Note: Descriptions are shown in the official language in which they were submitted.
CA 02168617 2002-11-21
CONSTITUTIVE PROMOTER FROM TOBACCO
FIELD OF INVENTION
The present invention relates to a constitutive promoter identified
from Nicotiana tabacum (tobacco). This invention further relates to the use of
said constitutive promoter to control the expression of exogenous genes in
transgenic plants of diverse plant species.
BACKGROUND OF THE INVENTION
Bacteria from the genus Agrobacterium have the ability to transfer
specific segments of DNA (T-DNA) to plant cells, where they stably integrate
into the nuclear chromosomes. Analyses of plants harbouring the T-DNA
have revealed that this genetic element may be integrated at numerous
locations, and can occasionally be found within genes. One strategy which
has been exploited to identify integration events within genes is to transform
plant cells with specially designed T-DNA vectors which contain a reporter
gene, devoid of cis-acting transcriptional and translational expression
signals
(i.e. promoterless), located at the end of the T-DNA. Upon integration, the
initiation codon of the promoterless gene (reporter gene) will be juxtaposed
to
plant sequences. The consequence of T-DNA insertion adjacent to, and
downstream of, gene promoter elements may be the activation of reporter
gene expression. The resulting hybrid genes, referred to as T-DNA-mediated
gene fusions, consist of unknown and thus un-characterized plant promoters
residing at their natural location within the chromosome, and the coding
sequence of a marker gene located on the inserted T-DNA (Fobert et al. ,
1991, Plant Mol. Biol. 17, 837-851).
It has generally been assumed that activation of promoterless or
enhancerless marker genes result from T-DNA insertions within or
immediately adjacent to genes. The recent isolation of several T-DNA
insertional mutants (Koncz et al., 1992, Plant Mol. Biol. 20, 963-976;
-2-
reviewed in Feldmann, 1991, Plant J. 1, 71-82; Van Lijsebettens et al., 1991,
Plant Sci. 80, 27-37; Walden et al., 1991, Plant J. 1: 281-288; Yanofsky et
al., 1990, Nature 346, 35-39), shows that this is the case for at least some
insertions. However, other possibilities exist. One of these possibilities is
that integration of the T-DNA activates silent regulatory sequences that are
not
associated with genes. Lindsey et al. (1993, Transgenic Res. 2, 33-47)
referred to such sequences as "pseudo-promoters" and suggested that they may
be responsible for activating marker genes in some transgenic lines. Fobert et
al. (1994, Plant J. 6, 567-577) have cloned such sequences and have referred
to these as "cryptic promoters" .
SUMMARY OF THE INVENTION
The present invention is directed to a constitutive promoter
identified from Nicotiana tabacum (tobacco).
The transgenic tobacco plant, T1275, contained a 4.2 kb
EcoRIlXbaI fragment containing the 2.2 kb promoterless GUS-nos gene and
2.0 kb of 5' flanking tobacco DNA. This 5' flanking DNA showed no
homology to known sequences. Expression of the cloned fragment in
transgenic tobacco is apparent on cultured leaf discs and in the early stages
of
shoot development.
Thus according to the present invention there is provided a
constitutive promoter from tobacco. The present invention is further directed
to a constitutive promoter having a DNA sequence, substantially homologous
to SEQ ID NO: 1.
This invention also relates to a chimeric gene construct comprising:
a constitutive promoter, having a DNA sequence substantially homologous to
SEQ ID NO: 1, and a gene encoding a protein, for which constitutive
expression is desired.
~16~~1~
-3-
This invention further relates to a cloning vector containing said
chimeric gene construct.
This invention also includes a plant cell which has been transformed
with said cloning vector.
This invention further relates to a transgenic plant containing a
constitutive promoter, having a DNA sequence substantially homologous to
SEQ ID NO: 1, operatively linked to a gene encoding a protein.
Also included in the present invention is a method of conferring
constitutive expression on a gene in a plant, comprising: operatively linking
an exogenous gene, for which constitutive expression is desired, with a
constitutive promoter, to produce a chimeric gene construct and introducing
the chimeric gene construct into a plant capable of expressing the chimeric
gene construct.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the invention will become more
apparent from the following description in which reference is made to the
appended drawings wherein:
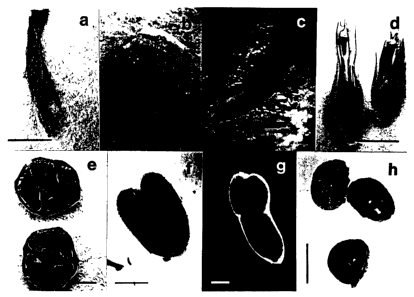
FIGURE 1 shows the constitutive expression of GUS in all tissues
of plant T1275, including leaf segments (a), stem cross-sections (b), roots
(c),
flower cross-sections (d), ovary cross-sections (e), immature embryos (f),
mature embryos (g), and seed cross-sections (h).
FIGURE 2 shows the GUS fluorogenic activity, which reveals that
the level of GUS expression in T1275 is comparable to levels in plants
expressing CaMV 35S - GUS - nos genes in leaf tissues.
_ ~16~~1'~
-4-
FIGURE 3 is the Southern blot analysis of Eco RI digested T1275
DNA with a GUS gene coding region probe (lane 1) and a nptll gene coding
region probe (lane 2).
FIGURE 4 shows the cloned GUS gene fusion pT1275.
FIGURE 5 shows the nucleotide sequence for the Xba I - Sal I
fragment of pT1275.
DESCRIPTION OF PREFERRED EMBODIMENT
The present invention relates to plant gene promoters. Specifically
this invention relates to a constitutive promoter, identified by T-DNA tagging
with a promoterless ~i-glucuronidase gene (GUS) to generate a transgenic N.
tabacum plant that expresses GUS activity constitutively.
In the context of this disclosure, the term "promoter" or "promoter
region" refers to a sequence of DNA, usually upstream (5') to the coding
sequence of a structural gene, which controls the expression of the coding
region by providing the recognition for RNA polymerase and/or other factors
required for transcription to start at the correct site.
There are generally two types of promoters, inducible and
constitutive promoters. An inducible promoter is a promoter that is capable of
directly or indirectly activating transcription of one or more DNA sequences
or genes in response to an inducer. In the absence of an inducer the DNA
sequences or genes will not be transcribed. Typically the protein factor, that
binds specifically to an inducible promoter to activate transcription, is
present
in an inactive form which is then directly or indirectly converted to the
active
form by the inducer. The inducer can be a chemical agent such as a protein,
metabolite, growth regulator, herbicide or phenolic compound or a
physiological stress imposed directly by heat, cold, salt, or toxic elements
or
~16~fi1
-5-
indirectly through the action of a pathogen or disease agent such as a virus.
A plant cell containing an inducible promoter may be exposed to an inducer
by externally applying the inducer to the cell or plant such as by spraying,
watering, heating or similar methods.
The present invention is directed to a constitutive promoter which
directs the expression of a gene, constitutively. Specifically, the present
invention is directed to a constitutive promoter isolated from N. tabacum. A
constitutive promoter directs the expression of a gene throughout the various
parts of a plant and continuously throughout plant development. Examples of
known constitutive promoters include those associated with the CaMV 35S
transcript and Agrobacterium Ti plasmid nopaline synthase gene (Sanders et
al. 1987, Nucleic Acids Res. 15, 1543-1558). The constitutive promoter of
the present invention demonstrated levels of expression greater than that
using
the CaMV 35S promoter.
The term "constitutive" as used herein does not necessarily indicate
that a gene is expressed at the same level in all cell types, but that the
gene is
expressed in a wide range of cell types, although some variation in abundance
is often observed.
The present invention is further directed to a chimeric gene
construct containing a gene of interest operatively linked to the constitutive
promoter of the present invention. Any exogenous gene can be used and
manipulated according to the present invention to result in the constitutive
expression of said exogenous gene.
The chimeric gene construct of the present invention can further
comprise a 3' untranslated region. A 3' untranslated region refers to that
portion of a gene comprising a DNA segment that contains a polyadenylation
signal and any other regulatory signals capable of effecting mRNA processing
or gene expression. The polyadenylation signal is usually characterized by
~16~61'~
-6-
effecting the addition of polyadenylic acid tracks to the 3' end of the mRNA
precursor. Polyadenylation signals are commonly recognized by the presence
of homology to the canonical form 5' AATAAA-3' although variations are not
uncommon.
Examples of suitable 3' regions are the 3' transcribed non-translated
regions containing a polyadenylation signal of Agrobacterium tumor inducing
(Ti) plasmid genes, such as the nopaline synthase (Nos gene) and plant genes
such as the soybean storage protein genes and the small subunit of the
ribulose-1, 5-bisphosphate carboxylase (ssRUBISCO) gene. The 3'
untranslated region from the structural gene of the present construct can
therefore be used to construct chimeric genes for expression in plants.
The chimeric gene construct of the present invention can also
include further enhancers, either translation or transcription enhancers, as
may
be required. These enhancer regions are well known to persons skilled in the
art, and can include the ATG initiation codon and adjacent sequences. The
initiation codon must be in phase with the reading frame of the coding
sequence to ensure translation of the entire sequence. The translation control
signals and initiation codons can be from a variety of origins, both natural
and
synthetic. Translational initiation regions may be provided from the source of
the transcriptional initiation region, or from the structural gene. The
sequence
can also be derived from the promoter selected to express the gene, and can
be specifically modified so as to increase translation of the mRNA.
To aid in identification of transformed plant cells, the constructs of
this invention may be further manipulated to include plant selectable markers.
Useful selectable markers include enzymes which provide for resistance to an
antibiotic such as gentamycin, hygromycin, kanamycin, and the like.
Similarly, enzymes providing for production of a compound identifiable by
colour change such as GUS (/3-glucuronidase), or luminescence, such as
luciferase are useful.
Also considered part of this invention are transgenic plants
containing the chimeric gene construct of the present invention. Methods of
regenerating whole plants from plant cells are known in the art, and the
method of obtaining transformed and regenerated plants is not critical to this
invention. In general, transformed plant cells are cultured in an appropriate
medium, which may contain selective agents such as antibiotics, where
selectable markers are used to facilitate identification of transformed plant
cells. Once callus forms, shoot formation can be encouraged by employing
the appropriate plant hormones in accordance with known methods and the
shoots transferred to rooting medium for regeneration of plants. The plants
may then be used to establish repetitive generations, either from seeds or
using vegetative propagation techniques.
The constructs of the present invention can be introduced into plant
cells using Ti plasmids, Ri plasmids, plant virus vectors, direct DNA
transformation, micro-injection, electroporation, etc. For reviews of such
techniques see for example Weissbach and Weissbach, Methods for Plant
Molecular Biology, Academy Press, New York VIII, pp. 421-463 (1988); and
Geierson and Corey, Plant Molecular Biology, 2d Ed. (1988). The present
invention further includes a suitable vector comprising the chimeric gene
construct.
When specific sequences are referred to in the present invention, it
is understood that these sequences include within their scope sequences that
are "substantially homologous" to said specific sequences. Sequences are
"substantially homologous" when at least about 80%, preferably at least about
90 % and most preferably at least about 95 % of the nucleotides match over a
defined length of the molecule. Sequences that are "substantially
homologous" include any substitution, deletion, or addition within the
sequence. DNA sequences that are substantially homologous can be identified
in Southern hybridization experiments, for example under stringent
CA 02168617 2004-12-06
_8_
hybridization conditions (see Maniatis et al., in Molecular Cloning (A
Laboratory Manual),
Cold Spring Harbor Laboratory (1982) p 387 to 389). For example, hybridization
is done in
6 x SSC, O.O1M EDTA, 5 x Denhardt's solution, 0.5% SDS and 100~g/ml denatured
salmon
sperm DNA, in addition to the DNA probe, at 68°C for 3-16 hours, as
needed. The filter is
S then washed for 5 minutes in 2 x SSC and 0.5% SDS at room temperature,
followed by a
second wash at room temperature in 0.1 x SSC and 0.1 % SDS for 1 S minutes. A
final wash
is done in 0.1 x SSC and 0.5% SDS at 68°C for 2 hours.
The specific sequences, referred to in the present invention, also include
sequences
which are "functionally equivalent" to said specific sequences. In the present
invention
functionally equivalent sequences refer to sequences which although not
identical to the
specific sequences provide the same or substantially the same function. DNA
sequences that
are functionally equivalent include any substitution, deletion or addition
within the sequence.
With reference to the present invention functionally equivalent sequences will
direct the
expression of an exogenous gene constitutively.
While this invention is described in detail with particular reference to
preferred
embodiments thereof, said embodiments are offered to illustrate but not limit
the invention.
EXAMPLES
Characterization of a Constitutive promoter - GUS Fusion
Transfer of binary constructs to Agrobacterium and leaf disc transformation of
N.
tabacum SR1 were performed as described by Fobert et al. (1991, Plant Mol.
Biol. 17, 837-
851). Plant tissue was maintained on 100 p,g/ml kanamycin sulfate (Sigma)
throughout in
vitro culture.
From the transgenic plants produced, one of these, T1275, was chosen for
detailed
CA 02168617 2004-12-06
-8A-
study because of its high level and constitutive expression of GUS.
Fluorogenic and histological GUS assays were performed according to Jefferson
(Plant Mol. Biol. Rep., 1987, 5, 387-405), as modified by Fobert et al. (Plant
Mol. Biol.,
1991, 17, 837-851). For initial screening, leaves were harvested from in vitro
grown
plantlets. Later nine different tissues:
216~61'~
-9-
leaf (L), stem (S), root (R), anther (A), petal (P), ovary (O), sepal (Se),
seeds
days post anthesis (S1) and seeds 20 days post-anthesis (S2), were collected
from plants grown in the greenhouse and analyzed. For detailed, quantitative
analysis of GUS activity, leaf, stem and root tissues were collected from
5 kanamycin resistant F1 progeny grown in vitro. Floral tissues were harvested
at developmental stages 8-10 (Koltunow et al., 1990, Plant Cell 2, 1201-1224)
from the original transgenic plants. Flowers were also tagged and developing
seeds were collected from capsules at 10 and 20 dpa. In all cases, tissue was
weighed, immediately frozen in liquid nitrogen, and stored at -80°C.
Tissues analyzed by histological assay were at the same
developmental stages as those listed above. Different hand-cut sections were
analyzed for each organ. For each plant, histological assays were performed
on at least two different occasions to ensure reproducibility. Except for
floral
organs, all tissues were assayed in phosphate buffer according to Jefferson
(1987, Plant Mol. Biol. Rep. 5, 387-405), with 1 mM X-Gluc (Sigma) as
substrate. Flowers were assayed in the same buffer containing 20% (v/v)
methanol (Kosugi et al., 1990, Plant Sci. 70, 133-140).
GUS activity in plant T1275 was found in all tissues. Figure 1
shows the constitutive expression of GUS by histochemical staining with X-
Gluc of T1275, including leaf (a), stem (b), root (c), flower (d), ovary (e),
embryos (f and g), and seed (h).
Constitutive GUS expression was confirmed with the more sensitive
fluorogenic assay of plant tissue from transformed plant T1275. These results
are shown in Figure 2. GUS expression was evident in all tissue types
including leaf (L), stem (S), root (R), anther (A), pistil (P), ovary (O),
sepal
(Se), seeds at 10 dpa (S1) and 20 dpa (S2). Furthermore, the level of GUS
expression is comparable to the level of expression in transformed plants
containing the constitutive promoter CaMV 35S in a GUS - nos fusion. As
reported by Fobert et al. (1991, Plant Molecular Biology, 17: 837-851) GUS
~1~~61'~
-lo-
activity in transformed plants containing pBI121 (Clontech), which contains a
CaMV 35S - GUS - nos chimeric gene, was as high as 18,770 ~ 2450 (pmole
MU per minute per mg protein).
Genetic Analysis of Transgenic Plant T1275
The T-DNA contains a kanamycin resistance gene. Seeds from
self pollinated transgenic plants were surface-sterilized in 70% ethanol for 1
min and in undiluted Javex bleach (6% sodium hypochloride) for 25 min.
Seeds were then washed several times with sterile distilled water, dried under
laminar flow, and placed in Petri dishes containing MSO medium
supplemented with 100 ~.g/ml kanamycin as described in Miki et al. (1993,
Methods in Plant Molecular Biology and Biotechnology, Eds., B.R. Glick and
J.E. Tompson, CRC Press, Boca Raton, 67-88). At least 90 plantlets were
counted for each transformant. The number of green (kanamycin-resistant)
and bleached (kanamycin-sensitive) plantlets were counted after 4-6 weeks,
and analyzed using the Chit test at a significance level of P<0.05.
The genetic analysis results are shown below in Table 1, which
demonstrates that the T-DNA loci segregated as a single locus of insertion.
TABLE 1
Genetic Analysis of Transgenic Plant T1275
No. of No. of Observed Expected Chit
Progeny Progeny Ratio Ratio
Km' Km'
262 88 3:1' 3:1 0
' Consistent
with a
single
dominant
gene
CA 02168617 2002-11-21
-11-
Southern Blot Analysis
The T-DNA in the transgenic plant T1275 was analyzed using either
a GUS gene coding region probe or a nptll gene coding region probe.
Genomic DNA was isolated from freeze-dried leaves using the
protocol of Sanders et al. (1987, Nucleic Acid Res. 15, 1543-1558). Ten
micrograms of T1275 DNA was digested for several hours with EcoRI using
the appropriate manufacturer-supplied buffer supplemented with 2.5 mM
spermidine. After electrophoresis through a 0. 8 % TAE agarose gel, Southern
blot analysis was conducted using standard protocols. As the T-DNA from
the construct containing the constitutive promoter - GUS - nos construct
contains only a single Eco RI recognition site the hybridizing fragments are
composed of both T-DNA and flanking tobacco DNA sequences. The length
of the fragment will vary depending on the location of the nearest Eco RI
site.
~ Using the GUS gene as a probe (Figure 3 - lane 1), the fragment to the
nearest Eco RI site in the plant DNA will be detected. With T1275, one such
fragment was located. Using the nptll coding region as a probe (Figure 3 -
lane 2), which hybridizes to sequences on the opposite side of the Eco RI
site,
again only one hybridization band was evident. As can also be seen in Figure
3, no major rearrangements occurred.
Cloning and Analysis of the Constitutive Promoter - GUS Vision
Genomic DNA was isolated from leaves according to Hattori et al.
(1987, Anal. Biochem. 165, 70-74). Ten ~cg of T1275 total DNA was
digested with EcoRI and XbaI according to the manufacturer's instructions.
The digested DNA was size-fractionated on a 0.7 % agarose gel. The DNA
fragments of about 4 to 6 kb were isolated from the gel using the Elu-Quick#
kit (Schleicher and Schuell) and ligated to lambdaGEM-2 arms previously
digested with EcoRI and XbaI and phosphatase-treated. About 40,000 plaques
were transferred to a nylon membrane (Hybond#Amersham) and screened
with the 3zP-labelled 2kb GUS insert isolated form pBIl2l, essentially as
Trademark
CA 02168617 2002-11-21
-12-
described in Rutledge et al. (1991, Mol. Gen Genet. 229, 31-40). The
positive clones were isolated. The XbaI-EcoRI fragment (Figure 4) was
isolated from the lambda phage and cloned into pTZl9R previously digested
with XbaI and EcoRI and treated with intestinal calf phosphatase.
The 4.2kb fragment containing about 2.2kb of the T1275 promoter
activity fused to the GUS gene and the nos 3' was isolated by digesting pTZ-
T1275 with HindIII and EcoRI. The isolated fragment was ligated into the
pRD400 vector (Datla et al. , 1992, Gene, 211:383-384) previously digested
with HindIII and EcoRI and treated with calf intestinal phosphatase. Transfer
of the binary vector to Agrobacterium tumefacierrs and leaf disc
transformation
of N. tabacum SR1 were performed as described above. Histochemical
analysis of GUS activity revealed staining of callus tissue after
transformation
and prior to shoot organogenesis and staining of hoots subsequently. Staining
~ was comparable for shoots transformed with a vector in which the T1275
promoter was replaced with the 3kb 35S promoter from pBI121.
The present invention has been described with regard to preferred
embodiments. However, it will be obvious to persons skilled in the art that a
number of .variations and modifications can be made without departing from
the scope of the invention as described in the following claims.
-13-
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
(A) NAME: BRIAN MIKI
(B) STREET: 1876 Dorset Drive
(C) CITY: Ottawa
(D) STATE: Ontario
(E) COUNTRY: Canada
(F) POSTAL CODE (ZIP): K1H 5V1
(A) NAME: JIRO HATTORI
(B) STREET: 763 Halstead Street
(C) CITY: Ottawa
(D) STATE: Ontario
(E) COUNTRY: Canada
(F) POSTAL CODE (ZIP): K1G 1M5
(A) NAME: PIERRE FOBERT
(B) STREET: 878 Kingsmere Blvd.
(C) CITY: Saskatoon
(D) STATE: Saskatchewan
(E) COUNTRY: Canada
(F) POSTAL CODE (ZIP): S7J 4J7
(A) NAME: VENKATRAN N. IYER
(B) STREET: 139 Iona Street
(C) CITY: Ottawa
(D) STATE: Ontario
(E) COUNTRY: Canada
(F) POSTAL CODE (ZIP): K1Y 3M2
(ii) TITLE OF INVENTION: Constitutive Promoter
(iii) NUMBER OF SEQUENCES: 1
(iv) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.30 (EPO)
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2255 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
TCTAGACTTACAGAAAGTCTCTAACACGTGAGGGAATGATCCCTTTCCTTACCTCCCTGT 60
AGAGATATTGGCTTTTCAACAACTAGTACATAAATATGCGACTTTGACCGTGTATCCCCA 120
GTCAAAAGGGAACTTCACCCTCCTAGTTCTTTATTTCCAACATACATGGGGAGTAATGCT 180
AAATTTACATAGAAGAATAATAAAATGAACTGTAACTAATGATGTACTGTTCCAAAGAGA 240
TGAGGACGTCAACATATTTATTCCTTCAGCCCTTTTCAGAATAATACCATAAGTAGAAGA 300
AATGGCACATAAAATGAAGTCCTCGGCAAGTCAAATGTAAATCTGAACCCACCCAGCTAA 360
21~'t ~ 1'~
-14-
CCCAGTGAACTCAACTTTCCTGGATAGATCAGCACTCCTTCATGACATTGCATGCCTTCT 420
CTTTAAAGAGCCGCTTGATCTCTGAAAACCAAATGAATCTCCACAGAGAGATTTCGAGCT 480
CCATGAGACGCCTTTTGGTTCTTGATTTACTAAACCTATAAAAATGAAAGGAAGTAGGAC 540
AACTGCATTTTGCCGCTTAAGATGCTTCGGCGCTTTGTGAATTTTAAGTCATGAGAAAGT 600
ACAATGTTGGAATCTCACATTAGAACAATGTATTTGTAATAACCTAGGAAAGCAAAGCTA 660
GAAGGGAGGTGCAGCTAAATCTTCTTCTACCTTGTTATCCTTGCATTTCTTGAGGAGGAG 720
GAACTGTCCTCGCAGGTGCAAAATCTGCAGTCGCCCAAAAGGATATTCAGAAGTATATTA 780
CAACATGTTTAATGGTTAACCAAGTGAAAGATCAAAATAGTCATTAGAACAAAATGCGTG 840
CTCAGAGCGTATCTACTAGTTCATCAACCCAGTACACATCTCTGAATTTCATCTCTTGCC 900
GTTGAACTAAGTCAATTGGTCAAAGACGCATAACATGAGAGACACTCATAAAACGGCTGA 960
ATAACATGCAGAAGACGTCATGCGCCTTAGGTCTCATTATGCATGAGATTATTAGTTATA 1020
TGCTCCTTCAGTTTGACTAGAAATGAAAAATCAGTTAAGCCTGTAACGAAATGATAACCT 1080
GCTTCAAGAAGATTAGACTATTTTTCATAAAATATGCAGTGCCGTGAAATAGATACTTAA 1140
TCTTAGGCAGGAAAAATCTTCTATTGGGCCATAATAAGAACTACCAATTAGAAAGGAGGT 1200 '
AGAAAGCTCCGATACTGTTATGAAGGCCATTCTAAGTGCTGATGTGAATTTCCCAATACA 1260
AAATGACAACAAAAACAAAAGCCTCAATCCTAAGCTAGTTGGGGTCGCTATATAAATCCT 1320
CGACATCCATTTAACTCCACTTGGACTCCTTTCTTTCCAATATTTTAATATTGTTAGATT 1380
AATCATAAAATTGCTTAGCTTTCTACTGGCACTTAACCTACTGCAACCCTCCTCTTCTGG 1440
GATTCCAACACAAACAACTAAGAGGAATTTGAAAAAAAGAAAGCAAATGTGAGAAGAGAC 1500
AAAATGTACAATGATACCTCTTCTTGCAGCAAAGGAGGCAGGTTCTCTGCTGAGACAAGG 1560
TTCTCTATTTCCTGCAAGACCTTCGTATCTTTTATTCGAGACCATGTATGTGGAGGTAAC 1620
GCCAGCAATAGTGCTGTCAGCACATCGTTGCTTGCAGGGGATCTTCTGCAAGCATCTCTA 1680
TTTCCTGAAGGTCTAACCTCGAAGATTTAAGATTTAATTACGTTTATAATTACAAAATTG 1740
ATTCTAGTATCTTTAATTTAATGCTTATACATTATTAATTAATTTAGTACTTTCAATTTG 1800
TTTTCAGAAATTATTTTACTATTTTTTATAAAATAAAAGGGAGAAAATGGCTATTTAAAT 1860
ACTAGCCTATTTTATTTCAATTTTAGCTTAAAATCAGCCCCAATTAGCCCCAATTTCAAA 1920
TTCAAATGGTCCAGCCCAATTCCTAAATAACCCACCCCTAACCCGCCCGGTTTCCCCTTT 1980
TGATCCAGGCCGTTGATCATTTTGATCAACGCCCAGAATTTCCCCTTTTCCTTTTTTAAT 2040
TCCCAAACACCCCTAACTCTATCCCATTTCTCACCAACCGCCACATATGAATCCTCTTAT 2100
CTCTCAAACTCTCTCGAACCTTCCCCTAACCCTAGCAGCCTCTCATCATCCTCACCTCAA 2160
AACCCACCGGAATACATGGCTTCTCAAGCCGTGGAAACCTTATACTCACCTCCCTTTGCT 2220
CTTACAGTACTCGGCCGTCGACCGCGGTACCCGGG 2255