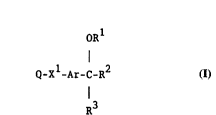Note: Descriptions are shown in the official language in which they were submitted.
~ w o 95/04055 ~ t 6 8 2 5 2 PCT~EP94/02408
THIAZOLE DERIVATIVES AS LIPOXYGENASE INHIBITORS
This invention concerns thiazole derivatives and more
particularly thiazole derivatives which are inhibitors of the enzyme
5-lipoxygenase (hereinafter referred to as 5-L0). The invention also
concerns processes for the manufacture of said thiazole derivatives
and novel pharmaceutical compositions cont~;ning them. Also included
in the invention is the use of said thiazole derivatives in the
treatment of various diseases such as inflammatory and/or allergic
diseases in which the direct or indirect products of 5-L0 catalysed
oxidation of arachidonic acid are involved, and the production of new
medicaments for such use.
As stated above the thiazole derivatives described
hereinafter are inhibitors of 5-L0, which enzyme is known to be
involved in catalysing the oxidation of arachidonic acid to give rise
via a cascade process to the physiologically active leukotrienes such
as leukotriene B4 (LTB4) and the peptido-lipid leukotrienes such as
leukotriene C4 (LTC4) and leukotriene D4 (LTD4) and various
metabolites.
The biosynthetic relationship and physiological properties
of the leukotrienes are summarised by G.W. Taylor and S.R. Clarke in
Trends in Pharmacolo~ical Sciences, 1986, 7, 100-103. The
leukotrienes and their metabolites have been implicated in the
production and development of various diseases, for example various
inflammatory and allergic diseases such as inflammation of the joints
(especially rheumatoid arthritis, osteoarthritis and gout),
inflammation of the gastrointestinal tract (especially inflammatory
bowel disease, ulcerative colitis and gastritis), skin diseases
(especially psoriasis, eczema and dermatitis), ocular conditions
(especially allergic conjunctivitis and uveitis) and respiratory
disease (especially asthma, bronchitis and allergic rhinitis), for
example in the production and development of various cardiovascular
and cerebrovascular disorders such as myocardial infarction, the
formation of atherosclerotic plaques, hypertension, platelet
aggregation, angina, stroke, reperfusion injury, vascular injury
including restenosis and peripheral vascular disease, for example in
W O 95/04055 2 ~ ~-8 2 5 ~ PCT~EP94/02408 ~
the formation of the conditions of shock or trauma such as can follow
burn injuries, toxaemia or surgery, and for example various disorders
of bone metabolism such as osteoporosis (including senile and
postmenopausal osteoporosis), Paget's disease, bone metastases,
hypercalcaemia, hyperparathyroidism, osteosclerosis, osteopetrosis and
periodontitis, and the abnormal changes in bone metabolism which may
accompany rheumatoid arthritis and osteoarthritis. In addition the
leukotrienes are mediators of inflammatory diseases by virtue of their
ability to modulate lymphocyte and leukocyte function. Other
physiologically active metabolites of arachidonic acid, such as the
prost~glanA;n~ and thromboxanes, arise via the action of the enzyme
cyclooxygenase on arachidonic acid.
It is disclosed in European Patent Applications Nos. 0385662
and 0420511 that certain heterocyclic derivatives possess inhibitory
properties against 5-LO. In particular, European Patent Application
No. 0462812 is also concerned with heterocyclene derivatives which
possess inhibitory properties against 5-LO. The heterocyclene
derivatives disclosed therein are of the formula
ORl
I
Arl_Al_xl_Ar2_c R2
I
R3
wherein Ar2 is a 5-membered heterocyclene moiety containing one
heteroatom selected from nitrogen, oxygen and sulphur such as
pyrroldiyl, furandiyl and thiophenediyl. Particular compounds
disclosed therein include the thiophenediyl derivatives:-
4-methoxy-4-[5-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-ylthio)-
thien-2-yl]tetrahydropyran (Example 7 therein); and (2S,4R)-4-methoxy-
2-methyl-4-[5-t1-methyl-2-thioxo-1,2,3,4-tetrahydroquinolin-6-ylthio)-
thien-2-yl]tetrahydropyran (Example 12 therein).
There is no disclosure in European Patent Application No.
0462812 of compounds wherein Ar is a 5-membered heterocyclene moiety
containing two heteroatoms selected from nitrogen, oxygen and sulphur.
~ W O 95/040$5 2 t ~ ~ 2 5 2 PCT~EP94/02408
-- 3 --
In particular there is no disclosure of a compound wherein Ar2 is a
thiazolediyl group.
It has been found that certain compounds disclosed in
European Patent Application No. 0462812 possess the undesirable
property of auto-induction i.e. the repeated dosing of such a compound
to a warm-blooded animal results in an increase in the efficiency with
which the animal's hepatic enzymes metabolise the compound. The
result is a decrease on repeat dosing of the quantity of the compound
present in the animal's blood stream as shown, for example, by a
decrease in the ~; concentration achieved (C max) or, for
example, a decrease in the exposure of the animal to the compound as
measured by the area under the curve (AUC) of a plot of the
concentration of the compound in the blood stream versus time after
dosing. The compound 4-methoxy-4-[5-(1-methyl-2-oxo-1,2,3,4-tetra-
hydroquinolin-6-ylthio)thien-2-yl~tetrahydropyran possesses the
undesirable property of auto-induction.
It has also been found that certain compounds disclosed in
European Patent Application No. 0462812 are non-cryst~lline, for
example they are formed in an oily or gummy state or they are isolated
as foams. Such non-crystalline compounds are undesirable when
consideration is given toward the preparation, purification, analysis,
h~n~l i ng and formulation of larger quantities of the compounds. The
compound (2S,4R)-4-methoxy-2-methyl-4-[5-(1-methyl-2-thioxo-1,2,3,4-
tetrahydroquinolin-6-ylthio)thien-2-yl]tetrahydropyran possesses the
undesirable property of being a viscous oil.
We have now discovered that certain thiazole derivatives are
preferred inhibitors of the enzyme 5-L0 and thus of leukotriene
biosyntheses. Thus such compounds are of value as therapeutic agents
in the treatment of, for example, allergic conditions, psoriasis,
asthma, cardiovascular and cerebrovascular disorders,
inflammatory and arthritic conditions, and/or disorders of bone
metabolism, mediated alone or in part by one or more leukotrienes.
According to the invention there is provided a
thiazole derivative of the formula I
W O 95/04055 2 1 6 8 2 5 2 PCT~EP94/02408 ~
ORl
Q_x1-Ar-C-R2
I
R3
wherein Q is 2-oxo-1,2,3,4-tetrahydroquinolin-6-yl or
2-oxo-1,2-dihydroquinolin-6-yl which bears on the nitrogen atom at the
1-position a (1-4C)alkyl substituent;
xl is thio, sulphinyl or sulphonyl;
Ar is thiazolediyl;
R1 is (1-4C)alkyl, (3-4C)alkenyl or (3-4C)alkynyl; and
R2 and R3 together form a group of the formula -A2-X2-A3- which
together with the carbon atom to which A2 and A3 are attached define a
ring having 5 or 6 ring atoms, wherein A2 and A3, which may be the
same or different, each is (1-3C)alkylene and x2 is oxy, and which
ring may bear one or two (1-4C)alkyl substituents;
or a ph~ ~ceutically-acceptable salt thereof.
In this specification the generic term "alkyl" includes both
straight-chain and branched-chain alkyl groups. However references to
individual alkyl groups such as "propyl" are specific for the
straight-chain version only and references to individual
branched-chain alkyl groups such as "isopropyl" are specific for the
branched-chain version only. An analogous convention applies to other
generic terms.
It is further to be understood that, insofar as certain of
the compounds of formula I defined above may exist in optically active
or racemic forms by virtue of one or more asymmetric carbon atoms, the
invention includes in its definition any such optically active or
racemic form which possesses the property of inhibiting 5-L0. The
synthesis of optically active forms may be carried out by standard
techniques of organic chemistry well known in the art, for example by
synthesis from optically active starting materials or by resolution of
a racemic form.
Suitable values for the generic terms referred to above
;ncl~l~e those set out below.
~ W O 95/04055 2 1 6 ~ 2 5 2 PCT~EP94/02408
A suitable value for the (1-4C)alkyl substituent which is
present on Q is, for example, methyl, ethyl or propyl.
A suitable value for Ar when it is thiazolediyl is, for
example, 2,4-thiazolediyl or 2,5-thiazolediyl.
A suitable value for R1 when it is (1-4C)alkyl is, for
example, methyl, ethyl or propyl; when it is (3-4C)alkenyl is, for
example, allyl; and when it is (3-4C)alkynyl is, for example,
2-propynyl.
When R2 and R3 together form a group of the formula
-A2-X2-A3- which together with the carbon atom to which A2 and A3 are
attached define a ring having 5 or 6 ring atoms then a suitable value
for A2 or A3, which may be the same or different, when each is
(1-3C)alkylene is, for example, methylene, ethylene or trimethylene.
Suitable values for the (1-4C)alkyl substituents which may be present
on said 5- or 6-membered ring ~ncl~lde, for example, methyl and ethyl.
A suitable pharmaceutically-acceptable salt of a compound of
the invention is, for example, an acid-addition salt of a compound of
the invention which is sufficiently basic, for example, an
acid-addition salt with, for example, an inorganic or organic acid,
for example hydrochloric, hydrobromic, sulphuric, phosphoric,
trifluoroacetic, citric or maleic acid. In addition a suitable
pharmaceutically-acceptable salt of a compound of the invention which
is sufficiently acidic is an alkali metal salt, for example a sodium
or potassium salt, an AlkAline earth metal salt, for example a calcium
or magnesium salt, an ammonium salt or a salt with an organic base
which affords a physiologically-acceptable cation, for example a salt
with methylamine, dimethylamine, trimethylamine, piperidine,
morpholine or tris-(2-hydroxyethyl)amine.
Particular compounds of the invention include, for example,
thiazole derivatives of the formula I, or phArr~ceutically-acceptable
salts thereof, wherein the variable groups Q, X1, Ar, R1, R2 and R3
have the values disclosed hereinbefore or hereinafter in this section
concerning particular compounds of the invention:-
(a) Q is 1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl;
(b) X is thio or sulphonyl;
W O 95/04055 ~ 1 ~ 8 2 5 2 PCT~EP94/02408
(c) Ar is 2,4-thiazolediyl (with the Xl group in the
2-position);
(d) Ar is 2,5-thiazolediyl (with the X group in the
2-position);
(e) Ar is 2,5-thiazolediyl (with the X group in the
5-position);
(f) Rl is methyl; or
(g) R2 and R3 together form a group of the formula -A2-X2-A3-
which together with the carbon atom to which A2 and A3 are attached
define a ring having 5 or 6 ring atoms, wherein A2 is methylene or
ethylene, A3 is ethylene and x2 is oxy, and which ring may bear a
methyl substituent alpha to X2.
A preferred compound of the invention comprises a
- thiazole derivative of the formula I
uherein Q is l-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl;
xl is thio or sulphonyl;
Ar is 2,5-thiazolediyl (with the Xl group in the 2-position);
Rl is methyl; and
R2 and R3 together form a group of the formula -A2-X2-A3- which
together with the carbon atom to which A2 and A3 are attached define a
ring having 6 ring atoms, wherein each of A2 and A3 is ethylene and x2
is oxy, and which ring may bear a methyl substituent alpha to X ;
or a pharmaceutically-acceptable salt thereof.
A further preferred compound of the inventi.on comprises a
thiazole derivative of the formula I wherein
Q is l-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl,
xl is thio or sulphonyl;
Ar is 2,5-thiazolediyl (with the Xl group in the 2-position);
Rl is methyl; and
R2 and R3 together form a group of the formula -CH2CH20CH(CH3)CH2-;
or a pharmaceutically-acceptable salt thereof.
A specific preferred compound of the invention is the
following compound of the formula I:-
(2S,4R)-4-methoxy-2-methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-
quinolin-6-ylthio)thiazol-5-yl]tetrahydropyran.
W O 9~/04055 ~ 8 2 5 2 PCT~EP94/02408
In a further aspect of the invention we have now discovered
that certain compounds of the invention lack to a substantial degree
the undesirable property of auto-induction. Thus such compounds are
of particular value in the treatment of various inflammatory and/or
allergic diseases in warm-blooded ~n;r~ls as they lack the
disadvantages which may arise as a result of auto-induction. Thus,
for example, the assessment of pharmacological and toxicological data
is made more complex if the test compound has been shown to possess a
significant degree of auto-induction. In addition auto-induction may
foreshadow the general induction of enzymes which could have
disadvantageous effects such as a detrimental increase in the rate of
metabolism of any co-administered drugs.
In another aspect of the invention we have discovered that
certain compounds of the invention are crystalline. Thus such
compounds are of value when their preparation on a larger scale is
required. The purification, analysis and h~n~ling of a material is
facilitated if it is formed in the crystalline state. It is known,
for example, that the removal of solvent residues from
non-crystalline, oily materials is problematical. In addition the
preparation of a pharmaceutical composition comprising a crystall;ne
material is a conventional procedure. The composition may, for
example, be in a form suitable for oral use such as a tablet or
capsule; or, for example, in a form suitable for ~ in;stration by
inhalation, for example as a finely divided powder or a
microcryst~ll;ne form. Such options for the formulation of the
material are precluded should it be formed in an oily state.
A compound of the invention comprising a thiazole derivative
of the formula I, or a pharmaceutically-acceptable salt thereof, may
be prepared by any process known to be applicable to the preparation
of structurally-related compounds. Such procedures are provided as a
further feature of the invention and are illustrated by the following
representative examples in which, unless otherwise stated, Q, X1, Ar,
A R1, R2 and R3 have any of the meanings defined hereinbefore.
(a) The coupling, conveniently in the presence of a suitable
base, of a compound of the formula Q-Xl-H with a compound of the
formula II
W O 95/04055 2 1 6 8 2 5 2 PCT~EP94/02408 ~
ORl
Z-Ar-C-R II
I
R3
wherein Z is a displaceable group.
A suitable displaceable group Z is, for example, a halogeno
or sulphonyloxy group, for example a fluoro, chloro, bromo, iodo,
methane- sulphonyloxy or toluene-4-sulphonyloxy group.
A suitable base for the coupling reaction is, for example,
an alkali or ~lk~line earth metal carbonate, (1-4C)alkoxide, hydroxide
or hydride, for example sodium carbonate, potassium carbonate, sodium
ethoxide, potassium butoxide, lithium hydroxide, sodium hydroxide,
potassium hydroxide, sodium hydride or potassium hydride, or an
organometallic base such as (1-4C)alkyl-lithium, for example
n-butyl-lithium. The coupling reaction is conveniently performed in a
suitable inert solvent or diluent, for example N,N-dimethylformamide,
N,N-dimethylacetamide, N-methylpyrrolidin-2-one, dimethylsulphoxide,
acetone, 1,2-dimethoxyethane or tetrahydrofuran, and at a temperature
in the range, for example, 10 to 150C, conveniently at or near 100C.
Conveniently the reaction may be performed in the presence
of a suitable catalyst, for example a metallic catalyst, for example
palladium(0) or copper(I) such as tetrakis(triphenylphosphine)-
palladium, cuprous chloride or cuprous bromide.
The starting materials of the formula Q-X1-H and of the
formula II may be obtained by standard procedures of organic
chemistry. The preparation of such starting materials is described
within the accompanying non-limiting Examples. Alternatively
necessary starting materials are obtainable by analogous procedures to
those illustrated which are within the ordinary skill of an organic
chemist.
(b) The coupling, conveniently in the presence of a suitable
base as defined hereinbefore, of a compound of the formula Q-Z,
wherein Z is a displaceable group as defined hereinbefore, uith a
compound of the formula III
~ WO 95/04055 ~ ~ ~ 8 ~ 5 2 PCT~EP94102408
O
I
H-xl-Ar-c-R2 III
I
R3
The coupling reaction is conveniently performed in a
suitable inert solvent as defined hereinbefore and at a temperature in
the range, for example, 10 to 150C, conveniently at or near 100C.
The reaction may conveniently be performed in the presence of a
suitable catalyst as defined hereinbefore.
The starting materials of the formula Q-Z and of the formula
III may be obtained by standard procedures of organic chemistry.
Alternatively necessary starting materials are obtainable by analogous
procedures to those illustrated which are within the ordinary skill of
an organic chemist. The disclosure of European Patent Application No.
0420511 is particularly relevant to the preparation of suitable
starting materials.
(c) The coupling of a compound of the formula Q-X1-Z, wherein Z
is a displaceable group as defined hereinbefore or, when X1 is a thio
group, Z may be a group of the formula Q-~1-,
with an organometallic reagent of the formula IV
ORl
I
M-Ar-C-R IV
I
R3
wherein M is an alkali metal or ~lk~line earth metal such as lithium
or calcium or M represents the magnesium halide portion of a
conventional Grignard reagent.
The coupling reaction is conveniently performed in a
suitable inert solvent or diluent as defined hereinbefore and at a
temperature in the range, for example, -80 to +50C, conveniently in
the range -80C to ambient temperature.
W O 95/04055 2 1 ~ 8 2 5 2 PCTnEP94/02408 ~
- 10 -
The preparation of the starting materials of the formula
Q-X1-Z and of the formula IV may be obtained by standard procedures of
organic chemistry. Alternatively necessary starting materials are
obtainable by analogous procedures to those illustrated which are
within the ordinary skill of an organic chemist. The disclosure of
the European Patent Applications set out hereinbefore are particularly
relevant to the preparation of suitable starting materials.
(d) The alkylation, conveniently in the presence of a suitable
base as defined hereinbefore, of a compound of the formula V
OH
I
Q-xl-Ar-C-R V
I
R3
with a compound of the formula R1-Z, wherein Z is a displaceable group
as defined hereinbefore.
The alkylation reaction is conveniently performed in a
suitable inert solvent or diluent as defined hereinbefore and at a
temperature in the range, for example, -20 to +70C, conveniently at
or near ambient temperature.
The preparation of the starting materials of the formula V
may be obtained by standard procedures of organic chemistry.
Alternatively necessary starting materials are obtainable by analogous
procedures to those illustrated in the European Patent Applications
set out hereinbefore.
(e) For the production of those compounds of the formula I
wherein Q bears an alkyl substituent on an available nitrogen atom,
the alkylation of a compound of the formula I wherein Q bears a
hydrogen atom on said available nitrogen atom.
A suitable alkylating agent is, for example, any agent known
in the art for the alkylation of an available nitrogen atom, for
example an alkyl halide, for example a (1-4C)alkyl chloride, bromide
or iodide, in the presence of a suitable base as defined hereinbefore.
The alkylation reaction is preferably performed in a suitable inert
~ W O 95/04055 2 1 ~ 8 ~ 5 2 PCT~EP94/02408
- 11 -
solvent or diluent, for example N,N-dimethylformamide,
N,N-dimethylacetamide, dimethylsulphoxide, acetone,
1,2-dimethoxyethane or tetrahydrofuran, and a~ a temperature in the
range, for example, 10 to 150C, conveniently at or near ambient
temperature.
(f) For the production of those compounds of the formula I
wherein Xl is a sulphinyl or sulphonyl group, the oxidation of a
compound of the formula I wherein Xl is a thio group.
A suitable oxidising agent is, for example, any agent known
in the art for the oxidation of thio to sulphinyl and/or sulphonyl,
for example, hydrogen peroxide, a peracid (such as 3-
chloroperoxybenzoic or peroxyacetic acid), an alkali metal
peroxysulphate (such as potassium peroxymonosulphate), chromium
trioxide or gaseous oxygen in the presence of platinum. The oxidation
is generally carried out under as mild conditions as possible and with
the required stoichiometric amount of oxidising agent in order to
reduce the risk of over oxidation and damage to other functional
groups. In general the reaction is carried out in a suitable solvent
or diluent such as methylene chloride, chloroform, acetone,
tetrahydrofuran or tert-butyl methyl ether and at a temperature, for
example, at or near ambient temperature, that is in the range 15 to
35C. When a compound carrying a sulphinyl group is required a milder
oxidising agent may also be used, for example sodium or potassium
metaperiodate, conveniently in a polar solvent such as acetic acid or
ethanol. It uill be appreciated that when a compound of the formula I
cont~;n;ng a sulphonyl group is required, it may be obtained by
oxidation of the corresponding sulphinyl compound as well as of the
corresponding thio compound.
When a pharmaceutically-acceptable salt of a novel compound
of the formula I is required, it may be obtained, for example, by
reaction of said compound with a suitable acid or base using a
conventional procedure. When an optically active form of a compound
of the formula I is required, it may be obtained by carrying out one
of the aforesaid procedures using an optically active starting
material, or by resolution of a racemic form of said compound using a
conventional procedure.
W O 95/0405~ 2 t 6 8 2 5 2 PCT~EP94/02408 ~
- 12 -
As stated previousl~, the compounds of the formula I are
inhibitors of the enzyme 5-LO. The effects of this inhibition may be
demonstrated using one or more of the standard procedures set out
below:-
a) An in vitro assay system involving incubating a test
compound with heparinised human blood, prior to challenge with the
calcium ionophore A23187 and then indirectly measuring the inhibitory
effects on 5-LO by assaying the amount of LTB4 using specific
radioimmunoassays described by Carey and Forder (F. Carey and R.A.
Forder, Prosta~landins, Leukotrienes Med., 1986, 22, 57;
Prosta~l~n~;nc~ 1984, 28, 666; Brit. J. Pharmacol. 1985, 84, 34P)
which involve the use of a protein-LTB4 conjugate produced using the
procedure of Young et alia (Prosta~l~n~ins, 1983, 26(4), 605-613).
The effects of a test compound on the enzyme cyclooxygenase (which is
involved in the alternative metabolic pathway for arachidonic acid and
gives rise to prostAgl~nd;ns, thromboxanes and related metabolites)
may be measured at the same time using the specific radioimmunoassay
for thromboxane B2(TxB2) described by Carey and Forder (see above).
This test provides an indication of the effects of a test compound
against 5-LO and also cyclooxygenase in the presence of blood cells
and proteins. It permits the selectivity of the inhibitory effect on
5-LO or cyclooxygenase to be assessed.
b) An ex vivo assay system, which is a variation of test a)
above, involving Al' nistration to a group of rats of a test compound
(usually orally as the suspension produced when a solution of the test
compound in dimethylsulphoxide is added to carboxymethylcellulose),
blood collection, heparinisation, challenge with A23187 and
radioimmunoassay of LTB4 and TxB2. This test provides an indication
of the bioavailability of a test compound as an inhibitor of 5-LO or
cyclooxygenase.
c) An in vivo system involving measuring the effects of a
test compound administered orally to a group of male rats against the
liberation of LTB4 induced by zymosan within an air pouch generated
within the subcutaneous tissue of the back of each rat. The rats are
anaesthetised and air pouches are formed by the injection of sterile
air (20ml). A further injection of air (lOml) is similarly given
WO 95/04055 ~ 2 5 2 PCT~EP94/02408
-- 13 --
after 3 days. At 6 days after the initial air injection the test
compound is administered (usually orally as the suspension produced
when a solution of the test compound in dimethylsulphoxide is added to
hydroxypropylmethylcellulose), followed by the intrapouch injection of
zymosan (lml of a 1% suspension in physiological saline). After 3
hours the rats are killed, the air pouches are lavaged with
physiological saline, and the specific radioimmunoassay described
above is used to assay LTB4 in the washings. This test provides an
indication of inhibitory effects against 5-LO in an inflammatory
milieu.
Although the pharmacological properties of the compounds of
the formula I vary with structural changes as expected, in general
compounds of the formula I possess 5-LO inhibitory effects at the
following concentrations or doses in at least one of the above tests
a)-c)~-
Test a): IC50 (LTB4) in the range, for example, 0.01-40~N
IC50 (TxB2) in the range, for example, 40-200~M;
Test b): oral ED50(LTB4) in the range, for example,
O.l-lOOmg/kg;
Test c): oral ED50(LTB4) in the range, for example,
O.l-lOOmg/kg.
No overt toxicity or other untoward effects are present in
tests b) and/or c) when compounds of the formula I are administered at
several multiples of their ini Im inhibitory dose or concentration.
Thus, by way of example, the compound
(2S,4R)-4-methoxy-2-methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-
quinolin-6-ylthio)thiazol-5-yl]tetrahydropyran has an IC50 of 0.06~M
against LTB4 in test a), and an IC50 of approximately 0.2 mg/kg
against LTB4 in test c).
These compounds are examples of compounds of the invention
which show selective inhibitory properties for 5-LO as opposed to
cyclooxygenase, which selective properties are expected to impart
improved therapeutic properties, for example, a reduction in or
freedom from the gastrointestinal side-effects frequently associated
with cyclooxygenase inhibitors such as indomethacin.
According to a further feature of the invention there is
W O 95/04055 2 ~ ~ 8 2 5 2 PCT~EP94/02408 ~
- 14 -
provided a pharmaceutical composition which comprises a thiazole
derivative of the formula I, or a pharmaceutically-acceptable salt
thereof, in association with a pharmaceuticalIy-acceptable diluent or
carrier.
The composition may be in a form suitable for oral use, for
example a tablet, capsule, aqueous or oily solution, suspension or
emulsion; for topical use, for example a cream, ointment, gel or
aqueous or oily solution or suspension; for nasal use, for example a
snuff, nasal spray or nasal drops; for vaginal or rectal use, for
example a suppository; for administration by inhalation, for example
as a finely divided powder such as a dry powder, a microcrystAll;ne
form or a liquid aerosol; for sub-lingual or buccal use, for example a
tablet or capsule; or for parenteral use (incl~l~;ng intravenous,
subcutaneous, intramuscular, intravascular or infusion), for example a
sterile aqueous or oily solution or suspension. In general the above
compositions may be prepared in a conventional manner using
conventional excipients.
The amount of active ingredient (that is a thiazole
derivative of the formula I, or a pharmaceutically-acceptable salt
thereof) that is combined with one or more excipients to produce a
single dosage form will necessarily vary depending upon the host
treated and the particular route of administration. For example, a
formulation intended for oral administration to humans will generally
contain, for example, from 0.5 mg to 2 g of active agent compounded
with an appropriate and convenient amount of excipients which may vary
from about 5 to about 98 percent by weight of the total composition.
Dosage unit forms will generally contain about 1 mg to about 500 mg of
an active ingredient.
According to a further feature of the invention there is
provided a thiazole derivative of the formula I, or a
phArr~ceutically-acceptable salt thereof, for use in a method of
treatment of the human or animal body by therapy.
The invention also includes a method of treating a disease
or medical condition mediated alone or in part by one or more
leukotrienes which comprises administering to a warm-blooded animal
requiring such treatment an effective amount of an active ingredient
_ WO 95/04055 PCT~EP94/02408
-- 2 1 68ZS~
- 15 -
as defined above. The invention also provides the use of such an
active ingredient in the production of a new medicament for use in a
leukotriene mediated disease or medical condition.
The size of the dose for therapeutic or prophylactic
purposes of a compound of the formula I will naturally vary according
to the nature and severity of the conditions, the age and sex of the
animal or patient and the route of administration, according to well
kno~n principles of medicine. As mentioned above, compounds of the
formula I are useful in treating those diseases such as allergic and
inflammatory conditions and disorders of bone metabolism which are due
alone or in part to the effects of the metabolites of arachidonic acid
arising by the linear (5-L0 catalysed) pathway and in particular the
leukotrienes, the production of which is mediated by 5-L0. As
previously mentioned, such conditions include, for example, various
inflammatory and allergic diseases such as inflammation of the joints
(especially rheumatoid arthritis, osteoarthritis and gout),
;nfl~ -tion of the gastrointestinal tract (especially ;nfl; ~tory
bowel disease, ulcerative colitis and gastritis), skin diseases
(especially psoriasis, eczema and dermatitis), ocular conditions
(especially allergic conjunctivitis and uveitis) and respiratory
disease (especially asthma, bronchitis and allergic rhinitis), for
example in the production and development of various cardiovascular
and cerebrovascular disorders such as myocardial infarction, the
formation of atherosclerotic plaques, hypertension, platelet
aggregation, angina, stroke, reperfusion injury, vascular injury
;nClu~;ng restenosis and peripheral vascular disease, for example in
the formation of the conditions of shock or trauma such as can follow
burn injuries, toxaemia or surgery, and for example various disorders
of bone metabolism such as osteoporosis (inc~ ;ng senile and
postmenopausal osteoporosis), Paget's disease, bone metastases,
hyperc~lc~e ;a, hyperparathyroidism, osteosclerosis, osteopetrosis and
periodontitis, and the abnormal changes in bone metabolism which may
accompany rheumatoid arthritis and osteoarthritis.
In using a compound of the formula I for therapeutic or
prophylactic purposes it will generally be administered so that a
daily dose in the range, for example, 0.5 mg to 75 mg per kg body
W O 95/04055 2 1 ~ 8 2 5 2 PCT~EP94/02408 ~
weight is received, given if required in divided doses. In general
lower doses will be A~' ;ni stered when a parenteral route is employed.
Thus, for example, for intravenous administration, a dose in the
range, for example, 0.5 mg to 30 mg per kg body weight will generally
be used. Similarly, for ~1 ;ni~tration by inhalation, a dose in the
range, for example, 0.5 mg to 25 mg per kg body weight will be used.
Although the compounds of the formula I are primarily of
value as therapeutic agents for use in warm-blooded ~n; ~ls (;nClll~;n~
man), they are also useful whenever it is required to inhibit the
enzyme 5-L0. Thus, they are useful as pharmacological standards for
use in the development of new biological tests and in the search for
new pharmacological agents.
By virtue of their effects on leukotriene production, the
compounds of the formula I have certain cytoprotective effects, for
example they are useful in reducing or suppressing certain of the
adverse gastrointestinal effects of the cyclooxygenase inhibitory non-
steroidal anti-inflammatory agents (NSAIA), such as indomethacin,
acetylsalicylic acid, ibuprofen, slll; nd~C ~ tolmetin and piroxicam.
Furthermore, co-~;n;stration of a 5-L0 inhibitor of the formula I
with a NSAIA can result in a reduction in the quantity of the latter
agent needed to produce a therapeutic effect, thereby reducing the
l~kelihood of adverse side-effects. According to a further feature of
the invention there is provided a pharmaceutical composition which
comprises a thiazole derivative of the formula I, or a
ph~ ~ceutically-acceptable salt thereof as defined hereinbefore, in
conjunction or admixture with a cyclooxygenase inhibitory
non-steroidal anti-inflammatory agent (such as those mentioned above),
and a ph~rr~ceutically-acceptable diluent or carrier.
The cytoprotective effects of the compounds of the formula I
may be demonstrated, for example in a standard laboratory model which
assesses protection against indomethacin-induced or ethanol-induced
ulceration in the gastrointestinal tract of rats.
The compositions of the invention may in addition contain
one or more therapeutic or prophylactic agents known to be of value
for the disease under treatment. Thus, for example a known platelet
aggregation inhibitor, hypolipidemic agent, anti-hypertensive agent,
W O 95/040$5 2 ~ 5 2 PCT~EP94/02408
beta-adrenergic blocker or a vasodilator may usefully also be present
in a pharmaceutical composition of the invention for use in treating a
heart or vascular disease or condition. Similarly, by way of example,
an anti-histamine, steroid (such as beclomethasone dipropionate),
- sodium cromoglycate, phosphodiesterase inhibitor or a beta-adrenergic
stimulant may usefully also be present in a pharmaceutical composition
of the invention for use in treating a plll cn~ry disease or condition.
The invention will now be illustrated in the following
non-limiting Examples in which, unless otherwise stated:-
(i) evaporations were carried out by rotary evaporation invacuo and work-up procedures were carried out after removal of
residual solids by filtration;
(ii) operations were carried out at room temperature, that
is in the range 18-25C and under an atmosphere of an inert gas such
as argon;
(iii) column chromatography (by the flash procedure) and
medium pressure liquid chromatography (MPLC) were performed on Nerck
Kieselgel silica (Art. 9385) or Merck Lichroprep RP-18 (Art. 9303)
reversed-phase silica obtained from E. Merck, Darmstadt, Germany;
(iv) yields are given for illustration only and are not
necessarily the maximum att~;n~ble;
(v) the end-products of the formula I have satisfactory
microanalyses and their structures were confirmed by nuclear magnetic
resonance (NNR) and mass spectral techniques; unless otherwise stated,
CDCl3 solutions of the end-products of the formula I were used for the
determination of NMR spectral data, chemical shift values were
measured on the delta scale; the following abbreviations have been
used: s, singlet; d, doublet; t, triplet; m, multiplet;
(vi) intermediates were not generally fully characterised
and purity was assessed by thin layer chromatographic, infra-red (IR)
or NMR analysis;
(vii) melting points are uncorrected and were determined
using a Mettler SP62 automatic melting point apparatus or an oil-bath
apparatus; melting points for the end-products of the formula I were
determined after crystallisation from a conventional organic solvent
such as ethanol, methanol, acetone, ether or hexane, alone or in
W O 9S/0405~ 2 5 2 PCT~EP94/02408
- 18 -
admixture; and
(viii) the following abbreviation has been used:-
NMP N-methylpyrrolidin-2-one;
THF tetrahydrofuran;
DNF N,N-dimethylformamide.
~ W O 95/04055 2 1 6 8 2 5 2 PCT~EP94/02408
- 19 -
Example 1
6-Mercapto-l-methyl-1,2,3,4-tetrahydroquinolin-2-one (0.166
g) was added to a mixture of 4-(2-chlorothiazol-5-yl)-4-methoxytetra-
hydropyran (0.2 g), potassium carbonate (0.13 g) and NMP (2 ml). The
mixture was stirred and heated to 100C for 3 hours. The mixture uas
cooled to ambient temperature and partitioned between ethyl acetate
and water. The organic phase was washed with brine, dried (MgS04) and
evaporated. The residue was purified by column chromatography using a
1:1 mixture of petroleum ether (b.p. 40-60C) and ethyl acetate as
eluent. There was thus obtained 4-methoxy-4-[2-(1-methyl-2-oxo-
1,2,3,4-tetrahydroquinolin-6-ylthio)thiazol-5-yl]tetrahydropyran (0.25
g), m.p. 114-115C (recrystallised from ethanol);
NMR Spectrum 1.97-2.01 (m, 4H), 2.69 (m, 2H), 2.94 (m, 2H), 3.05 (s,
3H), 3.38 (s, 3H), 3.7-3.8 (m, 4H), 7.02-7.04 (d, lH), 7.45 (s, lH),
7.46-7.47 (d, lH), 7.55-7.58 (m, lH).
The 6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-2-one
used as a starting material was obtained as follows:-
A mixture of concentrated hydrochloric acid (5 drops) andwater (50 ml) was added to a stirred mixture of di-(1-methyl-2-oxo-
1,2,3,4-tetrahydroquinolin-6-yl) disulphide (European Patent
Application No. 0462812, Example 7 thereof; 38.4 g),
triphenylphosphine (29 g) and 1,4-dioxan (300 ml). The mixture was
stirred at ambient temperature for 30 minutes. The mixture was
concentrated by evaporation to reduce the volume by approximately one
half. The residue was partitioned between ethyl acetate and 0.5N
aqueous sodium hydroxide solution. The aqueous phase was washed with
diethyl ether and then acidified to pH2 by the addition of dilute
aqueous hydrochloric acid. The acidic mixture was extracted with
ethyl acetate. The organic phase was dried (MgS04) and evaporated.
The residual oil was dissolved in diethyl ether and hexane was added.
There was thus obtained 6-mercapto-1-methyl-1,2,3,4-tetrahydro-
quinolin-2-one as a solid (35.5 g, 92%) which was used without further
purification.
The 4-(2-chlorothiazol-5-yl)-4-methoxytetrahydropyran used
as a starting material was obtained as follows:-
A saturated aqueous solution of sodium nitrite (6.9 g) was
wo gs/0405s ~ ~ ~$2 5 2 PCT~EP94/02408 ~
- 20 -
added dropuise to a stirred solution of 2-aminothiazole (10 g) in
concentrated hydrochloric acid (50 ml) which had been cooled to 0C.
The mixture was stirred at 0C for 75 minutes. Cuprous chloride (9.9
g) was added portionwise, the reaction temperature being maintained at
0C, and the mixture was stirred or 2.5 hours. The mixture was
neutralised by the addition of lON aqueous sodium hydroxide solution.
The mixture was partitioned between diethyl ether and water. The
organic phase was washed with brine, dried (MgS04) and evaporated.
The residue was purified by distillation. There was thus obtained
2-chlorothiazole (3.95 g, b.p. 68C at 68 mm of mercury).
A solution of 2-chlorothiazole (0.5 g) in diethyl ether (4
ml) and n-butyl-lithium (2.5M in hexane, 1.8 ml) were added
simultaneously to diethyl ether (5 ml) which had been cooled to -78C.
The mixture was stirred and allowed to warm to -20C. A solution of
tetrahydropyran-4-one (0.42 g) in diethyl ether (5 ml) was added
dropwise. The mixture was stirred for 1 hour and allowed to warm to
0C and then allowed to warm to ambient temperature. The mixture was
partitioned between diethyl ether and a saturated aqueous ammonium
chloride solution. The organic phase was washed with brine, dried
(MgS04) and evaporated. The residue was purified by column
chromatography using a 1:1 mixture of petroleum ether (b.p. 40-60C)
and ethyl acetate as eluent. There was thus obtained 4-(2-chloro-
thiazol-5-yl)-4-hydroxytetrahydropyran (0.25 g, 27%), m.p. 94C.
Sodium hydride lO.045 g (obtained after removal of the
mineral oil dispersant)l was added to a solution of
4-(2-chlorothiazol-5-yl)-4-hydroxytetrahydropyran (0.2 g) in THF (1.5
ml) and the mixture was stirred at ambient temperature for 1 hour.
Methyl iodide (0.17 ml) was added and the mixture was stirred at
ambient temperature for 12 hours. The mixture was evaporated and the
residue was partitioned between diethyl ether and brine. The organic
phase was dried (MgS04) and evaporated. The residue was purified by
column chromatography using a 1:1 mixture of petroleum ether (b.p.
40-60C) and ethyl acetate as eluent. There was thus obtained
4-(2-chlorothiazol-5-yl)-4-methoxytetrahydropyran (0.2 g, 94X) as an
oil.
~ W ~ 9~/04055 2 1 6 8 2 5 2 PCT~EP94/02408
Example 2
3-Chloroperoxybenzoic acid (0.141 g) was added to a stirred
solution of 4-methoxy-4-~2-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-
6-ylthio)thiazol-5-yl]tetrahydropyran (0.08 g) in methylene chloride
(1.5 ml). The mixture was stirred at ambient temperature for 5 hours.
A saturated aqueous sodium bicarbonate solution was added and the
mixture was extracted with ethyl acetate. The organic phase was
washed with brine, dried (NgS04) and evaporated. There was thus
obtained 4-methoxy-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-
ylsulphonyl)thiazol-5-yl]tetrahydropyran (0.071 g), m.p. 163-164C
(recrystallised from ethanol);
NNR Spectrum 2.03-2.05 (m, 4H), 2.70 (m, 2H), 3.0 (m, 2H), 3.1 (s,
3H), 3.38 (s, 3H), 3.78-3.81 (m, 4H), 7.12 (d, lH), 7.73 (s, lH), 7.9
(m, lH), 7.98-8.01 (m, lH).
r .le 3
Using an analogous procedure to that described in Example 1,
6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-2-one was reacted with
(2S,4R)-4-(2-chlorothiazol-5-yl)-4-methoxy-2-methyltetrahydropyran to
give (2S,4R)-4-methoxy-2-methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetra-
hydroquinolin-6-ylthio)thiazol-5-ylltetrahydropyran in 95X yield as a
foam;
NMR Spectrum 1.16-1.18 (d, 3H), 1.54-1.57 (m, lH), 1.81-2.02 (m, 3H),
2.67-2.71 (m, 2H), 2.92-2.96 (m, 2H), 3.04 (s, 3H), 3.38 (s, 3H),
3.77-3.82 (m, 3H), 7.02-7.04 (d, lH), 7.42 (s, lH), 7.46 (m, lH), 7.56
(m, lH).
The (2S,4R)-4-(2-chlorothiazol-5-yl)-4-methoxy-2-methyl-
tetrahydropyran used as a starting material was obtained as follows:-
A solution of 2-chlorothiazole (0.5 g) in diethyl ether (4
ml) and n-butyl-lithium (2.5M in hexane, 1.8 ml) were added
simultaneously during 10 minutes to diethyl ether (5 ml) which had
been cooled to -78C. The mixture was stirred for 3.5 hours and
allowed to warm to -20C. The mixture was recooled to -78C and a
solution of (2S)-2-methyltetrahydropyran-4-one [European Patent
Application No. 0385662 (Example 20 thereof); 0.43 g] in diethyl ether
(4 ml) was added. The mixture was stirred and allowed to warm to
W O 95/04n55 ~ PCT~EP94/02408
-lO~C. A 5% aqueous solution of ammonium chloride was added and the
mixture was extracted with diethyl ether. The organic phase was
washed with brine, dried (~gS04) and evaporated. The residue was
purified by column chromatography using a 1:1 mixture of petroleum
ether (b.p. 40-60C) and ethyl acetate as eluent. There was thus
obtained (2S,4R)-4-(2-chlorothiazol-5-yl)-4-hydroxy-2-methyltetra-
hydropyran (0.11 g, 13X) as an oil.
Sodium hydride (0.127 g, 5.27 mmol) was added to a solution
of the 4-hydroxy-2-methyltetrahydropyran so obtained in THF (2.5 ml)
and the mixture was stirred at ambient temperature for 1 hour. The
mixture was cooled to 0C and methyl iodide (0.33 ml) was added. The
mixture was stirred at ambient temperature for 2.5 hours. The mixture
was evaporated and the residue was partitioned between diethyl ether
and brine. The organic phase was dried (HgS04) and evaporated. The
residue was purified by column chromatography using a 1:1 mixture of
petroleum ether (b.p. 40-60C) and ethyl acetate as eluent. There was
thus obtained (2S,4R)-4-(2-chlorothiazol-5-yl)-4-methoxy-2-methyl-
tetrahydropyran (0.268 g, 71%) as an oil which crystallised on
stAntl; ng,
~xample 4
Using an analogous procedure to that described in Example 2,
(2S,4R)-4-methoxy-2-methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-
quinolin-6-ylthio)thiazol-5-yl]tetrahydropyran was oxidised to give
(2S,4R)-4-methoxy-2-methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-
quinolin-6-ylsulphonyl)thiazol-5-ylltetrahydropyran in 98% yield as a
foam;
NMR Spectrum 1.18-1.20 (d, 3H), 1.61-1.65 (m, lH), 1.94-2.10 (m, 3H),
2.68-2.72 (m, 2H), 2.98-3.02 (m, 2H), 3.09 (s, 3H), 3.38 (s, 3H),
3.81-3.87 (m, 3H), 7.11-7.13 (d, lH), 7.71 (s, lH), 7.89-7.90 (m, lH),
7.98-8.01 (m, lH).
Example 5
Potassium carbonate (15.5 g) was added to a solution of
6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-2-one (19.7 g) in N~P
(140 ml) and the mixture was stirred at ambient temperature for 10
~ W O 9~/04055 2 1 6 8 2 5 2 PCT~EP94/02408
- 23 -
minutes. (2S,4R)-4-(2-Chlorothiazol-5-yl)-4-methoxy-2-methyl-
tetrahydropyran (25.2 g) was added and the mixture was stirred and
heated to 100C for 2 hours. The mixture was cooled to ambient
temperature and partitioned between diethyl ether and water. The
organic phase was washed with water and with brine, dried (MgS04) and
evaporated. The residue was triturated under diethyl ether to give a
crystalline solid. There was thus obtained (2S,4R)-4-methoxy-2-
methyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-ylthio)-
thiazol-5-yl]tetrahydropyran (37.5 g, 91%), m.p. 78-79C
(recrystallised from diethyl ether).
The (2S,4R)-4-(2-chlorothiazol-5-yl)-4-methoxy-2-methyl-
tetrahydropyran used as starting material was obtained as follous:-
n-Butyl-lithium (2.5N in hexane, 57.5 ml) was dropwise added
during 30 minutes to a solution of di-isopropylamine (19.3 ml) in THF
(180 ml) which had been cooled to -78C. The mixture was stirred at
-78C for 1 hour. The reaction vessel was shielded from light and
2-chlorothiazole (15.0 g) was added dropwise during 20 minutes, whilst
keeping the temperature of the reaction mixture below -70C. The
mixture was stirred at -78C for 1.5 hours. (2S)-2-Methyl-
tetrahydropyran-4-one (12.9 g) was added during 20 minutes. The
mixture was stirred and allowed to warm briefly to -25C to obtain a
homogeneous solution. The mixture was recooled to -70C and stirred
at that temperature for 1 hour. A 5X aqueous solution of ammonium
chloride was added and the mixture was extracted with diethyl ether.
The organic solution was washed with brine, dried (MgS04) and
evaporated. There was thus obtained a mixture (29.4 g) of
(2S,4R)-4-(2-chlorothiazol-5-yl)-4-hydroxy-2-methyltetrahydropyran and
the corresponding (2S,4S)-isomer in a ratio of 1:4. The mixture so
obtained was dissolved in diethyl ether (250 ml) and the solution was
cooled to 0C. A concentrated (25% v/v) aqueous solution of sulphuric
acid was added and the mixture was stirred and allowed to warm to
ambient temperature. The mixture was stirred at ambient temperature
for 16 hours. The mixture was diluted with ethyl acetate (750 ml) and
neutralised to pH3 by the addition of sodium bicarbonate. The organic
solution was washed with brine, dried (MgS04) and evaporated to give a
mixture (26 g) of (2S,4R)-4-(2-chlorothiazol-5-yl)-4-hydroxy-2-methyl-
W O 9~/04055 2 ~ 6 8 2 5 2 PCT~EP94/02408 ~
tetrahydropyran and the corresponding (2S,4S)-isomer in a ratio of
9:1. The material was purified by column chromatography using a 1:1
mixture of petroleum ether (b.p. 40-60C) and ethyl acetate as eluent.
There was thus obtained (2S,4R)-4-(2-chlorothiazol-5-yl)-4-hydroxy-2-
methyltetrahydropyran (21 g, 79%).
Sodium hydride (60% dispersion in mineral oil, 14.5 g, 0.36
mol) was added portionwise during 15 minutes to a solution of
(2S,4R)-4-(2-chlorothiazol-5-yl)-4-hydroxy-2-methyltetrahydropyran
(42.15 g) in THF (150 ml) which had been cooled to 0C. The mixture
was stirred at 0C for 45 minutes. Hethyl iodide (22.5 ml) was added
dropwise and the mixture was stirred and allowed to warm to ambient
temperature during 2 hours. The mixture was recooled to 0C and a
solution of brine was added. The mixture was extracted with ethyl
acetate. The organic solution was washed with brine, dried (MgS04)
and evaporated. The residue was purified by column chromatography
using a 7:3 mixture of petroleum ether (b.p. 40-60C) and ethyl
acetate as eluent. There was thus obtained (2S,4R)-4-(2-chloro-
thiazol-5-yl)-4-methoxy-2-methyltetrahydropyran (40.9 g, 91%), m.p.
40-42C.
F. le 6
Using an analogous procedure to that described in Example 1
except that a catalytic amount (0.01 g) of potassium iodide was added,
6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-2-one was reacted with
4-(2-chlorothiazol-5-yl)-4-methoxy-2,2-dimethyltetrahydropyran to give
4-methoxy-2,2-dimethyl-4-[2-(1-methyl-2-oxo-1,2,3,4-tetrahydro-
quinolin-6-ylthio)thiazol-5-yl]tetrahydropyran in 80% yield as a foam;
NMR Spectrum 1.2 (s, 3H), 1.4 (s, 3H), 1.6-1.75 (m, 2H), 1.9-2.0 (m,
2H), 2.70 (m, 2H), 2.95 (m, 2H), 3.02 (s, 3H), 3.4 (s, 3H), 3.65 (m,
lH), 3.95 (m, lH), 7.03 (m, lH), 7.42 (s, lH), 7.46 (m, lH), 7.55 (m,
lH).
The 4-(2-chlorothiazol-5-yl)-4-methoxy-2,2-dimethyltetra-
hydropyran used as a starting material was obtained as follows:-
A solution of 2-chlorothiazole (0.75 g) in diethyl ether (8
ml) and n-butyl-lithium (1.4H in hexane, 4.5 ml) were added
simultaneously but separately to diethyl ether (8 ml) which had been
~ W O 95/04055 2 1 ~ ~ 2 ~ 2 PCT~EP94/02408
- 25 -
cooled to -80C. The mixture was stirred at -75C for 10 minutes and
then allowed to warm to -30C. The mixture was recooled to -80C and
a solution of 2,2-dimethyltetrahydropyran-4-one (0.76 g) in diethyl
ether (5 ml) was added. The mixture was stirred and allowed to warm
to -30C. The mixture was poured onto a mixture of ice and a
saturated aqueous ammonium chloride solution. The organic solution
was washed with brine, dried (MgS04) and evaporated. The residue was
purified by column chromatography using a 10:3 mixture of hexane and
ethyl acetate as eluent. There was thus obtained
4-(2-chlorothiazol-5-yl)-4-hydroxy-2,2-dimethyltetrahydropyran (0.67
g, 46%) as an oil;
NNR Spectrum 1.2 (s, 3H), 1.45 (s, 3H), 1.8-2.15 (m, 4H), 3.75 (m,
lH), 4.1 (m, lH), 7.38 (s, lH).
Using an analogous procedure to that described in the last
paragraph of the portion of Example 3 which is concerned with the
preparation of starting materials except that DMF was used as the
reaction solvent in place of THF, the 4-hydroxy-2,2-dimethyltetra-
hydropyran so obtained was methylated to give 4-(2-chlorothiazol-5-
yl)-4-methoxy-2,2-dimethyltetrahydropyran in 79% yield as an oil.
r le 7
Sodium hydride (60% dispersion in mineral oil, 0.06 g) was
added portionwise to a stirred solution of 4-hydroxy-2,6-dimethyl-4-
[2-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-ylthio)thiazol-5-yl~-
tetrahydropyran (0.27 g) in DMF (2 ml) which had been cooled to 0C.
The mixture was stirred at 0C for 30 minutes. Nethyl iodide (0.2 ml)
was added. The mixture was allowed to warm to ambient temperature and
was stirred for 16 hours. The mixture was partitioned between ethyl
acetate and a saturated aqueous ammonium chloride solution. The
organic solution was washed with brine, dried (NgS04) and evaporated.
The residue was purified by column chromatography using increasingly
polar mixtures of hexane and ethyl acetate as eluent. There was thus
obtained 4-methoxy-2,6-dimethyl-4-[2-(1-methyl-2-oxo-1,2,3,4-
tetrahydroquinolin-6-ylthio)thiazol-5-yl]tetrahydropyran (0.063 g) as
a gum;
NMR Spectrum 1.15 (d, 3H), 1.4 (d, 3H), 1.65 (m, 2H), 1.9-2.1 (m, 2H),
W O 95/04055 ~ ~ ~8 ~ 5 2 PCT~EP94/02408
- 26 -
2.70 (m, 2H), 2.95 (m, 2H), 3.0S (s, 3H), 3.37 (s, 3H), 4.1 (m, 2H),
7.03 (m, lH), 7.40 (s, lH), 7.47 (m, lH), 7.55 (m, lH).
The 4-hydroxy-2,6-dimethyl-4-~2-(1-methyl-2-oxo-1,2,3,4-
tetrahydroquinolin-6-ylthio)thiazol-5-yl]tetrahydropyran used as a
starting material was obtained as follows:-
The procedure described in the first paragraph of theportion of Example 6 which is concerned with the preparation of
starting materials was repeated except that 2,6-dimethyltetrahydro-
pyran-4-one was used in place of 2,2-dimethyltetrahydropyran-4-one.
There was thus obtained 4-(2-chlorothiazol-5-yl)-4-hydroxy-2,6-
dimethyltetrahydropyran in 77% yield.
NMR Spectrum 1.2 (s, 3H), 1.5 (s, 3H), 1.7-2.0 (m, 3H), 2.16 (m, 2H),
4.2 (m, 2H), 7.38 (s, lH).
A mixture of 6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-
2-one (0.32 g), 4-(2-chlorothiazol-5-yl)-4-hydroxy-2,6-dimethyltetra-
hydropyran (0.3 g), potassium carbonate (0.33 g), potassium iodide
(0.01 g) and DMF (3 ml) was stirred and heated to 100C for 4 hours.
The mixture was cooled to ambient temperature and partitioned between
ethyl acetate and a saturated aqueous ammonium chloride solution. The
organic solutiDn was washed with brine, dried (MgS04) and evaporated.
The residue was purified by column chromatography using increasingly
polar mixtures of hexane and ethyl acetate as eluent. There was thus
obtained 4-hydroxy-2,6-~ tl-yl-4-[2-(1-methyl-2-oxo-1,2,3,4-
tetrahydroquinolin-6-ylthio)thiazol-5-yl]tetrahydropyran (0.26 g, 53X)
as a foam;
NMR Spectrum 1.2 (s, 3H), 1.5 (s, 3H), 1.7-1.9 (m, 3H), 2.15 (m, lH),
2.66 (m, lH), 2.95 (m, 2H), 3.39 (s, 3H), 4.18 (m, 2H), 7.0 (d, lH),
7.5 (m, 2H), 7.55 (m, lH).
F. , 1 e 8
Using an analogous procedure to that described in Example 1
except that triphenylphosphine (0.04 g) was added,
6-mercapto-1-methyl-1,2,3,4-tetrahydroquinolin-2-one (0.153 g) was
reacted with (2S,4R)-4-(5-bromothiazol-2-yl)-4-methoxy-2-methyl-
tetrahydropyran (0.23 g) to give (2S,4R)-4-methoxy-2-methyl-4-[5-(1-
methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-ylthio)thiazol-2-yl]-
~ W O 95/04055 2 1 6 ~ 2 ~ 2 PCT~EP94/02408
- 27 -
tetrahydropyran in 72% yield as a foam;
NMR Spectrum 1.15-1.20 (d, 3H), 1.60 (m, lH), 1.90-2.05 (m, 3H),
2.65-2.75 (m, 2H), 2.90-3.00 (m, 2H), 3.05 (s, 3H), 3.4 (s, 3H),
3.75-3.85 (m, 3H), 7.05 (d, lH), 7.4 (s, lH), 7.45 (m, lH), 7.55
(m, lH).
The (2S,4R)-4-(5-bromothiazol-2-yl)-4-methoxy-2-methyl-
tetrahydropyran used as starting material was obtained as follows:-
A solution of 2,5-dibromothiazole (2 g, J. Chem. Soc.,
Perkin Trans. I, 1992, 215-219) in diethyl ether (3 ml) was added
dropwise to a solution of n-butyl-lithium (2.5M in hexane, 3.7 ml) in
diethyl ether (80 ml) which has been cooled to -100C. The mixture
was stirred for 5 minutes and (2S)-2-methyltetrahydropyran-4-one
(0.845 g) added, whilst maintaining the temperature of the reaction
mixture at -100C. The mixture was stirred for 45 minutes and
quenched with a 5Z aqueous solution of ammonium chloride. The mixture
was extracted twice with diethyl ether, washed with brine, dried
(MgS04), filtered and evaporated. The oil was purified by column
chromatography using a 1:1 mixture of petroleum ether and ethyl
acetate as eluent. There was thus obtained a mixture (1.177 g, 46%)
of (2S,4S)- and (2S,4R)-4-(5-bromothiazol-2-yl)-4-hydroxy-2-methyl-
tetrahydropyran as an oil.
The mixture so obtained (1 g) was dissolved in diethyl ether
(15 ml) and the solution cooled to 0C. A concentrated (30Z v/v)
aqueous solution of sulphuric acid (10 ml) was added and the mixture
was stirred and allowed to warm to ambient temperature. The mixture
was stirred at ambient temperature for 16 hours. The mixture was
neutralised by the addition of sodium bicarbonate and extracted three
times with diethyl ether, washed with brine, dried (MgS04), filtered
and evaporated. The oil was purified by column chromatography using a
1:1 mixture of petroleum ether and ethyl acetate as eluent. There was
thus obtained (2S,4R)-4-(5-bromothiazol-2-yl)-4-hydroxy-2-methyltetra-
hydropyran as a solid (0.707 g, 70%).
Sodium hydride (60% dispersion in mineral oil, 0.045 g,
0.0018 mol) was added to a solution of (2S,4R)-4-(5-bromothiazol-2-
yl)-4-hydroxy-2-methyltetrahydropyran (0.25 g) in THF (2.5 ml) which
had been cooled to 0C. The mixture was stirred at 0C for 45
W O 95/0405~ 2 1~ ~ g; ~ 5 2 PCT~EP94/02408 ~
- 28 -
minutes. Methyl iodide tO.13 ml) was added dropwise and the mixture
was stirred and allowed to warm to ambient temperature during 2 hours.
A solution of brine was added and the organic solution extracted with
ethyl acetate, dried (MgS04), filtered and evaporated. The oil was
purified by column chromatography using a 1:2 mixture of petroleum
ether and ethyl acetate as eluent. There was thus obtained (2S,4R)-4-
(5-bromothiazol-2-yl)-4-methoxy-2-methyltetrahydropyran as an oil
(0.233 g, 89X).
xample 9
The following illustrate representative phA ~ceutical
dosage forms contA;n;ng the compound of formula I, or a
phA ~ceutically-acceptable salt thereof (hereafter compound X), for
therapeutic or prophylactic use in humans:
(a) Tablet I m~/tablet
Compound X..................................... 100
Lactose Ph.Eur................................ 182.75
Croscarmellose sodium.......................... 12.0
Maize starch paste (5X w/v paste).............. 2.25
Nagnesium stearate............................. 3.0
(b) Tablet II mg/tablet
Compound g...................................... 50
Lactose Ph.Eur................................ 223.75
Croscarmellose sodium.......................... 6.0
Maize starch................................... 15.0
Polyvinylpyrrolidone (5X w/v paste)............ 2.25
Magnesium stearate............................. 3.0
(c) Tablet III mg/tablet
Compound ~..................................... 1.0
Lactose Ph.Eur................................ 93.25
CroscA -llose sodium........................... 4.0
Maize starch paste (5% w/v paste).............. 0.75
Magnesium stearate............................. 1.0
~ WO 9S/04055 2 1 6 8 2 5 2 PCT~EP94/02408
- 29 -
(d) Capsule m~/capsule
Compound g.................................... lO
Lactose Ph.Eur ............................... 488.5
Magnesium stearate ........................... 1.5
(e) Injection I (50 m~/ml)
Compound ~ ................................... 5.0X w/v
lN Sodium hydroxide solution ................. 15.0X v/v
O.lM Hydrochloric acid
(to adjust pH to 7.6)
Polyethylene glycol 400....................... 4.5% w/v
Uater for injection to 100%
(f) Injection II (10 mg/ml)
Compound X ................................... l.OX w/v
Sodium phosphate BP .......................... 3.6% w/v
O.lM Sodium hydroxide solution ............... 15.0% v/v
Water for injection to lOOX
(g) Injection III t1m~/ml.buffered to pH6)
Compound X ................................... 0.1% w/v
Sodium phosphate BP .......................... 2.26% w/v
Citric acid .................................. 0.38X w/v
Polyethylene glycol 400 ...................... 3.5% w/v
~ater for injection to 100X
(h) Aerosol I m~/ml
Compound X ................................... 10.0
Sorbitan trioleate ........................... 13.5
Trichlorofluoromethane ....................... 910.0
Dichlorodifluoromethane ...................... 490.0
W O 95/04055 PCT~EP94/02408 ~
2 ~ ~252
- 30 -
(i) Aerosol II mg/ml
Compound X .................................. 0.2
Sorbitan trioleate ................ ~............ 0.27
Trichlorofluoromethane ...................... 70.0
Dichlorodifluoromethane ..................... 280.0
Dichlorotetrafluoroethane ................... 1094.0
(;) Aerosol III m~/ml
Compound X .................................. 2.5
Sorbitan trioleate .......................... 3.38
Trichlorofluoromethane ...................... 67.5
Dichlorodifluoromethane ..................... 1086.0
Dichlorotetrafluoroethane ................... 191.6
(k) Aerosol IV mg/ml
Compound X .................................. 2.5
Soya lecithin ............................... 2.7
Trichlorofluoromethane ...................... 67.5
Dichlorodifluoromethane ..................... 1086.0
Dichlorotetrafluoroethane ................... 191.6
Note
The above fo lAtions may be obtained by conventional
procedures well known in the phA ?ce~lt;c~Al art. The tablets ~a)-(c)
may be enteric coated by conventional means, for example to provide a
coating of cellulose acetate phthalate. The aerosol formulations
(h)-(k) may be used in conjunction with standard, metered dose aerosol
dispensers, and the suspending agents sorbitan trioleate and soya
lecithin may be replaced by an alternative suspending agent such as
sorbitan monooleate, sorbitan sesquioleate, polysorbate 80,
polyglycerol oleate or oleic acid.
IS37684
27JUN94
IGB/MB