Note: Descriptions are shown in the official language in which they were submitted.
CA 02166696 2004-06-28
Production of Paper from a Suspension with High Electrolyte
Content using Non-ionic or Anionic Retention Aids
This invention relates to the production of paper
(including paper-board) by processes in which a cellulosic
suspension is drained through a screen to form a sheet,
which is then dried.
It is well known to add high molecular weight
polymeric retention aid to the cellulosic suspension during
the paper-making process. Usually the retention aid is
added after the last point of high shear, generally
immediately prior to drainage. It is also known to include
particulate inorganic material such as bentonite, for
instance it may be added to thick stock to reduce pitch
problems.
Thera have been a few instances where it has been
proposed to use a substantially non-ionic retention aid,
but more usually the retention aid is ionic, most usually
cationic.
US 3,052,595 discloses a method of making paper
comprising the addition of filler, bentonite and non-ionic
acrylamide to the cellulosic suspension. It is disclosed
that the polymer can be added to the suspension either
before or after the addition of fillers, but the preferred
process involves adding bentonite to a cellulosic
suspension containing filler, and then adding the polymer.
The disclosure is concerned with conventional suspensions
and the production of filled, good quality, paper, and the
discovery that the inclusion of bentonite with the filler
enhances the activity of a non-ionic polymeric retention
aid.
34 US 4,305,781 discloses a method of making paper from
a pulp having a high cationic demand by adding bentonite to
the stock followed by a substantially non-ionic
polyacrylamide as a retention aid. Hentonfte is added so
as to modify the suspension to render it amenable to
treatment by the substantially non-ionic retention aid.
In US 4,7<9,44~, a low molecular weight cationic polymer is
added after the bentonite and before the non-ionic
PCT/GB94/0145
WO 95/02088 . -
2
retention aid, so as to modify the formation properties of
the paper.
In US 4,643,801, cationic starch is mixed into the
suspension and thereafter an electro-neutralising amount of
anionic polymer and dispersed silica are added, generally
as a mixture, but it is also mentioned that the anionic '
polymer may be added followed by the dispersed silica.
In US 4,795,531 low molecular weight cationic polymer
is added to the cellulosic suspension to neutralise the
charge in the suspension, and subsequently high molecular
weight polymer and colloidal silica are added in either
order. The high molecular weight polymer can be anionic
or cationic.
Despite some usage of non-ionic or anionic retention
aids, as indicated above, it is more common to use cationic
retention aids. The amount of cationic retention aid that
is required generally increases with increasing anionic
charge in the suspension.
The cationic polymer that is to be used as a retention
aid is normally added after the last point of high shear,
but in US 4,753,710 and US 4,913,775 we describe processes
in which a cationic polymer is added, the suspension is
then subjected to shearing, and bentonite is then added
prior to drainage. It is explained that microflocs are
formed by the shearing and that the amount of cationic
polymer should be sufficient to render parts at least of
the surfaces of the microflocs sufficiently cationically
charged, but it is acknowledged that the Zeta potential of
the stock prior to addition of the bentonite can be either
cationic or anionic. It is stated to be essential to use
a cationic polymer rather than a non-ionic or anionic
polymer. It is stated that the flocs carry sufficient
cationic charge to interact with the bentonite.
These processes have been commercialised very
successfully under the trade name "Hydrocol" and they are
effective for a wide range of cellulosic suspensions. It
is explained in US 4,753,710 that the retention aid should
s
95/02088 . .. PCT/GB94/01457
3
be cationic and is a fact that other retention aids are
generally unsatisfactory in that process.
It is alleged in U.S. 5,234,548 (not published until
' after the priority date of this application) that good
results are obtained when the retention aid is an anionic
" or nonionic polymer but the only detailed proposal for when
this might apply is when the suspension is initially dosed
with a cationic donor such as alum or a low molecular
weight cationic polymer.
The reality is that conventional suspensions can
advantageously be dosed with low molecular weight cationic
polymer and still be suitably treated with cationic high
molecular polymer in the process of US 4,753,710. However
the use of cationic retention aid followed by bentonite,
as
in the Hydrocol process, does prove less satisfactory with
some suspensions, and in particular with those have a
substantial amount of electrolyte in them, which may arise
from the presence of anionic trash, recycling or added
materials. Thus, for instance, the processes have been
less successful for the treatment of mechanical pulps such
as groundwood and thermo-mechanical pulps; dirty pulps such
as crude pulps traditionally used for newsprint
manufacture; and recycled pulps such as de-inked waste;
and for the treatment of suspensions in closed mills
wherein the whitewater is repeatedly recycled with the
introduction of only low amounts of fresh water into the
process. The anionic trash arises from impurities in the
mechanical pulps. The high electrolyte content may
alternatively arise from, for instance, the use of filler
that is liable to render the white water alkaline due to
partial dissolution of the filler, for instance calcium
' sulphate or calcium carbonate.
Suspensions which contain high electrolyte levels are
generally anionic and conventional thinking would suggest
that increased amounts of cationic polymer should be added
to reduce or eliminate the anionic nature of the
suspension.
S
t w
WO 95/02088 . ~ PCT/GB94/0145
4
Processes involving the application of cationic starch
and colloidal silicic acid or other modified silicas are
described in U.S. 4,388,150 and have been commercialised
under the trade name "Composil". In general, these '
processes are applicable to a narrower range of suspensions
than the "Hydrocol" process.
It would be desirable to be able to devise a
dewatering process for the manufacture of paper that can
have, in particular, good dewatering performance
(retention, drainage and/or drying) and formation
properties as good as the "Hydrocol" process, using a pulp
having a high electrolyte content rather than a
conventional pulp that typically works with the "Hydrocol"
process using cationic retention aid. In particular, it
would be desirable to be able to obtain benefits similar to
those of the "Hydrocol" process in a cost-effective manner
when treating a cellulosic suspension that contains
significant amounts of anionic trash.
According to the present invention a process for
making paper (including paper-board) is provided, the
process comprising the steps of
forming an aqueous cellulosic suspension,
adding to the suspension a polymeric retention aid
having an IV of at least 6d1/g to form flocs,
shearing the suspension to break down the flocs to
form microflocs,
aggregating the microflocs by adding to the suspension
-an anionic particulate material, and
draining the suspension to form a sheet and white
water which drains through the screen and
drying the sheet, wherein
the polymeric retention aid is a substantially non
ionic or anionic polymer formed of non-ionic monomer units
and less than 2 mole percent cationic units or less than 30
mole (preferably 10 mole) percent anionic units, and
the suspension to which the retention aid is added is
a suspension that contains a high electrolyte content. The
2~.~~69~
WO 95/02088 . PCT/GB94/01457
high electrolyte content is manifested by high
conductivity. The polymers are formed from ethylenically
unsaturated monomers. Anionic monomer provided anionic
units, cationic monomer provides cationic units and
5 nonionic monomer provides nonionic units.
' In other processes of the invention the same process
is applied but the suspension, instead of containing high
electrolyte, has been treated with an excess of cationic
starch or low molecular weight cationic polymer, so that
it
has a near zero or positive zeta potential before addition
of the retention aid.
In the invention we surprisingly find that good
results can be obtained using a substantially nonionic or
anionic polymeric retention aid when the suspension, at
the
time of the addition of that retention aid, has a high
amount of electrolyte. If the overall process of the
invention involves adding, for instance, a cationic polymer
before the defined nonionic or anionic polymeric retention
aid, then the suitability of the nonionic or anionic
polymeric retention aid will be dependent upon the
properties of the suspension after the addition of the
cationic polymer and so the polymer must be selected having
regard to the properties of the suspension containing that
polymer.
The amount of electrolyte and the other properties of
the suspension are generally such that, after treatment
with the said retention aid at a dose of 400 grams per
tonne dry weight, the suspension gives a Schopper Riegler
drainage time that is shorter than the drainage time
obtained when the same suspension is treated with the same
dosage of each of cationic and anionic test retention aids
of substantially the same IV as the substantially non-ionic
retention aid, wherein the cationic test retention aid
contains 5 mole percent cationic units and the anionic test
retention aid contains up to 25 mole percent (usually 15
mole percent) anionic units.
PCT/GB94/01457
WO 95/02088
6
The invention includes processes in which the choice
of polymeric retention aid is made, at least in part, on
the basis of conducting a test as described.
The amounts of retention aid and particulate material
must of course be such that useful results are obtained.
For instance processes that use so little bentonite (or '
other anionic particulate material) that poor retention is
obtained are unsatisfactory. The amount of bentonite
should usually be about (e.g., within 25% or 50%) of the
l0 amount that gives optimum retention. -
The Schopper Riegler drain test which, if desired, can
be used in the invention is conducted by mixing the chosen
amount of the dissolved polymer dissolved in water with
500m1 of the cellulose suspension in a measuring cylinder
filled with the suspension, inverting the cylinder four
times to cause flocculation, transferring the flocculated
suspension to a Schopper Riegler beating and freeness
tester modified by blockage of its back drain, and
measuring the time for collecting 230m1 of drain liquor,
and expressing this time as a percentage of the drainage
time in the absence of the polymer addition.
The cationic test retention aids that are used are
copolymers of acrylamide and dimethylaminoethyl quaternary
salt while the anionic test retention aids are copolymers
of acrylamide and sodium acrylate.
The Schopper Riegler drainage test is conducted on the
suspension to which the substantially non-ionic retention
aid or the anionic retention aid is to be added or on a
suspension substantially the same as that suspension.
Accordingly the retention aid may be selected on the basis
of tests conducted on the actual suspension or on the basis
of tests conducted on a sample suspension made up in the
laboratory from ingredients that will simulate the actual
suspension, for instance after,prolonged recycling. If the
properties of the suspension change during prolonged use,
fresh tests may be required to select the polymer that is
then necessary. If a chemical pre-treatment of the
~WO 95/02088 PCT/GB94/01457
7
suspension is to be made (for instance the addition of a
low molecular weight cationic polymer) before the addition
of the substantially non-ionic polymer, the Schopper
Riegler test is conducted on the suspension after such
chemical treatment.
- The test can be conducted on various suspensions using
polymers of various types ranging from anionic through
substantially non-ionic to cationic. When the results for
any individual suspension are plotted with the drainage
time on the vertical axis against the ionic characteristics
of the polymer on the horizontal axis for any particular
suspension the curve generally follows an approximately V-
shape or U-shape. The bottom of the curve indicates the
ionic characteristic of the polymer at which the fastest
drainage occurs. The position of this varies from one
suspension to another. We find that with most paper-
making pulps the optimum value is in the cationic range,
but that with pulps containing a substantial amount of
electrolyte the optimum performance is in the range of
2o substantially non-ionic or anionic polymers.
The electrolyte in the suspension can be of organic
origin and so can be anionic trash from the original
cellulosic pulp or recycled cellulosic suspension.
Alternatively or additionally it can be of inorganic origin
and so it can be due to partial dissolution of an alkaline
filler such as calcium sulphate or carbonate, or the
hardness of the water. Electrolyte can be added
deliberately.
By referring to a suspension having a high electrolyte
content we mean that the white water has high conducitivty.
The invention is of particular value when the'conductivity
of the white water is above 1500 microsiemens, often 2000-
3000 microsiemens or more. The conductivity can be
measured by conventional techniques.
The suspension will often contain a high amount of
anionic trash if it is to be treated usefully in the
invention and so may have been formed from crude pulp.
,2~.~~~9~
WO 95102088 , PCTIGB94101457
8
Thus the cellulosic component of the suspension may contain
a significant amount of a mechanical pulp (such as ground
wood) and/or a thermo-mechanical pulp and/or a de-inked
waste. Preferably the total amount of mechanical pulp
and/or thermo-mechanical pulp and/or de-inked waste is at
least 50% and generally at least 80% and preferably
substantially the entire amount of the cellulosic material
in the suspension.
The electrolyte content can, alternatively or
additionally, arise from alkaline filler, especially
calcium sulphate, that dissolves slightly into 'the
suspension. Accordingly other suspensions to which the
invention is usually applied are suspensions that contain
at least 5%, and generally 10-50% (based on the dry solids
content of the suspension) of calcium sulphate or other
very slightly soluble alkaline filler.
The invention is of particular value when using such
cellulosic material and/or filler in a closed mill in which
white water from the drainage stage is repeatedly recycled
for diluting thick stock to make the thin stock suspension
that is treated with the retention aid and subsequently
drained, to form paper such as newsprint. Prolonged
recycling of the white water, as a result of the mill being
substantially entirely closed, can cause accumulation of
electrolyte and therefore high conductivity. When there is
very little recycling of white water, a mill may typically
require 100 tons water or more to make a ton of paper.
When there is very extensive recycling, a mill may only
require 5-10 tons water per ton paper. The invention is
preferably applied to mills where there is extensive
recycling, e.g., 50 that the mill uses less than 30,
preferably less than 20 and most preferably 2-15 tons
freshly introduced water per ton of paper produced.
The invention is also of value when electrolyte is
deliberately added to the suspension, which may be
subjected to prolonged recycling. For instance sodium
chloride or other monovalent metal salt (or any other water
_216~~9
WO 95/02088 . PCT/GB94/01457
9
soluble electrolyte) can be added to a suspension or thick
stock to provide a conductivity value such that the anionic
or nonionic retention aid is then suitable. For instance
sodium chloride may be added when the pulp is a dirty pulp
having high cationic demand, thereby suppressing the
- cationic demand (as measured by titration against a
cationic polymer) and making it suitable for use in the
invention.
Another instance when the invention is of particular
value is in the production of liner board from a suspension
that has been treated with large amounts of alum.
The invention is also of value when the suspension,
regardless of anionic trash or other electrolyte content,
has been pre-treated with low molecular weight (intrinsic
viscosity below 3dl/g) cationic polymer and/or cationic
starch in an amount sufficient to give a near zero, or
positive zeta potential. Suitable low molecular weight
polymers are described in US 4,913,775. Alum or other
inorganic coagulant can be used in place of part or all of
the cationic polymer.
The suspensions to~which the invention is applicable
include those where the optimum performance (.i. e. , shortest
drain time) is obtained with a polymer falling within the
range 25, preferably 20 or 15, mole percent anionic groups
to 5 mole percent cationic groups. Preferably the minimum
is obtained at less than 2 mole percent cationic groups,
and preferably the minimum is obtained with less than 10
mole percent, and most preferably less than 6 mole percent,
anionic groups. These values all assume that there is no
deliberate intention to produce an amphoteric polymer. If
the polymer is amphoteric then an appropriate adjustment
in
the quantitative amounts of the anionic and cationic groups
may be appropriate. For instance similar performance may
be obtained from a polymer made by charging 2 mole percent
cationic monomer and 98% acrylamide as would be obtained
from charging 7 mole percent cationic monomer, 5 mole
percent anionic monomer and 88 mole percent acrylamide.
WO 95/02088 . PCT/GB94/01457
It is often preferred that the retention aid that is
used in the process of the invention should be the one that
gives optimum performance in the described Schopper Riegler
drain test. However economic or other considerations
5 sometimes make it preferable to use a slightly different
polymer. Generally the polymer that is actually used
contains from -2 mole percent to +1 mole percent of the
ionic content of the optimum polymer, that is to say if the
optimum polymer is wholly non-ionic the used polymer
10 contains from 2 mole percent anionic groups to-1 mole
percent cationic group, and if the optimum polymer contains
2 mole percent anionic groups then the polymer that is used
contains from 4 to 1 mole percent anionic groups.
An additional or alternative way of defining a
suitable suspension is by determining the drainage time of
it or of a substantially similar suspension as described
above when using 400g/t of a standard substantially non
ionic test retention aid consisting of a non-ionic
polyacrylamide having intrinsic viscosity 13 to 16d1/g and
formed from about 99 to 100% acrylamide and about 0 to 1%
sodium acrylate (on a molar basis). The drainage time
with such a polymer should be below 50%, preferably below
30% and most preferably below 15% of the drainage time of
the suspension without the addition of the polymer.
Instead of or in addition to meeting this criterion,
the drainage time with the non-ionic test retention aid may
be below 80% and preferably below 50% of the drainage time
obtained with the 15 mole percent anionic test retention
aid and below 90%, and preferably below 70% of the drainage
time obtained with the 5 mole percent cationic test
retention aid.
As a generality the suspension can be substantially ,
unfilled, for instance containing no filler other than
filler that may be recycled in the white water, or it may
be filled as a result of deliberate filler addition. Often
relatively crude pulps are used in which event the amount
of filler in the suspension is generally low, for instance
~~ s~~~6
WO 95102088 . " PCT/GB94/01457
11
in the range 0 to 20 or 30% by weight based on dry solids,
and the amount of filler in the resultant paper is
generally in the range 0 to 15%, often around 5 to 10%,
by
weight of the paper.
When filler is used it can be any conventional
- papermaking filler but, as mentioned above,, the invention
is of particular value when the filler is an alkaline
filler having some solubility, sufficient to build up
alkalinity in the white water during prolonged recycling.
Such a filler is calcium sulphate or carbonate.
In conventional processes the interaction between the
retention aid and the solids (fibre and filler) is often
essentially counter-ionic. Thus a cationic retention aid
is appropriate wa.th conventional anionic fibre and filler
particles and an anionic retention aid is appropriate when
the fibre and filler particles have been overdosed with
cationic donor, as in US 5, 234, 548, 4, 643, 801 or 4,
795, 531.
However in the high electrolyte, high conductivity
suspensions of the invention, this electrostatic
interaction mechanism probably does not apply and instead
we believe that hydrogen bonding is the main mechanism for
interaction of the polymeric retention aid with the
cellulosic fibres and filler particles if present, and
between the microflocs and the anionic particulate
material. The hydrogen bonding capability of the nonionic
or anionic polymer is unaffected by the electrolyte content
in the suspension whereas the electrostatic bonding
capability of cationic retention aid is neutralised or
rendered relatively ineffective by the anionic and
electrolyte content of the suspension.
When the retention aid polymer is wholly non-ionic
(i.e., when no deliberate addition of anionic or cationic
groups has been made) the polymer is preferably
polyethylene oxide or polyacrylamide formed from acrylamide
without any deliberate addition of anionic monomer.
However acrylamide is frequently contaminated with a small
amount of anionic monomer and so this polyacrylamide may
be
~~.6~~9
WO 95/02088 . PCT/GB94/01457
12
found to be formed from up to about 1 mole percent
(typically 1.5 mole percent maximum) sodium acrylate, with
the remainder being acrylamide.
However it is not essential in the invention to use a
wholly non-ionic polymer as the retention aid.
Substantially non-ionic polymers used in the invention are
preferably copolymers of acrylamide (or other non-ionic
ethylenically unsaturated monomer that does not render the
polymer insoluble in water) with less than 2 (and usually
not more than 1 or 1.5) mole percent cationic monomer
and/or up to 10 (and usually not more than 5, and usually
not more than 3 ) mole percent anionic monomer. However, in
some instances best results are obtained with polymers
having a higher anionic content, for instance up to 20 mole
percent or even 30 mole percent anionic monomer.
Suitable cationic monomers include nitrogen-containing
ethylenically unsaturated monomers, such as
dialkylaminoalkyl -(meth) acrylamides and -(meth)
acrylates, usually as their acid salts or quaternary
derivatives. ' Suitable anionic monomers include
ethylenically unsaturated carboxylic or sulfonic acids,
which may be present as the free acid or as the water
soluble salt, for instance with ammonium or sodium or other
alkali metal. The preferred monomers include sodium
acrylate as the anionic monomer and dimethylaminoethyl
acrylate quaternary salt as the cationic monomer.
Useful results can be obtained in the invention, on
-appropriate suspensions, with deliberately anionic
polymers, especially in the production of liner board and
with high conductivity white water process and with low
amounts of fresh water. However the invention is of
particular value on suspensions where the polymeric
retention aid is what we consider to be a "substantially
nonionic polymer'°, that is to say a polymer formed of
nonionic monomer units optionally with less than 2 mole
percent cationic units and/or less than 10 mole percent
anionic units.
WO 95/02088 . PCT/GB94/01457
13
The retention aid and test polymers generally have an
intrinsic viscosity above 6d1/g and preferably above 8d1/g.
It can be up to for instance 18d1/g or higher. Often it
is
' in the range 13 to l6dl/g but when making cationic test
polymers at higher cationic contents it may be suitable
to
' use test polymers having IV values in the range, for
instance, 6 to lOdl/g even though the retention aid may
have higher IV. Intrinsic viscosity values quoted herein
are measured by a suspended level viscometer at 25C in
buffered 1% sodium chloride solution.
The anionic particulate material can be any material
that has a sufficiently large and sufficiently hydrophillic
surface area to permit appropriate aggregation of the
microflocs. Preferably the material has a surface area of
at least 200 to 800 square metres per gram. The material
can be colloidal silicic acid or derivatives thereof (for
instance as described in U.S. 4,388,150) or it can be an
emulsion (preferably a micro-emulsion) of an anionic
hydrophillic polymer in water, or zeolite or a silica gel
material as in US 4,927,498. Preferably it is an anionic
swelling clay as described in US 4,753,710. Suitable
swelling clays are generally classed as bentonite but this
term embraces smectites and include hectorites and
montmorillonites.
The amount of the substantially non-ionic retention
aid that is added will be selected having regard to the
particular suspension that is being treated, and will be
influenced by whether or not the suspension has already
been treated by the addition of other polymeric material.
Routine tests, such as the Schopper Riegler test, can be
used to determine a suitable amount, which is usually about
the optimum amount. This is generally in the range 100 to
2,o00g/t (grams per ton dry weight of the suspension),
preferably in the range 300 to 1,OOOg/t.
Routine testing establishes the amount that is optimum
for a particular process (i.e., with a predetermined amount
of polymer), and this is the preferred amount. However
~.~6~~9~
WO 95/02088 PCTlGB94/01457
14
greater or lesser amounts (e.g., ~50% and preferably ~25%)
of this amount can be used.
When performance is determined over a range of polymer
dosages (at constant particulate dosage), it will generally '
be found that performance increases with increasing dosage
to a maximum, but that further increase in dosage results '
in no further increase or in deterioration, in properties.
If insufficient polymer is used poor drainage and/or
retention properties will be obtained either because the
microflocs are so unstable that they break down to the
component fibres and filler particles or because there is
insufficient aggregation of the microflocs by the anionic
particulate material. Preferably the amount of polymer is
such that the initial flocs are easily broken down to
microflocs by the shearing, but that the microflocs are
less easily degraded by continuation of the shearing.
The shearing may be provided merely by turbulent flow
of the flocculated suspension along a duct to the point at
which the anionic particulate material is applied, or the
shearing may be provided by a high shear process step such
as passage through a pump (e.g., a fan pump) or a screening
device such as a centriscreen. The non-ionic polymeric
material may be added at a single point of addition or at
two or more points of addition, for instance with each
addition point being followed by a shearing stage.
The bentonite or other anionic particulate material is
usually added in an amount of 300 to 10,OOOg/t, often
around 1,000 to 3,o00g/t. However when the anionic
material is less efficient as an aggregating aid than
bentonite, larger amounts may be useful, for instance up to
20,OOOg/t.
The anionic particulate material is usually added
after the last point of high shear, e.g., at the headbox,
but it can be added at an earlier stage if desired.
The following are examples.
Hxample 1
WO 95/02088 . .. ~ PCT/GB94/01457
This is a laboratory test conducted using a modified
Britt Jar and a modified Canadian Standard Freeness tester
(CSF). Thus a standard, baffled Britt Dynamic Drainage Jar
a is modified by removing the wire and support mesh, and
5 replacing these with a solid plastic disc. This creates a
baffled stirring pot.
A CSF tester is modified by blocking its back drain,
and a measuring cylinder is placed under its front drain to
create a drainage tester.
10 A 500m1 sample of a thinstock comprising a thermo-
mechanical pulp furnish obtained from a newsprint machine
and having a consistency of 0.95% (by weight dry solids in
aqueous medium) is added to the modified Britt Jar. The
sample is stirred at 1, 500 rpm for 5 seconds. A sample
15 polymer is then added, as a solution, at a dosage level of
O.S g/t. The treated sample is stirred at 1,500 rpm for 1
minute, and is then transferred to a 500m1 measuring
cylinder. Bentonite is added to the sample at a dosage
level of 6kg/t. The open end of the cylinder is then
sealed and its contents mixed by inverting the cylinder
four times.
The sample is then transferred to the modified CSF
tester, and the drainage time measured by recording the
time taken for 200m1 of backwater to drain from the 500m1
sample and collect in the measuring cylinder under the
front drain of the CSF tester.
A blank test is performed according to the above
procedure in the absence of both added polymer and added
bentonite. The drainage times recorded for each of the
polymer samples are then normalised by expressing them as
a percentage of the blank drainage time.
The dosage levels of the polymer and the bentonite are
expressed in terms of kg/t which is kg of dry polymer or
bentonite per tonne of dry fibre.
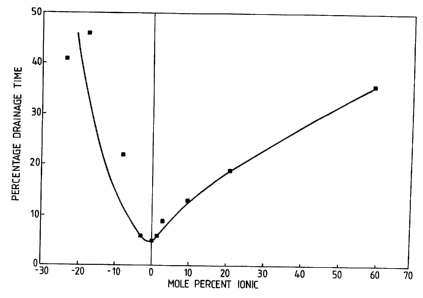
The sample polymers are as follows:
WO 95/02088 . PCT/GB94/01457
16
sample Polymer Ionic Content Intrinsic
a (si n and mole%) viscosit (dl )
ACM NaAC -23.6 17.0 ,
ACM NaAC -17.6 12.0
ACM NaAC -7.9 12.0
ACM NaAC -3.0 13.0
ACM 0 12.0
ACM DMAEA MeCl +1.4 11.0
ACM DMAEA MeCl +2.7 6.0
ACM DMAEA MeCl +9.7 8.0
ACM DMAEA MeCl +20.9 6.5
ACM DMAEA MeCl +59.5 7.0
Where ACM/NaAC is a copolymer of acrylamide and sodium
acrylate, ACM is acrylamide homopolymer and ACM/DMAEAqMeCl
is a copolymer of acrylamide and dimethylaminoethylacrylate
quaternised with methyl chloride.
Figure 1 is a graph of percentage drainage time (%
seconds) vs ionic content (mole %), and shows the results
obtained by use of the sample polymers in the above
described test in the form of a relatively smooth curve.
The results illustrate that the optimum polymer has an
ionic content of about 0% of those tested, this was
represented by the acrylamide homopolymer.
Although the curve is relatively smooth, in practice
there can be irregularities. It may sometimes be observed
that the performance at exactly zero percent ionic content
is slightly worse than the performance on either side.
However this may be due to a difference in, for instance,
the solubility or molecular weight of the non-ionic polymer
compared to the slightly anionic or slightly cationic
polymers with which it was compared. Accordingly when
interpreting plots of the performance of different polymers '
it is desirable either to ensure that the polymers are
directly comparable, as regards molecular weight and
WO 95/02088 . - ~ PCT/GB94/01457
17
solubility, or to study the overall shape of the curve
rather than to rely upon any particular individual point.
The following examples demonstrate processes broadly
' as described in US 4,753,710 but with different polymers.
Example 2
Test results from a Light Weight Coated furnish.
A comparison of polymers of the same molecular weight,
(IV 7.Od1/g) but differing cationic contents.
Constant additions for the tests: Polymer 800g/T and
l0 Bentonite 2kg/T.
Polymer Ionic Content Total Retention
(mole %) (%)
0 57.7
0.37 59.7
0.74 59.2
1.12 57.4
1.50 55.6
1.89 54.9
2.28 55.8
2.68 55.3
3.08 56.4
n i
This shows best results are obtained at 0-1% cationic.
Example 3
Test results from a saturating base kraft furnish.
A comparison of polymers of the same molecular weight,
(IV 7.Odl/g) but differing cationic contents.
Constant additions for the test: Polymer 800g/T
Bentonite 2kg/T.
PCT/GB94/01457
WO 95/02088
18
Polymer Ionic Content Total Retention
(mole %) (%)
0 81.3
+ 0.37 84.3
+ 0.74 81.7
+ 1.12 81.0
+ 1.50 77.9
+ 1.89 77.4
+ 2.28 76.6
+ 2.68 78.4
+ 3.08 77.1
This again shows best results at 0-1% cationic.
Example 4
Test results from a fine furnish.
A comparison of cationic and anionic polymers.
Constant additions for the tests: Polymer 500g/t
Bentonite 2kg/T.
Polymer Ionic Content Percentage Drain Time
(mole %) (% seconds)
+ 26.8 57
- 7.74 34
- 33.5 35
The fine furnish is a relatively pure suspension
having low electrolyte content. This shows that, on such
a suspension, better results are obtained using cationic
retention aid than with the nonionic or anionic retention
aids of the invention.
Example 5
Paper is made by a process as described generally in
Example 1 of US 4,753,710except that the drained white
water has a conductivity of above 2000 microsiemens (as a
result of having been formulated to represent white water
obtained in a process that utilised 10 tons fresh water per
WO 95/02088
PCT/GB94/01457
19
ton paper) and the cationic retention aid is replaced by a
copolymer of 95% acrylamide and 5% (molar) sodium acrylate
having intrinsic viscosity above 8d1/g.
s Example 5
A paper furnish having 20% CaSO4 filler is formed with
a headbox consistency of 0.5%. A Britt Jar, tester is used
to determine retention. The total retention in the absence
of polymer is 79.8% and the ash retention is 9.1%.
400g/t of 90% acrylamide 10% sodium acrylate polymer IV
12d1/g is added and gives total retention 89.4% and ash
retention of 74.4%. The same system with subsequent
addition of 4kg/t bentonite gives total retention 96.9% and
ash retention 91.7%.
As indicated, the process of the invention is best
performed using suspensions that give a white water
conductivity above 1500 microsiemens, preferably above 2000
microsiemens. The suspension is preferably such that it
would have these high conductivity values irrespective of
whether or not cationic starch or low molecular weight
synthetic cationic polymer (or even alum) has been added to
the suspension.