converter, a
molten iron of the next charge is charged under the
conditions that a T.Fe concentration and a Mn0
concentration in the slag and a slag temperature satisfy
the following formula (1):
3.038 x 103 x [(oT.Fe) + (~MnO)JZ
x exp(-91400/(TS + Tm -546)) <_ 0.1 (1)
wherein (%T.Fe) is a weight proportion of iron in the
decarbonizinc~ slag (sum of the iron concentrations of Fe0
and Fe203) , (%Mn0) is a weight proportion (°s) of
manganese oxide in the decarbonizing slag, TS is a
decarbonizing slag temperature (°C), and Tm is a molten
iron tempe>rature (°C) to be charged, and
dephosphoriz;~tion and decarbonization are conducted
again.
BRIEF DESCRIIPTION OF THE DRAWINGS
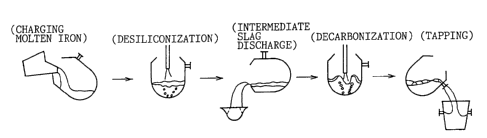
Fig. 1 is a view showing the process flow of the present
invention.
Fig. 2 is a graph showing the relationship between the
bottom-blowing agitation energy and the slag discharge
ratio.
Fig. 3 is a graph showing the relationship between the
bottom-blowing agitation power and an equilibrium
accomplishment degree of dephosphorization.
Fig. 4 is a graph showing the relationship between
burnt lime consumption in dephosphorization refining and
the dephosphorized amount.
Fig. 5 is a graph showing the relationship between the
molten iron temperature subsequent to treatment to obtain a
dephosphorization ratio of 80% and the slag basicity.
Fig. 6 is a graph showing the relationships between
the molten iron temperature subsequent to dephosphorization
refining, the slag basicity and the slag discharge ratio.
Fig. 7 is a graph showing the relationship between the
discharge ratio of dephosphorizing slag and the consumption
of total burnt lime, to obtain the same [%P] in blowing-off
in the decarbonization stage.
Fig. 8 is a graph showing the relationship between the
sum of a T.Fe concentration and the Mn0 concentration in
slag, and a (%P)/[%P] ratio.
Fig. 9 is a graph showing the change with time of the
[P] concentration in a molten iron.
Fig. 10 is a graph showing the relationship between
the feed rate of top-blown oxygen and the primary
dephosphorization rate constant.
Fig. 11 is a graph showing the relationship between
the sum of the iron oxide concentration and the Mn0
concentration in decarbonizing slag and the bumping-
critical decarbonizing slag temperature.
Fig. 12 is a graph showing the relationship between
the sum of the iron oxide concentration and the Mn0
concentration in decarbonizing slag and the bumping-
critical decarbonizing slag temperature.
Fig. 13 is a graph showing the relationship between
the sum of the iron oxide concentration and the Mn0
concentration in decarbonizing slag and the bumping-
critical decarbonizing slag temperature.
_ g _
~~~609~
~" Fig. 14 is a view showing a state for rapidly
discharging dephosphorizing slag.
BEST MODE FOR Cr~RRYING OUT THE INVENTION
The present invention has been achieved by combine the
desiliconization step and the dephosphorization step for
a
molten iron in a converter process. In order to maintain
the capacity of a process for producing a steel having a
low phosphorus content comparable to that of a steel
produced by the current separate refining, rapid and
complete discharge of dephosphorization refining slag
becomes an essential condition. That is, discharging slag
subsequently to the molten iron treating steps causes
problems such as described below: (1) a molten metal flows
out during slag discharge, and as a result the yield
lowers; (2) the productivity lowers due to the increase
in
the discharge time; and (3) ensuring a high slag discharge
ratio is extremely difficult, and a rephosphorization
phenomenon takes place when there remains dephosphorizing
slag containing P205 at a high concentration.
The present inventors have done research and
development to improve the discharge efficiency of slag
after desiliconizing and dephosphorizing a molten iron by
utilizing a converter, combine pretreatment steps of the
molten iron in a converter process, greatly improve a heat
margin, and reduce flux costs.
First, the present inventors conducted experiments
wherein a 300-ton converter having a bottom-blowing
function in a practical installation scale was used, about
290 ton of a molten iron was charged thereinto, burnt lime
for dephosphorization and iron ore were added, top-blown
oxygen was supplied while bottom-blowing agitation was
being conducted to effect desiliconization and
dephosphorization, intermediate slag discharge was
practiced by once interrupting blowing after
dephosphorization and tilting the converter, and
decarbonization blowing was continuously conducted. The
molten iron had contained 0.40% b~' mass ~f Si and G.1G0~
of
the average before the treatment, and a desired temperature
of the molten iron subsequent to dephosphorization had been
- 9 _ ~1~60~7
determined to be 1,350°C on the basis of a conventional
knowledge for the purpose of achieving efficient
dephosphorization reaction. Consequently, the present
inventors have paid attention to the fact that the
agitation force of bottom-blown gas and the slag
composition subsequent to dephosphorization greatly
influence a dephosphorization ratio and a slag discharge
efficiency, and have found that there is an optimum
composition of the slag satisfying both factors.
That is, it can be seen from Fig. 2 that the slag
discharge ratio is influenced by the agitation force of
bottom-blown gas, and that the slag discharge ratio is
sharply improved at an agitation energy of bottom-blown gas
of at least 0.5 kW/ton even when the slag composition is
the same. The slag discharge efficiency is improved
because the bottom-blown gas enhances the slag-foaming
level and slag discharge is conducted at a stage much
earlier than that of intermediate slag discharge.
Furthermore, the present inventors have conducted
various experiments on dephosphorization, and found that
the apparent dephosphorization equilibrium in a molten iron
is expressed by the following formula:
log(%P)/[%P]=2.51og[(%T. Fe)+(%Mn0)]+0.0715[(%Ca0)+
0.25(%Mg0)+7710.2/T-8.55+(105.1/T+0.0723)[%C] (2)
wherein (%P) is a phosphorus concentration in the slag, and
[%P] is a phosphorus concentration in the molten iron.
The relationship between the bottom-blowing agitation
energy and the apparent equilibrium accomplishment degree
was investigated using the formula (2).
Concretely, dephosphorization experiments were
conducted using an 8-ton test converter. About 6 tons of a
molten iron which had an initial temperature of 1,180 to
1,300°C and contained from 4 to 4.8% of C, from 0.1 to
0.15% of P and about 0.3% of Si was refined for 8 to 10
minutes. The molten iron was refined, with a predetermined
amount of Ca0 charged as a flux, under the following
conditions: a top-blown oxygen feed rate of 1.1 to 3.6
Nm3/min/ton, and a bottom-blown N2 gas feed rate of 3 to 350
Nm3/h (0.03 to 3.7 kW/ton). The Ca0/Si02 ratio in the slag
- 10 - ~166~~'~
was from 0.6 to 2.5, and the molten iron temperature was
from 1,250 to 1,400°C after the treatment.
Fig. 3 shows the relationship between the bottom-
blowing agitation power and an equilibrium accomplishment
degree (ratio of a record (P)/[P] ratio to a (P)/[P] ratio
obtained from the formula (2)).
It has become evident from Fig. 3 that the
dephosphorization reaction substantially proceeds to an
equilibrium when the bottom-blowing agitation energy of at
least 1 kW/ton is ensured. Although the bottom-blowing
agitation power increases with the flow rate of bottom-
blown gas, the gas is blown through the molten iron and
spitting greatly increases when the gas flow rate becomes
excessively large. The upper limit of the agitation energy
is, therefore, determined in accordance with the bath depth
of the molten iron and the diameter of a bottom-blowing
tuyere, and that the blown gas has such an agitation energy
that it is not blown through the molten iron.
An agitation energy is obtained from the following
formula (3):
E = 0.0285 x Q x 103 x T x log(1+Lo/1.48)/W (3)
wherein ~ is the agitation energy (Watt/T-S), Q is the flow
rate of bottom-blown gas (Nm3/min), T is the bath
temperature (K), Lo is the bath depth (m), and W is the
weight of the molten iron (ton)
(reference: Agitation Strength and Metallurgical Reaction
in a Composite Converter (1980), a document submitted to
Japan Society for the Promotion of Science, Steel Making,
No. 19 Committee, 3rd Section, Steel Making Reaction
Conference).
Fig. 4 shows the relationship between burnt lime
consumption and a dephosphorization amount in
dephosphorization refining when a bottom-blowing agitation
power of at least 1.0 kW/ton is practically applied. The
relationship therebetween, in the conventional process
wherein a torpedo car and a molten-iron ladle are used, is
also shown for comparison. It is seen from Fig. 4 that the
burnt lime consumption can be decreased by about 15 kg/ton
compared with the conventional process.
- 11
Next, the present inventors variously investigated the
relationship (for achieving a dephosphorization ratio of
80~) between a molten steel-treating temperature and a
Ca0/Si02 ratio in slag subsequent to treatment while the
flow rate of bottom-blown gas was adjusted so that the
agitation energy became at least 0.5 kW/ton. The results
thus obtained are shown in Fig. 5. The present inventors
carried out an intermediate slag discharge test by changing
the temperature and the Ca0/Si02 ratio in slag subsequent
to the treatment, and investigated variously the
relationship between the Ca0/Si02 ratio and the slag
discharge ratio. The results thus obtained are shown in
Fia. 6.
Furthermore, the following converter operation was
repeated using the same converter: a molten iron was
dephosphorization refined; slag was discharged by tilting
the converter; the converter was then made to stand
vertically, and the molten iron was decarbonization
refined; the steel thus obtained was tapped from the tap
hole of the converter; and a molten iron was charged into
the converter again while the decarbonizing slag was left
therein. The relationship between a slag discharge ratio
and an amount of Ca0 (sum of an amount of Ca0 used in the
dephosphorization stage and an amount thereof used in the
decarbonization stage) necessary for refining 1 ton of a
molten iron was investigated. The results thus obtained
are shown in Fig. 7.
It is evident from Fig. 7 that discharging slag as
much as possible subsequent to dephosphorization is
necessary for preventing rephosphorization, caused by low
burnt lime consumption, and improving the yield of Mn ore
in the decarbonization stage, and that although bringing a
slag discharge ratio close to 100 as much as possible is
effective in improving the yield of Mn ore, the decreasing
ratio of the burnt lime consumption becomes small at a slag
discharge ratio of at least 60~ when viewed from the
standpoint of decreasing dephosphorizing flux, and that the
slag discharge ratio of at least 60~ is, therefore, the
minimum necessary one. It is seen from Fig. 7 that when
~1~6a9'~
- 12 -
the slag discharge ratio is at least 60~, the total amount
of the burnt lime used in the dephosphorization stage-and
in the decarbonization stage may be made to amount to up to
kg/ton by recycling the decarbonizing slag. On the
5 other hand, when the decarbonizing slag is not recycled,
the sum of a consumption unit in the dephosphorization
stage and in the decarbonization stage is about 15 kg/ton.
Accordingly, recycling the decarbonizing slag may reduce a
burnt lime consumption by about at least 5 kg/ton.
10 Furthermore, it is evident from Fig. 6 that when the
temperature subsequent to the treating is less than
1,200°C, the slag discharge ratio does not reach 60°s at any
Ca0/SiOz ratio subsequent to the treatment, and that when
the temperature subsequent to the treatment exceeds
1,450°C, the slag discharge ratio also does not reach 60~
at a Ca0/Si02 ratio of at least the necessary one obtained
from Fig. 5. Accordingly, in order to obtain a high
dephosphorization efficiency and a high slag discharge
efficiency, dephosphorization is required to be carried out
so that the molten iron temperature subsequent to the
treatment becomes at least 1,200°C and up to 1,450°C and
the Ca0/Si02 ratio in the slag subsequent thereto becomes
at least 0.7 and up to 2.5.
The Ca0/Si02 ratio in the slag subsequent to the
treatment herein can be freely controlled by the amount of
flux charged during dephosphorization refining, and the
molten steel temperature subsequent to the treatment can
also be freely controlled by coolants (scrap and iron ore)
charged during dephosphorization refining.
That is, the desired slag discharge ratio of 60~ as
well as the desired dephosphorization amount can be
sufficiently achieved at a Ca0/Si02 ratio in the slag
subsequent to the treatment of 0.7 to 2.5 in accordance
with the molten iron temperature subsequent to the
treatment which is from 1,200 to 1,450°C, under the
condition of a bottom-blowing agitation power of at least
0.5 kW/ton.
Furthermore, Fig. 8 shows the relationship between the
sum of a T.Fe concentration and a Mn0 concentration and a
CA 02166097 1999-07-06
- 13 -
(%P)/[%P] ratio at a molten iron temperature of 1,350°C
subsequent to the treatment, with the Ca0/Si02 ratio in the
slag subsequent to the treatment being 1.0, 1.5 or 2Ø It
is seen from Fig. 8 that in any of the Ca0/Si02 ratios,
when the T.Fe becomes less than 10%, the (%P)/(%P] ratio
falls sharply, and that the (%P)/[%P] ratio does not
increase or rather falls when the T.Fe exceeds 35% ((%P)
herein designates the concentration of P in the slag, and
[%P] designates the concentration of P in the molten iron).
The phenomena take place for the reasons described
below. When th.e sum of a T.Fe concentration and a Mn0
concentration in the slag becomes less than 10%, the
(%P)/[%P] ratio falls sharply due to an insufficient oxygen
potential. When the sum exceeds 35%, the (%P)/[%P] ratio
also falls due to the dilution of a basic component
concentration in the slag.
Accordingly, in order to obtain a high (%P)/[%P] ratio
while the iron yield is being maintained, the sum of the
T.Fe concentration and the Mn0 concentration subsequent to
the treatment is desirably maintained at least at 10% and
up to 35% as a better control parameter by operating the
converter while: adjusting a top-blown oxygen feed rate, a
bottom-blown ga.s flow rate or the height of a top-blowing
lance.
As a method for controlling the T.Fe subsequent to
the treatment b~y adjusting the feed conditions of top-
blown oxygen, there is an operation method wherein the
L/Lo ratio ((depth of the recess of the molten
steel)/bath depth)) is utilized as an index.
The L/Lo ratio herein is represented by the
following formula:
:L/Lo = Lh exp (-0 . 78h/Lh) /Lo
wherein Lo is a bath depth (m), h is the height of a top-
blowing lance i:or oxygen, L is the depth of a molten
steel recess and is represented by the formula
L,, exp (-0.78h/Lh) /Lo, and Lh is represented by the formula
63.0 x (k/Qo2/n.d) z~3 (wherein ~ is the flow rate of
oxygen (Nm3/h), n is a number of nozzles, d is the
diameter of each of the nozzles (mm), and K is a constant
determined by the ejecting angle of the nozzles).
~1~~~9'~
- 14 -
Basically, when the L/Lo ratio is made smaller, the
(~FeO) concentration in the slag increases, and
dephosphorization becomes advantageous. Concretely, in
order to lower the L/Lo ratio, the lance height is required
to be elevated. As the lance is elevated, the secondary
combustion ratio within the furnace is increased, and the
recovery amount of LDG is lowered or heat damage to the
bricks in the inclined portions of the converter increases.
Accordingly, the increase in the lance height is
restricted. Moreover, when the L/Lo ratio becomes smaller,
slag foaming increases, and slopping which hinders the
converter operation during blowing becomes more likely to
take place. In view of what has been mentioned above, the
minimum L/Lo ratio is restricted to at least 0.1. Moreover,
as the L/Lo ratio increases, the (~T.Fe) in the slag is
decreased, and the dephosphorization capacity is lowered.
Accordingly, in order to ensure (the sum of the T.Fe
concentration and the Mn0 concentration) of at least 10~ in
the slag during dephosphorization refining so that
efficient dephosphorization refining can be practiced, the
L/Lo ratio is required to be restricted to up to 0.3. The
following advantages can be obtained when the L/Lo ratio is
controlled to satisfy the conditions 0.1<_L/Lo<_0.3:
excessive slopping can be controlled during
dephosphorization refining; and the [~P] in the molten iron
can be stably controlled to be up to 0.030 while an
extraordinary increase in the secondary combustion ratio of
the exhaust gas is suppressed.
On the other hand, when the converter is operated
while the bottom-blowing agitation energy, the Ca0/Si02
ratio in slag subsequent to the treatment and the molten
steel temperature subsequent thereto are adjusted in the
ranges mentioned above, the dephosphorization time can be
decreased with an increase in an oxygen feed rate.
Fig. 9 shows a change of the [P] concentration in the
molten iron with time at different oxygen-blowing rates
under the condition that the slag composition and the slag
temperature subsequent to the treatment are each
approximately constant. When oxygen is fed at a rate of at
_ 15 _ ~1G~~~'~
least 2.5 Nm3/min/ton, the treating time can be decreased
by about 4 minutes compared with the operation wherein
oxygen is fed at a rate of 1.1 Nm3/min/ton.
Fig. 10 shows the relationship between an oxygen feed
rate and a primary dephosphorization rate constant (Kp').
Fig. 10 also shows the relationship in conventional
processes (1), (2) and (3) in actual installations. Even
when the Ca0/Si02 ratio is lowered to 0.6 to 1.1 subsequent
to the refining to decrease burnt lime consumption, a
dephosphorization rate constant equivalent to that of the
conventional process (1) using a torpedo car or that of the
conventional process (2) using a ladle can be obtained by
increasing the oxygen feed rate. 4~lhen the Ca0/Si02 ratio
is at least 1.1 and up to 2.5, it is confirmed that a
dephosphorization rate constant about twice as much as that
of the conventional process (3) using the same converter
can be obtained.
When proper dephosphorization satisfying conditions,
such as the bottom-blowing agitation energy, the Ca0/Si02
ratio in slag subsequent to the treatment and the molten
steel temperature subsequent thereto, are present, rapid
and complete discharge of the dephosphorization refining
slag becomes possible, and the steps of desiliconization,
dephosphorization and decarbonization can thus be combined
in the converter.
That is, after proper dephosphorization, the converter
is tilted, and the slag is discharged. As to steps
subsequent to the slag discharge, the converter is
immediately made to stand vertically, and flux such as
burnt lime and light burned dolomite in the necessary and
lowest amounts in accordance with a slag discharge ratio, a
state of the melt loss of the furnace, a desired [P]
concentration, etc. is charged in addition, followed by
decarbonizing the molten iron by blowing oxygen until the
molten iron has a desired end point [C]. Scrap, iron ore,
Mn ore corresponding to a desired [Mn] concentration, and
the like may optionally be charged.
When the decarbonizing slag is recycled by leaving it
in the converter and charging a molten iron of the next
- 16 -
charge thereinto, the burnt lime consumption may greatly be
cut as shown in Fig. 7. However, in some cases, C in~the
molten iron drastically reacts with oxygen sources in the
converter decarbonizing slag, namely FeO, Fe203 and Mn0
according to the reaction formulas (4), (5) and (6):
Fe0 + C -~ Fe + CO (4)
Fe203 + 3C -~ 2Fe + 3C0 (5)
Mn0 + C -~ Mn + CO ( 6 )
to form a large amount of a CO gas. The CO gas makes the
slag and charged molten iron jump out from the converter
and produces slag foaming so that the slag flows out of the
converter. Thus, the CO gas generation in a large amount
results not only in that the yield of iron is lowered but
also that the operation may be obliged to be interrupted.
The amount of a CO gas produced by the reaction of the
formulas (4) to (6) increases with a FeO, a Fe203 or Mn0
concentration in the slag. Moreover, the rates of these
reactions increase with a temperature of the slag or molten
iron. That is, the reaction becomes more drastic when the
temperature is higher. However, even when the
concentration of FeO, Fe203 or Mn0 in the slag is high, the
reaction rates become slow at a low slag temperature or a
low molten iron temperature. As a result, bumping or slag
foaming may not take place sometimes.
As the result of investigating in detail the effects
of concentrations of FeO, Fe203 and MnO, the slag
temperature and the molten iron temperature on bumping and
slag foaming, the present inventors have discovered that in
order to prevent bumping and slag foaming, the formula (1)
mentioned above must be satisfied. The formula (1)
signifies that when the relationship of T.Fe (sum of the
concentrations of iron in Fe0 and Fe203), a Mn0
concentration, a slag and a molten iron on the left side is
up to 0.1, bumping and slag foaming do not take place.
That is, the slag temperature or molten iron temperature is
selected so that they match the concentrations of FeO, Fe203
and Mn0 in the slag, and as a result the value of the left
side of the formula (1) becomes up to 0.1. When the molten
iron is then charged, bumping and slag foaming may be
~lG~a~'~
- 17 -
prevented. Moreover, on the contrary, bumping and slag
foaming may also be prevented by adjusting the
concentrations of T.Fe and Mn0 in the slag on the basis of
the slag temperature and the molten iron temperature so
that the relationship of the formula (1) is satisfied, and
by charging the molten iron.
In addition, there is a procedure wherein charging a
molten iron is delayed until the decarbonizing slag
temperature becomes the temperature determined by the sum
of the concentrations of iron oxide and manganese oxide in
the decarbonizing slag and a molten iron temperature of the
next charge so that the formula (1) is satisfied. However,
there may also be another procedure wherein a coolant such
as CaC03 or a mixture of the coolant and a deoxidizing
agent such as coke and smokeless coal is added to forcibly
satisfy the formula (1).
For example, when CaC03 is used as the coolant, CaC03
is decomposed into Ca0 and CO2. Since the decomposition
reaction is endothermic, the decarbonizing slag temperature
is lowered, and the conditions of the formula (1) can be
satisfied in a short period of time. Moreover, since Ca0
produced by decomposition acts as a flux in
dephosphorization reaction, flux for dephosphorization in
the dephosphorization stage can be advantageously reduced.
The sum of the concentrations of iron oxide and
manganese oxide in the decarbonizing slag is determined
either by sampling a slag sample and rapidly analyzing it
or by obtaining in advance the relationship between a
carbon concentration in the molten steel and the sum of an
iron oxide concentration and a manganese oxide
concentration in the decarbonizing slag and calculating the
sum from the analytical results of the carbon concentration
in the molten steel of the previous charge after
decarbonization. Moreover, the decarbonizing slag
temperature is measured by a radiation thermometer, etc.
Fig. 1 shows the outline of the entire process.
The present invention has been illustrated above on
the basis of the cases where a molten iron having been
predesulfurized outside a converter is used. When
~1~~~9"~
- 18 -
predesulfurization of high degree is not required, the
molten iron can be desulfurized within a converter before
dephosphorization as described above. That is,
desulfurizing flux which is one or at least two substances
selected from CaO, Na2C03 and Mg is charged by top charging
or bottom-blowing injection, and then desulfurization is
conducted in a short period of time of 2 to 5 minutes.
Dephosphorization as mentioned above is subsequently
conducted. Since from 40 to 60~ of S in the slag is then
vaporized and desulfurized, desulfurization of from 30 to
50% of [S] in the molten iron at the initial stage in
combination with dephosphorization becomes possible by
adjusting the flux amount.
In addition, when slag is discharged by tilting the
converter, the converter is desirably turned in a short
period of time such as within 1 minute (as short as
possible) while the slag is being prevented from scattering
with a slag-preventive plate in front of the converter as
shown in Fig. 11.
The present invention will be explained in detail on
the basis of examples.
EXAMPLES
Example 1
Into an 8-ton test converter having a bottom-blowing
function was charged about 6 tons of a molten iron which
had been predesulfurized. The molten iron was
dephosphorized for about 8 minutes by controlling the
amounts of charged flux and charged scrap so that the
Ca0/Si02 ratio in the slag became at least 0.7 and up to
2.5 and the molten steel temperature became at least
1,200°C and up to 1,450°C after the treatment, while the
flow rate of bottom-blown gas was controlled so that the
agitation energy became at least 0.5 kW/ton. The furnace
was subsequently tilted, and intermediate slag discharge
was conducted for about 3 minutes. The furnace was made to
stand vertically, and decarbonization was immediately
carried out for about 9 minutes, followed by tapping the
resulting steel.
- 19 -
Table 1 shows concrete conditions, chemical
compositions of molten steels, and temperature changes of
the steels.
The molten iron subsequent to dephosphorization had
[P] of 0.025%, and the resulting molten steel subsequent to
decarbonization had [P] of 0.019. The total amount of
burnt lime added in both the predesulfurization stage and
dephosphorization and decarbonization stage in the
converter was about 20 kg/ton. The consumption could thus
be significantly cut compared with the average total burnt
lime consumption of 34 kg/ton in a conventional process
(desulfurization and dephosphorization of the molten iron +
decarbonization in the converter) for obtaining refining
effects equivalent to those in the present invention.
The results could be obtained due to the application
of dephosphorization operation conditions of the present
invention which were consistent with a high slag discharge
ratio and a high dephosphorization efficiency.
Table 1
~ Principal Conditions of Practice
Amount of charged molten iron 6180 kg
Dephosphorization Stage Decarbonization Stage
Flow rate of 1000 Nm3/h Flow rate of 1500 Nm3/h
top-blown 02 top-blown 02
Flow rate of 350 Nm3/h Flow rate of 02 200 Nm3/h
3 0 bottom-blown bottom-blown gas Ar 125 Nm3/h
N2
LPG 20 Nm3/h
Amount of 1200 kg
charged scrap Amount of 50 kg
charged burnt lime
3 5 Amount of 70 kg
charged burnt lime Treating time 8.9 min
Treating time 7.8 min
21G~0~'~
- 20 -
~ Chemical Composition of Metal, Temperature Change
[~C) ($Si) [$Mn) [~Pl [~Sl Temp.
(°C)
Before treatment 4.52 0.31 0.30 0.104 0.010 1350
After dephosphorization 3.62 0.01 0.09 0.025 0.010 1352
1 0 After decarbonization 0.037 <0.01 0.05 0.019 0.010 1648
Example 2
Into an 8-ton test converter having a bottom-blowing
function was charged about 6 tons of a molten iron which
had been predesulfurized. The molten iron was
dephosphorized for about 8 minutes by controlling the
amounts of charged flux and charged scrap so that the
Ca0/Si02 ratio in the slag became at least 0.7 and up to
2.5 and the molten steel temperature became at least
1,200°C and up to 1,450°C after the treatment, while the
flow rate of bottom-blown gas was being controlled so that
the agitation energy became at least 0.5 kW/ton. The
converter was subsequently tilted, and intermediate slag
discharge was conducted in about 3 minutes. The converter
was made to stand vertically, and decarbonization was
immediately carried out for about 9 minutes, followed by
tapping the resulting steel. Four charges of the molten
iron were subjected to the refining operation while amounts
of scrap charged were changed.
Table 2 shows conditions such as the chemical
composition, the temperature, etc. of each of the charges.
It can be seen from the results that scrap in a large
amount of about 17~ could be charged according to the
process of the present invention having a high heat margin,
whereas scrap only in an amount of about 7~ could be
charged in the conventional process where dephosphorization
and decarbonization were conducted in a torpedo car and in
a converter, respectively.
Furthermore, it can also be seen from the results that
when [Si] in the molten iron is increased, the molten iron
may be dephosphorized at a lower basicity due to an
increase in the amount of slag formed in the
dephosphorization stage, and that as a result the burnt
2166~~7
- 21 -
lime consumption
unit does not increase
much. Even when
[Si] in the molten iron is increased,
the operation
is
stabilized without drastic sloppingdue to an operation
with a low basicity
and at low temperatures.
The operation
may be conducted of 25~ using a molten
with a scrap ratio
iron having an [Si] content of 1~.
Table 2
Molten Iron before Treatment
1 Weight [$Cj [$Sij [$Mn] [~P][$S] Temp.
0
Charge No. (kg) (C)
1 6050 4.52 0.31 0.30 0.104 0.020 1350
1 2 5990 4.52 0.52 0.29 0.099 0.020 1352
5
3 6020 4.45 0.65 0.29 0.101 0.020 1345
4 6010 4.53 0.95 0.31 0.102 0.020 1348
20
~ Chemical Composition of Metal, Temperature Change,
Burnt
Lime
Consumption
Unit
2 Charge AfterAfter Decarbonization Burnt lime
5
No. dephosphorization consumption
Temp.[~Cl I$Pl Temp. [~Cj[$P]
(C) (C) (kg/ton)
30
1 1345 3.52 0.018 1648 0.0340.021 19.7
2 1353 3.43 0.019 1640 0.0420.019 24.8
3 3 1352 3.55 0.020 1652 0.0370.019 27.3
5
4 1352 3.51 0.020 1650 0.0380.019 31.3
40 ~ Amount of Molten Scrap
Charge No. Amount of molten scrap(kg) Scrap ratio ()
1 1220 16.8
2 1360 18.5
3 1525 20.2
4 1970 24.7
Prior art - about 7$
([Sij in molten
iron 0.3$)
Example 3
- 22 -
Into an 8-ton test converter having a bottom-blowing
function was charged about 6 tons of a molten iron which
had not been desulfurized, and the molten iron was
desulfurized by adding a desulfurizing agent thereto. The
molten iron was dephosphorized for about 8 minutes by
controlling the amounts of charged flux and charged scrap
so that the Ca0/Si02 ratio in the slag became at least 0.7
and up to 2.5 and the molten steel temperature became at
least 1,200°C and up to 1,450°C after the treatment, while
the flow rate of bottom-blown gas was controlled so that
the agitation energy became at least 0.5 kW/ton. The
converter was subsequently tilted, and intermediate slag
discharge was conducted for about 3 minutes. The converter
was made to stand vertically, and decarbonization was
immediately carried out for about 9 minutes, followed by
tapping the resulting steel..
Table 3 shows concrete conditions, chemical
compositions of molten steels, and temperature changes of
the steels.
[S] of 0.030 in the molten iron at the initial stage
became 0.010 after desulfurization, 0.015 after
dephosphorization and 0.014 after decarbonization. It
was, therefore, found that the molten iron could be
sufficiently desulfurized to the level of an ordinary
steel.
Table 3
~ Auxiliary Raw Material and Treating Time
Desulfurization Dephosphorization Intermediate Decarbonization
slag discharge
Consumption Desulfurizing agent burnt lime - burnt lime
unit of auxi- 4.9 kg/ton* 10.1 kg/ton 7.3 kg/ton
liary raw
3 5 material
Treating time 3.2 min 8.0 min 3.1 min 8.8 min
4 0 Note: * desulfurizing agent: 50~Ca0+308Na2C03+20$Mg
~1~~~~~
- 23 -
~ Chemical Composition of Metal, Temperature Change
[~Cl [$Si1[$Mnl[~P1 [$Sl Temp.
(C)
Before treatment 4.46 0.31 0.31 0.101 0.0301350
After desulfurization 4.41 0.30 0.30 0.090 0.0101335
1 0 After dephosphorization3.49 0.01 0.09 0.021 0.0151351
After decarbonization 0.037<0.010.05 0.019 0.0141648
Examt~le 4
Table 4 shows each of the examples wherein a molten
iron was charged into a 300-ton top- and bottom-blowing
converter equipped with a bottom-blowing tuyere at the
bottom in an amount of 290 to 300 ton, C02 and 02 were blown
thereinto from the bottom-blowing tuyere and the top-
blowing lance, respectively.
Comparative Examples 1 to 3 are instances wherein the
slag basicity subsequent to dephosphorization was at least
2.0, or a molten iron was refined with a decreased
agitation force. Examples 4 to 7 were carried out
according to the present invention. The basicity of a
molten iron could be easily adjusted by charging burnt lime
in an amount in accordance with an amount of Si02 to be
formed from Si in the molten iron before the treatment, and
an amount of Si02 remaining in the slag in the furnace,
etc.
It is seen from the results of the examples that the
intermediate slag discharge ratio subsequent to
dephosphorization can be greatly improved by applying the
present invention compared with conventional processes,
that rephosphorization can be inhibited in the
decarbonization step continuously carried out after slag
discharge, and that carrying out desiliconization,
dephosphorization and decarbonization refining in one
furnace may be satisfactorily carried out.
~1~~Q~9~
- 24 -
Table 4
Test No. Amount Chemical compsn.+ of molten iron($) Dephos# Dephos#
of molten time ratio
iron C Si Mn P S Temp.
(ton) (°C) (min) ($)
Comp.Ex.l 289.8 before T* 4.37 0.39 0.21 0.094 0.030 1249 8 83.0
after D# 3.66 0.03 0.08 0.016 0.029 1342
Comp.Ex.2 294.7 before T* 4.20 0.36 0.12 0.105 0.015 1241 7 85.7
after D# 3.71 0.02 0.03 0.015 0.014 1348
1 afterD# 3.770.02 0.050.0180.0121350
5
Ex.4 304.3 beforeT* 4.430.42 0.170.0970.0121236 6 84.5
afterD# 3.680.02 0.020.0150.0131341
2 Ex.S 307.6 beforeT* 4.330.37 0.230.0960.0141252 7 86.5
0
afterD# 3.660.01 0.040.0130.0141360
Ex.6 291.5 beforeT* 4.390.28 0.160.0940.0171298 9 80.9
afterD# 3.750.01 0.060.0180.0151390
25
Ex.7 298.9 beforeT* 4.420.34 0.260.1130.0241306 8 86.7
afterD# 3.730.02 0.040.0150.0221371
3 Note:
0 compsn.+
= composition
T* = Treatment
Dephos# = D# = Dephosphorization
Table (continued)
4
35
Test No. Slag after DephosphorizationFlow Bath Energy of
rate depth
of bottom- bottom-blowing
Basicity Iron oxide blown agitation
(Mn0) gas
($) ($) Nm3/min (m) (kW/ton)
40
Comp.Ex.l2.34 12.7 1.50 12.0 2.1 0.73
Comp.Ex.23.65 12.1 0.95 - 2.2 0
4 Comp.Ex.31.72 16.8 1.52 6.0 2.2 0.37
5
Ex. 4 1.68 13.4 1.30 11.0 2.3 0.71
Ex. 5 1.82 14.1 1.70 10.7 2.3 0.69
50
Ex. 6 1.75 10.5 1.31 19.5 2.2 1.26
Ex. 7 1.56 7.2 3.50 22.0 2.2 1.41
2166097
Table 4 (further continued)
Test No. Time Amount of molten metal flowingAmount of Slag
for out slag
dischargi ng during slag discharge formed discharge
5 slag ratio
(min) (ton) (ton) (~)
Comp.Ex.l 5.5 0.7 12.0 41.0
1 0 Comp.Ex.2 1.1 14.3 26.4
3.5
Comp.Ex.3 4.0 0.6 15.6 58.2
Ex. 4 5.0 0.3 14.0 86.0
Ex. 5 3.2 0.3 15.6 93.2
Ex. 6 4.3 0.1 13.7 89.4
2 0 Ex. 7 4.5 0.2 11.3 80.4
Example 5
Using a 300-ton converter, decarbonizing slag formed
in the preceding decarbonization step was left therein
without discharging, and a molten iron of the next charge
was charged thereinto. The converter was then operated by
reutilizing the slag as flux for dephosphorization.
When the decarbonizing slag left in the furnace came
to have a temperature defined by the molten iron
temperature and the (%T.Fe+Mn0) concentration of the
decarbonizing slag so that conditions of the formula (1)
were satisfied, the molten iron in an amount of 300 ton
having a temperature of 1) 1,290 to 1,310°C, 2) 1,340 to
1,360°C or 3) 1,390 to 1,410°C was charged thereinto.
In addition, the chemical composition of the molten
iron was as follows: a [C] concentration of 4.5 to 4.8%, a
[Si] concentration of 0.39 to 0.41%, and a [P]
concentration of 0.099 to 0.103%. The amount of the
decarbonizing slag which had been left in the converter was
about 30 kg/ton. Moreover, even a molten iron which did
not satisfy conditions of the formula (1) was also charged
for comparison. Whether bumping or rapid foaming took
place or not after the charging is shown in Fig. 11 to Fig.
13 at respective molten iron temperatures.
Each of the slant line portions in Fig. 11 to Fig. 13
is a region where the conditions of the formula (1) are
satisfied. The mark o designates the case where bumping
2~sss97
- 26 -
and slag foaming did not take place when the molten iron
was charged. The mark x designates the case where bumping
and slag foaming took place when the molten iron was
charged. When a molten iron was charged without satisfying
the conditions of the formula (1), bumping and slag foaming
took place without fail. On the other hand, when a molten
iron was charged while the conditions of the formula (1)
were satisfied, neither bumping nor slag foaming took
place, and the operation was not hindered.
Furthermore, there was practiced a comparative test
wherein decarbonizing slag was discharged once from the
converter, and the slag was crushed and used as
dephosphorizing flux for a molten iron. However, in the
present invention, the scrap ratio increased by 5~ on the
average and the heat margin was increased compared with the
comparative test.
Dephosphorization was subsequently practiced, and the
results were as follows: the reutilized decarbonizing slag
acted as dephosphorizing flux; the Ca0 component in the
decarbonizing slag was effectively used for
dephosphorization; and the consumption unit of Ca0 to be
charged in the dephosphorization stage could be reduced
compared with the case where the decarbonizing slag was not
reused.
INDUSTRIAL APPLICABILITY
It is evident from the examples as mentioned above
that the present invention has the effects described below.
(1) The conventional dephosphorization step or
conventional desulfurization and dephosphorization steps
outside a converter can be done in the converter, and the
fixed cost may be cut greatly.
(2) The variable cost may also be cut by cutting the
flux consumption unit.
(3) Since the heat margin is improved by doing the
steps in the converter, the practice of the present
invention has optional operation advantages such as
described below: 1) an improvement of the capacity of
melting scrap, 2) an improvement of the yield of molten
steel due to an increase in the reduction amount of iron
2166~~'~
- 27 -
ore, and 3) a decrease in the flux cost by substituting
limestone for burnt lime.
(4) The total amount of slag discharged from the
converter refining steps can be decreased to 2/3 of the
amount in the conventional refining steps due to a decrease
in the consumption unit of flux used.