Note: Descriptions are shown in the official language in which they were submitted.
CA 02167500 1999-06-10
1
METHOD FOR REPRODUCING CONIFERS BY SOMATIC
EMBRYOGENESIS USING MIXED GROW TH HORMONES
FOR EMBRYO CULTURE
10
BACKGROUND OF THE INVENTION
The present invention is a method for reproducing coniferous plants
by somatic embryogenesis using the techniques of plant tissue culture. More
specifically, it relates to the use of particular mixtures of growth hormones
in the
culture media used during the various stages of somatic embryo development.
The
invention is especially suited for producing large clones of superior
selections useful
for reforestation.
Loblolly pine (Pines taeda L.), its closely related southern pines, and
Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) are probably the most
important
commercial species of temperate North American trees. Similarly, Norway spruce
(Picea abies (L.) Karst.) is probably the most important European softwood
species.
Since the early 1940s, when serious private reforestation efforts began,
literally
billions of one and two year old nursery-grown trees have been planted on
cutover
or burned forest lands. For many years these seedling trees were grown using
naturally produced seed from cones collected as a part time effort of
individuals
seeking to supplement their incomes. As early as 1957 forest geneticists began
to
plant seed orchards using either seed or grafted scions obtained from superior
trees
discovered in the forests. These trees were selected for such inheritable
characteristics as rapid growth, straightness of bole, wood density, etc. Now
in both
WO 95/05070 PCT/US93/07803
2
the southern pine and Douglas fir regions the bulk of the seed is produced
from
selected trees grown in seed orchards, some of them now second and third
generation ,
orchards.
Despite the fact that the orchards were stocked with superior trees,
pollination often cannot be carefully controlled and frequently the seed trees
are
fertilized by wild pollen of unknown characteristics. For this reason, the
characteristics of the progeny produced by sexual reproduction have not bean
as
predictable as hoped and genetic gain could not be attained as rapidly as
desired.
Beginning about 1960, techniques were developed for reproducing
some species of plants by tissue culture. These were predominately angiosperms
and
usually ornamental house plants. The method employed use of a suitable explant
or
donor tissue from a desirable plant. This was placed on a series of culture
media in
which nutrients and growth hormones were carefully controlled from step to
step.
The usual progression was growth from the explant to a callus. The callus was
placed on a budding medium where adventitious buds formed. These, in turn,
were
separated, elongated, and rooted to ultimately form plantlets. A plantlet has
the
nature of a seedling but is genetically identical to the explant donor plant.
Gymnosperms in general, and most forest tree species in particular,
proved to be much more difficult to reproduce by tissue culture. It was not
until
about 1975 that Douglas fir was successfully reproduced by organogenesis.
Loblolly
pine was successfully reproduced about two years later.
A brief review of some of the most important work relating to the
present invention will follow. This is intended to be representative only and
is not
fully inclusive of all the work in the field. Literature citations in the text
are given
in abbreviated form. Reference should be made to the bibliography at the end
of the
specification for full citations of the literature noted herein.
Culture by organogenesis is tedious and expensive due to the large '
amount of delicate manual handling necessary. It was soon recognized that
embryogenesis was potentially a much more desirable method from the
standpoints
of quantity of plantlets produced, cost, potential genetic gain, and much
lower
probability of mutations. Work on embryogenesis of forest species began in the
late
SUBSTITUTE SHEET
CA 02167500 1999-06-10
3
1970s. U.S. Patent 4,217,730 to El-Nil describes one early attempt at somatic
embryogenesis of Douglas fir. This approach was later set aside because
advanced
stage embryos and plantlets could not be readily obtained. However, other
workers
entered the field in increasing numbers and progress has been rapid even if it
has
not, until the present time, reached the commercial stage.
Our earlier U.S. Patents 4,957,866, 5,034,326, 5,036,007, describe
improved methods of conifer embryogenesis. These also include extensive
reviews
of the most closely related literature. In the methods described in all of
these patents,
late stage proembryos, defined as totipotent embryonic structures having at
least about
100 cells, are transferred to and further cultured in a cotyledonary embryo
develop-
ment medium containing abscisic acid (ABA) as an essential growth hormone. It
appears to be highly desirable during this stage to gradually reduce the level
of
exogenous ABA so that little or none is ultimately present. Other growth
hormones;
e.g. auxins, cytokinins, and gibberellins were not used at this time. The
ultimate
product of this culturing step is somatic embryos resembling natural mature
zygotic
embryos in morphology.
It is well accepted that plant tissue culture is a highly unpredictable
science. Sondahl et al., in published European Patent Application 293,598,
speak
directly to this point.
"Since each plant species appears to possess a unique
optimal set of media requirements, the successful
preparation and regeneration of a new species cannot be
necessarily inferred from the successful regimens
applied to unrelated plant species.
This statement can be carried even farther. Rangaswamy ( 1986) notes
that the potential for embryogenesis is even genotype specific within any
given
species.
Composition of the media used to initiate embryogenesis and induce
embryo maturation are critical to success, regardless of the species being
propagated.
In particular, the type and level of the nitrogen source in the media and the
presence
or absence, composition, level, and timing of availability of growth hormones
have
WO 95/05070 PCTIUS93107803
216~~~~~
4
been key to success. It is also these very factors, particularly the hormones,
that
have proved to be so unpredictable. As one example, Ammirato (1977), conducted
,
a study examining the effects of zeatin (a cytokinin), ABA, and gibberellic
acid
(GA3) on the yield and morphology of caraway (Carom carvi) somatic embryos.
These hormones were present singly and in all possible combinations in the
media
used for the later stages of embryo development. He concluded that a change in
level or presence/absence of any one of the hormones caused a ripple effect
felt
throughout the system due to unpredictable inter-actions between the various
hormones. The same problem is again discussed by Evans (1984) who notes that
growth hormones which affect the same process can either act independently or
may
interact in some fashion.
The Ammirato (1977) paper is midrange in time between the first
successful plant embryogenesis and the present. Much has been learned since
then.
However, this paper is useful in its clear and still valid presentation and
characterization of the various growth hormones as promoters (or stimulators)
and
inhibitors. Evans (1984) once again expands Ammiratto's discussion. Auxins are
seen by these investigators as promoting cell elongation, especially in shoot
tissues,
and in lower concentrations, in roots. Gibberellic acid (GA3) also promotes
cell
elongation in shoots but is either without effect or inhibitory in root
tissues. ABA
and ethylene are seen as inhibitors and tend to counteract the promotive
effects of
auxins and GA. Cytokinins appear to be more difficult to characterize. They
generally tend to inhibit auxin induced cell elongation in stem and root
tissues but
act as promoters of leaf cell expansion.
In general, as far as conifer species are concerned, it appears that at
least one exogenous auxin and usually a cytokinin are necessary hormones in a
medium for the initiation of embryogenesis. Exogenous ABA is normally not used
at this point nor is gibberellic acid (GA3) or its related gibberellins. The
concentration of the growth hormones used in the initiation medium is
typically then
reduced or they are removed entirely as embryo development proceeds. However,
auxins in particular may be beneficial at the stage of cotyledonary embryo
development.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
In most cases gibberellic acid appears to suppress embryogenesis;
e.g., Kochba et al. (1978), Tisserat et al. (1977), Rajasekaran et al. (1987),
Rangaswamy (1986). However, there are certainly exceptions. Chalupa (1990a,
1990b) refers to induction of embryogenesis in two QuercuS species on a medium
' S containing the cytokinin N6-benzylaminopurine and gibberellic acid.
Gmitter et al.
(1990) appear to require gibberellic acid in their initiation medium for
selected
triploid hybrid citrus plants. Lakshmi Sita (1985) summarizes her earlier work
and
that of others in promoting embryogenesis of sandalwood (Santalum sp.).
Gibberellic Acid was found to be useful in inducing embryogenesis using shoot
explants in either solid or liquid suspension cultures. Despite her success,
which
included successful production of converted plants, she again points to the
lack of
predictability of embryogenesis.
"Despite progress, our knowledge of embryogenesis is
still fragmentary. At present we cannot yet define the
conditions necessary for embryogenesis. . . . . "
Gibberellic acid has more frequently been used to promote late stage
somatic embryo development and germination; e.g., Cruz et al. (1990), Eapen et
al.
(1990), Ghosh et al. (1991), Manrique et al. (1987), Trolinder et al. (1988).
Nolan
et al. (1988) and Garcia-Maya et al. (1990) note that gibberellins are
involved in the
synthesis of -amylase. This enzyme is necessary for conversion of starches
into
sugars during germination.
Much less frequently various gibberellins have been used in
combination with ABA at the late stage of embryo development and for
stimulating
germination; e.g., Eapen et al. (1989), Feffeira et al. (1990), Sondahl et al.
(1988).
Noriega et al. (1991) describe the first successful regeneration of hybrid tea
rose by
embryogenesis. Among the various media employed is an embryo maturation
medium containing low concentrations of GA, and ABA. Ammirato (1977), working
with caraway (Carom carvi L.), reported that the beneficial effects of ABA on
embryo maturation could be enhanced by gibberellic acid (GA3). However, in an
SUBSTITUTE SHEET
WO 95105070 PCT/US93/07803
6
extension of this work investigating the effect of mode of agitation, he noted
that
"the addition of GA3. alone or with ABA, had little effect on these cultures"
(Ammirato (1983). All of the reported work with this particular hormone
combination has been exclusively with various angiosperm species.
The present inventors are aware of only one reported instance in which
gibberellic acid has been examined in conifer somatic embryogenesis. Hakman
and
von Arnold (1985) tried adding ABA, IAA (indoleacetic acid), GA3, GA4/~, and
activated charcoal singly to media used for further development of Acea abies
(Norway spruce) proembryos. All of these were found to be ineffective and only
a
cytokinin-containing medium was useful under the conditions they employed.
Techniques to promote embryogenesis of numerous conifer genera are
now well established. Research emphasis is now shifting to development of ways
to scale up laboratory knowledge and techniques so that the process may become
field operational on large scale. Yet many problems of a relatively
fundamental
nature still remain to be solved. One of these is improving somatic embryo
quality
and vigor. This is necessary so that germination to hardy plantlets and
ultimate
conversion to growing trees can be achieved at much higher percentages than
has
heretofore been possible. The present invention is directed to this end.
SUMMARY OF THE INVENTION
The present invention is directed to the use of various gibberellins in
the media at the different stages of conifer embryogenesis. It is particularly
directed
to new combinations of growth hormones during the cotyledonary embryo
development stage of conifer somatic embryogenesis. We have also found that
the
inclusion of gibberellins in the medium used for proembryo maintenance results
in
more robust proembryos. Addition of gibberellins at this stage enables further
development of many genotypes that were previously found difficult to culture.
We '
have also found that a combination of abscisic acid and various gibberellins
is
advantageous in promoting the growth of more robust somatic embryos of
coniferous
species when used at the cotyledonary embryo stage of development. These
embryos
closely resemble natural mature zygotic embryos in external and internal
SUBSTITUTE SHEET
WO 95105070 PCT/US93/07803
7
morphology. Conversion success of embryos produced using the new hormone
regimen has been significantly improved over those made using previous
culturing
techniques.
. The present method is especially suitable for reproducing woody
gymnosperms of the order Coniferales. It is particularly well suited for
generating
large clones of superior forest trees for reforestation, including species
within
families Pinaceae, Cupressaceae, and Taxodiaceae. Most or all species within
the
genera Abies, Pinus, Picea, Tsuga, Pseudotsuga, Thuja, Juniperis, Larix, Taxus
and
Sequoia are believed to be amenable to multiplication by the present method.
The method is particularly advantageous in that it enables more robust
somatic embryos to be produced. This results in higher numbers of embryos that
can
be successfully converted into plants growing in soil. Costs per plant can be
significantly reduced over prior known tissue culture methods. In addition,
use of
the method generates proembryos that can be retained for extended periods of
time
in cryogenic storage. Alternatively, cotyledonary embryos are produced that
can be
held in cold storage for prolonged periods without the need to transfer them
from the
development medium.
A number of terms are known to have differing meanings when used
in the literature. The following definitions are believed to be the ones most
generally
used in the field of botany and are consistent with the usage of the terms in
the
present specification.
"Auxins" are plant growth hormones that promote cell division and
growth.
"Cytokinins" are plant growth hormones that affect the organization
of dividing cells.
"Callus" is generally considered to be a growth of unorganized and
' either unconnected or loosely connected plant cells generally produced from
culturing
an explant.
"Embryogenic callus" is a translucent white mucilaginous mass that
contains early stage proembryos attached to suspensors. This is also referred
to as
an "embryonal-suspensor mass" or "ESM" by some investigators.
SUBSTITUTE SHEET
WO 95105070 PCT/US93107803
8
A "proembryo" is a cell or group of cells having the potential to
become a plant but lacking defined meristematic organ primordia.
An "early stage proembryo" is a mass generally of 1-10 cells with
dense cytoplasm and large nuclei that have the potential of forming a plant.
The
early stage proembryo is normally found as a head associated at the end of a
long
thin-walled suspensor cell (FIG. 1).
A "late stage proembryo" is a proembryo with a smooth embryonal
head of at least about 100 cells associated with multiple suspensor cells. The
late
stage proembryo is a very robust advanced proembryo (FIG. 2). Many
investigators
refer to these as "globular embryos."
A "cotyledonary embryo", sometimes simply referred to as an
"embryo" , has a well defined elongated bipolar structure with latent
meristematic
centers having cotyledonary primordia at one end and a potential radicle at
the
opposite end. The cotyledonary structure frequently appears as a smakl "crown"
at
one end of the embryo (FIGS. 3 and 5). A cotykedonary somatic embryo is
analogous to a developed zygotic embryo.
An "explant" is a piece of tissue taken from a donor plant for
culturing.
"Gibberellins" are plant growth hormones which serve to promote cell
elongation. They are closely related members in a chemical genus of over 70
known
compounds. Gibberellins GA3, GA4, GA7, and mixtures of the latter two
designated
GA417 are the most commonly used due to their relatively greater availability
and
much lower cost.
"Gibberellic acid" is an unspecified mixture of gibberellins. When
referred to in the more recent literature it usually designates a composition
with at
least 70 k GA3. In the present application the term "GA" or "GAs" without a
specific numeric designation is used in a generic sense to represent an active
gibberellin.
An "active gibberellin" is one that is biologically active in the
particular plant species being cultured.
SUBSTITUTE SHEET
WO 95105070 PCT/LTS93/07803
~' 2i6?5~0
9
A "meristem" or "meristematic center" is a group of tissue forming
cells capable of further development into plant organs; e.g., shoots and
roots.
An "osmoticant" or "osmoticum" is a chemical material used for con-
trolling the osmotic potential of a solution. In the present context the
solution would
be a culture medium.
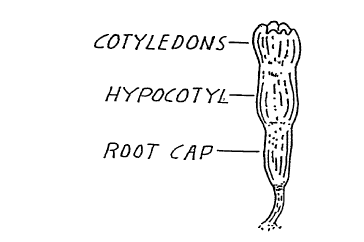
A "plantlet" is a plant asexually reproduced by tissue culture (FIG. 4).
A "converted embryo" is an embryo that has germinated and been
established as a plant growing in soil.
"Somatic embryogenesis" is the process using tissue culture techniques
for generating multiple embryos from an explant. The embryos generated from a
given tissue source are presumed to be genetically identical.
The present method as a whole comprises a multistage culturing
process. A suitable explant is first placed on an induction or initiation
culture
medium. This will usually contain relatively high quantities of growth
hormones
including at least one auxin and frequently one or more cytokinins. However,
growth hormones at this initial stage are not always necessary or desirable
for
induction of early stage proembryos. A number of sources of explants may
ultimately prove to be satisfactory for culturing. These include, but are not
limited
to, tissue from cotyledons, hypocotyls, epicotyls, buds, meristematic centers
for buds
or roots, and seed embryos. Zygotic embryos removed from seeds are presently
preferred. In particular, for species which in the past have proved to be very
diffiicult or impossible to propagate by somatic embryogenesis, the embryos
from
immature seeds may be preferred. In the case of Douglas fir, an embryo
selected
between the time that an apical dome begins to form but before the first
appearance
of cotyledon primordia appears to be optimum.
The first stage or induction medium will normally be one of those well
known from past work which contain a balanced concentration of inorganic salts
and
organic nutrient materials, with plant growth hormones included as noted
above.
Auxins are normally present in concentrations which may initially be as high
as
about 600 ~,M/L, more typically not exceeding about 500 ~cM/L. Cytokinins, if
present, may initially be as high as 500 ~,M/L. The plant growth hormones may
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
r
include at least one auxin and one cytokinin in a combined initial
concentration not
exceeding about 1100 ~,M/L, more typically not exceeding about 900 ~cM/L. The
particular auxins and cytokinins used and their exact concentrations, or
whether they
are used at all, will depend somewhat on the species being cultured and even
on the
5 particular genotype within that species. This is something that cannot be
easily
predicted but can be readily determined experimentally. These very high levels
of
growth hormones assume the presence in the medium of an adsorbent material,
such
as activated charcoal. Where charcoal is not present the levels of growth
hormones
would normally be much lower; e.g., a full order of magnitude, than those just
10 noted.
Culturing during this stage may be canned out in the dark, under very
low light conditions, or in full light until an embryogenic mass forms.
Lighting
conditions will depend in large part on the composition of the particular
medium
selected. In general, initiation in full dark is preferred. This embryogenic
mass has
been described by various other names by researchers who have reported it in
the
past; e.g., embryogenic callus (Hakman and von Arnold 1985) or embryonal-
suspensor (Durzan and Gupta 1987). It has the appearance of a whitish,
translucent,
mucilaginous mass containing early stage proembryos which are readily apparent
by
low power light microscopy. In the case of Douglas fir the presence of
activated
charcoal or a similar adsorbent in the initiation medium appears to be quite
advantageous. It should be noted here that Douglas fir does not experience
polyembryony as do most other coniferous species. The reasons for this are not
well
understood but one hypothesis suggests that Douglas fir seeds contain a high
endogenous level of abscisic acid which suppresses polyembryony. Activated
charcoal in the initiation medium may remove this endogenous ABA, as well as
other
undesirable metabolic byproducts, to allow polyembryony to occur in vitro.
Because
the charcoal will also gradually remove growth hormones over time the initial
'
concentrations of these materials are necessarily higher than might otherwise
be the
case. The preferred induction medium for Douglas fir will preferably contain
an
auxin or auxins in amounts of about 400-600 ~M/L and a cytokinin or cytokinins
in
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
~1d75~0
11
the amount of about 240-500 ~.M/L in combination with 0.05-1.0% activated
charcoal.
Early stage proembryos from the first culture may be directly
transferred to a late proembryo development culture medium having
significantly
reduced plant growth hormones and, for some species, a higher concentration of
osmoticants. However, they are preferably first subcultured in a maintenance
medium of similar or slightly higher osmotic potential than the induction
medium for
multiplication. This multiplication medium will also usually have the
concentration
of plant hormones significantly reduced below that of the induction medium. By
"significantly reduced" adsorbent is meant lowered by a factor which may
typically
be one whole order of magnitude. In the case of Douglas fir it may be two full
orders of magnitude below that initially present in a charcoal containing
induction
medium. No hormone adsorbent is usually necessary or desirable at this time.
The
osmotic potential of the induction and maintenance medium will most usually
not
exceed about 160 mM/kg.
The composition and use of the late proembryo development culture
medium is important to the success of the present process. It differs from the
induction medium by having a similar level of plant growth hormones to those
present in the maintenance and multiplication medium. However, for many
species
such as Pinus taeda and Pseudotsuga menziesii, the late proembryo development
media should have a concentration of osmoticants that is significantly raised
above
that of the induction or multiplication media. The optimum osmoticant levels
at each
stage will usually differ for each species and often for individual genotypes
within
a species. For loblolly pine the osmotic level should typically be of the
magnitude
of at least 200 mM/kg and preferably about 240 mM/kg or even higher. However,
lower levels of about 170 mM/kg minimum will suffice for most genotypes of
Douglas fir. The key advantage of this osmotic "pulse" is that proembryo
quality
and/or size can be significantly improved. Some species such as Picea abies,
which
are relatively easy to reproduce, may not require this raised osmotic level,
or it may
only be necessary for some genotypes In these cases late proembryo development
SUBSTITUTE SHEET
WO 95!05070 PCTlUS93l07803
12
may usually be achieved without a change in medium composition from the
maintenance and multiplication medium.
It appears now that the inclusion of between 0.05 and 15 mglL,
preferably about 0.1-5 mg/L, of selected active gibberellins and/or abscisic
acid in .
the maintenance and/or late proembryo development media is also beneficial for
improvement of proembryo quality.
Incubation at this stage is usually carried out in the dark or in greatly
reduced light until robust late stage proembryos have formed. These may then
be
transferred to an embryo development medium which usually lacks auxins and
cytokinins entirely.
Many investigators refer to cotyledonary embryo development from
pr~mbryos simply as a "development" stage and that usage will be understood
herein unless the word "development" is otherwise qualified.
Douglas fir generally requires an intermediate step between the late
proembryo growth stage and the final cotyledonary embryo development stage
which
is not necessary for other species. The proembryos tend to form in tight
clumps or
clusters which must first be singulated before going to the development stage.
This
singulation is carried out in a series of liquid shake cultures which lack
auxins and
cytokinins but have exogenous abscisic acid as a necessary new hormone. The
level
of osmotic potential is also reduced from that of the late stage proembryo
development medium. ABA will initially usually be within the range of 5-15
mg/L
(20-60 ~,M/L) with osmotic potential levels in the range of 130-160 mM/kg.
Typically the singulation process will encompass two or three transfers at
weekly
intervals following the initial singulation treatment. A preferred procedure
uses an
initial treatment with ABA at a 10 mg/L level followed by two treatments at
weekly
intervals with ABA at a 5 mg/L concentration. It now appears to be beneficial
to
include from 0.05-15 mg/L, preferably about 0.1-10 mg/L, of an active
gibberellin '
the singulation medium.
Further development and enlargement of the proembryos will occur
during the singulation stage for Douglas fir.
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
216750
13
The singulated late stage proembryos can then be transferred to a solid
or pad-on-liquid cotyledonary embryo development medium. If the embryos are
not
singulated they will develop into a tight clump of cotyledonary embryos which
cannot
be readily separated and are difficult to use for further germination.
Significantly, species other than Douglas fir can be advantageously
cultured by beginning early cotyledonary embryo development in a series of
media
similar to those used for Douglas fir singulation.
The singulation stages are preferably carried out in Douglas fir culture
in liquid media under gently agitated conditions. Other species may produce
better
results if the early development cultures are made on solid medium or on pad
systems using liquid medium. However, for all species it is most desirable for
a
final development stage or stages to be carried out on either solid medium or
with
liquid medium using a pad system. For reasons not perfectly understood, far
more
vigorous embryos are normally obtained when they are exposed to air in the
final
development stages.
Especially when Douglas fir is being cultured, but also with some
genotypes of loblolly pine and other species, the osmotic potential of the
later stage
cotyledonary development medium should be sharply raised above that of any of
the
preceding media. Initially levels may be in the 300-350 mM/kg range but these
should be increased to levels of at least about 400 mM/kg as development
proceeds.
If development is started at levels around 300-350 mM/kg, the osmotic level
may be
increased during development by a complete medium change, a partial change in
which some old medium is replaced, or by adding an appropriate form, such as a
solution, of osmodcants to the medium without replacement of any of the
original
~25 medium. Any of these changes may be considered a transfer to a "new"
medium.
With Douglas fir, it is preferred that the osmotic levels at the end of the
development
period should be at least about 450 mM/kg although with some genotypes lower
levels are acceptable. With some Douglas fir genotypes final osmotic levels as
high
as 600 mM/kg have given superior results. These higher levels tend to prevent
deterioration and callusing of the embryos.
SUBSTITUTE SHEET
CA 02167500 1999-06-10
14
Osmotic potential in the later stages of cotyledonary development is
best controlled by a combination of osmoticants. One of these should be a
readily
metabolized carbohydrate energy source, preferably a sugar such as sucrose,
glucose,
fructose, maltose, or galactose. Sucrose is a preferred ingredient and may be
present
in amounts in the range of 2-6 % . The other is a poorly metabolized
osmoticant of
which sorbitol, lactose, or a polyalkylene glycol would be examples. In a
solid
development medium, a combination of sorbitol, lactose and polyethylene glycol
has
proved very effective. Polyethylene glycol (PEG) alone, in concentrations of
about
15-30% of the medium, has worked very well IN liquid development media. The
molecular weight of the PEG is not critical and may fall in the range of
several
hundred to several thousand. While the salts and organic components of the
medium
make a small contribution to the osmolality, the osmotic potential is
primarily
controlled by the energy-providing sugar and the other osmoticants. It is
within the
scope of the invention to use one combination of osmoticants at the beginning
of
development and transfer to a medium having a different combination at some
point
during the development stage.
In some cases where transfers to fresh media a.re made during the
cotyledonary embryo development stage, especially when culturing Douglas fir,
at
least the final and most preferably the penultimate media should have osmotic
potential of at least about 350 mM/kg, preferably about 400 mM/kg or higher.
For virtually all coniferous species a supply of exogenous abscisic acid
is a useful hormone and media component in the development from proembryos to
cotyledonary embryos. As described in our earlier U.S. Patents 5,034,326 and
5,036,007, this was always used in combination with an adsorbent, such as
activated
charcoal. The adsorbent was present in a sufficient amount and form to slowly
reduce the abscisic acid and remove metabolic waste products. It could not be
present in such a high concentration as to deplete the abscisic acid in a very
short
time; e.g., in a matter of days. The combination of abscisic acid with the
adsorbent
usually required a higher initial concentration of abscisic acid than was the
case if
no adsorbent was present in the medium. United States Patent No. 5,236,841,
describes cotyledonary embryo development by the use
WO 95105070 PCT/US93/07803
2i~75~~
is
of stepwise media changes with progressively lower ABA contents. Activated
charcoal or other adsorbents are not necessary using this procedure.
In the particular case of Douglas fir, but with other species as well, we
have found that the level of exogenous abscisic acid should be generally
continuously
s lowered over time from the s-is mg/L normally found necessary at the
beginning
of the singulation step or cotyledonary embryo development stage to a level
perhaps
of, about 1-2 mg/L, or even to zero, at the end of the development stage.
Accurate
measurements of abscisic acid present in the development stage have not yet
been
made due to the extreme difficulties of analyzing the medium. It is possible
in some
cases to produce cotyledonary embryos without exogenous ABA in the development
medium. However, the embryos so produced are usually of inferior quality.
A significant discovery of the present invention is the advantage of
including an active gibberellin along with abscisic acid in the development
medium.
The use of the gibberellins at this stage results in the development of larger
and more
is mature cotyledonary embryos. Concentrations in the range of about O.s-s0
mg/L,
preferably about 2.s-10 mg/L, have been found to be effective.
Following embryo development the embryos (FIG. s) may be placed
directly on a germination medium for conversion into plantlets. Alternatively,
they
may be converted into artificial seeds by any of a number of published
processes.
The germination medium has no exogenous hormones, a lowered
organic nitrogen content, and a reduced level of osmoticants. After a
sufficient time
in darkness followed by light, or a 16 hour light and 8 hour dark photoperiod,
the
cotyledonary embryos will have developed into plantlets. Douglas fir does not
require an initial dark period although an initial four day dark period is
usually more
2s satisfactory. A one week dark period is useful for Norway spruce. The time
period
for germination will be about 1-2 months. The resulting plantlets will have a
well
developed radicle and cotyledonary structure with a growing epicotyl and are
ready
for planting in soil.
The present invention is primarily concerned with the composition of
the proembryo maintenance medium and the cotyledonary embryo development
media and method of their use. In the case of Douglas fir, the composition of
the
SUBSTITUTE SHEET
WO 95/05070 PCTIUS93/07803
16
proembryo singulation medium is also a concern. In particular the addition of
at
least one active gibberellin to the maintenance medium and the combination of
the .
growth hormone abscisic acid with selected gibberellins in the maintenance
and/or
development media gives improved size, vigor, and maturity of somatic embryos
and
further reduces tendency to precocious germination.
It is an object of the present invention to produce coniferous plantlets
by somatic embryogenesis.
It is another object to produce a large clone of a genetically selected
forest species for reforestation using the methods of somatic embryogenesis
and plant
tissue culture.
It is a further object to provide a method of somatic embryogenesis that
will dependably and consistently provide coniferous plantlets in large
quantities.
It is yet another object to provide a method of somatic embryogenesis
that can dependably and consistently reproduce large clones of selected
individuals
of forest species that heretofore have not been successfully reproduced by
this
method.
It is still a further object to provide a method whereby superior
genotypes of coniferous trees can be multiplied by tissue culture in the large
quantities needed for reforestation.
It is also an object to provide a method that will produce somatic
embryos in large quantities with improved robust morphology for conversion
into
plandets.
It is a particular object to provide a method and suitable culture media
for somatic embryogenesis that produces robust somatic embryos with a high
percentage of conversion to plants growing in soil.
It still another object to provide a method that generates robust somatic
proembryos capable of withstanding extended periods of cryogenic preservation.
'
These and many other objects will become readily apparent to those
skilled in the art by reading the following detailed description, taken in
conjunction
with the drawings.
SUBSTITUTE SHEET
WO 95/05070
PCT/ITS93/07803
17
BRIEF DESCRIPTION OF THE DRAWINGS
The figures show various stages of plant embryogenesis in which:
FIGURE 1 shows early stage proembryos.
FIGURE 2 shows late stage proembryos.
FIGURE 3 depicts cotyledonary stage embryos.
FIGURE 4 shows a plantlet ready for transfer to soil.
FIGURE 5 is a photomicrograph of a high quality Douglas fir
cotyledonary embryo.
FIGURE 6A is a drawing showing a conifer embryo typical of those
produced prior to using the treatments of the present invention.
FIGURE 6B is a drawing showing improvement in embryo morphology
using the treatments of the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The process of the present invention is not limited to any single basal
culture medium or to the use of specific growth hormones other than those
defined
in the claims. Any of a number of well known basal media, such as that of
Murashige and Skoog (1962), may be used. However, the present inventors have
found the basal media described in Table 1 to give excellent results,
particularly
when used for culturing Douglas fir (Pseudotsuga menziesii). The basal media
are
modified for each of the various culturing stages as shown in Table 2. Similar
media
particularly preferred for Norway spruce (Picea abies) are given in Tables 7
and 8.
Gibberellins are diterpenoid acids, all which have the same basic ent-
gibberellane ring structure. As Sponsel (1987) describes them, there are two
basic
types, one having 20 carbon atoms and the other having only 19, one carbon
atom
having been lost by metabolism. There are 72 known gibberellins, only a small
portion of which have biological activity. It is not uncommon to find as many
as 20
different gibberellins in given species of vascular plants. Only a few of the
gibberellins are readily available commercially. These are most usually
prepared by
isolation from the fungus Gibberella fujikuroi in which they occur in some
quantity
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
t
18
as metabolites. Of the known gibberellins, 25 have been found in Gibberella.
Gibberellic acid (GA3) is the end product of gibberellin metabolism in G.
fujikuroi.
DOUGLAS FIR CULTURE
As noted in the background discussion of our earlier U.S. Patent
5,036,007, the embryogeny of Douglas fir is quite different from trees such as
the
spruces or pines. One of these differences is seen when early stage proembryos
are
placed in or on a late stage proembryo development medium. Instead of single
late
stage embryos, Douglas fir develops tight clumps of these embryos. Upon
further
development into cotyledonary embryos, these clumps remain united and the
resulting product is difficult to work with for further conversion. This
phenomenon
had apparently been recognized earlier by Durzan and Gupta (198'n who, while
they
did not discuss it specifically, transferred their embryonal-suspensor masses
to a
liquid shake culture containing 0.5 ~cM abscisic acid. They note that under
the
influence of ABA, individual bipolar embryos were produced which were then
transferred to a development medium without ABA. The present method utilizes a
series of liquid shake cultures with reduced osmotic level and added abscisic
acid
between late proembryo development and cotyledonary embryo development stages
to achieve the necessary singulation. Osmotic level is again raised to levels
generally
above about 450 mM/kg during the final cotyledonary embryo development stage
or
stages.
A basal culture medium has been developed by the present inventors
specifically to give more successful initiation and multiplication of Douglas
fir.
Preferred media compositions are given in the following tables. A number of
ingredients may be varied in quantity, such as those that affect the level and
balance
between organic and inorganic nitrogen, depending on the response of
individual
genotypes. This response cannot be readily predicted and media optimization
must ,
largely be achieved by a combination of intuition and trial and error.
A number of abbreviations are used in the following text. These are
in common use in the field of tissue culture.
SUBSTITUTE SHEET
WO 95!05070 PCT/US93/07803
19
BAP -- N6-benzylaminopurine (or IV6-benzyladenine), a cytokinin.
KIN -- kinetin (6-furfurylaminopurine), also a cytokinin
2,4-D -- 2,4-dichlorophenoxyacetic acid, an auxin
NAA -- 2-naphthylacetic acid (naphthalene-2-acetic acid), also an
auxin.
ABA -- abscisic acid (5-(1-hydroxy-2,6,b-trimethyl-4-oxo-2-
cyclohexen-1-yl)-3-methyl-2,4-pentadienoic acid), a maturation promoter.
GA or GAs -- a generic term referring to one or more of the more than
70 closely related compounds formerly known as gibberellic acid, Gibberellins
promote cell growth and elongation.
GAn; e.g., GA3 -- refers to a specific gibberellin.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
20
Table 1
Pseudotsuga Menziesii Basic Culture a '
Medi
S Constituent Conc entration. m~/L
WTC~I) BMG~2)
EASAL SALTS
NH4N03 ~'' --- 206.3
KN03 Varies~l) 1170.0
CaC 12 6H20 200.0 220.0
Ca(N03)2 2H20 Varies~l) ___
KH2P04 340.0 85.0
MgS04 7H20 400.0 185.0
MnS04 H20 20.8 8.45
ZnS04 7H20 8.0 4.30
CuS04 SH20 0.024 0.013
FeS04 7H20 27.85 13.93
Na2EDTA 37.25 18.63
H3B03 5.0 3.10
NaMo04 2H20 0.20 0.0125
CoCl2 6H20 0.025 0.0125
KI 1.00 0.42
ORGANIC ADDITIVES
myo-Inositol Varies~l) 100.0
Thiamine HCI 1.00 1.00
Nicotinic acid 0.50 0.50
Pyridoxine HCI 0.50 0.50
Glycine 2.00 2.00
L-Glutamine Varies 450.0
Casamino acids 500.0 ---
Sucrose Varies 20,000.
pH 5.7 5.7
~l)Usage varies according to culturing stage and genotype. ,
~2)1VIVIodified Gupta and Durzan medium BM3 (1986). Medium BMA of
U.S. Patent 5,034,326.
SUBSTITUTE SHEET
WO 95/05070
PCT/US93/07803
21
~s c7 0
N~.~ ~ t~ i g ~ ~ ~ i ~ ~ i i ~ i
i ~ ~ ~ ~ i i ~ ,
CO
C/~ N pp
N
> E E-r g g ~ g ~n g r~
p~p~,O U N ; ~'~ ~ M i ; ; N
3 $ .-, ~°~n N ~ ~ 0 0
M
.-w O O
M
C
O
bhp ~ U ~O ~ ; ; i ; ; "-~ ~ i i , O
c~ dp ~ .~ N ~' .~ p ~ ~ ~ i i N
N
N
"~ U ~..a
~ C U O ~ ~ N N O
cd ~ ; M ~ ~ O ; N N ; ; ; ; ; N
N O ~-~ O O
M ..O'.,
H
a
i
b4 0 U N ; V7 i ; N N ; ; ; ;
.-.a et ~ '-'' O O ~ N
VI.,~. ~ N ..Ø~
""', C
~.
a
'-' o ~ a
o ; ~ ; o ~ ~ ; ; g 52 ;
~' ~ ~ " N ~~n5 ~ , o
~ ~n
_c ~ ~ ;v~
aQ~
.~.N .'~.. ~ ~~ a
,., , o
V7 E ~ '' ~ a~ C ~ U
O C .~ O N V .NS", C .'v-' 4N., '~' T.""',' ~' ~ O
~'; ~'~" ...~.~ p Q., A ~ 'i.~', ~ V ~ ~ ice.. ~ O r.a ~
a ~ ~ ~ U C. .~. ~ ~ ~ :fl U by N a ~ ~-r H O
~x U s .~ a ~ ~ N ~z x a ~ a a
v ~ ~i yr
WO 95/05070 PCT/US93107803
2I~~~~~
22
It will be seen by reference to the media compositions that the features
of the earlier inventions described in our parent applications are
advantageously used ._
with Douglas fir. A raised osmotic level following initiation is desirable for
good
quality late proembryo development. This level will differ somewhat between
genotypes within each species as it does between species. Similarly, the level
of
abscisic acid present should be gradually reduced during the singulation stage
and
also during the cotyledonary embryo development period, if exogenous ABA is
used
in that stage. This may be done either by the inclusion of activated charcoal
in the
medium or by a stepwise reduction effected by multiple transfers to media of
successively lower ABA concentration. The exogenous ABA level is preferably
gradually reduced from that present at the beginning of the singulation stage
so that
little or none is available at the end of the development period. Selected
gibberellins
may also be advantageous when added during the singulation stage in an amount
of
about 0.05-15 mg/L, preferably about 0.1-10 mglL. However, they should be
present in the medium used for cotyledonary embryo development in a
concentration
of about 0.5-50 mg/L, preferably at initial levels between about 2.5 mg/L and
mg/L.
The examples that follow represent the best mode known at present for
culturing Douglas fir by somatic embryogenesis. With two exceptions, these
20 examples are principally directed to the cotyledonary embryo development
stage.
The steps prior to that time will first be briefly outlined in the following
example.
Exam In a 1
A preferred explant for Douglas fir is an immature zygotic embryo.
25 Best results have been realized with embryos selected in the interval just
prior to the
development of an apical dome up to the time just before cotyledon primordia
become visible. The cones are split longitudinally and seeds isolated from
young '
ovuliferous scales. Seeds are sterilized by first being agitated in 10~ Liqui-
Nox
laboratory cleaner (Alconox, Inc., New York, New York) with a small additional
amount of liquid surfactant for about 10 minutes. They are then rinsed in
running
tap water for 30 minutes. At this time they are transferred to a sterile hood
and
SUBSTITUTE SHEET
WO 95!05070 PCT/LTS93/07803
21 b75~~
23
agitated in 20 % H202 for 10 minutes. Following five rinses in sterile
deionized
water the seed coat is split and the female gametophyte removed. This is split
on
one side and the embryo teased out while still remaining attached to the
gametophyte
by the suspensor. An explant so prepared is placed on the Stage I solid
initiation
medium in a 50 mm petri dish. The explants are incubated in the dark from 4-8
weeks. Success in forming an embryonal-suspensor mass (ESM) containing
proembryos varies from about 1-10% depending on a number of variable factors
which presently are not well understood.
All stages of culture are carried out at temperatures which may vary
between about 20°-25°C. Temperature is not generally critical
and may, on
occasion be varied so as to fall outside this range.
The embryonal-suspensor masses containing early stage proembryos
are transferred to a solid Stage II maintenance and multiplication medium
containing
greatly reduced plant growth hormones and preferably a somewhat raised osmotic
level. Again, culturing is carried out in the dark with subcultures made at no
greater
than about two week intervals. The clone can be maintained at this stage for
long
periods of time. Low concentrations of a gibberellin and/or abscisic acid are
frequently beneficial at this stage of culture.
Early stage proembryos from the multiplication step are preferably then
transferred to a liquid Stage III second maintenance medium having a
significantly
raised osmotic level. An osmotic level of at least about 170 mM/kg will
usually
suffice for Douglas fir although some genotypes may require levels as high as
240 mM/kg. Myo-inositol, which will normally be around 5000 mg/L, may need to
be adjusted somewhat depending on the needs of the particular genotype in
order to
obtain optimum results. Culture is carried out in the dark and is periodically
subcultured, usually weekly. Robust late stage proembryos having 100 or more
cells
will develop during this time, normally 3-4 weeks.
Following late proembryo development, the cultures are transferred to
a Stage IV liquid medium for the singulation step referred to earlier. This
has a
reduced osmotic level and is free of auxins and cytokinins. Abscisic acid is a
newly
added hormone in an initial amount in the range of about 5-15 mg/L, more
usually
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
24
about 5-10 mg/L. Cultures are again carried out in the dark. From two to four
subcultures are made on a weekly basis. The level of exogenous abscisic acid
will
drop somewhat during each subculture. It is generally preferred that the level
of
abscisic acid at the beginning of a new subculture should not be significantly
higher .
than the level used in the previous subculture. A preferred schedule is one
week on
a medium containing 10 mg/L ABA, a second week on a medium containing 5 mg/L
ABA, and a third week on a medium also with 5 mg/L ABA. This gradual decrease
in ABA level will continue through the development period. As with the
maintenance medium, a low concentration of an active gibberellin appears to be
advantageous in the singulation media.
Following the singulation period the embryos are ready to complete
their development to cotyledonary embryos. They are transferred to either a
solid
medium or supported on a liquid medium. This will normally contain exogenous
ABA which may be present up to about 50 mg/L. More typically, ABA will not
generally exceed about 10 mg/L and most usually will not initially exceed 5
mg/L
and may be considerably lower. In some cases it is not necessary to add any
exogenous ABA to the development medium since a sufficient amount will be
carried
over with the residual singulation or rinse medium accompanying the embryos
when
the transfer is made from the last singulation stage. The development medium
will
also contain from 0.5-50 mg/L of a selected gibberellin. This is preferably
GA4~7-
GA3 is also useful although it is somewhat less effective in most cases. Other
active
gibberellin would also be expected to be beneficial at this stage. In cases
where an
adsorbent such as activated charcoal is not used in the development medium
concentrations of GA and ABA will be significant lower than the maximum levels
just noted; e.g., by a full order of magnitude.
It has been found preferable for Douglas fir to carry out development
cultures entirely in the dark. Activated charcoal is preferably used in the
development medium to effect ABA reduction over time but it is not essential.
Particularly for Douglas fir, a raised osmotic level in the development medium
is
very highly desirable. Osmotic levels should be above about 400 mM/kg and for
SUBSTITUTE SHEET
WO 95!05070 PCT/US93/07803
some genotypes may advantageously be considerably higher. The effect of
osmotic
. level is discussed in detail in our,earlier U.S. Patent 5,036,007.
Example 2
5 This experiment was designed to test the effect of the gibberellin GA3
on embryo development and subsequent germination. Late stage Douglas fir
proembryos were singulated in a three-step liquid shake culture as outlined
above in
media containing respectively 10, 5, and 5 mg/L ABA and lacking any other
growth
hormones. The medium from the last stage was from the embryos and they were
10 rinsed with a development medium lacking activated charcoal and containing
2.5 mg/L ABA, 20.0 g/L (2 % ) sucrose and no supplemental carbohydrate. The
rinsed embryos were then transferred onto a development medium having 750 mg/L
glutamine, 50.0 g/L (5 % ) sucrose, 175 g/L ( 17.5 % ) polyethylene glycol
7000,
24.5 g/L (2.45 % ) lactose, and 1 g/L (0.1 % ) activated charcoal and without
ABA.
15 GA3 levels were varied from 0-25 mg/L. The media were absorbed into a
double
thickness pad of polyester batting and the rinsed proembryos placed on top of
the
pad. Embryos from five different genotypes were used. One of the five
genotypes
did not form cotyledonary embryos under any of the conditions employed here.
Embryos without GA3 averaged 1.8 mm in length. Those with 5 mg/1
20 GA3 had a mean length of 1.95 mm and those with 25 mg/L GA3 averaged 2.1 mm
in length. The differences between no hormone and the use of 5 or 25 mg/L GA3
was statistically significant. The embryo shape of the gibberellin treated
embryos,
when observed under low power magnification, was improved over that lacking
this .
growth hormone. By improved it is meant that the embryos were more robust and
25 more closely resembled zygotic embryos in size and overall morphology.
Specifically, the hypocotyl and root cap length were increased in comparison
to
embryos cultured without GA in the medium.
Further tests of the above embryos showed improved germination
percentages of the embryos cultured with 25 mg/L GA3 when compared with those
lacldng this hormone.
SUBSTITUTE SHEET
WO 95/05070 PCT/L1S93107803
26
x m 1
In view of the improved embryo morphology noted in the previous
example, additional experiments were conducted to further determine the
effects of
GA3 used by itself and in combination with ABA. Earlier work has shown that
ABA
alone caused a very significant yield increase and that the embryos so
cultured were
of similar or somewhat smaller size compared with those grown in A-BA free
cultures. An additional trial was made using a GA4~7 mixture without ABA.
GA4~7
was tried because it is known to be more active than GA3 in some conifers when
used for inducement of flowering. Singulation conditions were identical to
those
used in the previous example as was the rinse prior to transfer of the
proembryos to
the development medium. Four genotypes were tested. Three of the genotypes
were
those used in the previous example and were among those that had produced
cotyledonary embryos. The other genotype was new to this trial. Except for the
variables of GA and ABA, other components of the development media used in
this
test were identical to those used in Example 2. GA3 concentration was varied
using
0, 1, 2.5, 5, and 10 mg/L. Trials were also made using 10 and 20 mg/L ABA in
combination with 5 mg/L GA3. A single trial was made using GA417 by itself at
5 mg/L. Two genotypes responded well and produced embryos under all
conditions.
The other two genotypes generally responded poorly under all conditions.
In those genotypes that responded well, significant improvement in
embryo length and morphology were seen using 5 or 10 mg/L GA3 or 5 mg/L GA417
compared to the control samples without any added growth hormones. Average
length without hormones was 2.1 mm compared with 2.3-2.4 mm when 5 and
10 mg/L GA3 or GA4~ was used. Embryos developed using the GA3lABA
combinations averaged a bit over 1.9 mm in length. Visual evaluation of the
GA~l7
embryos showed them to be slightly more tapered in the hypocotyl and root cap
regions than those grown on the GA3 media. While the trials using the
combination
of 5 mg/L GA with ABA showed a decrease in embryo length, there was a ,
significant change in visual morphology. The suspensor/rootcap region was more
similar to that seen in zygotic embryos in seeds. The suspensor elongated and
remained narrow and the embryo tapered to flow smoothly into the suspensor, as
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
~~6~5~~
27
seen in FIG. 6B. Without hormones, or with either GA3 or ABA alone, the
suspensor cells proliferate and the embryo appears to emerge from a stumpy
mass
of cells, as shown in FIG. 6A.
What appears here to be a morphological synergism between GA and
ABA is interesting because these hormones are reported to be antagonists in
most of
the literature relating to angiosperms.
x m 1 4
This factorial experiment employed five levels of GA4/~, 0, 1, 2.5, 5,
and 7.5 mg/L, each with 0 or 10 mg/L ABA. Singulation and rinsing were as de
scribed in Example 2. The basal development medium was modified slightly,
howev
er. Sucrose was reduced to 40,000 mg/L (4 % ) and lactose was raised to 33.0
g/L
(3.3090). Five genotypes of Douglas fir were tested. Three had been used in
either
the trials of Examples 2 or 3 and two were new to this trial. One of the new
genotypes performed poorly. The others produced good cotyledonary embryos.
The following conclusions can be drawn from this test. GA4/7 alone
in all concentrations increased embryo elongation relative to the control
samples
lacking GA. All combinations of GA and ABA also showed increased length. ABA
alone at 10 mg/L produced embryos of comparable length to the control sample.
These lengths were measured as follows:
SUBSTITUTE SHEET
WO 95/05070 PCT/US93107803
28
Table 3
ABA GA4~7 Embryo length, mm
0 0 1.95
0 1 2.2
0 2.5 2.3
0 5 2.35
0 7.5 2.45
0 2.0
10 10 1 2.4
10 2.5 2.45
10 5 2.4
10 7.5 2.35
The differences in length between the control and 10 mg/L ABA sam-
ples are not statistically different nor are the differences between any of
the GA
treated samples. However, differences between the samples with and without GA
treatment are highly significant.
Visual observation showed embryo shape to be improved in treatments
combining GA and ABA with the best treatment qualitatively being 10 mg/L ABA
and 7.5 mg/L GA4~7. Since these were the highest levels used of both hormones
the
suggestion is present to explore the effect of still increased levels.
ml
To follow up the possibility of even better performance at higher levels
of hormones another factorial experiment was conducted using 0, 10, and 20
mg/L
ABA and 0, 5, 7.5, and 10 mg/L GA~~7- Other than the hormone levels, media
composition and earlier treatments were the same as those of Example 4. Four ,
genotypes were used. Three of these were common with those of the previous
trials.
The one genotype that did not produce embryos in Example 2 yielded good
embryos
in this trial.
SUBSTITUTE SHEET
WO 95/05070
PCTlUS93/07803
29
Results were similar to those of the previous Example. All treatments
using GA4/7 in any concentration produced longer embryos than control cultures
lacking this hormone. ABA did not appear to have any effect on embryo length
although with no GA present embryo length trended downward with increasing ABA
concentration. Embryos grown on any of the GA4/7 media averaged about 2.4 mm
while those without were about 1.9-2.0 mm in length. GA4/7 alone improved
embryo shape. Combinations of ABA and GA4n produced the best embryos. These
embryos are similar to those sketched in FIG. 6B. In particular, embryos with
the
combined treatment showed longer cotyledons and greater taper and were most
similar to zygotic embryos. All of the embryos were classified as to one of
five
shapes. A nonparametric statistical test showed the shape differences noted
above
to be significant. There did not appear to be any advantage to hormone levels
higher
than those of Example 4. Again, the best appearing embryos were those
developed
on medium containing 10 mg/L ABA and 7-5 mg/L GA4/7.
A representative sample of the embryos were sectioned for organ meas-
urement. The "Control" sample had no hormones present in the development
medium. "GA Alone" used 7.5 mg/L of GA4/7 in the development medium. "GA
+ ABA" had 7.5 mg/L GA4~ and 10 mg/L ABA in the development medium.
Results appear in the following table.
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
216~5~~
Tabs ,
CotyledonHypocotyl Root Cap HypocotylTotal
5 Treatment Length. Length, L,~ngth. Width. Length. mm
mm mm mm mm
Zygotic 0.89 1.51 1.17 0.49 3.57
Control 0.18 0.55 0.49 0.51 1.22
GA Alone 0.39 0.69 0.55 0.49 1.63
GA + ABA 0.33 0.60 0.60 0.44 1.52
Observation and measurement of the apical dome can provide an
indicator of expected successful germination. These measurements were also
made
on the above sections and are reported in the following table.
Table 5
Mean Dome Maximum Dome Minimum Dome Embryos
Treatment Height. Height. mm Height, mm With Dome,
mm %
Zygotic . 0.112 0.184 0.0490 100
Control 0.33 0.061 0 82.2
GA Alone 0.039 0.084 0 97.2
GA + ABA 0.037 0.069 0.015 100
While not yet equal in size to zygotic embryos, the improvements in
quality brought about by incorporation of GA or GA and ABA in the development
media are clearly evident from the above tables.
It would appear that each hormone plays an important role in embryo '
development. GA seems to increase embryo length and improve cotyledon and
apical dome development. ABA may be critical to development in the root
cap/suspensor region in addition to its role in prevention of premature
germination
and accumulation of storage products.
SUBSTITUTE SHEET
WO 95105070 PCT/US93/07803
31
Selected embryos from two genotypes were placed on a germination
medium as described in Table 2. Embryos showing a root at least 3 mm long and
an epicotyl were considered germinated. There was a statistically significant
increase
in germination of embryos grown on media containing only GA4/7 at all levels
as
was also the case for those grown on ABA/GA containing media. ABA alone in
either concentration did not increase germination percentage.
It would appear to be beneficial to somatic embryo development to
begin addition of gibberellins even earlier in the culturing process than has
been
heretofore described. In the case of Douglas fir, this benefit may accrue by
addition
of GAs to at least the final and probably the earlier singuladon stages. There
are
further indications that GAs may have beneficial effects for all coniferous
species
when added to the late stage proembryo development medium or even to the
maintenance medium for the early stage proembryo cultures. On the, other hand,
it appears that the benefit of GAs is not fully realized, or not realized at
all, when
added later than about the midpoint of the cotyledonary embryo development
period.
This will be illustrated by the following examples.
Douglas fir embryos from two genotypes were cultured as in the
previous examples but with the following modifications in the third
singulation
shake. In one set of cultures the nominal 5 mg/L ABA was supplemented with 0,
1, 2.5, and 5 mg/L GA4/~. In the other set the ABA was supplemented with 0, 1,
2.59 and 5 mg/L GA4n. After the one week shake period the cells were rinsed as
in the earlier examples and plated on one cotyledonary embryo development
medium
which lacked growth hormones entirely, and on another which contained
10 mg/L ABA and 7.5 mg/L GA4/7. Otherwise the development media were similar
in composition to that of Examples 4 and 5.
At the end of the development period, embryos of both genotypes
grown using the modified hormone regimen in the third singulation shake were
not
significantly different in size or appearance from the control embryos using 5
mg/L
ABA alone in the third singulation shake culture. The embryos were placed on
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
32
germination medium and left for three weeks in the dark. At that time it
became
apparent that there were differences in germination. Statistically significant
increases ,
in root length occurred with embryos cultured with 2.5 or 5 mg/L GA4~~ plus
mg/L ABA in the third shake and then further developed on a medium containing
5 10 mg/L ABA and 7.5 mg/L GA4/7. Thus, while not initially visually apparent,
there appears to be ultimate benefit in extending GA treatment back into the
singulation stage.
m le 7
Since it was shown to be advantageous in Douglas fir culture to add
GA to the media of the singulation stage used prior to the cotyledonary embryo
development medium, an additional set of trials was run to investigate the
effect of
GA used even earlier in the process. In the present example GA4~ was added to
the
Stage II maintenance medium (refer to Table 2) for Douglas fir early
proembryos.
GA levels were 0, 0.1, 0.5, and 1.0 mg/L. These were combined with ABA at 0
and 0.5 mg/L in a factorial experiment design. Subcultures to fresh media were
made on a weekly basis for eight weeks. It is noted that neither ABA or GA is
ordinarily used in the maintenance media. Three genotypes were used in the
trial.
Embryos were rated visually every week for each genotype and the
ratings were averaged over the trial. A rating of 0 indicates that no embryos
are
visible among the cell mass. A rating of 1 indicates that embryonic heads are
clearly
visible. A 2 rating is given when the heads and attached suspensors have
achieved
at least a late stage proembryo form. A rating of 3 indicates further
enlargement of
the head and attached suspensors to a size typical of the embryos at the end
of the
singulation process. Results are given in the following table.
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
Z167~~
33
P roembryo e
Ratin
Tri o. ABA. mg/LA m L keno. 711 Geno 917.28Geno 925.22
1 0 0 1.9 1.6 1.4
2 0 0.1 2.1 1.7 1.5
3 0 0.5 - 2.2 1.9 1.6-
4 0 1.0 2.2 1.8 1.6
5 0.5 0 2.3 1.9 1.7
6 0.5 0.1 2.4 1.8 1.7
7 0.5 0.5 2.4 1.8 1.8
8 0.5 1.0 2.5 1.8 1.8
Results clearly show that either GA alone or ABA alone improve
embryo quality. The combination of GA and ABA appears to have additive effect,
at least in two of the genotypes. It has been generally established that
larger
proembryos entering the development stage will most usually result in larger
and
more robust cotyledonary embryos.
NORWAY SPRUCE CULTURE
While the media compositions and growth hormone usages described
in the previous examples of this application are those that we presently
regard as
optimum for Douglas fir, different concentrations and mixtures appear more
suitable
for other species. The following tables show preferred media for culture of
Norway
spruce by somatic embryogenesis.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
34
Table 7
Picea Abies Basic Culture Media '
Constituent Concentration. mgt
An) B(2)
BASAL SALTS
NH4NO3 -- 206.3
KCl 372.5 --
KN03 50.0 2340.0
KH2PO4 85.0 85.0
MgS04 7H20 160.0 185.0
CaCI2 6H20 220.0 220.0
KI 0.415 0.415
H3B03 3.10 3.10
MnS04 H20 8.45 8.45
ZnS04 7H20 4.30 4.30
NaMo04 2H20 0.125 0.125
CuS04 SH20 0.0125 0.0125
CoCl2 6H20 0.0125 0.0125
FeS04 7H20 13.90 13.93
Na2EDTA 18.65 18.63
ORGANIC ADDITIVES
Sucrose 10,000 30,000
myo-Inositol 50.0 1000.0
Casamino acids -- 500.0
L-Glutamine 750.0 450.0
Thiamine HC 1 0.05 1.00
Pyridoxine HC 1 0.05 0.50
Nicotinic acid 0.25 0.50
Glycine -- 2.00
L-Asparagine 50.0 --
pH 5.8 5.7
(1) Institute of Paper Chemistry medium (Verhagen and, Wann 1989)
~) Gupta and Durzan medium BM3 (1986b).
SUBSTITUTE SHEET
WO 95!05070 PCT/I1S93/07803
~1675~0
Table 8
Composition of Picea AbieS Media for Different Stage Treatments
5 BMI -- Induction Medium
BMp~I~ + NAA~31 (10.8~,M) + BAP~4~ (4.4~,M) + 7.Og/L Difco
agar.
BMM -- Maintenance and Multiplication Medium
10 BMB~2> + 2,4-D(~ (5 ~cM) + BAP (2 ~cM) + KIN~6> (2 ~cM).
6.0 g/L Difco agar added if solid medium is desired.
BMD -- Cotyledonary Embryo Development Medium
BMp + 40.0 mg/L Arginine + l~ mg/L Asparagine + 6.0 g/L
15 Tissue Culture Agar + Abscisic acid (as specified) + Activated
charcoal 1.25 g/L. KN03 is reduced to 1170 mg/L in basal salts.
BMG -- Germination Medium
BMB with KN03 reduced to 1170 mg/L, myo-Inositol reduced to
20 100 mg/L, Sucrose reduced to 20.0 g/L, and L-Glutamine and
Casamino acids removed. 2.5 g/L of Adsorbent and 6.0 g/L of
Tissue Culture Agar are added.
~l~Basa1 medium A from Table 4
25 ~2>Basic medium B from Table 4
~~2-Naphthylacetic acid (Naphthalene-2-acetic acid)
~4>PI~-Benzylaminopurine
«2,4-Dichlorophenoxyacetic acid
~6>Kinetin
E»am 1D a $
Norway spruce late stage proembryos were plated directly from a
maintenance medium onto solid development media containing 50 mg/L ABA and
concentrations of GA4n of 0, 1, 2.5, 5, 7.5, 10, and 15 mg/L. After six weeks
development it was noted that embryo yield was significantly reduced in all
cultures
where GA4/7 was present. Little difference in yield was seen at any
concentration
of GA4/7. The magnitude of the differences was from an average of about 57
embryos per plate when no GA was present to about 33 embryos per plate for the
GA containing cultures. However, as early as three weeks after plating on the
development media, visible differences were seen between embryos on the GA
SUBSTITUTE SHEET
WO 95105070 PCT/US93/07803
21~~~~0
36
treated media and those in the control culture lacking GA. Ultimately the
cotyledonary embryos from the GA containing cultures showed increased length
and
greater taper and generally had greater resemblance to zygotic embryos.
Concentrations of 5 and 10 mglL GA41~ were most effective.
m1
This trial was made with some development media having activated
charcoal removed in order to better understand the specific individual and
combined
effects of ABA and GA. In those media lacking charcoal, hormones were reduced
by a factor of 10 and transfers to fresh media were made on a biweekly basis.
At
the time of the first transfer, inorganic nitrogen (KN03) was halved and amino
acid
concentration increased (see Table 9 footnotes). ABA and GA417 concentration
was
held constant during the entire development time for any given treatment.
Development media were modified as shown in the following table.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
37
Table 9
Media Initial First Second ABA GA4/7 Activated
N~. Medium Transfer Transfer mg/L m L Charcoal.
~
1 a No transfers made 50 0 0.125
2 a No transfers made 50 15 0.125
3 b c c 0 0.1 0
4 b c c 0 0.5 0
5 b c c 0 1.5 0
6 b c c 5 0 0
7 b c c 5 0.5 0
8 b c c 5 1.0 0
9 b c c 5 1.5 0
10 b c c 0 0 0
Basal Medium Inorganic Nitrogen/Amino Acid Nitrogen
a. 2340 mg/L KN03, 40 mg/L arginine, 100 mg/L asparagine,
b. 2340 mg/L KN03, 0 mg/L arginine, 0 mg/L asparagine.
c. 1170 mg/L KN03, 40 mg/L arginine, 100 mg/L asparagine.
Average embryo lengths were measured as follows:
Table 10
Treatment Embryo Yield, Embryo
No. Number Per Plate Length.
mm
1 25 2.77
2 28.5 3.05
3 2 --
4 2 --
5 2 --
6 12 2.93
7 12 3.12
8 13 3.13
9 14 2.92
10 2 --
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
38
Treatments 3, 4, 5, and 10, lacking both ABA and activated charcoal,
developed only a few poor quality precociously germinating embryos. Thus,
either .,
no hormones, or GA alone, were sufficient to generate useful quantities of
good
quality embryos. Yields improved, as did embryo size and shape, when GA and
ABA were used together, even when charcoal was absent (Treatments 7-9). Shape
of these embryos was improved over Treatment 6 which had ABA but lacked GA.
Yields of normal cotyledonary embryos were significantly higher in the two
treatments ( 1 and 2) containing activated charcoal. Treatment 2, containing
activated
charcoal, GA, and ABA produced the largest embryos. These also had a
significantly improved shape; i. e. , more closely resembled zygotic embryos
in
morphology.
As with Douglas fir, gibberellins appear to have a role in determining
embryo size and interact with ABA in determining shape and yield. This is
contrary
to the findings of others that GA and ABA are antagonistic.
Following embryo development the somatic embryos may be retained
for some period of time in cold storage. They may be converted into artificial
seeds
for field or nursery planting. Alternatively, they may be placed immediately
on a
germination medium for conversion into plantlets prior to planting in soil.
It should be recognized that there is not one single set of culturing
conditions that will be suitable for achieving somatic embryogenesis of all
species
or for all genotypes within a species. Tissue culture as a whole is a highly
unpredictable science. This statement has even greater applicability to
somatic
embryogenesis'. Adjustments in the mineral and plant hormone constituents of
the
culture media must frequently be made depending on the particular species and
genotype being cultured. This applies to each of the various stages of
culturing from
explants to plantlets. These adjustments are considered to be within the
routine
experimental capability of those skilled in the art of tissue culture. The
important
discovery of the present invention is the usefulness of various gibberellins
for ,
improvement of embryo quality. Gibberellins have been found beneficial by
including them in the process as early as the proembryo maintenance cultures.
They
are particularly useful when combined with abscisic acid in the cotyledonary
embryo
SUBSTITUTE SHEET
WO 95/05070 PCT/LTS93/07803
2167500
39
development media. These procedures have given results that are far superior
in
terms of success and consistency than any process reported heretofore. The
process
has been successfully applied to several species and many genotypes of
coniferous
plants studied to date and appears to be of general use for all coniferous
species.
It will be understood that many variations can be made in the
procedures described for the various culturing stages while still remaining
within the
spirit of the present invention. It is the intention of the inventors that
such variations
should be included within the scope of their invention if found defined within
the
following claims.
BIBLIOGRAPHY
Abo El- Nil, Mostafa M.
1980 Embryogenesis of gymnosperm forest trees. U.S. Patent 4,217,730.
Ammirato, Philip V.
1977 Hormonal control of somatic embryo development from cultured cells
of caraway: interactions of abscisic acid, zeatin, and gibberellic
acid. Plans Physiology 59: 579-586.
1983 The regulation of somatic embryo development in plant cell cultures:
suspension culture techniques and hormone requirements.
BiolTechnology 1(3): 68-74.
Chalupa, V.
1990a Somatic embryogenesis and plant regeneration in Quercus petraea
(Matt.) Liebl., ~lia platyphyllos Scop. and Aesculus hippocastanum
L. Lesnictvi (Prague) 36: 599-604.
1990b Plant regeneration by somatic embryogenesis from cultured immature
embryos of oak (Quercus robur L.) and linden (Tilia cordate Mill.
Plant Cell Reports 9: 398-401.
Cruz, Gil S., Jorge M. Canhoto, and Maria Alexandra V. Abreu
1990 Somatic embryogenesis and plant regeneration from zygotic embryos
of Feijoa sellowiana Berg. Plant Science 66: 263-270.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
21~7~~0
Durzan, D. J. and P.K. Gupta
1987 Somatic embryogenesis and polyembryogenesis in Douglas fir cell ,
suspension cultures. Plans Science 52:229-235.
Eapen, S., V. Abraham, M. Gerdemann, and O. Schieder '
5 1989 Direct somatic embryogenesis, plant regeneration and evaluation of
plants obtained from mesophyll protoplasts of Brassica juncea.
Annals of Botany 63: 369-372.
Eapen, Susan, and Leela George
1990 Ontogeny of somotic embryos of Ygna aconitifolia, Ygna mungo and
10 Vigna radiata. Annals of Botany 66: 219-226.
Evans, M.L.
1984 Functions of hormones at the cellular level of organization. In
Hormonal Regulation ofDevelopment II, Tom K. Scott Ed., pp 23-79,
Springer-Verlag, New York.
15 Ferriera, Alfredo Gui, Ching Yeh-Hu, and Elaine Romanato Santarem
1990 Somatic embryogenesis of soybean Glycine max (1.) Merril, Brazilian
cultivars Ivory and IAS-5. Phyton (Buenos Aires) 51(2): 139-144.
Garcia-Maya, M., J. M. Chapman, and M. Black
1990 Regulation of a-amylase formation and gene expression in the
20 developing wheat embryo: role of abscisic acid, the osmotic
environment and gibberellin. Planta 181: 296-303.
Ghosh, Biswajit and Sumitra Sen
1991 Plant regeneration through somatic embryogenesis from spear callus
culture of Asparagus cooperi Baker. Plant Cell Reports 9: 667-670.
25 Gmitter, F. G. Jr., X. B. Ling, and X. X. Deng
1990 Induction of triploid citurs plants from endosperm calli in vitro.
Theoretical and Applied Genetics 80: 785-790.
SUBSTITUTE SHEET
WO 95105070 PCT/US93/07803
216750
41
Gupta, Pramod K and Don J. Durjan
1986 Plantlet regeneration via somatic embryogenesis from subcultured
callus of mature embryos of Picea abies (Norway spruce). In Yrtro
Cellular & Development Biology 22: 684-688.
Gupta, Pramod K. and Gerald S. Pullman
1990 Method for reproducing coniferous plants by somatic embryogenesis.
U.S. Patent 4,957,866.
1991 Method for reproducing coniferous plants by somatic embryogenesis
using abscisic acid and asmotic potential variation. U.S. Patent
5, 036, 007.
Hakman, Inger and Sara von Arnold
1985 Plantlet regeneration through somatic embryogenesis in Picea abies.
Journal of Plaru Physiology 121: 149-158.
Kochba, J., P. Spiegel-Roy, H. Neumann, and S. Saad.
1978 Stimulation of embryogenesis in citrus ovular callus by abscisic acid,
ethepon, 2-chloroethyltrimethyl ammonium chloride, and Alar and its
suppression by gibberellic acid. Z. P,flanzenphysiol. Bd. 89: S. 427-
432.
Lakshmi Sita, G.
1985 Sandalwood (Santalum album). In Biotechnology in Agriculture and
Forestry I: Trees l, Y.P.S. Bajaj, ed. Springer-Verlag, New York.
Manrique, S.L. and W. Roca
1987 Effect of the photoperiod and the culture medium on somatic
embryogenesis and a histological analysis of the process in the cassava
Manihot esculenta Crantz. Acta Agronomira (Palmira) 37(2): 7-18.
Murashihe, Toshio and Folke Skoog
1962 A revised medium for rapid growth and bio assays with tobacco tissue
cultures. Physiologia Plantarum 15: 473-493.
SUBSTITUTE SHEET
WO 95/05070 PCT/US93/07803
9
42
Nolan, Randall C. and Tuan-Hua David Ho
1988 Hormonal regulation of «-amylase expression in barley aleurone
layers: the effects of gibberellic acid removal and abscisic acid and
phaseic acid treatments. Plant Physiology 88: 588-593. ,
Noriega, Clemencia and Maro R. Sondahl
1991 Somatic embryogenesis in hybrid tea roses. BiolTechnology
9: 991-993.
Pullman, Gerald S. and Pramod K. Gupta
1991 Method for reproducing coniferous plants by somatic embryogenesis
using adsorbent materials in the development stage media. U. S.
Patent 5,034,326.
Rajasekaran, K., M. B. Hein, and 1. K. Vasil
1987 Endogenous abscisic acid and IAA and somatic embryogenesis in
cultured leaf explants of Pennisetum purpureum Schum.: effects in
vivo and in vitro of glyphosphate fluridone, and paclobutrazol. Plant
Physiology (Bethesda) 84: 47-S 1.
Rangaswamy, N. S.
1986 Somatic embryogenesis in angiosperm cell tissue and organ
cultures. Proceedings Indian Academy of Sciences (Plant Sciences)
96(4): 247-271.
Sondahl, Maro R., T. B. Sereduk, Claudia M. Bellato, and Zhenghua Chen
1988 Somatic embryogenesis and plant regeneration of cacao. European
Patent Application A 0 293 598.
Sponsel, Valerie M.
1987 Gibberellin biosynthesis and metabolism. In Plant Hormones and
their Role in Plant Growh and Development, Peter J. Davies, Ed., pp
43-69, Martinus Nijhoff, Boston.
Tisserat, Brent and Toshio Murashige
1977 Probable identity of substances in citrus that repress asexual
embryogenesis. In Vitro 13: 785-789.
SUBSTITUTE SHEET
WO 95!05070
PCT/US93/07803
43
Trolinder, Norma L. , and J. R. Goodin
1988 Somatic embryogenesis in cotton (Gossypium). II: requirements for
embryo development and plant regeneration. Plant Cell, Tissue and
' Organ Culture 12: 43-53.
Verhagen, Shirley A. and Steven R. Warm
1989 Norway spruce somatic embryogenesis: high-frequency initiation from
light cultured mature embryos. Plant Cell, Issue and Organ Culture
16: 103-111
SUBSTITUTE SHEET