Note: Descriptions are shown in the official language in which they were submitted.
WO 95121626
21 b 917 3 PCT~S95101829
1
Description
Methods for stimulating erythropoiesis using thrombopoietin.
Bac round of the Invention
Hematopoiesis is the process by which blood
cells develop and differentiate from pluripotent stem
cells in the bone marrow. This process involves a complex
interplay of polypeptide growth factors (cytokines) acting
via membrane-bound receptors on the target cells.
Cytokine action results in cellular proliferation and
differentiation, with response to a particular cytokine
often being lineage-specific and/or stage-specific.
Development of a single cell type, such as a platelet or
erythrocyte, from a stem cell may require the coordinated
action of a plurality of cytokines acting in the proper
sequence.
The known cytokines include the interleukins,
such as IL-1, IL-2, IL-3, IL-6, IL-8, etc. ; and the colony
stimulating factors, such as G-CSF, M-CSF, GM-CSF,
erythropoietin (EPO), etc. In general, the interleukins
act as mediators of immune and inflammatory responses.
The colony stimulating factors stimulate the proliferation
of marrow-derived cells, activate mature leukocytes, and
otherwise form an integral part of the host's response to
inflammatory, infectious, and immunologic challenges.
Various cytokines have been developed as
therapeutic agents. Several of the colony stimulating
factors have been used in conjunction with cancer
chemotherapy to speed the recovery of patients' immune
systems. Interleukin-2,.a-interferon and ~-interferon are
used in the treatment of certain cancers. EPO, which
stimulates the development of erythrocytes, is used in the
treatment of anemia arising from renal failure. Factors
W J yS;=Il~2f~ f,~. S9SIUlY29
.
2
21691 j3
responsible for stimulation of megakaryocytopoiesis and
thrombocytopoiesis resisted definitive characterization,
due in part to Iack of a good source, a lack of good
assays, and a lack of knowledge as to the sites) of
production until recently, despite three decades of work
to isolate and characterize them. The
megakaryocytopoietic factor, referred to in the literature
as "thrombopoietin" (recently reviewed by McDonald, Exp.
Hematol. 16:201-205, 1988; and McDonald, Am. J. Ped.
Hematol. Oncol. 14:8-21, 1992) has now been identified and
isolated (see copending PCT/W095/2I920;
Lok et al., Nature 369:565-568, 1994; and
Kaushansky et al., ature 369:568-571, 1994.
Mild bleeding disorders (MBDs) associated with
platelet dysfunctions are relatively common (Bachmann,
.Seminars in Hematology ~7: 292-305, 1980), as are a number
of congenital disorders of platelet function, including :.
Bernard-Soulier syndrome (deficiency in platelet GPIb),
Glanzmann's thrombasthenia (deficiency of GPIIb and
GPIIIa), congenital afibrinogenemia (diminished or absent
levels of fibrinogen in plasma and platelets), and gray
platelet syndrome (absence of a-granules). In addition
there are a number of disorders associated with platelet
secretion, storage pool deficiency, abnormalities in
platelet arachidonic acid pathway, deficiencies of
platelet cyclooxygenase and thromboxane synthetase and
defects in platelet activation (reviewed by Rao and
Holmsen, Seminars in Hematolocxy 23: 102-118, 1986). At
present, the molecular basis for most of these defects is
not well understood.
Anemias are deficiencies in the production of
red blood cells (erythrocytes) and result in a reduction
in the level of oxygen transported by blood to the tissues
of the body. Hypoxia may be caused by loss of large
amounts of blood through hemorrhage, destruction of red
a
t' '
r~. WO 95121626 ~ ~ PCT/US95/01829
3
blood cells from exposure to autoantibodies, radiation or
chemicals, reduction in oxygen intake due to high
altitudes or prolonged unconsciousness. When hypoxia is
present in tissue, EPO production is stimulated and
increases red blood cell production. EPO promotes the
conversion of primitive precursor cells in the bone marrow
into pro-erythrocytes which subsequently mature,
synthesize hemoglobin and are released into the
circulation as red blood cells. When the number of red
blood cells in circulation is greater than needed for
normal tissue oxygen requirements, the level of EPO in
circulation is decreased.
Severe reductions in both megakaryocyte and
erythrocyte levels can be associated with the treatment of
various cancers with chemotherapy and radiation and
diseases such as AIDS, aplastic anemia and
myelodysplasias. Levels of megakaryocytes and/or
erythrocytes that become too low, for example, platelet
counts below 25,000 to 50,000 and hematocrits of less than
25 are likely to produce considerable morbidity and in
certain circumstances these levels are life-threatening.
In addition to treating the underlying disease, specific
treatments include platelet transfusions for
thrombocytopenia (low megakaryocyte levels) and
stimulation of erythropoiesis using EPO or transfusion of
red blood cells for anemia.
Recent advances in molecular biology have
greatly increased our understanding of hematopoiesis, but
at the same time have shown the process to be extremely
3o complex. While many cytokines have been characterized and
some have proven clinical applications, there remains a
need in the art for additional agents that stimulate
proliferation and differentiation of myeloid and lymphoid
precursors and the production of mature blood cells.
There is a particular need for agents that stimulate the
development and proliferation of cells of the
WO 95121626 21 b 917 3 pCT~s95/01829 .,."
4
megakaryocytic and erythroid lineages, including platelets
and red blood cells. There is a further need in the art
for agents that can be used in the simultaneous treatment
of cytopenias and anemias such as those caused by
destruction of hematopoietic cells in bone marrow such as
in the treatment of cancer with chemotherapy and
radiation, and pathological conditions such as
myelodysplasia, AIDS, aplastic anemia, autoimmune disease
or inflammatory conditions. The present invention
fulfills these needs and provides other, related
advantages.
Summary of the Invention
It is an object of the present invention to
provide methods for stimulating erythropoiesis by
culturing bone marrow or peripheral blood cells in the
presence of TPO and EPO in amount sufficient to produce an
increase in the number of erythrocytes or erythrocyte
precursors as compared to cells cultured without TPO.
It is a further object of the invention to
provide methods for stimulating erythropoiesis by
culturing bone marrow or peripheral blood cells in the
presence of a composition comprising TPO in an amount
sufficient to produce an increase in the number of
erythrocytes or erythrocyte precursors as compared to
cells cultured without TPO.
It is a further object of the invention to
provide methods for stimulating erythropoiesis in a mammal
by administering a composition comprising TPO in a
pharmaceutically acceptable vehicle to produce an increase
in proliferation or differentiation of erythroid cells.
It is a further object of the invention to
provide methods for stimulating erythropoiesis in a mammal
by administering a composition comprising EPO and TPO in a
pharmaceutically acceptable vehicle to produce an increase
in proliferation or differentiation of erythroid cells.
7 3 pC'I'~1S95/01829
~-- WO 95/21626
It is a further object of the invention to
provide methods for stimulating erythropoiesis in a
patient by administering a composition comprising EPO and
TPO in amount sufficient to increase reticulocyte counts
5 and erythroid colony formation.
It is a further object of the invention to
provide methods for stimulating erythropoiesis in a
patient by administering a composition comprising TPO in
an amount sufficient for increasing reticulocyte counts at
least 2-fold over baseline reticulocyte counts.
It is a further object of the invention to
provide methods for stimulating erythropoiesis in a
patient by administering a composition comprising TPO and
EPO in an amount sufficient for increasing reticulocyte
counts at least 2-fold over baseline reticulocyte counts.
Within one aspect, the present invention
provides that the TPO is human TPO. In another
embodiment, the TPO comprises of a sequence of amino acids
selected from group consisting of: the sequence of amino
acids shown in SEQ ID N0:2 from amino acid residue 1 to
residue 172; the sequence of amino acids shown in SEQ ID
N0:2 from amino acid residue 1 to residue 173; the
sequence of amino acids shown in SEQ ID N0:2 from amino
acid residue 1 to residue 175; the sequence of amino acids
shown in SEQ ID N0:2 from amino acid residue 1 to amino
acid residue 353; the sequence of amino acids shown in SEQ
ID N0:2 from amino acid residue 22 to residue 353; the
sequence of amino acids shown in SEQ ID N0:2 from amino
acid residue 22 to residue 172; the sequence of amino
acids shown in SEQ ID N0:2 from amino acid residue 22 to
residue 173; the sequence of amino acids shown in SEQ ID
N0:2 from amino acid residue 22 to residue 175; the
sequence of amino acids shown in SEQ ID N0:2 from amino
acid residue 28 to residue 172; the sequence of amino
acids shown in SEQ ID N0:2 from amino acid residue 28 to
residue 173; the sequence of amino acids shown in SEQ ID
PCTIUS95/01829
W O 95/21626
6 '
No:2 from amino acid residue 28 to residue 175; and the
sequence of amino acids shown in SEQ ID N0:2 from amino
acid residue 28 to residue 353.
Within another aspect, the invention provides
methods where a mammal is administered TPO of 1 x 105 to
100 x 105 units TPO/kg/day, preferably 5 x 105 to 50 x 105
units TPO/kg/day.
In another embodiment, the invention provides
methods where a mammal is administered TPO of 1 x 105 to
100 x 105 units TPO/kg/day, preferably 5 x 105 to 50 x 105
units TPO/kg/day and EPO of 1 to 150 units EPO/kg/day.
RriPf Description of the Drawings
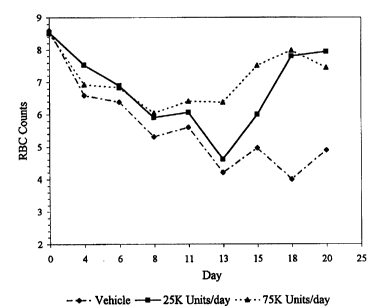
Figure 1 illustrates that following the addition
of TPO and EPO to cultured bone marrow cells, erythroid
colony formation is enhanced relative to addition of EPO
alone.
Figure 2 illustrates that following the addition
of TPO to animals made pancytopenic with prior irradiation
and chemotherapy, the decline in red blood cell count is
not as severe, and returns to normal sooner in animals
given TPO.
WO 95/21626 t ~ ~ 6 917 3 PCTlUS95l01829
7
Detailed Description of the Invention
Prior to describing the present invention in
detail, it may be helpful to define certain terms used
herein:
Allelic variant: An alternative form of a gene
that arises through mutation, or an altered polypeptide
encoded by the mutated gene. Gene mutations can be silent
(no change in the encoded polypeptide) or may encode
polypeptides having altered amino acid sequence.
cDNA: Complementary DNA, prepared by reverse
transcription of a messenger RNA template, or a clone or
amplified copy of such a molecule. Complementary DNA can
be single-stranded or double-stranded.
Expression vector: A DNA molecule, linear or
circular, that comprises a segment encoding a polypeptide
of interest operably linked to additional segments that
provide for its transcription. Such additional segments
include promoter and terminator sequences, and may also
include one or more origins of replication, one or more
selectable markers, an enhancer, a polyadenylation signal,
etc. Expression vectors are generally derived from
plasmid or viral DNA, or may contain elements of both.
The term "operably linked" indicates that the segments are
arranged so that they function in concert for their
intended purposes, e.g. transcription initiates in the
promoter and proceeds through the coding segment to the
terminator.
Gene: A segment of chromosomal DNA that encodes
a polypeptide chain. A gene includes one or more regions
3o encoding amino acids, which in some cases are interspersed
with non-coding "intervening sequences" ("introns"),
together with flanking, non-coding regions which provide
for transcription of the coding sequence.
Molecules complementary to~ Polynucleotide
molecules having a complementary base sequence and reverse
orientation as compared to a reference sequence. For
11'C:'J~i=1!,'_O PCB '~~llll.'S?9
8
2169173
example, the sequence 5' ATGCACGGG 3' is complementary to
5' CCCGTGCAT 3'.
Promoter: The portion of a gene at which RNA
polymerase binds and mRNA synthesis is initiated.
As noted above, the present invention provides
methods for stimulating thrombopoiesis and erythropoiesis
using proteins having hematopoietic activity. As used
herein, the term "hematopoietic" denotes the ability to
stimulate the proliferation and/or differentiation of
myeloid or lymphoid precursors as determined by standard
assays. See, for example, Metcalf, Proc. Natl. Acad. Sci.
USA 77: 5327-5330, 1980; Metcalf et al. , J. Cell. Physiol .
198-206, 1983; and Metcalf et al., ~xp. Hematol.
288-295, 1987. Typically, marrow cells are incubated in '
the presence of a test sample and a control sample. The . .
cultures are then scored for cell proliferation and
.differentiation by visual examination and/or staining. A
particularly preferred assay is the MTT colorimetric assay
of Mosman (J. Immunol. Meth. 65: 55-63, 1983.
As used herein, the terra "erythropoiesis"
denotes the proliferation and/or differentiation of
erythroid precursor cells. Standard measures of erythroid
cell proliferation and differentiation include hematocrit
and reticulocyte counts. Hematocrit is a measurement of
red blood cells, and is commonly expressed as the
percentage of total blood volume which consists of
erythrocytes. Reticulocyte counts measure 1-2 day-old
cells that contain mRNA (absent in mature erythrocytes)
and aggregates of ribosomes as demonstrated by staining
(Erslev, A., "Reticulocyte Enumeration", in Hematology,
McGraw-Hill, NY, 1990). A reticulocyte count is the
percentage of such cells per 500 or 1000 cells counted.
An average range for reticulocyte counts is 0.8% to 1.2%.
EPO is commercially available (R & D Systems, Minneapolis,
MN and Amgen, Thousand oaks, CA) and activity is measured
-.--. WO 95/21626 .. ~ 16 917 3 PCT~S95/01829
9
by calibration against the second international reference
preparation of erythropoietin (Annable et al., Bull. Wld.
Hlth. Orcr. x:99, 1972) using an in vivo assay which
measures the incorporation of 56Fe into red blood cells of
exhypoxic polycythemic mice (Cotes et al., Nature
1~9 :1065, 1961) or by in vitro cell proliferation assay
that uses a factor-dependent human erythroleukemic cell
line, TF-1 (Kitamura et al., J. Cell. Physiol. 140:323,
1989) .
The present invention is based in part upon the
discovery that thrombopoietin (TPO) stimulates erythroid
cell growth. When the present inventors administered TPO
to thrombocytopenic mammals, in addition to an increase in
platelets, surprisingly TPO was found to augment the
recovery of red blood cells and produce a rapid increase
in hematocrit levels.
The sequences of cDNA clones encoding
representative human and mouse TPO proteins are shown in
SEQ ID NO:1 and SEQ ID N0:3, respectively and the
corresponding amino acid sequence are shown in SEQ ID N0:2
and SEQ ID N0:4, respectively. Those skilled in the art
will recognize that the sequences shown in SEQ ID NOS: 1
and 2, and the human genomic sequence shown in SEQ ID
NOS:5 and 6, correspond to single alleles of the human
gene, and that allelic variation is expected to exist. It
will also be evident that one skilled in the art could
engineer sites that would facilitate manipulation of the
nucleotide sequence using alternative codons.
The present invention provides methods for
stimulating erythropoiesis using proteins that are
substantially homologous to the proteins of SEQ ID NO: 2
and their species homologs. By "isolated" is meant a
protein which is found in a condition other than its
native environment, such as apart from blood and animal
tissue. In a preferred form, the isolated protein is
substantially free of other proteins, particularly other
WO 95121626
216 9 ~ ~ 3 p~~S95101829
proteins of animal origin. It is preferred to provide the
proteins in a highly purified form, i.e. greater than 95%
pure, more preferably greater than 99% pure. The term
"substantially homologous" is used herein to denote
5 proteins having 50%, preferably 60%, more preferably at
least 80%, sequence identity to the sequences shown in SEQ
ID NO: 2 or their species homologs. Such proteins will
more preferably be at least 90% identical, and most
preferably 95% or more identical to SEQ ID NO: 2 or their
10 species homologs. Percent sequence identity is determined
by conventional methods. See, for example, Altschul et
al., Bull. Math. Bio. 48: 603-616, 1986 and Henikoff and
Henikoff, Proc. Natl. Acad. Sci. USA $x:10915-10919, 1992.
Briefly, two amino acid sequences are aligned to optimize
the alignment scores using a gap opening penalty of 10, a
gap extension penalty of 1, and the "blosum 62" scoring
matrix of Henikoff and Henikoff (ibid.) as shown in Table
1 (amino acids are indicated by the standard one-letter
codes). The percent identity is then calculated as:
Total number of identical matches
x 100
[length of the longer sequence plus the
number of gaps introduced into the longer
sequence in order to align the two
sequences]
..~~.. WO95/21626 _- ' ~ ~ ~~ PCT/US95l01829
-11-
n
I
ri N M
I
H InN N O
I I
~ ~P rlM N N
I I 1
1~~-1rld' M N
1 I 1 1 1
~O d'N N rl M v-I
I I I 1
InO N rl r-Ie-Irir-I
I 1 I 1 1
!l1v-1M rlO riM N N
I I 1 1 I I I
a d' N N O M N riN riv-1
1 1 1 1 I I
1"~ d'N M rlO M N e~iM ~-1M
la
I I I I I 1
x 00 M M ~-iN ~-1N r-1N N N M
1 1 1 1 1 I I I 1 I
~DN d'e!'N i M N O N N i M
w lf1N O M C7 ~-iN M rlO r-IM N N
1 I 1 I I i I ( I I
tl1N N O M N ~ O M rlO e-~N rlN
I 1 ( 1 1 1 I I I
U 01 M styM M e-1~i M rlN M eW -iN N r-I
1 ( 1 1 1 I 1 1 1 I 1 1 I I I
O 10 M O N rie-)M er ~ M M e-1O rle1'M M
I 1 I I 1 1 1 1 I I I I 1
Z ~Oe-1M O O O r-iM M O N M N v-1O tl'IVM
1 1 I I I I 1 1 1
Qi In O N M riO N O M N N e-1M N ~-I~-iM N M
1 1 I I I 1 I I 1 1 1 1 1
R,' d'~ N N O e-1r-IO N riri ~-i~ N rlrl O M N O
1 I I I I 1 1 I I I I 1 1 1
x z o a a w c~x H a x ~ w w cn H 3 ?~
W J')~;=10~G p[' :')~llll~i'_')
'~'' 12
2169173
Substantially homologous proteins are
characterized as having one or more amino acid
substitutions, deletions or additions. These changes are
preferably of a minor nature, that is conservative amino
acid substitutions that do not significantly affect the
folding or activity of the protein (see Table 2); small
deletions, typically of one to about 30 amino acids; and
small amino- or carboxyl-terminal extensions, such as an
amino-terminal methionine residue, a small linker peptide
of up to about 20-25 residues, or a small extension that
facilitates purification, such as a poly-histidine tract,
an antigenic epitope or a binding domain. See, in general
Ford et al., Protein Expression and Purification 2: 95-
107, 1991,
Table 2
Conservative amino acid substitutions
Basic: arginine
._. f.
lysine - '
2o histidine
Acidic: glutamic acid
aspartic acid
Polar: glutamine
asparagine
Hydrophobic: leucine
isoleucine
valine
Aromatic: phenylalanine
tryptophan
3o tyrosine
Small: glycine
alanine
serine .
threonine
methionine
216 917 3 PGTlUS95101829
WO 95121b26 .
13
Essential amino acids in TPO and EPO may be
identified according to procedures known in the art, such
as site-directed mutagenesis or alanine-scanning
mutagenesis (Cunningham and Wells, Science ~4 , 1081-1085,
1989). In the latter technique, single alanine mutations
are introduced at every residue in the molecule, and the
resultant mutant molecules are tested for biological
activity (e. g. receptor binding, in vitro or in vivo
proliferative activity) to identify amino acid residues
that are critical to the activity of the molecule. Sites
of ligand-receptor interaction can also be determined by
analysis of crystal structure as determined by such
techniques as nuclear magnetic resonance, crystallography
or photoaffinity labeling. See, for example, de Vos et
al., Science 255:306-312, 1992; Smith et al., J. Mol.
io 224:899-904, 1992; Wlodaver et al., FEBS Lett.
309:59-64, 1992.
Biologically active muteins of EPO based on
elucidation of structure-function relationships have
recently been identified (Boissel et al., J. of Biol.
C em. x:15983-15993, 1993 and Higuchi et al., J. Biol.
Chem. X67:7703-7709, 1992). EPO isoforms having different
sialic acid compositions are disclosed by Strickland et
al. EP 0428267.
In general, cytokines are predicted to have a
four-alpha helix structure, with the first and fourth
helices being most important in ligand-receptor
interactions and more highly conserved among members of
the family. Referring to the human TPO amino acid
sequence shown in SEQ ID N0:2, alignment of cytokine
sequences suggests that these helices are bounded by amino
acid residues 29 and 53, 80 and 99, 108 and 130, and 144
and 168, respectively (boundaries are ~ 4 residues).
Helix boundaries of the mouse and other non-human TPOs can
be determined by alignment with the human sequence. Other
WO 95121626 14 ~ ~ ~ ~ ~ ~ ~ p~'~595101829
important structural aspects of TPO include the cysteine
residues at positions 28, 50, 106 and 172 of SEQ ID N0:2.
In addition to the hematopoietic proteins
disclosed above, the methods of the present invention
include utilization of fragments of these proteins and
isolated polynucleotide molecules encoding the fragments.
Of particular interest are fragments of at least 10 amino
acids in length that bind to an MPL receptor, and
polynucleotide molecules of at least 30 nucleotides in
l0 length encoding such polypeptides. Polypeptides of this
type are identified by known screening methods, such as by
digesting the intact protein or synthesizing small,
overlapping polypeptides or polynucleotides (and
expressing the latter), optionally in combination with the
techniques of structural analysis disclosed above. The
resultant polypeptides are then tested for the ability to
specifically bind the MPL receptor and stimulate cell
proliferation via the MPL receptor. Binding is determined
by conventional methods, such as that disclosed by Klotz,
Science 217: 1247, 1982 ("Scatchard analysis"). Briefly,
a radiolabeled test polypeptide is incubated with MPL
receptor-bearing cells in the presence of increasing
concentrations of unlabeled TPO. Cell-bound, labeled
polypeptide is separated from free labeled polypeptide by
centrifugation through phthalate oil. The binding
affinity of the test polypeptide is determined by plotting
the ratio of bound to free label on the ordinate versus
bound label on the abscissa. Binding specificity is
determined by competition with cytokines other than TPO.
Receptor binding can also be determined by precipitation
of the test compound by immobilized MPL receptor (or the
ligand-binding extracellular domain thereof). Briefly,
the receptor or portion thereof is immobilized on an
insoluble support. The test compound is labeled, e.g. by
metabolically labeling of the host cells in the case of a
recombinant test compound, or by conventional, in vitro
WO 95121626 216 917 3 pCT~s95/01829
labeling methods (e. g. radio-iodination). The labeled
compound is then combined with the immobilized receptor,
unbound material is removed, and bound, labeled compound
is detected. Methods for detecting a variety of labels
5 are known in the art. Stimulation of proliferation is
conveniently determined using the MTT colorimetric or 3H-
thymidine incorporation assay with MPL receptor-bearing
cells. Polypeptides are assayed for activity at various
concentrations, typically over a range of 1 nm to 1 ffaMM.
10 Larger polypeptides of up to 50 or more
residues, preferably 100 or more residues, more preferably
about 140 or more residues, up to the size of the entire
mature protein are also provided. For example, analysis
and modeling of the amino acid sequence shown in SEQ ID
15 N0:2 from residue 28 to residue 172, inclusive, suggest
that these portions of the molecules are cytokine-like
domains capable of self assembly. Also of interest are
molecules containing this core cytokine-like domain plus
one or more additional segments or domains of the primary
translation product. Thus, other polypeptides of interest
include those shown in Table 3.
2 I 6 917 3 p~~S95101829
WO 95/21626
16
Table 3
Mouse TPO (SEQ ID N0:4):
Cys (residue 51)--Val (residue 196)
Cys (51)--Pro (206)
Cys (51)--Thr (379)
Ser (45)--Cys (195)
Ser (45)--Val (196)
Ser (45)--Pro (206)
Ser (45)--Thr (379)
Met (24)--Cys (195)
Met (24)--Val (196)
Met (24)--Pro (206)
Met (24)--Thr (379)
Met (1)--Cys (195)
Met (1)--Val (196)
Met (1)--Pro (206)
Met (1)--Thr (379)
Human TPO (SEQ ID N0:2)
Cys (28)--Val (173)
Cys (28)--Arg (175)
Cys (28)--Gly (353)
Ser (22)--Cys (172)
Ser (22)--Val (173)
Ser (22)--Arg (175)
Ser (22)--Gly (353)
Met (1)--Cys (172)
Met (1)--Val (173)
Met (1)--Arg (175)
Met (1)--Gly (353)
Those skilled in the art will recognize that
intermediate forms of the molecules (e.g those having C-
termini between residues 196 and 206 of SEQ ID N0:4 or
those having N-termini between residues 22 and 28 of SEQ
W~'»nIG2(~ PG S9S~W1129
17
2169173
ID N0:2j are also of interest; as are polypeptides having
one or more amino acid substitutions, deletions,
insertions, or N- or C-terminal extensions as disclosed
above. Thus, the present invention provides hematopoietic
polypeptides of at Ieast 10 amino acid residues,
preferably at least 50 residues, more preferably at least
100 residues and most preferably at least about 140
residues in length, wherein said polypeptides are
substantially homologous to like-size polypeptides of SEQ
ID N0:2.
The proteins used in the present invention for
stimulating erythropoiesis can be produced in genetically
engineered host cells according to conventional
techniques. Suitable host cells are those cell types that
can be transformed or transfected with exogenous DNA and
grown in culture, and include bacteria, fungal cells, and
cultured higher eukaryotic cells. Techniques for
manipulating cloned DNA molecules and introducing _
exogenous DNA into a variety of host cells are disclosed
by Sambrook et al., trlolecular Cloning~ A Laboratory
anual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY, 1989, and Ausubel et al., ibid.
Production of
recombinant EPO has been described in Lin et al., EP
0148'05; Fritsch et al., EP 0411678; Fritsch et al., EP
0205564; Hegwick et al., EP 0209539; Lin et al., WO
85/02610; U.S. Patent No. 4,677,195 and U.S. Patent No.
4,703,008. Production of recombinant TPO has been
described in Lok et al. Nature 369:565-568, 1994; Bartley
et al., Cell 77:1117-1124, 1994 and Sauvage et al., Nature
369:533-538, 1994.
In general, a DNA sequence encoding a cytokine
is operably linked to a transcription promoter and
terminator within an expression vector. The vector will
commonly contain one or more selectable markers and one or
more origins of replication, although those skilled in the
WO')5I'"~~~(~ 18
PC'T:r ;rU18~9
216917
art will recognize that within certain systems selectable
markers may be provided on separate vectors, and
replication of the exogenous DNA may be provided by
integration into the host cell genome. Selection of
promoters, terminators, selectable markers, vectors and
other elements is a matter of routine design within the
level of ordinary skill in the art. Many such elements
are described in the literature and are available through
commercial suppliers.
To direct a protein into the secretory pathway
of the host cells, a secretory signal sequence (also known
as a leader sequence, prepro sequence or pre sequence) is
provided in the expression vector. The secretory signal
sequence is joined to the DNA sequence encoding a protein
of interest in the correct reading frame. Secretory
signal sequences are commonly positioned 5' to the DNA
sequence encoding the protein of interest, although
certain signal sequences may be positioned elsewhere in
the DNA sequence of interest (see, e.g., Welch et al.,
ZO U.S. Patent No. 5,037,?43; Holland et al., U.S. Patent No.
5,143,830). The secretory signal sequence may be that
normally associated with a protein of interest, or may be
from a gene encoding another secreted protein.
Yeast 'cells, particularly cells of the genus
Saccharomyces, are a preferred host for producing
cytokines for use within the present invention. Methods
for transforming yeast cells with exogenous DNA and
producing recombinant proteins therefrom are disclosed by,
for example, Kawasaki, U.S. Patent No. 4,599,311; Kawasaki
et al., U.S. Patent No. 4,931,373; Brake, U.S. Patent No.
4,870,008; Welch et al., U.S. Patent No. 5,037,743; and
Murray et al., U.S. Patent No. 4,845,075.
'Transformed cells are
selected by phenotype determined by the selectable marker,
commonly drug resistance or the ability to grow in the
absence of a particular nutrient (e.g. leucine). A
WO'1.5~2tG~G PC' S?~~UtY?9
,.~ 19
2169173
preferred vector system for use in yeast is the POT1
vector system disclosed by Kawasaki et al. (U. S. Patent
No. 4,931,373), which allows transformed cells to be
selected by growth in glucose-containing media. A
preferred secretory signal sequence for use in yeast is
that of the S. cerevisiae MFal gene (Brake, ibid.; Kurjan
et al., U.S. Patent No. 4,546,082). Suitable promoters
and terminators for use in yeast include those from
glycolytic enzyme genes (see, e.g., Kawasaki, U.S. Patent
No. 4,599,311; Kingsman et al., U.S. Patent No. 4,615,974;
and Bitter, U..S. Patent No. 4,977,092,
and alcohol
dehydrogenase genes. See also U.S. Patents Nos.
4,990,446; 5,063,154; 5,139,936 and 4,661,454.
Transformation systems
for other yeasts, including Hansenula polymorpha,
Schizosaccharomyces pombe, Kluyveromyces lactis,
Kluyveromyces fragilis, Ustilago maydis, Pichia pastoris,
Pichia guillermondii and Candida maltosa are known in the
art. See, for example, Gleeson et al., J. Gen. Microbiol.
132:3459-3465, 1986 and Cregg, U.S. Patent No. 4,882,279.
Other fungal cells are also suitable as host
cells. For example, Aspergillus cells may be utilized
according to the methods of McKnight et al., U.S. Patent
No. 4, 935, 349.
Methods for transforming Acremonium chrysogenum are
disclosed by Sumino et al., U.S. Patent No. 5,162,228.
Methods for
transforming Neurospora are disclosed by Lambowitz, U.S.
Patent No. 4,486,533.
Cultured mammalian cells are also preferred
hosts. Methods for introducing exogenous DNA into
mammalian host cells include calcium phosphate-mediated
transfection (Wigler et al., Cell 14:725, 1978; Corsaro
and Pearson, Somatic Cell Genetics 7:603, 1981: Graham and
;..F
w t~ ~~~~~=n
Btu'; ~:~~mn=v
2169173
Van der -Eb, ViroloQY 52:456, 1973), electroporation
(Neumann _et al., EMBO J. 1:841-845, 1982) and DEAE-dextran
mediated transfection (Ausubel et al., eds., Current
protocols in Molecular BiologY, John Wiley and Sons, Inc.,
5 NY, 1987),
The production of recombinant proteins in cultured
mammalian cells is disclosed, for example, by Levinson et
al., U.S. Patent No. 4,713,339; Hagen et al., U.S. Patent
No. 4,784,950; Palmiter et al., U.S. Patent No. 4,579,821;
10 and Ringold, U.S. Patent No. 4,656,134,
Preferred cultured
mammalian cells include the COS-1 (ATCC No. CRL 1650),
COS-? (ATCC No. CRL 1651), BHK (ATCC No. CRL 1632), BHK
570 (ATCC No. CRL 10314), 293 (ATCC No. CRL 1573; Graham
15 et al., J Gen. Virol. 36:59-72, 1977) and Chinese hamster
ovary (e. g. CHO-K1; ATCC No. CCL 61) cell lines.
Additional suitable cell lines are known in the art and
available from public depositories such as the American
Type Culture Collection, Rockville, Maryland. In general,
20 strong transcription promoters are preferred, such as
promoters from SV-40 or cytomegalavirus. See, e.g., U.S.
Patent No. 4,956,288. Other suitable promoters include
those from metallothionein genes (U. S. Patent Nos.
4,579,821 and 4,601,978
and the adenovirus major late promoter.
Drug selection is generally used to select for
cultured mammalian cells into which foreign DNA has been
inserted. Such cells are commonly referred to as
"transfectants". Cells that have been cultured in the
presence of the selective agent and are able to pass the
gene of interest to their progeny are referred to as
"stable transfectants." A preferred selectable marker is
a gene encoding resistance to the antibiotic neomycin.
Selection is carried out in the presence of a neomycin-
type drug, such as G-418 or the like. Selection systems
may also be used to increase the expression level of the
k
".~~~~pIG'_6 pf :<)«(11319
,"." , 21
2169173
gene of interest, a process referred to as
"amplification." Amplification is carried out by
culturing transfectants in~the presence of a low level of
the selective agent and then increasing the amount of
selective agent to select for cells that produce high
levels of the products of the introduced genes. A
preferred amplifiable selectable marker is dihydrofolate
reductase, which confers resistance to methotrexate.
Other drug resistance genes (e. g. hygromycin resistance,
multi-drug resistance, puromycin acetyltransferase) can
also be used.
Other higher eukaryotic cells can also be used
as hosts, including insect cells, plant cells and avian
cells. Transformation of insect cells and production of
foreign proteins therein is disclosed by Guarino et al.,
U.S. Patent No. 5,162,222; Bang et al., U.S. Patent No.
4,775,624; and WIPO publication WO 94/06463.
The use of
Agrobacteriurrc rhizogenes as a vector for expressing genes
in plant cells has been reviewed by Sinkar et al., J.
Biosci. (Bangalore) 11:47-58, 1987.
Preferred prokaryotic host cells are strains of
the bacteria Escherichia coli, although Bacillus and other
genera are also useful. Techniques for transforming these
hosts and expressing foreign DNA sequences cloned therein
are well known in the art (see, e.g., Sambrook et al.,
ibid.). When expressing the proteins in bacteria such as
E. coli, the protein may be retained in the cytoplasm,
typically as insoluble granules, or may be directed to the
periplasmic space by a bacterial secretion sequence. In
the former case, the cells are lysed, and the granules are
recovered and denatured using, for example, guanidine
isothiocyanate. The denatured protein is then refolded by
diluting the denaturant. In the latter case, the protein
can be recovered from the periplasmic space in a soluble
and functional form by disrupting the cells (by, for
c
WO 95121626 2 2 ~ ~ ~ ~ ~ ~ ~ PCT/US95101829
example, sonication or osmotic shock) to release the
contents of the periplasmic space and recovering the
protein.
Transformed or transfected host cells are
cultured according to conventional procedures in a culture
medium containing nutrients and other components required
for the growth of the chosen host cells. A variety of
suitable media, including defined media and complex media,
are known in the art and generally include a carbon
source, a nitrogen source, essential amino acids, vitamins
and minerals. Media may also contain such components as
growth factors or serum, as required. The growth medium
will generally select for cells containing the exogenously
added DNA by, for example, drug selection or deficiency in
an essential nutrient which is complemented by the
selectable marker carried on the expression vector or co-
transfected into the host cell.
Transgenic animal technology may be employed to
produce TPO and EPO for use in the present invention. It
is preferred to produce the proteins within the mammary
glands of a host female mammal. Expression in the mammary
gland and subsequent secretion of the protein of interest
into the milk overcomes many difficulties encountered in
isolating proteins from other sources. Milk is readily
collected, available in large quantities, and well
characterized biochemically. Furthermore, the major milk
proteins are present in milk at high concentrations (from
about 1 to 15 g/1).
From a commercial point of view, it is clearly
preferable to use as the host a species that has a large
milk yield. While smaller animals such as mice and rats
can be used (and are preferred at the proof-of-concept
stage), it is preferred to use livestock mammals
including, but not limited to, pigs, goats, sheep and
cattle. Sheep are particularly preferred due to such
factors as the previous history of transgenesis in this
w0 v~iz~r,:r~ Yc-~ ;o;ns~"~
23
2169173
species, milk yield, cost and the ready availability of
equipment for collecting sheep milk. See WIPO Publication
WO 88/00239 for a comparison of factors influencing the
choice of host species. It is generally desirable to -
select a breed of host animal that has been bred for dairy
use, such as East Friesland sheep, or to introduce dairy
stock by breeding of the transgenic line at a later date.
In any event, animals of known, good health status should
be used.
l0 To obtain expression in the mammary gland, a
transcription promoter from a milk protein gene is used.
Milk protein genes include those genes encoding caseins
(see U.S. Patent No. 5,304,489.
beta-lactoglobulin, a-lactalbumin, and whey
acidic protein. The beta-lactoglobulin (BLG) promoter is
preferred. In the case of the ovine beta-lactoglobulin
gene, a region of at least the proximal 406 by of 5'
flanking sequence of the gene will generally be used,
although larger portions of the 5' flanking sequence, up
to about 5 kbp, are preferred, such as a -4.25 kbp DNA
segment encompassing the 5' flanking promoter and non
coding portion of the beta-lactoglobulin gene. See
Whitelaw et al., Biochem J. 286: 31-39, 1992. Similar
fragments of promoter DNA from other species are also
suitable.
Other regions of the beta-lactoglobulin gene may w
also be incorporated in constructs, as may genomic regions
of the gene to be expressed. It is generally accepted in
the art that constructs lacking introns, for example,
express poorly in comparison with those that contain such
DNA sequences (see Brinster et al., Proc. Natl. Acad. Sci.
USA 85: 836-840, 1988; Palmiter et al., Proc. Natl. Acad.
Sci. USA 88: 478-482, 1991; Whitelaw et al., Transcxenic . .y;;:
Res. 1: 3-13, 1991; WO 89/01343; WO 91/02318). In this '
regard, it is generally preferred, where possible, to use
genomic sequences containing all or some of the native
W J')Sr?1WG P(.:'t' ~~~~UI!1~~)
24
21fi9173:
introns of a gene encoding the protein or polypeptide of
interest, thus the further inclusion of at least some .
introns from, e.g, the beta-lactoglobulin gene, is
preferred. One such region is a DNA segment which
provides for intron splicing and RNA polyadenylation from
the 3' non-coding region of the ovine beta-lactoglobulin
gene. When substituted for the natural 3' non-coding
sequences of a gene, this ovine beta-lactoglobulin segment
can both enhance and stabilize expression levels of the
protein or polypeptide of interest. Within other
embodiments, the region surrounding the initiation ATG of
the cytokine sequence is replaced with corresponding
sequences from a milk specific protein gene. Such
replacement provides a putative tissue-specific initiation
environment to enhance expression. It is convenient to
replace the entire cytokine pre-prv and 5' non-codingw
sequences with those of, far example, the- BLG gene,
although smaller regions may be replaced.
For expression of cytflkines in transgenic
animals, a DNA segment encoding the cytokine is operably
linked to additional BNA segments reguired for its
expression to produce expression units. Such additional
segments include the above-mentioned promoter, as well as
sequences which provide for termination of transcription
and polyadenylation of mRNA. The expression units will
further include a DNA segment encoding a secretory signal
sequence operably linked to the segment encoding the
cytokine. The secretory signal sequence may be a native
cytokine secretory signal sequence or may be that of
another protein, such as a milk protein. See, for
example, von Heinje, Nuc. Acids Res. I4: 4683-4690, 1986;
and Meade et al., U.S. Patent No. 4,873,316.
Construction of expression units for use in
transgenic animals is conveniently carried out by
inserting a cytokine-encoding sequence into a plasmid or
t
r
,,.",, WO 95121626 . . ~ ~ ~ 917 3 PCT/US95J01829
phage vector containing the additional DNA segments,
although the expression unit may be constructed by
essentially any sequence of ligations. It is particularly
convenient to provide a vector containing a DNA segment
5 encoding a milk protein and to replace the coding sequence
for the milk protein with that of the cytokine of
interest, thereby creating a gene fusion that includes the
expression control sequences of the milk protein gene. In
any event, cloning of the expression units in plasmids or
10 other vectors facilitates the amplification of the
cytokine sequence. Amplification is conveniently carried
out in bacterial (e.g. E. coli) host cells, thus the
vectors will typically include an origin of replication
and a selectable marker functional in bacterial host
15 cells.
The expression unit is then introduced into
fertilized eggs (including early-stage embryos) of the
chosen host species. Introduction of heterologous DNA can
be accomplished by one of several routes, including
20 microinjection (e. g. U.S. Patent No. 4,873,191),
retroviral infection (Jaenisch, Science X40,: 1468-1474,
1988) or site-directed integration using embryonic stem
(ES) cells (reviewed by Bradley et al. , Bio/Technoloqy ,~0:
534-539, 1992). The eggs are then implanted into the
25 oviducts or uteri of pseudopregnant females and allowed to
develop to term. Offspring carrying the introduced DNA in
their germ line can pass the DNA on to their progeny in
the normal, Mendelian fashion, allowing the development of
transgenic herds.
General procedures far producing transgenic
animals are known in the art. See, for example, Hogan et
al., Manipulating the Mouse Embryo: A Laboratory Manual,
Cold Spring Harbor Laboratory, 1986; Simons et al.,
Bio~/Technoloqy 6_: 179-183, 1988; Wall et al., Biol.
Reprod. ~: 645-651, 1985; Buhler et al. , Bio,ITechnoloqy
$: 140-143, 1990; Ebert et al. , BiofTechnology 9: 835-838,
wo~mzn~:.~ ~c :v;;m~~v
~ 26
2169173
1991; Krimpenfort et al., Bio/Technoloqy 9: 844-847, 1991;
Wall et al., J. Cell. Biochem. 49: 113-120, 1992; U.S.
Patents Nos. 4,873,191 and 4,873,316; WIPO publications WO
88/00239, WO 90/05188, WO 92/I1757; and GB 87/00458.
Techniques for
introducing foreign DNA sequences into mammals and their
germ cells were originally developed in the mouse. See,
e. g. , Gordon et al. , Proc. Natl. Acad . Sci . USA 77: 7380-
7384, 1980; Gordon and Ruddle, Science 214: 1244-1246,
1981; Palmiter and Brinster, Cell 41: 343-345, 1985;
Brinster et al., Proc. Natl. Acad. Sci. USA 82: 4438-4442,
1985; and Hogan et al. (ibid.}. These techniques were
subsequently adapted for use with larger animals,
including livestock species (see e.g., WIPO publications
WO x8/00239, WO 90/05188, and WO 92/11757; and Simons et
al., Bio/TechnoloqY 6: 1?9-183, 1988}. To summarize, in
the most efficient route used to date in the generation of
~transgenic mice or livestock, several hundred linear
molecules of the DNA of interest are injected into one of
the pro-nuclei of a fertilized egg according to techniques
which have become standard in the art. Injection ~of DNA
into the cytoplasm of a zygote can also be employed.
Production in transgenic plants may also be
employed. Expression may be generalized or directed to a
particular organ, such as a tuber. See, Hiatt, Nature
344:469-479, 1990; Edelbaum et al., J. Interferon Res.
12:449-453, 1992; Sijmons et al., Bio/Technology 8:217
221, 1990; and European Patent Office Publication EP
255,378.
TPO and EPO are purified using methods generally
known in the art, such as affinity purification and
separations based on size, charge, solubility and other
properties of the protein. When the protein is produced
in cultured mammalian cells, it is preferred to culture
the cells in a serum-free culture medium in order to limit
the amount of contaminating protein. The medium is
9
WO 95/21626 2~ 16 917 3 pCT~S95/01829
27
harvested and fractionated. Preferred methods of
fractionation include affinity chromatography on
concanavalin A or other lectin, thereby making use of the
carbohydrate present on the protein. TPO can also be
purified using an immobilized MPL receptor protein or
ligand-binding portion thereof or through the use of an
affinity tag (e. g. polyhistidine, substance P or other
polypeptide or protein for which an antibody or other
specific binding agent is available) . A specific cleavage
site may be provided between the protein of interest and
the affinity tag. EPO has been purified from uremic
patients exhibiting elevated EPO levels, see U.S. Patent
Nos. 4,397,840, 4,303,650 and 3,865,801 and Miyake et al.
J. Biol. Chem. 252:5558, 1977. EPO obtained from both
uremic patients and recombinant methods have been purified
using reverse-phase HPLC (Hewick et al. U.S. Patent No.
4,677,195).
TPO proteins can be used therapeutically
wherever it is desirable to increase proliferation of
hematopoietic cells in the bone marrow, such as in the
treatment of cytopenia and anemia, such as that induced by
aplastic anemia, myelodysplastic syndromes, autoimmune
diseases, AIDS, chemotherapy or radiation.
Compositions containing TPO will have useful
application in the treatment of disorders characterized by
low red blood cell production (anemia), particularly when
accompanied by low platelet production (thrombocytopenia).
Various chemotherapeutic treatments of cancers and disease
states are known to result in a combination of low
platelet and erythrocyte levels in patients.
Compositions of TPO have been found effective
for increasing the level of circulating erythrocytes and
erythrocyte precursor cells. Reduction in the circulating
levels of these cells are known as anemia. The
erythrocyte level in blood is measured as the amount of
hemoglobin per 100 ml or as the volume of packed red blood
W0 95/21626 ~ 21 ~ 91 T 3 PCTIUS95/01829
28
cells per 100 ml of blood. Patients are diagnosed as
anemic if their hematocrit levels fall below 11-13 gm/100
ml of blood (depending upon the age and sex of the
patient). The methods of the present invention are
particularly useful for treatment of anemias associated
with bone marrow failure, where a decrease in blood cell
formation is associated with, for example, the toxic
effects of chemotherapy.
TPO proteins have been found useful for
simultaneous treatment of thrombocytopenia and anemia by
increasing platelet production with a concurrent increase
erythroid cell levels. Anemia and thrombocytopenia are
associated with a diverse group of diseases and clinical
situations that may act alone or in concert to produce the
condition. Lowered platelet counts may be associated with
anemia, for example, by dilutional losses due to massive
transfusions, or abnormal destruction of bone marrow. For
example, chemotherapeutic drugs used in cancer therapy may
suppress development of platelet and erythroid progenitor
cells in the bone marrow, and the resulting
thrombocytopenia and anemia limit the chemotherapy and may
necessitate transfusions. In addition, certain
malignancies can impair platelet and erythrocyte
production and distribution. Radiation therapy used to
kill malignant cells also kills platelet and erythroid
progenitor cells. Abnormal destruction of platelets and
erythrocytes can result from hematologic disorders such as
leukemia and lymphoma or metastatic cancers involving bone
marrow. Other indications for the proteins of the present
invention to treat concurrent anemia and thrombocytopenia
include aplastic anemia and drug-induced marrow
suppression resulting from, for example, chemotherapy or
treatment of HIV infection with AZT.
Thrombocytopenia is manifested as increased
bleeding, such as mucosal bleedings from the nasal-oral
area or the gastrointestinal tract, as well as oozing from
W09Sr21r~~G PC ;95rUt5~u
,~'"~ 2 9
21 69 1 y3
wounds, ulcers or injection sites. Symptoms of anemia
include dyspnea with exertion, dizziness, fatigue, and
pallor of the skin and mucous membranes. When associated
with thrombocytopenia, retinal hemorrhage can be present.
EPO has been used for stimulating erythrocyte
production. EPO is a an acidic glycoprotein of
approximately 34,000 dalton molecular weight and may occur
in three forms: a, ~, and asialo. The a and ~ forms differ
slightly in carbohydrate components, but have the same
potency, biological activity and molecular weight. The
asialo form is an a or ~ form with the terminal
carbohydrate (sialic acid) removed. Erythropoietin is
present in very low concentrations in plasma when the body
is in a healthy state and tissues are receiving sufficient
oxygenation from the existing number of erythrocytes.
See, for example, Lin et al., U.S. Patent 4,703,008; Lin
et al. , WO 85/02610; Fritsch et al. EP 0411678; Hewick et
al., EP 0209539 and Hewick et al., U.S. Patent 4,67?,195.
In normal individuals, red blood cell production
is precisely controlled to sufficiently oxygenate tissue
without producing an overabundance of red blood cells and
impeding circulation. A reduction in red blood cell
production, resulting in tissue hypoxia, stimulates EPO
expression and increases endogenous EPO found in plasma.
EPO increases red blood cell production by stimulating the
conversion of primitive precursor cells in the bone marrow
into pro-erythroblasts which subsequently mature,
synthesize hemoglobin and are released into the
circulation as red blood cells.
To provide for the stimulatory effect of TPO and
EPO for erythropoiesis, the present invention does not
always require the administration of exogenous EPO. As
stated previously, a reduction in the level of red blood
cells will in same cases result in an elevation in the
endogenous levels of EPO (greater than 500 mU/ml of
~1
CA 02169173 2001-11-02
~lf''.~~In:G I I ~n(~~~)
;J
plasma) and administration of TFO alone may be su:~ficient.
In cases where expre:_--lion of e:ythropoie'~in is not
elevated, then a~y ..,ro;ooiet.n is advant_ageoulsy
administered with compcsitions of TFO.
As a therapeutic, EPO is administered to uremic
patients where the hemoglobin concentration is less than
gm/100 ml of blood. The route of administration can be
either intravenous (ICI) or subcutaneous (SC) and frequency
variea from daily to weekly depending upon patient's
10 physical condition (De Marchi et <~i. Ciin. and ~'xperim.
Rheumatol. 11:429-444, 1993; Miller et al., N. Enq. J. of
Med. 322:1689-1692, 1990; Nissenson et al., Annals of Int.
Mea. 114:402-416, 1991; Erslev, Sem. Oncol. 19181 Suppl.
x:14-18, 1992 and PROCIT* Epotin-alfa package insert,
Amgen,. Thousand Oaks, CA).
For pharmaceutical use, TPO and EPO are
formulated for parenteral, particularly intravenous or
subcutaneous, delivery according to conventional methods.
Intravenous administration will be by bolus injection or ~ '
infusion over a typical period of one to several hours.
Zn general, pharmaceutical formulations will include the
hematopoietic proteins in combination with a
pharmaceutically acceptable vehicle, such as saline,
buffered saline, 5% dextrose in water or the like.
Formulations may further include one or more excipients,
preservatives, solubilizers, buffering agents, albumin to
prevent protein loss on vial surfaces, etc. In addition,
TPO and EPO may be combined with other cytokines,
particularly early-acting cytokines such as sta_m cell
factor, IL-3, IL-6, IL-11 or GM-CSF. When utilizing such
a combination therapy, the cytokines may be combined in a
single formulation or may be administered in :separate
formulations. Methods of formulation are well known in
the ant and are disclosed, for example, in Rem:inqton's
Pharmaceutical Sciences, Gennaro, ed., Mack Publishing
Co., Easton PA, 1990,
* Trademark
CA 02169173 2001-11-02
1vp')Sl?1G2G P(~- ;9~;UIH~9
31
Therapeutic doses of TPO will generally be in _
the range of 1 x 105 to 100 x 105 units/kg of patient
weight per day, preferably 5 x 105 to 50 x 105 units!kg
per day. Therapeutic doses of EPO will generally be in w
the range of 10-150 tl/kg of patient weight per day,
preferably 50-150 U/kg per day.. For both TPO and EPO, the
exact dose will be determined by the clinician according
to accepted standards, taking into account the nature and
severity of the condition to be treated, patient traits,
etc. Determination of dose is within the level of
ordinary skill in the art. The proteins will commonly be
administered over a period of up to 28 days following
chemotherapy, radiation therapy or bone-marrow transplant
or until a platelet count of >20,000/mm3, preferably
>50,000/mm3, a hematocrit of 30-33% and reticulocyte
counts that are at least 2-fold over. baseline are
achieved. More commonly, the proteins will be
administered over one week or more, often over a period of
seven to fourteen days. In general, a therapeutically -
effective amount of TPO or EPO is an amount sufficient to
produce a clinically significant increase in the
proliferation and/or differentiation of lymphoid or
myeloid progenitor cells, which will be manifested as an
increase in circulating levels of mature cells (e. g.
platelets or erythrocytes). Treatment of platelet
disorders will thus be continued until a platelet count of
at least 20,000/mm3, preferably 50,000/mm3, is reached.
Treatment of anemias will continued until hematocrit
levels of 30-33% and a reticulocyte count of at least 2-
fold over baseline, a level that adequate to have a
significant impact upon hematocrit, are reached. As
stated previously, a normal range for reticulocyte counts
is 0.8% to 1.2%. TPO and EPO can also be administered in
combination with other cytokines such as IL-3, -6 and -11;
stem cell factor; G-CSF and GM-CSF. Within regimens of
combination therapy, daily doses of other cyt.okines will
WO 95/21626
3 2 ~ ~ ~ ~ ~ ~ ~ p~~S95/01829
in general be: GM-CSF, 5-15 ~tg/kg; IL-3, 1-5 ~tg/kg; and G-
CSF, 1-25 ~tg/kg. Combination therapy with GM-CSF, for
example, is indicated in patients with low neutrophil
levels.
TPO and EPO can also be used ex vivo, such as in
autologous marrow culture. Briefly, bone marrow is
removed from a patient prior to chemotherapy and treated
with TPO, optionally in combination with EPO, optionally
in combination with one or more additional cytokines. The
treated marrow is then returned to the patient after
chemotherapy to speed the recovery of the marrow. In
addition, TPO, alone and in combination with EPO, can also
be used for the ex vivo expansion of marrow or peripheral
blood progenitor (PBPC) cells. Prior to chemotherapy
treatment, marrow can be stimulated with stem cell factor
(SCF) or G-CSF to release early progenitor cells into
peripheral circulation. These progenitors can be
collected and concentrated from peripheral blood and then
treated in culture with TPO and EPO, optionally in
combination with one or more other cytokines, including
but not limited to SCF, G-CSF, IL-3, GM-CSF, IL-6 or IL-
il, to differentiate and proliferate into high-density
megakaryocyte cultures, which can then be returned to the
patient following high-dose chemotherapy.
The invention is further illustrated by the
following non-limiting examples.
Example I. Induction of Red Blood Cell Colonv Formation
At physiological levels of EPO, the addition of
TPO stimulates the production of erythroid colony forming
units (CFU-E) above levels of production seen with EPO
alone.
Bone marrow cells were isolated from BDF1 mice
(Jackson Labs, Bar Harbor, ME) by femoral flushing. The
cells (2 x 104/100 ul clot) were resuspended in medium
containing a medium (Flow Laboratories, McLean, VA)
W O 95121 G26 PC' S9slO l3=9
,,..", 3 3
2169173
supplemented with 30% fetal calf serum (Hyclone, Logan,
UT), 1% bovine serum albumin, 5 x 10-5 M ~-mercaptoethanol;
and 2 x 10-5 M CaCl2. One hundred-twenty U/ml recombinant
mouse TPO were added to select for early erythroid
progenitors (BFU-E) and late erythroid progenitor (CFU-E)
colonies.
Units of TPO activity were determined using the
following assay. A crude BHK/pZGmpl-1 transfectant cell
line that produces mouse TPO as~ described in copending
PCT/W095/21920; filed June 1,
1994, was growr in serum-free medium. An asymptotic
mitogenic activity curve was generated using this standard
solution (conditioned culture medium) and BaF3/MPLR1.1 . ...
cells (IL-3-dependent cells expressing a stably
transfected Type I mouse MPL receptor). The point of 1/2
maximal activity (average of 16 curves) was assigned the
value of 50 U/ml. The original standard solution was
calculated to contain 26,600 U/ml mouse TPO.
For test samples, a culture supernatant or
purified protein preparation was diluted in RPMI 1640
medium supplemented with 57 ~tM 2-mercaptoethanol, 2 mM L
glutamine, 1 mM sodium pyruvate, PSN, 10 mM HEPES and 10%
heat inactivated fetal bovine serum, generally using 8-24
dilutions. Briefly, 100 ~tl of diluted test sample or
standard sample and 100 ~C1 BaF3 cells (final cell number
added - about 500-10,000 cells/well) were combined in
wells of a 96 well plate. Internal standards included
eight 2-fold dilutions of 100 U/ml mouse TPO for mouse TPO
assays, or eight 2-fold dilutions of 150 U/ml mouse TPO
for human TPO assays. To each well was added 2 X11 3H-
thymidine {1 ;CCi/ul; Amersham), and the plates were
incubated overnight at 37'C.
The contents of each well of each plate were
*: . . .
transferred to a filter/plate using a Packard apparatus. -
The filters were washed 8 times with water, and the
filters were dried and counted. Units of TPO activity in
* Trademark
95121626 ~ ~ ~ ~ ~ PCTJUS95101829
wo
34 '
each sample well were determined by comparison to the
standard curve.
Human EPO (Amgen Inc., Thousand Oaks, CA) was
added at varying concentrations in the range from 0 to 300
mUnits/ml with or without 120 units TPO. Clotting was
initiated by the addition of 10% citrated bovine plasma.
The bone marrow cultures were incubated for two
days at 37°C in a fully humidified atmosphere containing 5%
Co2. Erythroid colonies contained greater than 40 cells.
After incubation, the clots were harvested, dried, stained
with benzidine and erythroid colonies were counted (Broudy
et al. Arch. of Biochem. and Biophvs. X5:329-336, 1988).
The results have been indexed to that of the maximal
colony growth and represent the mean of at least three
separate experiments of two to three replicate plates.
Figure 1 shows that at physiological
concentrations of EPO, in the range of 0-100 mUnits/ml,
the addition of 120 U/ml TPO results in a significant
increase the number of erythroid progenitor cell colonies.
Example II. TPO-Induced Increase in Reticulocyte Counts
TPO-treated animals have elevated reticulocyte
counts when compared to untreated animals.
Ten male BALB/c mice (Simonsen Labs, Gilroy, CA;
approximately 8 weeks old) were divided into a TPO-treated
group of five animals and a sham group of five animals. A
12.5 kU dose of mouse recombinant TPO was prepared in 20
mM Tris (pH 8.1), 0.9% NaCl and 0.25% rabbit serum albumin
(RSA). The sham animals were treated with buffer alone.
Each animal was given a 0.2 ml intraperitoneal injection
once daily with either 12.5 kU TPO or buffer for six
consecutive days. On day=0, the animals were bled, and
complete blood counts (CBC), including reticulocyte
counts, were determined for each animal. On day=6, the
animals were bled and sacrificed, and CBCs and
reticulocyte counts were measured. For the sham treated
WO 9«21626 P(- 'SJSlUiR29
2169173
animals, the reticulocyte counts went from a baseline at
d=0 of 4.5% to 8.7% at d=6, and for the TPO-treated
animals, the reticulocyte counts went from a baseline at
d=0 of 5.3% to I2.0% at d=6.
5
Example III. Increase in Erythropoiesis in TPO-
and EFO-Treated Animals
TPO administered to animals that had been
treated with radiation and a chemotherapeutic drug showed
10 increased erythropoietic recovery when compared to
untreated animals.
Four to six-week old, female BDF1 mice (Simonsen
Labs) were irradiated by exposure to I37Cs using a
Gammacell 40 irradiator (Nordion International Inc.,
15 Kanata, Ontario, Canada) and treated with 1.2 mg of
carboplatin (Bristol Laboratories, Princeton, NJ) injected
intraperitoneally on day=0. The mice were treated either
with TPO or TPO buffer only. TPO or TPO buffer was
administered on day=I through day=14. The mice were
20 divided to three groups as follows:
Group 1: 8 mice treated with 500 cGy radiation +
1.2 mg carboplatin + TPO buffer
Group 2: 8 mice treated with 500 cGy radiation +
1.2 mg carboplatin + 25 kU TPO/day for 14 days
25 Group 3: 8 mice treated with 5Q0 cGY radiation +
1.2 mg carboplatin + 75 kU TPO/day for l4 days
TPO was prepared in a buffer containing 20 mM
Tris (pH 8.1), 0.9o NaCl and 0.25% RSA. The mice were bled
and CBCs were measured on days 0 (to establish baseline),
30 4, 6, 8, 10, 11 (CBC and reticulocyte counts), 13 (CBC and
reticulocyte counts), 15, 18, 20, 22 and 25 (CBC and
reticulocyte counts) and then sacrificed.
Figure 2 demonstrates that Group 2 and Group 3, ,..
TPO-treated animals, had a statistically shorter period of
35 red blood cell nadir and their red blood cell levels
* Trademark
PCT/US95/01829 ,, w,
W O 95121626
36
recovered to baseline significantly faster than animals
treated with buffer only.
From the foregoing, it will be appreciated that,
although specific embodiments of the invention have been
described herein for purposes of illustration, various
modifications may be made without deviating from the
spirit and scope of the invention. Accordingly, the
invention is not limited except as by the appended claims.
WO y~l2IG2G F'(-- ''S9~Itltg29
37
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: University of Washington
Seattle
WA
98195
(ii) TITLE OF INVENTION: Methods of Stimulating Erythropoiesis
Using Hematopoietic Proteins.
(iii) NUMBER OF SEQUENCES: 6
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: ZymoGenetics, Inc.
(8) STREET: 1201 Eastlake Avenue East
(C) CITY: Seattle
(D) STATE: WA
(E) COUNTRY: USA
(F) ZIP: 98102
(v) COMPUTER READABLE. FORM:
(A) MEDIUM TYPE~Floppy disk
(B) COMPUTER: IBM PC *compatible
(C) OPERATING SYSTEM: *PC-DOS%MS-DOS
(D) SOFTWARE: PatentIn Release ;~1.0, Version #1.25
(vi) CURRENT APPLICATION DATA:
(A} APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Parker, Gary E
(B) REGISTRATION NUMBER: 31-648
(C) REFERENCE/DOCKET NUMBER: 94-09PC
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: Z06-442-6673 ,
(B) TELEFAX: 206-442-6678
* Trademark
WO 95121626 . 2 ~ b 9 ~ ~ ~ PCTlUS95101829
38
(2) INFORMATION FOR SEQ ID N0:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1062 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LOCATION: 1..1059
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:1:
ATG GAG CTG ACT GAA TTG CTC CTC GTG GTC ATG CTT CTC CTA ACT GCA 48
Met Glu Leu Thr Glu Leu Leu Leu Val Yal Met Leu Leu Leu Thr Ala
1 5 10 15
AGG CTA ACG CTG TCC AGC CCG GCT CCT CCT GCT TGT GAC CTC CGA GTC 96
Arg Leu Thr Leu Ser Ser Pro Ala Pro Pro Ala Cys Asp Leu Arg Val
20 25 30
CTC AGT AAA CTG CTT CGT GAC TCC CAT GTC CTT CAC AGC AGA CTG AGC 144
Leu Ser Lys Leu Leu Arg Asp Ser His Val Leu His Ser Arg Leu Ser
35 40 45
CAG TGC CCA GAG GTT CAC CCT TTG CCT ACA CCT GTC CTG CTG CCT GCT 192
Gln Cys Pro Glu Val His Pro Leu Pro Thr Pro Val Leu Leu Pro Ala
50 55 60
GTG GAC TTT AGC TTG GGA GAA TGG AAA ACC CAG ATG GAG GAG ACC AAG 240
Val Asp Phe Ser Leu Gly Glu Trp Lys Thr Gln Met Glu Glu Thr Lys
65 70 75 80
GCA CAG GAC ATT CTG GGA GCA GTG ACC CTT CTG CTG GAG GGA GTG ATG 288
Ala Gln Asp Ile Leu Gly Ala Val Thr Leu Leu Leu Glu Gly Val Met
85 90 95
GCA GCA CGG GGA CAA CTG GGA CCC ACT TGC CTC TCA TCC CTC CTG GGG 336
Ala Ala Arg Gly Gln Leu Gly Pro Thr Cys Leu Ser Ser Leu Leu Gly
100 105 110
,... WO 95/21626 21 b 917 3 p~~S95/01829
39
CAG CTT TCT GGA CAG GTC CGT CTC CTC CTT GGG GCC CTG CAG AGC CTC 384
Gln Leu Ser Gly Gln Val Arg Leu Leu Leu Gly Ala Leu Gln Ser Leu
115 120 125
CTT GGA ACC CAG CTT CCT CCA CAG GGC AGG ACC ACA GCT CAC AAG GAT 432
Leu Gly Thr Gln Leu Pro Pro Gln Gly Arg Thr Thr Ala His Lys Asp
130 135 140
CCC AAT GCC ATC TTC CTG AGC TTC CAA CAC CTG CTC CGA GGA AAG GTG 480
Pro Asn Ala Ile Phe Leu Ser Phe Gln His Leu Leu Arg Gly Lys Val
145 150 155 160
CGT TTC CTG ATG CTT GTA GGA GGG TCC ACC CTC TGC GTC AGG CGG GCC 528
Arg Phe Leu Met Leu Val Gly Gly Ser Thr Leu Cys Val Arg Arg Ala
165 '170 175
CCA CCC ACC ACA GCT GTC CCC AGC AGA ACC TCT CTA GTC CTC ACA CTG 576
Pro Pro Thr Thr Ala Val Pro Ser Arg Thr Ser Leu Val Leu Thr Leu
180 185 190
AAC GAG CTC CCA AAC AGG ACT TCT GGA TTG TTG GAG ACA AAC TTC ACT 624
Asn Glu Leu Pro Asn Arg Thr Ser Gly Leu Leu Glu Thr Asn Phe Thr
195 200 205
GCC TCA GCC AGA ACT ACT GGC TCT GGG CTT CTG AAG TGG CAG CAG GGA 672
Ala Ser Ala Arg Thr Thr Gly Ser Gly Leu Leu Lys Trp Gln Gln Gly
210 215 220
TTC AGA GCC AAG ATT CCT GGT CTG CTG AAC CAA ACC TCC AGG TCC CTG 720
Phe Arg Ala Lys Ile Pro Gly Leu Leu Asn Gln Thr Ser Arg Ser Leu
225 230 235 240
GAC CAA ATC CCC GGA TAC CTG AAC AGG ATA CAC GAA CTC TTG AAT GGA 768
Asp Gln Ile Pro Gly Tyr Leu Asn Arg Ile His Glu Leu Leu Asn Gly
245 250 255
ACT CGT GGA CTC TTT CCT GGA CCC TCA CGC AGG ACC CTA GGA GCC CCG 816
Thr Arg Gly Leu Phe Pro Gly Pro Ser Arg Arg Thr Leu Gly Ala Pro
260 265 270
GAC ATT TCC TCA GGA ACA TCA GAC ACA GGC TCC CTG CCA CCC AAC CTC 864
Asp Ile Ser Ser Gly Thr Ser Asp Thr Gly Ser Leu Pro Pro Asn Leu
275 280 285
WO 95121626 4 O ~ ; ~ g ~ ~ 3 PCT/US95I01829
CAG CCT GGA TAT TCT CCT TCC CCA ACC CAT CCT CCT ACT GGA CAG TAT 912
Gln Pro Gly Tyr Ser Pro Ser Pro Thr His Pro Pro Thr Gly Gln Tyr
290 295 300
ACG CTC TTC CCT CTT CCA CCC ACC TTG CCC ACC CCT GTG GTC CAG CTC 960
Thr Leu Phe Pro Leu Pro Pro Thr Leu Pro Thr Pro Val Val Gln Leu
305 310 315 320
CAC CCC CTG CTT CCT GAC CCT TCT GCT CCA ACG CCC ACC CCT ACC AGC 1008
His Pro Leu Leu Pro Asp Pro Ser Ala Pro Thr Pro Thr Pro Thr Ser
325 330 335
CCT CTT CTA AAC ACA TCC TAC ACC CAC TCC CAG AAT CTG TCT CAG GAA 1056
Pro Leu Leu Asn Thr Ser Tyr Thr His Ser Gln Asn Leu Ser Gln Glu
340 345 350
GGG TAA 1062
Gly
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 353 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Met Glu Leu Thr Glu Leu Leu Leu Val Val Met Leu Leu Leu Thr Ala
1 5 10 15
Arg Leu Thr Leu Ser Ser Pro Ala Pro Pro Ala Cys Asp Leu Arg Val
20 25 30
Leu Ser Lys Leu Leu Arg Asp Ser His Val Leu His Ser Arg Leu Ser
35 40 45
Gln Cys Pro Glu Val His Pro Leu Pro Thr Pro Val Leu Leu Pro Ala
50 55 60
~... WO 95121626 216 9 i 7 3 pCT~s95/01829
41
Val Asp Phe Ser Leu Gly Glu Trp Lys Thr Gln Met Glu Glu Thr Lys
65 70 75 80
Ala Gln Asp Ile Leu Gly Ala Val Thr Leu Leu Leu Glu Gly Val Met
85 90 95
Ala Ala Arg Gly Gln Leu Gly Pro Thr Cys Leu Ser Ser Leu Leu Gly
100 105 110
Gln Leu Ser Gly Gln Val Arg Leu Leu Leu Gly Ala Leu Gln Ser Leu
115 120 125
Leu Gly Thr Gln Leu Pro Pro Gln Gly Arg Thr Thr Ala His Lys Asp
130 135 140
Pro Asn Ala Ile Phe Leu Ser Phe Gln His Leu Leu Arg Gly Lys Val
145 150 155 160
Arg Phe Leu Met Leu Val Gly Gly Ser Thr Leu Cys Val Arg Arg Ala
165 170 175
Pro Pro Thr Thr Ala Val Pro Ser Arg Thr Ser Leu Val Leu Thr Leu
180 185 190
Asn Glu Leu Pro Asn Arg Thr Ser Gly Leu Leu Glu Thr Asn Phe Thr
195 200 205
Ala Ser Ala Arg Thr Thr Gly Ser Gly Leu Leu Lys Trp Gln Gln Gly
210 215 220
Phe Arg Ala Lys Ile Pro Gly Leu Leu Asn Gln Thr Ser Arg Ser Leu
225 230 235 240
Asp Gln Ile Pro Gly Tyr Leu Asn Arg Ile His Glu Leu Leu Asn Gly
245 250 255
Thr Arg Gly Leu Phe Pro Gly Pro Ser Arg Arg Thr Leu Gly Ala Pro
260 265 270
Asp Ile Ser Ser Gly Thr Ser Asp Thr Gly Ser Leu Pro Pro Asn Leu
275 280 285
Gln Pro Gly Tyr Ser Pro Ser Pro Thr His Pro Pro Thr Gly Gln Tyr
290 295 300
WO 95121626 4 2 ~ ~ ~ ~ ~ ~ ~ PCTIUS95/01829
Thr Leu Phe Pro Leu Pro Pro Thr Leu Pro Thr Pro 11a1 Nal Gln Leu
305 310 315 320
His Pro Leu Leu Pro Asp Pro Ser Ala Pro Thr Pro Thr Pro Thr Ser
325 330 335
Pro Leu Leu Asn Thr Ser Tyr Thr His Ser Gln Asn Leu Ser Gln Glu
340 345 350
Gly
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1486 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(vii) IMMEDIATE SOURCE:
(B) CLONE: 1081
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LOCATION: 105..1241
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:3:
CCTCGTGCCG GTCCTGAGGC CCTTCTCCAC CCGGACAGAG TCCTTGGCCC ACCTCTCTCC 60
CACCCGACTC TGCCGAAAGA AGCACAGAAG CTCAAGCCGC CTCC ATG GCC CCA GGA 116
Met Ala Pro Gly
1
AAG ATT CAG GGG AGA GGC CCC ATA CAG GGA GCC ACT TCA GTT AGA CAC 164
Lys Ile Gln Gly Arg Gly Pro Ile Gln Gly Ala Thr Ser 11a1 Arg His
10 15 20
,e~~. WO 95121626 ~ z I 6 9 i 7 3 pCT~S95/01829
43
CTG GCC AGA ATG GAG CTG ACT GAT TTG CTC CTG GCG GCC ATG CTT CTT 212
Leu Ala Arg Met Glu Leu Thr Asp Leu Leu Leu Ala Ala Met Leu Leu
25 30 35
GCA GTG GCA AGA CTA ACT CTG TCC AGC CCC GTA GCT CCT GCC TGT GAC 260
Ala Val Ala Arg Leu Thr Leu Ser Ser Pro Val Ala Pro Ala Cys Asp
40 45 50
CCC AGA CTC CTA AAT AAA CTG CTG CGT GAC TCC CAC CTC CTT CAC AGC 308
Pro Arg Leu Leu Asn Lys Leu Leu Arg Asp Ser His Leu Leu His Ser
55 60 65
CGA CTG AGT CAG TGT CCC GAC GTC GAC CCT TTG TCT ATC CCT GTT CTG 356
Arg Leu Ser Gln Cys Pro Asp Val Asp Pro Leu Ser Ile Pro Val Leu
70 75 80
CTG CCT GCT GTG GAC TTT AGC CTG GGA GAA TGG AAA ACC CAG ACG GAA 404
Leu Pro Ala Val Asp Phe Ser Leu Gly Glu Trp Lys Thr Gln Thr Glu
85 90 95 100
CAG AGC AAG GCA CAG GAC ATT CTA GGG GCA GTG TCC CTT CTA CTG GAG 452
Gln Ser Lys Ala Gln Asp Ile Leu Gly Ala Yal Ser Leu Leu Leu Glu
105 110 115
GGA GTG ATG GCA GCA CGA GGA CAG TTG GAA CCC TCC TGC CTC TCA TCC 500
Gly Yal Met Ala Ala Arg Gly Gln Leu Glu Pro Ser Cys Leu Ser Ser
120 125 130
CTC CTG GGA CAG CTT TCT GGG CAG GTT CGC CTC CTC TTG GGG GCC CTG 548
Leu Leu Gly Gln Leu Ser Gly Gln Val Arg Leu Leu Leu Gly Ala Leu
135 140 145
CAG GGC CTC CTA GGA ACC CAG CTT CCT CTA CAG GGC AGG ACC ACA GCT 596
Gln Gly Leu Leu Gly Thr Gln Leu Pro Leu Gln Gly Arg Thr Thr Ala
150 155 160
CAC AAG GAC CCC AAT GCC CTC TTC TTG AGC TTG CAA CAA CTG CTT CGG 644
His Lys Asp Pro Asn Ala Leu Phe Leu Ser Leu Gln Gln Leu Leu Arg
165 170 175 180
GGA AAG GTG CGC TTC CTG CTT CTG GTA GAA GGT CCC ACC CTC TGT GTC 692
Gly Lys Yal Arg Phe Leu Leu Leu Val Glu Gly Pro Thr Leu Cys Val
185 190 195
WO 95121626 44 ~ ~ ~ ~ ~ ~ PCTIUS95101829
AGA CGG ACC CTG CCA ACC ACA GCT GTC CCA AGC AGT ACT TCT CAA CTC 740
Arg Arg Thr Leu Pro Thr Thr Ala Val Pro Ser Ser Thr Ser Gln Leu
200 205 210
CTC ACA CTA AAC AAG TTC CCA AAC AGG ACT TCT GGA TTG TTG GAG ACG 788
Leu Thr Leu Asn Lys Phe Pro Asn Arg Thr Ser Gly Leu Leu Glu Thr
215 220 225
AAC TTC AGT GTC ACA GCC AGA ACT GCT GGC CCT GGA CTT CTG AGC AGG 836
Asn Phe Ser Val Thr Ala Arg Thr Ala Gly Pro Gly Leu Leu Ser Arg
230 235 240
CTT CAG GGA TTC AGA GTC AAG ATT ACT CCT GGT CAG CTA AAT CAA ACC 884
Leu G1n Gly Phe Arg Val Lys Ile Thr Pro Gly Gln Leu Asn Gln Thr
245 250 255 260
TCC AGG TCC CCA GTC CAA ATC TCT GGA TAC CTG AAC AGG ACA CAC GGA 932
Ser Arg Ser Pro Val Gln Ile Ser Gly Tyr Leu Asn Arg Thr His Giy
265 270 275
CCT GTG AAT GGA ACT CAT GGG CTC TTT GCT GGA ACC TCA CTT CAG ACC 980
Pro Val Asn Gly Thr His Gly Leu Phe Ala Gly Thr Ser Leu Gln Thr
280 285 290
CTG GAA GCC TCA GAC ATC TCG CCC GGA GCT TTC AAC AAA GGC TCC CTG 1028
Leu Glu Ala Ser Asp Ile Ser Pro Gly Ala Phe Asn Lys Gly Ser Leu
295 300 305
GCA TTC AAC CTC CAG GGT GGA CTT CCT CCT TCT CCA AGC CTT GCT CCT 1076
Ala Phe Asn Leu Gln Gly ~Gly Leu Pro Pro Ser Pro Ser Leu Ala Pro
310 315 320
GAT GGA CAC ACA CCC TTC CCT CCT TCA CCT GCC TTG CCC ACC ACC CAT 1124
Asp Gly His Thr Pro Phe Pro Pro Ser Pro Ala Leu Pro Thr Thr His
325 330 335 340
GGA TCT CCA CCC CAG CTC CAC CCC CTG TTT CCT GAC CCT TCC ACC ACC 1172
Gly Ser Pro Pro Gln Leu His Pro Leu Phe Pro Asp Pro Ser Thr Thr
345 350 355
ATG CCT AAC TCT ACC GCC CCT CAT CCA GTC ACA ATG TAC CCT CAT CCC 1220
Met Pro Asn Ser Thr Ala Pro His Pro Val Thr Met Tyr Pro His Pro
360 365 370
2 l 6 917 3 PCT/US95101829
,..... WO 95121626
AGG AAT TTG TCT CAG GAA ACA TAGCGCGGGC ACTGGCCCAG TGAGCGTCTG 1271
Arg Asn Leu Ser Gln Glu Thr
375
CAGCTTCTCT CGGGGACAAG CTTCCCCAGG AAGGCTGAGA GGCAGCTGCA TCTGCTCCAG 1331
ATGTTCTGCT TTCACCTAAA AGGCCCTGGG GAAGGGATAC ACAGCACTGG AGATTGTAAA 1391
ATTTTAGGAG CTATTTTTTT TTAACCTATC AGCAATATTC ATCAGAGCAG CTAGCGATCT 1451
TTGGTCTATT TTCGGTATAA ATTTGAAAAT CACTA 1486
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 379 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Met Ala Pro Gly Lys Ile Gln Gly Arg Gly Pro Ile Gln Gly Ala Thr
1 5 10 15
Ser Val Arg His Leu Ala Arg Met Glu Leu Thr Asp Leu Leu Leu Ala
20 25 30
Ala Met Leu Leu Ala Val Ala Arg Leu Thr Leu Ser Ser Pro Val Ala
35 40 45
Pro Ala Cys Asp Pro Arg Leu Leu Asn Lys Leu Leu Arg Asp Ser His
55 60
Leu Leu His Ser Arg Leu Ser Gln Cys Pro Asp Val Asp Pro Leu Ser
65 70 75 80
Ile Pro Val Leu Leu Pro Ala Val Asp Phe Ser Leu Gly Glu Trp Lys
85 90 95
Thr Gln Thr Glu Gln Ser Lys Ala Gln Asp Ile Leu Gly Ala Val Ser
100 105 110
WO 95!21626 ~ ~,~ PCTIUS95/01829
46
Leu Leu Leu Glu Gly Val Met Ala Ala Arg Gly Gln Leu Glu Pro Ser
115 120 125
Cys Leu Ser Ser Leu Leu Gly Gln Leu Ser Gly Gln Val Arg Leu Leu
130 135 140
Leu Gly Ala Leu Gln Gly Leu Leu Gly Thr Gln Leu Pro Leu Gln Gly
145 150 155 160
Arg Thr Thr Ala His Lys Asp Pro Asn Ala Leu Phe Leu Ser Leu Gln
165 170 175
Gln Leu Leu Arg Gly Lys Val Arg Phe Leu Leu Leu Val Glu Gly Pro
180 185 190
Thr Leu Cys Val Arg Arg Thr Leu Pro Thr Thr Ala Yal Pro Ser Ser
195 200 205
Thr Ser Gln Leu Leu Thr Leu Asn Lys Phe Pro Asn Arg Thr Ser Gly
210 215 220
Leu Leu Glu Thr Asn Phe Ser Val Thr Ala Arg Thr Ala Gly Pro Gly
225 230 235 240
Leu Leu Ser Arg Leu Gln Gly Phe Arg Val Lys Ile Thr Pro Gly Gln
245 250 255
Leu Asn Gln Thr Ser Arg Ser Pro Val Gln Ile Ser Gly Tyr Leu Asn
260 265 270
Arg Thr His Gly Pro Val Asn Gly Thr His Gly Leu Phe Ala Gly Thr
275 280 285
Ser Leu Gln Thr Leu Glu Ala Ser Asp Ile Ser Pro Gly Ala Phe Asn
290 295 300
Lys Gly Ser Leu Ala Phe Asn Leu Gln Gly Gly Leu Pro Pro Ser Pro
305 310 315 320
Ser Leu Ala Pro Asp Gly His Thr Pro Phe Pro Pro Ser Pro Ala Leu
325 330 335
Pro Thr Thr His Gly Ser Pro Pro Gln Leu His Pro Leu Phe Pro Asp
340 345 350
,....,, WO 95!21626 ~ ~ ~ ~ PCTIU895101829
47
Pro Ser Thr Thr Met Pro Asn 5er Thr Ala Pro His Pro 11a1 Thr Met
355 360 365
Tyr Pro His Pro Arg Asn Leu Ser Gln Glu Thr
370 375
(2) INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4823 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LOCATION: join(632..644, 876..1003, 1290..1376, 3309..3476,
3713..4375)
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
CTTTCTTGCT TTCTTTCTTT CTTTCTTTCT TTCTTTTTTT TTTTTGAGAC GGAGTTTCAC 60
TCTTATTGCC CAGGCTGGAG TGCAATGGTG CGATCTCGGC TCACCACAAC CTCCGCCTCC 120
CAGGTACAAG CGATTCTCCT GTCTCAGCCT CCCAAGTAGC TTGGATTACA GGCATGAACC 180
ACCACACCCT GCTAGTTTTT TTGTATTTCG TAGAGCCGGG GTTTCACCAT GTTAGTGAGG 240
CTGGTGGCGA ACTCCTGACC TCAGGTGATC CACCCGCCTT GGACTCCCAA AGTGCTGGGA 300
TTACAGGCAT GAGCCACTGC ACCCGGCACA CCATATGCTT TCATCACAAG AAAATGTGAG 360
AGAATTCAGG GCTTTGGCAG TTCCAGGCTG GTCAGCATCT CAAGCCCTCC CCAGCATCTG 420
TTCACCCTGC CAGGCAGTCT CTTCCTAGAA ACTTGGTTAA ATGTTCACTC TTCTTGCTAC 480
TTTCAGGATA GATTCTTCAC CCTTGGTCCG CCTTTGCCCC ACCCTACTCT GCCCAGAAGT 540
GCAAGAGCCT AAGCCGCCTC CATGGCCCCA GGAAGGATTC AGGGGAGAGG CCCCAAACAG 600
WO 95121626 ~ ~ PCTIUS95/01829
48
GGAGCCACGC CAGCCAGACA CCCCGGCCAG A ATG GAG CTG ACT G GTGAGAACAC 654
Met Glu Leu Thr
1
ACCTGAGGGG CTAGGGCCAT ATGGAAACAT GACAGAAGGG GAGAGAGAAA GGAGACACGC 714
TGCAGGGGGC AGGAAGCTGG GGGAACCCAT TCTCCCAAAA ATAAGGGGTC TGAGGGGTGG 774
ATTCCCTGGG TTTCAGGTCT GGGTCCTGAA TGGGAATTCC TGGAATACCA GCTGACAATG 834
ATTTCCTCCT CATCTTTCAA CCTCACCTCT CCTCATCTAA G AA TTG CTC CTC 886
Glu Leu Leu Leu
GTG GTC ATG CTT CTC CTA ACT GCA AGG CTA ACG CTG TCC AGC CCG GCT 934
Val Val Met Leu Leu Leu Thr Ala Arg Leu Thr Leu Ser Ser Pro Ala
15 20
CCT CCT GCT TGT GAC CTC CGA GTC CTC AGT AAA CTG CTT CGT GAC TCC 982
Pro Pro Ala Cys Asp Leu Arg Val Leu Ser Lys Leu Leu Arg Asp Ser
25 30 35 40
CAT GTC CTT CAC AGC AGA CTG GTGAGAACTC CCAACATTAT CCCCTTTATC 1033
His Val Leu His Ser Arg Leu
CGCGTAACTG GTAAGACACC CATACTCCCA GGAAGACACC ATCACTTCCT CTAACTCCTT 1093
GACCCAATGA CTATTCTTCC CATATTGTCC CCACCTACTG ATCACACTCT CTGACAAGGA 1153
TTATTCTTCA CAATACAGCC CGCATTTAAA AGCTCTCGTC TAGAGATAGT ACTCATGGAG 1213
GACTAGCCTG CTTATTAGGC TACCATAGCT CTCTCTATTT CAGCTCCCTT CTCCCCCCAC 1273
CAATCTTTTT CAACAG AGC CAG TGC CCA GAG GTT CAC CCT TTG CCT ACA 1322
Ser Gln Cys Pro Glu Val His Pro Leu Pro Thr
55
CCT GTC CTG CTG CCT GCT GTG GAC TTT AGC TTG GGA GAA TGG AAA ACC 1370
Pro Val Leu Leu Pro Ala Val Asp Phe Ser Leu Gly Glu Trp Lys Thr
65 70
CAG ATG GTAAGAAAGC CATCCCTAAC CTTGGCTTCC CTAAGTCCTG TCTTCAGTTT 1426
Gln Met
WO 95!21626 ~ ~' ~ PGTIUS95101829
49
CCCACTGCTTCCCATGGATTCTCCAACATTCTTGAGCTTTTTAAAAATATCTCACCTTCA1486
GCTTGGCCACCCTAACCCAATCTACATTCACCTATGATGATAGCCTGTGGATAAGATGAT1546
GGCTTGCAGGTCCAATATGTGAATAGATTTGAAGCTGAACACCATGAAAAGCTGGAGAGA1606
AATCGCTCATGGCCATGCCTTTGACCTATTCCCGTTCAGTCTTCTTAAATTGGCATGAAG1666
AAGCAAGACTCATATGTCATCCACAGATGACACAAAGCTGGGAAGTACCACTAAAATAAC1726
AAAAGACTGAATCAAGATTCAAATCACTGAAAGACTAGGTCAAAAACAAGGTGAAACAAC1786
AGAGATATAAACTTCTACATGTGGGCCGGGGGCTCACGCCTGTAATCCCAGCACTTTGGG1846
AGGCCGAGGCAGGCAGATCACCTGAGGGCAGGAGTTTGAGAGCAGCCTGGCCAACATGGC1906
GAAACCCCGTCTCTACTAAGAATACAGAATTAGCCGGGCATGGTAGTGCATGCCTGTAAT1966
CCCAGCTACTTGGAAGGCTGAAGCAGGAGAATCCCTTGAACCCAGGAGGTGGAGGTTGTA2026
GTGAGCTGAGATCATGCCAATGCACTCCAGCCTGGGTGACAAGAGCAAAACTCCGTCTCA2086
AAAAGAAAAAAAAATTCTACATGTGTAAATTAATGAGTAAAGTCCTATTCCAGCTTTCAG2146
GCCACAATGCCCTGCTTCCATCATTTAAGCCTCTGGCCCTAGCACTTCCTACGAAAAGGA2206
TCTGAGAGAATTAAATTGCCCCCAAACTTACCATGTAACATTACTGAAGCTGCTATTCTT2266
AAAGCTAGTAATTCTTGTCTGTTTGATGTTTAGCATCCCCATTGTGGAAATGCTCGTACA2326
GAACTCTATTCCGAGTGGACTACACTTAAATATACTGGCCTGAACACCGGACATCCCCCT2386
GAAGACATATGCTAATTTATTAAGAGGGACCATATTAAACTAACATGTGTCTAGAAAGCA2446
GCAGCCTGAACAGAAAGAGACTAGAAGCATGTTTTATGGGCAATAGTTTAAAAAACTAAA2506
ATCTATCCTCAAGAACCCTAGCGTCCCTTCTTCCTTCAGGACTGAGTCAGGGAAGAAGGG2566
CAGTTCCTATGGGTCCCTTCTAGTCCTTTCTTTTCATCCTTATGATCATTATGGTAGAGT2626
CTCATACCTACATTTAGTTTATTTATTATTATTATTTGAGACGGAGTCTCACTCTATCCC2686
CCAGGCTGGAGTGCAGTGGCATGATCTCAACTCACTGCAACCTCAGCCTCCCGGATTCAA2746
WO 95!21626 , ~ ~ ~ PCTlUS95101829
GCGATTCTCC TGTCTCAGTC TCCCAAGTAG CTGGGATTAC AGGTGCCCAC CACCATGCCC 2806
AGCTAATTTG TGTATTTGTG GTAGAGATGG GGTTTCACCA TGTTGGGCAG GCTGATCTTG 2866
AACTCCTGAC CTCAGGTGAT CCACCTGCCT CAGCCTCCCA AAGTGCTGGG ATTACAGGCG 2926
TGAGCCACTG CACCCAGCCT TCATTCAGTT TAAAAATCAA ATGATCCTAA GGTTTTGCAG 2986
CAGAAAGAGT AAATTTGCAG CACTAGAACC AAGAGGTAAA AGCTGTAACA GGGCAGATTT 3046
CAGCAACGTA AGAAAAAAGG AGCTCTTCTC ACTGAAACCA AGTGTAAGAC CAGGCTGGAC 3106
TAGAGGACAC GGGAGTTTTT GAAGCAGAGG CTGATGACCA GCTGTCGGGA GACTGTGAAG 3166
GAATTCCTGC CCTGGGTGGG ACCTTGGTCC TGTCCAGTTC TCAGCCTGTA TGATTCACTC 3226
TGCTGGCTAC TCCTAAGGCT CCCCACCCGC TTTTAGTGTG CCCTTTGAGG CAGTGCGCTT 3286
CTCTCTTCCA TCTCTTTCTC AG GAG GAG ACC AAG GCA CAG GAC ATT CTG GGA 3338
Glu Glu Thr Lys Ala Gln Asp Ile Leu Gly
80 85
GCA GTG ACC CTT CTG CTG GAG GGA GTG ATG GCA GCA CGG GGA CAA CTG 3386
Ala Val Thr Leu Leu Leu Glu Gly Val Met Ala Ala Arg Gly Gln Leu
90 95 100
GGA CCC ACT TGC CTC TCA TCC CTC CTG GGG CAG CTT TCT GGA CAG GTC 3434
Gly Pro Thr Cys Leu Ser Ser Leu Leu Gly Gln Leu Ser Gly Gln Val
105 110 115
CGT CTC CTC CTT GGG GCC CTG CAG AGC CTC CTT GGA ACC CAG 3476
Arg Leu Leu Leu Gly Ala Leu Gln Ser Leu Leu Gly Thr Gln
120 125 130
GTAAGTCCCC AGTCAAGGGA TCTGTAGAAA CTGTTCTTTT CTGACTCAGT CCCCCTAGAA 3536
GACCTGAGGG AAGAAGGGCT CTTCCAGGGA GCTCAAGGGC AGAAGAGCTG ATCTACTAAG 3596
AGTGCTCCCT GCCAGCCACA ATGCCTGGGT ACTGGCATCC TGTCTTTCCT ACTTAGACAA 3656
GGGAGGCCTG AGATCTGGCC CTGGTGTTTG GCCTCAGGAC CATCCTCTGC CCTCAG 3712
CTT CCT CCA CAG GGC AGG ACC ACA GCT CAC AAG GAT CCC AAT GCC ATC 3760
Leu Pro Pro Gln Gly Arg Thr Thr Ala His Lys Asp Pro Asn Ala Ile
135 140 145
PCTIUS95101829
..~.A Wp 95121626
51
TTC CTG AGC TTC CAA CAC CTG CTC CGA GGA AAG GTG CGT TTC CTG ATG 3808
Phe Leu Ser Phe Gln His Leu Leu Arg Gly Lys Yal Arg Phe Leu Met
150 155 160
CTT GTA GGA GGG TCC ACC CTC TGC GTC AGG CGG GCC CCA CCC ACC ACA 3856
Leu Yal Gly Gly Ser Thr Leu Cys Yal Arg Arg Ala Pro Pro Thr Thr
165 170 175 180
GCT GTC CCC AGC AGA ACC TCT CTA GTC CTC ACA CTG AAC GAG CTC CCA 3904
Ala Yal Pro Ser Arg Thr Ser Leu Yal Leu Thr Leu Asn Glu Leu Pro
185 190 195
AAC AGG ACT TCT GGA TTG TTG GAG ACA AAC TTC ACT GCC TCA GCC AGA 3952
Asn Arg Thr Ser Gly Leu Leu Glu Thr Asn Phe Thr Ala Ser Ala Arg
200 205 210
ACT ACT GGC TCT GGG CTT CTG AAG TGG CAG CAG GGA TTC AGA GCC AAG 4000
Thr Thr Gly Ser Gly Leu Leu Lys Trp Gln Gln Gly Phe Arg Ala Lys
215 220 225
ATT CCT GGT CTG CTG AAC CAA ACC TCC AGG TCC CTG GAC CAA ATC CCC 4048
Ile Pro Gly Leu Leu Asn Gln Thr Ser Arg Ser Leu Asp Gln Ile Pro
230 235 240
GGA TAC CTG AAC AGG ATA CAC GAA CTC TTG AAT GGA ACT CGT GGA CTC 4096
Gly Tyr Leu Asn Arg Ile His Glu Leu Leu Asn Gly Thr Arg Gly Leu
245 250 255 260
TTT CCT GGA CCC TCA CGC AGG ACC CTA GGA GCC CCG GAC ATT TCC TCA 4144
Phe Pro Gly Pro Ser Arg Arg Thr Leu Gly Ala Pro Asp Ile Ser Ser
265 270 275
GGA ACA TCA GAC ACA GGC TCC CTG CCA CCC AAC CTC CAG CCT GGA TAT 4192
Gly Thr Ser Asp Thr Gly Ser Leu Pro Pro Asn Leu Gln Pro Gly Tyr
280 285 290
TCT CCT TCC CCA ACC CAT CCT CCT ACT GGA CAG TAT ACG CTC TTC CCT 4240
Ser Pro Ser Pro Thr His Pro Pro Thr Gly Gln Tyr Thr Leu Phe Pro
295 300 305
CTT CCA CCC ACC TTG CCC ACC CCT GTG GTC CAG CTC CAC CCC CTG CTT 4288
Leu Pro Pro Thr Leu Pro Thr Pro Yal Yal Gln Leu His Pro Leu Leu
310 315 320
WO 95!21626 ' 21 s 9' l 3 PCT1US95/01829
52
CCT GAC CCT TCT GCT CCA ACG CCC ACC CCT ACC AGC CCT CTT CTA AAC 4336
Pro Asp Pro Ser Ala Pro Thr Pro Thr Pro Thr Ser Pro Leu Leu Asn
325 330 335 340
ACA TCC TAC ACC CAC TCC CAG AAT CTG TCT CAG GAA GGG TAAGGTTCTC 4385
Thr Ser Tyr Thr His Ser Gln Asn Leu Ser Gln Glu Gly
345 350
AGACACTGCC GACATCAGCA TTGTCTCGTG TACAGCTCCC TTCCCTGCAG GGCGCCCCTG 4445
GGAGACAACT GGACAAGATT TCCTACTTTC TCCTGAAACC CAAAGCCCTG GTAAAAGGGA 4505
TACACAGGAC TGAAAAGGGA ATCATTTTTC ACTGTACATT ATAAACCTTC AGAAGCTATT 4565
TTTTTAAGCT ATCAGCAATA CTCATCAGAG CAGCTAGCTC TTTGGTCTAT TTTCTGCAGA 4625
AATTTGCAAC TCACTGATTC TCAACATGCT CTTTTTCTGT GATAACTCTG CAAAGACCTG 4685
GGCTGGCCTG GCAGTTGAAC AGAGGGAGAG ACTAACCTTG AGTCAGAAAA CAGAGGAAGG 4745
GTAATTTCCT TTGCTTCAAA TTCAAGGCCT TCCAACGCCC CCATCCCCTT TACTATCATT 4805
CTCAGTGGGA CTCTGATC 4823
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 353 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
Met Glu Leu Thr Glu Leu Leu Leu Yal Ual Met Leu Leu Leu Thr Ala
1 5 10 15
Arg Leu Thr Leu Ser Ser Pro Ala Pro Pro Ala Cys Asp Leu Arg Yal
20 25 30
Leu Ser Lys Leu Leu Arg Asp Ser His 11a1 Leu His Ser Arg Leu Ser
35 40 45
PCTIUS95/01829
,..~~~,. W O 95!21626
53
Gln Cys Pro Glu Val His Pro Leu Pro Thr Pro Val Leu Leu Pro Ala
50 55 60
Val Asp Phe Ser Leu Gly Glu Trp Lys Thr Gln Met Glu Glu Thr Lys
65 70 75 80
Ala Gln Asp Ile Leu Gly Ala Val Thr Leu Leu Leu Glu Gly Val Met
85 90 95
Ala Ala Arg Gly Gln Leu Gly Pro Thr Cys Leu Ser Ser Leu Leu Gly
100 105 110
Gln Leu Ser Gly Gln Val Arg Leu Leu Leu Gly Ala Leu Gln Ser Leu
115 120 125
Leu Gly Thr Gln Leu Pro Pro Gln Gly Arg Thr Thr Ala His Lys Asp
130 135 140
Pro Asn Ala Ile Phe Leu Ser Phe Gln His Leu Leu Arg Gly Lys Val
145 150 155 160
Arg Phe Leu Met Leu Val Gly Gly Ser Thr Leu Cys Val Arg Arg Ala
165 170 175
Pro Pro Thr Thr Ala Val Pro Ser Arg Thr Ser Leu Val Leu Thr Leu
180 185 190
Asn Glu Leu Pro Asn Arg Thr Ser Gly Leu Leu Glu Thr Asn Phe Thr
195 200 205
Ala Ser Ala Arg Thr Thr Gly Ser Gly Leu Leu Lys Trp Gln Gln Gly
210 215 220
Phe Arg Ala Lys Ile Pro Gly Leu Leu Asn Gln Thr Ser Arg Ser Leu
225 230 235 240
Asp Gln Ile Pro Gly Tyr Leu Asn Arg Ile His Glu Leu Leu Asn Gly
245 250 255
Thr Arg Gly Leu Phe Pro Gly Pro Ser Arg Arg Thr Leu Gly Ala Pro
260 265 270
Asp Ile Ser Ser Gly Thr Ser Asp Thr Gly Ser Leu Pro Pro Asn Leu
275 280 285
WO 95121626 f " z ~ ~ ~ ~ ~ 3 PCT/US95101829
54
Gln Pro Gly Tyr Ser Pro Ser Pro Thr His Pro Pro Thr Gly Gln Tyr
290 295 300
Thr Leu Phe Pro Leu Pro Pro Thr Leu Pro Thr Pro Val Val Gln Leu
305 310 315 320
His Pro Leu Leu Pro Asp Pro Ser Ala Pro Thr Pro Thr Pro Thr Ser
325 330 335
Pro Leu Leu Asn Thr Ser Tyr Thr His Ser Gln Asn Leu Ser Gln Glu
340 345 350
Gly