Note: Descriptions are shown in the official language in which they were submitted.
217080~
P-3032
BLOOD COLLECTION TUBE ASSEMBLY
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a multi-layer barrier coating for
providing an effective barrier against gas and water permeability for
containers, especially plastic blood collection tubes.
2. Description of the Related Art
, With the increased emphasis on the use of plastic medical
products, a special need exists for improving the barrier properties of
20 articles made of polymers.
Such medical products that would derive a considerable benefit
from improv ng their ba~ier properties include, but are not limited to,
collection tubes and particularlv those used for blood collection.
Blood collection tubes require certain performance standards to
be acceptable for use in medical applications. Such performance
standards include the ability to m~int~in greater than about 90%
original draw volume over a one year period, to be radiation
30 sterilizable and to be non-interfering in tests and analysis.
Iherefore, a need exists to improve ~e barrier properties of
articles made of polymers and in particular plastic evacuated blood
collection tubes wherein certain pe,rollllance standards would be met
35 and the article would be effective and usable in medical applications.
I
21708~
-
P-3032
SUMMARY OF THE INVENTION
The present invention is a plastic composite container with a
s multi-layer barrier coating comprising at least two barrier materials
disposed over the outer surface of the previously formed composite
cont~in~r. Desirably, the barrier materials comprise a first layer of a
polymeric material and a second layer of an inorganic material applied
over the first layer.
The first layer, a primer coating, is preferably a highly crossed
linked acrylate polymer. The coating may be formed either on an
interior surface portion, on an exterior surface portion, or both of the
container.
The second layer of the barrier coating may ~le~l~bly be a
silicon oxide based composition, such as SiOx wherein x is from 1.0 to
about 2.0; or an aluminium oxide based composition. Most l~lefelably,
the second layer is a silicon oxide based composition applied over the
20 first layer.
I'reÇe,ably, the primer coating is a blend of monoacrylate (i.e.,
isobornyl acrylate) and diacrylate monomers (i.e., an epoxy diacrylate
or a ur~ e diacrylate) as described in U.S. Patent Nos. 4,490,774,
25 4,696,719, 4,647,818, 4,842,893, 4,954,371 and 5,032,461, the
disclosures of which are herein incorporated by lefel-ellce. The primer
coating is cured by an electron beam or by a source of ultraviolet
radiation.
Desirably, the first layer is formed of a substantially cross
linked component selected from the group con~i~1in~ of polyacrylates
and n~ixlures of polyacrylates and monacrylates having an average
molecular weight of between 150 and 1,000 and a vapor pressure in the
range of Ix10-6 to IxlO-l Torr at standard temperature and pressure.
2170809
P-3032
Most preferably, the material is a diacrylate.
The process for applying the primer coating to a container is
preferably carried out in a vacuum chamber wherein a curable
s monomer component is metered to a heated vapoIizer system where
the material is atomized, vaporized and condensed on the surface of the
container. Following deposit of the monomer onto the surface of the
container, it is cured by suitable means such as electron beam curing.
The deposition and curing steps may be repeated until the desired
o number of layers has been achieved.
Preferably, the thickness of the acrylate primer coating is about
.1 to about 10 microns and most plefcl~bly from about .5 to about 5
microns.
A desirable second layer which is disposed over the first layer
prefelably comprises a silicon oxide based composition, such as SiOx.
Such a film desirably is derived from volatile organosilicon
compounds.
A method for depositing a silicon oxide based film is as
follows: (a) pretreating the first layer on the container with a first
plasma coating of oxygen; ~b) controllably flowing a gas stream
including an organosilicon compound into a plasma; and (c) depositing
2s a silicon oxide onto the first layer while m~;"l~ g a pressure of less
than about 500 microns Hg during the depositing.
It is believed that the pretreatment step provides for improved
adherence qualities between the second layer and the primer coating.
The organosilicon compound is llreferably combined with
oxygen and optionally helium or another inert gas such as argon or
nitrogen and at least a portion of the plasma is ~refelably magnetically
confined adjacent to the surface of the first layer during the depositing,
2170~09
P-3032
most preferably by an unbalanced magnetron.
The silicon oxide based composition provides a dense, vapor-
impervious coating over the primer coating. Preferably, the thickness
s of the silicon oxide based layer is about 100 to about 10,000
Angstroms (~) and most l~refel~bly from about 1,000 to about 3,000
. A coating above 10,000 ~ may crack and therefore be ineffective
as a barrier.
o Plastic tubes coated with the multi-layer barrier coating,
comprising the primer coating and an oxide layer are able to m~int~in
substantially far better vacuum retention and draw volume retention
than previous tubes comprised of polymer compositions and blends
thereof without a coating of barrier materials or of tubes comprising
S only an oxide coating. In addition, the tube's resistance to impact is
much better than that of glass. Most notably is the clarity of the multi-
layer coating and its durability to subst~nti~lly withstand resistance to
impact.
Most plefelably, the container of the present invention is a
blood collection device. The blood collection device can be either an
evacuated blood collection tube or a non-evacuated blood collection
tube. The blood collection tube is desirably made of polyethylene
t~rep~ te, polypropylene, polyethylene napthalate or copolymers
thereof.
Printing may be placed on the multi-layer barrier coating
applied to the container of interest. For example, a product
identification, bar code, brand name, company logo, lot number,
ex~iration date and other data and information may all be included on
the barrier coating. Moreover, a matte finish or a corona discharged
surface may be developed on the barrier coating so as to make the
surface appropriate for writing additional information on the label.
Furthermore, a pressure sensitive adhesive label may be placed over
` ` ~17Q80~
P-3032
the barrier coating so as to accommodate various hospital over-labels,
for example.
Preferably, the multi-layer barrier coating of the present
s invention provides a transparelll or colorless appearance and may have
printe~l matter applied thereon.
A further advantage is that the method of the present invention
provides a reduction in the gas permeability of three-dimensional
lO objects that has not been achieved with conventional deposition
method typically used with thin fil~ns.
Surprisingly, it has been found that a primer coating of acrylate
on a plastic surface followed by a coating of SiOx will result in an
5 oxide coating co.~ reduced amounts of elemental carbon, thereby
resulting in a better barrier coating as col~aled to SiOx coated directly
over the plastic. It is believed that it is difficult to prepare a ~iro~
continuous gas-impervious coating of SiOx with good adhesion
properties and minim~l defects on plastic surfaces. It is believed, that
20 poor adhesion propetties are due to the presence of low molecular
weight hydrocarbon moieties at the surface of the plastic, such as dust
particles and imperfections, formed du~ng injection molding. The
amorphous regions at the surface of the polyolefin, having high chain
mobility, also cause low adhesion of SiOx to a polymer. Such plastic
25 surfaces include polyolefins such as polypropylene and polyethylene.
It has been found that a highly crosslinked layer of acrylate
improves the adhesion between a plastic surface and SiOx and overall
improves the thermomechanical stability of the coated system. In
30 addition, acrylate primer coating has a role of a planarization (leveling)
layer, covering the particles and imperfections on the suface of a
polymer and reducing the defect density in the deposited inorganic
coatings. The good bonding properties of the acrylate are also due to
the fact that arcylate is polar and the polar propertiy provides means
s
` ` 2170809
P-3032
for good bond formation between the SiOx and the acrylate. In
addition, it hæ been found that a good bond formation is made
between plastic tubes made of polypropylene and acrylate. Thus, the
present invention provides the means of subst~nti~lly improving the
5 barrier properties of polypropylene tubes. The adhesion properties of
both the acrylate coating and the oxide coating can be further
subst~nti~lly improved by surface pretreatment methods such as flame
or oxygen plasma. Therefole, a significant reduction in permeability of
the article is due to the subst~nti~lly improved SiOx surface coverage
10 that is obtained by the use of a primer coating of acrylate on the plastic
article surface.
A plastic blood collection tube coated with the multi-layer
barrier coating of the present invention will not interfere with testing
5 and analysis that is typically performed on blood in a tube. Such tests
include but are not limited to, routine chemical analysis, biological
inertness, hematology, blood chemistry, blood typing, toxicology
analysis or therapeutic drug monitoring and other clinical tests
involving body fluids. Furthermore, a plastic blood collection tube
20 coated with the barrier coating is capable of being subjected to
automated machinery such as centrifuges and may be exposed to
certain levels of readiation in the sterilization process with subst~nti~lly
no change in optical or mechanical and functional properties.
2s DESCRIPTION OF THE DRAWINGS
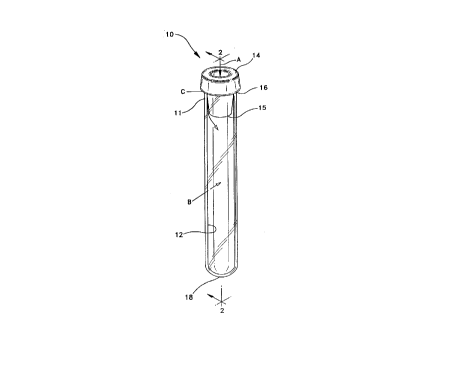
FIG. 1 is a perspective view of a typical blood collection tube
with a stopper.
30FIG. 2 is a longit~ltlin~l sectional view of the tube of FIG. I
taken along line 2-2.
FIG. 3 is a longitll-lin~l sectional view of a tube-shaped
container similar to the tube of FIG. I without a stopper, comprising a
21708~9
,,
P-3032
multi-layer barrier coating.
FIG. 4 is a longitlltlin~l sectional view of a tube-shaped
container, similar to the tube of FIG. 1 with a stopper, comprising a
s multi-layer barrier coating.
FIG. 5 is a lon~itllflin~l sectional view of a further embodiment
of the invention illustrating the tube with a stopper similar to FIG. 1
and with the multi-layer barrier coating encompassing both the tube
10 and stopper thereof.
FIG. 6 illustrates an enlarged partially sectioned, diagram of a
flash evaporator apparatus.
s FIG. 7 illustrates a plasma deposition system.
FIG. 8 illustrates atom percent versus etch time of
polypropylene plaque, acrylate and SiOx.
FIG. 9 illustrates atom percent versus etch time of
polypropylene plaque and SiOx.
DET~ll,Fn DESCRIPTION
The present invention may be embodied in other specific forms
and is not limited to any specific embodiment described in detail which
is merely exemplary. Various o~er modif;cations will be apparent to
and readily made by those skilled in the art without dep~~ g from the
scope and spirit of the invention. The scope of the invention will be
30 measured by the appended claims and their equivalents.
Referring to the drawings in which like refel-ellce characters
refer to like parts throughout the several views thereof, FIGS. 1 and 2
show a typical blood collection tube 10, having a sidewall 11 extending
~1 708~9
P-3032
from an open end 16 to a closed end 18 and a stopper 14 which
includes a lower ~nmll~r portion or skirt 15 which extends into and
presses ~in~t the inner surface 12 of the sidewall for m~ i";"~?;
stopper 14 in place.
s
FIG. 2 schematically illustrates that there are three mech~ni~m~
for a change in vacuum in a blood collection tube: (A~ gas permeation
through the stopper material; (B) gas permeation through the tube and
(C) leak at the stopper tube interface. Thelefore, when there is
o subst~nti~lly no gas permeation and no leak, there is good vacuum
retention and good draw volume retention.
FIG. 3 shows the prefelred embodiment of the invention, a
plastic tube coated with at least two layers of barrier materials. The
lS prerelled embodiment includes many components which are
subst~nti~lly identical to the components of FIGS. 1 and 2.
Accordingly, similar components performing similar functions will be
numbered identically to those components of FIGS. 1 and 2, except
that a suffix "a" will be used to identify those components in FIG. 3.
Referrmg now to FIG. 3, the l~rere,led embodiment of the
invention, collection tube assembly 20 comprises a plastic tube lOa,
having a sidewall 11a extending from an opened end 16a to a closed
end 18a. A barrier coating 25 extends over a substantial portion of the
25 outer surface of the tube with the exception of open end 16a. Barrier
coating 25 comprises a first layer 26 of an acrylate polymer material
and a second layer 27 of a silicon oxide based composition.
FIG. 4 illustrates an alternate embodiment of the invention,
30 wherein collection tube assembly 40 comprises stopper 48 in place for
closing open end 41 of tube 42. As can be seen, sidewall 43 extends
from open end 41 to closed end 44 and stopper 48 includes an annular
upper portion 50 which extends over the top edge of tube 42. Stopper
48 includes a lower annular portion or skirt 49 which extends into and
21708~
P-3032
presses ~in~t the inside inner surface 46 of sidewall 43 for
m~;"l~ stopper 48 in place. Also, the stopper has a septum
portion 52 for receiving a cannula therethrough.
s Thus, the user, once receiving a container such as that shown in
FIG. 4 with a sample contained therein, may insert a cannula through
septum 52 for receiving part or all of the contents in tube 42 to p~rolln
various tests on a sample. Covering a substantial portion of the length
of the tube is a multi-layer barrier coating 45. Multi-layer barrier
coating 45 covers subst~nti~lly most of the tube with the exception of
open end 41 thereo Multi-layer barrier coating 45 comprises a first
layer 54 of a poly_er material and a second layer 56 of an inorganic
material. FIG. 4 differs from the embodiment in FIG. 3 in that the tube
may be evacuated with the simultaneous placement of stopper 48
lS therein after the application of layers 54 and 56 over the tube.
Alternatively, the multi-layer barrier coating may be applied to the tube
after it has been evacuated.
FIG. 5 shows an additional embodiment of the barrier coating
and a tube. The alternate embodiment functions in a similar manner to
the embodiment illustrated in FIG. 4. Accordingly, similar components
pelrol~ g simil~r functions will be numbered identically to those
components in the embodirnent of FIG. 4, except that a suffix "a" will
be used to identify those components in FIG. 5.
2s
Referring now to FIG. 5, a further embodiment 60 of the
invention wherein multi-layer barrier coating 45a incorporates both
upper portion 50a of stopper 48a, as well as the entire outer surface of
tube 42a. Multi-layer barrier coating 45a includes serrations 62 at the
tube, stopper interface. The serrations are registered so that it can be
determined if the sealed container has been tampered with. Such an
embodiment may be utilized, for example, for sealing the container
with the stopper in place. Once a sample has been placed in the tube,
the sample cannot be tampered with by removal of the stopper.
2~70809
P-3032
Additionally, the serrations may be registered so that it can be
~ietç. ~ ed if the sealed container has been tampered with. Such an
arrangement may be a~ o~Jliate, for example, in drug abuse testing,
specimen identification and quality control.
s
In an altetn~te embodiment of the invention, multi-layer barrier
coating 45 is repeatedly applied to the inner and/or outer surface of the
tube. Preferably, the coating is applied at least twice.
o It will be understood by practitioners-in-the-art, that such tubes
may contain reagents in the form of additives or coatings on the inner
wall of the tube.
The multi-layer barrier coating forms a subst~nti~lly clear or
translucent barrier. Thele~ole, the contents of a plastic tube with a
multi-layer barrier coating comprising at least two layers of barrier
materials are subst~nti~lly visible to the observer at the same time
identifying il~lmation may be displayed over the multi-layer barrier
coating after it is applied to the plastic tube.
The first layer of the multi-layer barrier coating may be formed
on the tube by dip-coating, roll-coating or spraying an aqueous
emulsion of the acrylate polymer, followed by air drying.
The acrylate polymer material may also be applied to the tube
by an evaporation and curing process carried out as described in U.S.
Patent No. 5,032,461, the disclosure of which is herein incorporated by
leferellce.
The acrylate evaporation and curing process involves first
atomi7ing the acrylate monomer into about 50 micron droplets and then
fl~hing them off of a heated surface. This produces an acrylate
molecular vapor which has the same chemis~y as the starting
monomer.
21708~
P-3032
Acrylates are available with almost any chemistry desired.
They usually have either one, two or three acrylate groups per
molecule. Various mixtures of mono, di and tri acrylates are useful in
s the present invention. Most prefelable are monoacrylates and
diacrylates.
Acrylates form one of the most reactive classes of chemicals.
They cure rapidly when exposed to UV or electron beam radiation to
IO form a cross-linked structure. This imparts high tempe~ e and
abrasion resistant properties in the coating.
The monomer materials utilized a-re relatively low in molecular
weight, between 150 and 1,000 and ~ref~bly in the range of 200 to
15 300 and have vapor pressures between about lxlO~6Torr and lxlO-1
Torr at standard temperature and pressure (i.e., relatively low boiling
materials). A vapor pressure of about lx10-2 Torr is prefelled.
Polyfunctional acrylates are especially ~refelled. The monomers
employed have at least two double bonds (i.e., a plurality of olefinic
20 groups). The high-vapor-pressure monomers used in the present
invention can be vaporized at low temperatures and thus are not
degraded (cracked) by the heating process. The absence of unreactive
degradation products means that films formed from these low
molecular weight, high-vapor-pressure monomers have reduced volatile
2s levels of components. As a result, subst~nti~lly all of the deposited
monomer is reactive and will cure to form an integral film when
exposed to a source of radiation. These properties make it possible to
provide subst~nti~lly continuous coating despite the fact that the film is
very thin. The cured film exhibits excellent adhesion and are resistant
30 to chemical attack by organic solvents and inorganic salts.
Because of their reactivity, physical properties and the
properties of cured films formed from such components, polyfunctional
acrylates are particularly useful monomeric materials. The general
11
2170809
-
P-3032
formula for such polyfunctional acrylates is:
Rl-(OC-C=CH2)n
I
R2
wherein:
Rl is an aliphatic, alicyclic or mixed aliphatic-alicyclic radical;
R2 is a hydrogen, methyl, ethyl, propyl, butyl or pentyl; and
nisfrom2to4.
Such polyfunctional acrylates may also be used in combination
with various monacrylates, such as those having the formula:
., Xl
I
CH3(CH2)rC-(CH2)SX3
I
CH2oC-C=CH2
Il I
O R2
5
wherein:
R2, is as def~ned above;
xl is H, epoxy, 1,6-hexanediol, ~ ol~yleneglycol or urethane;0 and
r, s are 1-18.
o
12
21708~3
P-3032
CH2OC-C=CH2; and
I
R2
X3 is CN or CoOR3 wherein R3 is an alkyl radical co.,l~;..i~.g
l~ carbon atoms. Most often, X3 is CN or COOCH3.
Diacrylates of the formula below are particularly l~iefelled:
o
Il
CH3(CH2)rCXl (cH2)scH2oc-cH=cH2
I
CH20C-CH=CH2
O
wherein:
0
Xl, r and s are as defined above.
Culing is accomplished by opening the double bonds of the
reactant molecules. This can be accomplished by means of an energy
25 source such as ~palalus which emits infrared, electrons or ultraviolet
radiation.
FIG. 6 illustrates the process for applying an acrylate coating.
An acrylate monomer 100 is directed through a dielectric evaporator
30 102 and then through an ultrasonic atomizer 104 and into a vacuum
chamber 106. The monomer droplets are atomized ultrasonically and
the droplets vaporized where they condense on the tube or film that is
loaded on a drum 108.
21708~
P-3032
The condensed monomer liquid subsequently is radiation cured
by means of an electron beam gun 110.
The second layer of the multi-layer barrier coating, an inorganic
s material, may be formed over the acrylate coating by radio frequency
discharge, direct or dual ion beam deposition, sputtering or plasma
chemical vapor deposition, as described in U.S. Patent Nos. 4,698,256,
4,809,876, 4,992,298 and 5,055,318, the disclosures of which are
herein incorporated by refe.~ ce.
For example, a method of depositing an oxide coating is
provided by establi~hinp; a glow discharge plasma in a previously
evacuated chamber. The plasma is derived from one or more of the
gas stream components, and l~rerel~bly is derived from the gas stream
S itself. The article is positioned in the pl~m~, plerel~bly adjacent the
confined plasma, and the gas stream is controllably flowed into the
plasma. A silicon oxide based film is deposited on the substrate to a
desired thickness. The thickness of the oxide coating is about 100
Angstroms (~) to about 10,000 ~. A thickness of less than about
20 10,000 ~ may not provide sufficient barrier and a thickness of greater
than about 10,000 A may crack, thus decreasing the effective barrier.
Most ~refelably, t~e thickness of the oxide coating is about 1,000 A
to about 3,000 ~.
Another method of depositing an oxide coating is by
confinin~ a plasma with magnets. I~e~erably, the magnetically
enhanced method for depositing a silicon oxide based film on a
substrate is prefelably conducted in a previously evacuated chamber
of glow discharge from a gas stream. The gas stream ~refe,~bly
30 comprises at least two components: a volatilized organosilicon
component, an oxidizer component such as oxygen, nitrous oxide,
carbon dioxide or air and an optionally inert gas component.
Examples of suitable organosilicon compounds useful for the
14
21708~
~,
P-3032
gas stream in ~e plasma deposition methods are liquid or gas at
about ~mbient tempe~ e and when vol~tili7e~1 have a boiling point
about 0C to about 150C and include dimethysilane, trimethylsilane,
diethyl~ ne, propylsilane, phenylsilane, hexamethyldisilane, 1,1,2,2-
s tetramethyldisilane, bis (trimethylsilane)methane, bis (dimethylsilyl)methane, hexamethyldisiloxane, vinyl trimethoxy silane, vinyl
triethyoxysilane, ethylmethoxysilane, ethyltrimethoxysilane,
divinyltetramethyldisiloxane, hexamethyklsil~7~ne divinyl-
hexamethyltrisiloxane, trivinylpentamethyltrisiloxa_ane,
o tetraethoxysilane and tetramethoxysilane.
Among the ~refelled organosilicons are 1,1,3,3-
tetramethyldisiloxane, trimethylsilane, hexamethyldisiloxane,
vinyltrimethylsilane, methyltrimethoxysilane, vinyltrimethoxysilane
S and hexamethyl~ 7~ne. These IJrerelled organosilicon compounds
have boiling points of 71C, 55.5C, 102C, 123C and 127C
respectively.
The optional inert gas of the gas stream preferably is helium,
20 argon or nitrogen.
The vol~tili7e~1 organosilicon component is preferably
admixed with the oxygen component and the inert gas component
before being flowed into the chamber. The quantities of these gases
25 being so admixed are controlled by flow controllers so as to
adjustably control the flow rate ratio of the gas stream components.
Various optical methods known in the art may be used to
determine the thickness of the deposited film while in the deposition
30 chamber, or the film thickness can be determined after the article is
removed from the deposition chamber.
The deposition method of the present invention is prerelably
practiced at relatively high power and quite low pressure. A pressure
21708~
P-3032
less than about 500 millitorr (mTorr) should be m~int~ined during ~e
deposition, and ~ref~,lably the chamber is at a pressure between about
43 to about 490 millitorr during the deposition of film. Low system
pressure results in lower deposition rates whereas higher system
5 pressure provides faster deposition rates. When the plastic article to
be coated is heat sensitive, a higher system pressure may be used to
minimi7e the amount of heat the substrate is exposed to during
deposition because high substrate tempera~ s are to be avoided for
low Tg polymers such as polypropylene and PET (Tg is -10C and
lO 60C respectively).
The substrate is electrically isolated from the deposition
system (except for electrical contact with the plasma) and is at a
tempe.alure of less than about 80C during the depositing. That is,
the substrate is not deliberately heated.
Referring to FIG. 7, the system for depositing a silicon oxide
based film comprises an enclosed reaction chamber 170 in which a
plasma is formed and in which a substrate or tube 171,is placed for
~o depositing a thin film of material on a sample holder 172. The
substrate can be any vacuum compatible material, such as plastic.
One or more gases are supplied to the reaction chamber by a gas
supply system 173. An elec~ic field is created by a power supply
174.
2s
The reaction chamber can be of an approllliate type to
perform any of the plasma-enhanced chemical vapor deposition
(PECVD) or plasma polymer~zation process. Furthermore, the
reaction chamber may be modified so that one or more articles may
30 be coated with an oxide layer simultaneously within the chamber.
The pressure of the chamber is controlled by a mechanical
pump 188 connected to chamber 170 by a valve 190.
16
217080~
l~
P-3032
The tube to be coated is first loaded into chamber 170 in
sample holder 172. The pressure of the ch~mber is reduced to about
Sm Torr by mechanical pump 188. The operating pressure of the
chamber is about 90 to about 140 mTorr for a PECVD or plasma
s polymerization process and is achieved by flowing the process gases,
oxygen and trimethyl silane, into the chamber through monomer inlet
176.
The thin film is deposited on the outer surface of the tube and
o has a desired uniform thickness or the deposition process may be
interrupted periodically to ~ i"~i7e heating of the substrate and/or
electrodes and/or physically remove particulate matter from the
articles.
Magnets 196 and 198 are positioned behind electrode 200 to
create an appropriate combination of magnetic and electrical fields in
the plasma region around the tube.
The system is suitable for low frequency operation. An
20 example frequency is 40kHz. However, there can be some
advantages from operating at a much high frequency, such as in the
radio frequency range of several megahertz.
The silicon oxide based film or blends thereof used in
2s accordance with this disclosure, may contain conventional additives
and ingredients which do not adversely affect the properties of
articles made therefrom.
A variety of substrates can be coated with a barrier coating by
30 the process of the present invention. Such substrates include, but are
not limited to pack~ing containers, bottles, jars, tubes and medical
devlces.
A plastic blood collection tube coated with the multi-layer
17
21708~
P-3032
barrier coating will not interfere with testing and analysis that is
typically pclro~ ed on blood in a tube. Such tests include but are not
limited to, routine chemical analysis, biological inertness, hematology,
blood chemistry, blood typing, toxicology analysis or therapeutic drug
s monitoring and other clinical tests involving body fluids. Furthermore,
a plastic blood collection tube coated with the barrier coating is
capable of being subjected to automated m~chinery such as ce~ iruges
and may be exposed to certain levels of radiation in the sterilization
process with subs~nh~lly no change in optical or mechanical and
o functional properties.
A plastic blood collection tube coated with the multi-layer
barrier coating is able to m~int~in 90% original draw volume over a
period of one year. Draw volume retention depends on the existence of
5 a particle vacuum, or reduced pressure, inside the tube. The draw
volume changes in direct proportion to the change in vacuum (reduced
pressure). Therefore, draw volume retention is dependent on good
vacuum retention. A plastic tube coated with a barrier coating
subst~nti~lly prevents gas permeation through the tube material so as to
20 m~int~in and enhance the vacuum retention and draw volume retention
of the tube. Plastic tubes without the multi-layer coating of the present
invention may m~int~in about 90% draw volume for about 3 to 4
months.
If the multi-layer barrier coating is also coated or applied on the
inner surface of the plastic blood collection tube, the barrier coating
may be hemorepellent and/or have characteristics of a clot activator.
It will be understood that it makes no difference whether the
30 plastic composite container is evacuated or not evacuated in
accordance with this invention. The presence of a barrier coating on
the outer surface of the container has the effect of maillt; ;.~ g the
general integrity of the container holding a sample so that it may be
properly disposed of without any cont~min~tion to the user. Notable is
~170809
P-3032
the clarity of the barrier coating as coated or applied on the container
and its abrasion and scratch re~i~t~nce.
The barrier coating used in accordance with this disclosure,
5 may container conventional additives and ingredients which do not
adversely affect the properties of articles made ther~lio~
The following examples are not limited to any specific
embodiment of the invention, but are only exemplary.
EXAMPLE 1
METHOD FOR COATING PLASTIC SUBSTRATES
TUBES WITH MULTI-LAYER BARRIER COATING
An acrylate coating was applied to polypropylene tubes and
films (substrates) of various thickness in a chamber wherein
T~ opylene Glycol Diacrylate (TPGDA) was fed into the evaporator
and was flash vaporized at about 343C onto the substrate in the
20 chamber and condensed. The condensed monomer film was then E-
beam cured by an electron beam gun.
The substrate coated with the acrylate coating (TPGDA) was
then cleaned with a nlixlule comprising equal parts of a micro
2s detergent and de-ionized (DI) water solution. The substrate was rinsed
thoroughly in DI water and allowed to air dry. The cleaned substrate
was then stored in a vacuum oven at room temperature until it was to
be coated.
The cleaned substrate was then attached to a holder which lSts
midway between the electrodes in the glass vacuum chamber. The
chamber was closed and a mechanical pump was used to achieve a
base pressure of 5 mTorr.
19
2170~09
P-3032
The electrode configuration is internally capacitively coupled
with permanent magnets on the backside of the lila~ electrodes.
This special configuration provides the ability to confine the glow
between the electrodes because of the increase in collision probability
s between electrons and reacting gas molecules. The net result of
applying a magnetic field is similar to increasing the power applied to
the electrodes, but without the disadvantages of higher bombardment
energies and increæed substrate heating. The use of magnetron
discharge allows operation in the low pressure region and a substantial
10 increase in polymer deposition rate.
The monomer which consists of a mixture of trimethylsilane
(TMS) and oxygen was introduced through stainless steel tubing near
the electrodes. The gases were mixed in the monomer inlet line before
introduction into the chamber. Flow rates were manually controlled by
stainless steel metering valves. A power supply operating at an audio
frequency of 40 kHz was used to supply power to the electrodes. The
system parameters used for thin film deposition of plasma polymerized
TMS/O2 on the polymer substrate were as follows:
Surface Pretreatment: TMS Flow = 0 sccm
Base Pressure = 5 mTorr
Oxygen Flow = 10 sccm
System Pressure = 140 mTorr
Power = 50 watts
Time = 2 minutes
OxideDeposition: TMS Flow = 0.75 -1.0 sccm
Oxygen Flow = 2.5 = 3 0 sccm
System Pressure = 90 - 100 mTorr
Power = 30 watts
Deposition Time = 5 minutes
After the thin film was deposited, the reactor was allowed to
2170~
P-3032
cool. The reactor was then opened, and the substrate with a multi-
layer barrier coating was removed.
EXAMPLE 2
s
METHOD FOR COATING PLASTIC SUBSTRATES
WITH MULTI-LAYER BARRIER COATING
An acrylate coating was applied to polypropylene tubes and
10 films (substrates) in a chamber wherein a 60:40 ~ e of isobornyl
acrylate: epoxydiacrylate (IBA:EDA) was fed into the evaporator and
flash vaporized at about 343C onto the substrate in the chamber and
condensed. The condensed monomer film was then UV cured by an
actinic light source of 365 nm.
The substrate coated with the acrylate coating (IBA:EDA) was
then cleaned with a n~ixlure comprising equal parts of a micro
detergent and de-ionized (DI) water solution. The substrate was rinsed
thoroughly in DI water and allowed to air dry. The cleaned substrate
20 was then stored in a vacuum oven at room temperature until it was to
be coated.
The cleaned substrate was then attached to a holder which fits
midway between the electrodes in the glass vacuum chamber. The
2s chamber was closed and a mechanical pump was used to achieve a
base pressure of 5 mTorr.
The electrode configuration is internally capacitively coupled
with permanent magnets on the backside of the titanium electrodes.
30 The special configuration provides the ability to confine the glow
between the electrodes because of the increase in collision probability
between electrons and reacting gas molecules. The net result of
applying a magnetic field is simil~r to increasing the power applied to
the electrodes, but without the disadvantages of higher bombardment
21
217081D~
P-3032
energies and increased substrate heating. The use of magnetron
discharge allows ol,e alion in the low pressure region and a substantial
increase in polymer deposition rate.
s The monomer which consists of a ~ ure of trimethylsilane
(TMS) and oxygen was introduced through stainless steel tubing near
the electrodes. The gases were mixed in the monomer inlet line before
introduction into the chamber. Flow rates were manually controlled by
stainless steel metering valves. A power supply operating at an audio
frequency of 40 kHz was used to supply power to the electrodes. The
system parameters used for thin film deposition of plasma polymerized
TMS/02 on the polymer substrate were as follows:
Surface Pretreatment: TMS Flow = 0 sccm
Base Pressure = 5 mTorr
Oxygen Flow = 10 sccm
System Pressure = 140 mTorr
Power = 50 watts
Time = 2 minutes
2~
Oxide Deposition: TMS Flow = 0.75 - 1.0 sccm
Oxygen Flow = 2.5 = 3.0 sccm
System Pressure = 90 - 100 mTorr
Power = 30 watts
DepositionTime = 5 minutes
After the thin film was deposited, the reactor was allowed to
cool. The reactor was then opened, and the substrate with a multi-
layer barrier coating was removed
3Q
EXAMPLE 3
COMPARISON OF SUBSTRATES WITH AND WITHOUT
MULTI-LAYER BARRIER COATINGS
22
217080~
P-3032
All of the substrates ~repaled in accordance with Examples 1
and 2 above were evaluated for oxygen permeance (OTR) in the oxide
coatings.
s
(i) Oxygen permeance (OTR):
Film or plaque samples were tested for oxygen permeance
(OTR) using a MO CON Ox-TRAN 2/20 (sold by Modern Controls,
o Inc., 7500 Boone Avenue N., Minneapolis, MN 55428). A single
side of the film sample was exposed to l atm of 100% oxygen
atmosphere. Oxygen permeating through the sample film was
entrained in a nitrogen carrier gas stream on the opposite side of the
film, and detected by a coulmetric sensor. An electrical signal was
15 produced in proportion to the amount of oxygen permeating through
the sample. Samples were tested at 30C and 0% relative hutnidity
(R.H.). Samples were conditioned for 1 to 20 hours prior to
determinin~ oxygen permeance. The results are reported in Table I in
units of cc/m2-atm-day.
Tube samples were tested for oxygen permeance (OTR) using
a MOCON OX-TRAN 1,000 (sold by Modern Controls, Lnc., 7500
Boone Avenue N., Minneapolis, MN 55428). A package adapter was
used for mounting the tubes in a manner that allowed the outside of the
2s tube to be immersed in a l OO% 2 atrnosphere while the inside of tube
is flushed with a nitrogen carrier gas The tubes were then tested at
20C and 50% R.H. The tubes were allowed to equilibrate for 2-14
days before a steady state permeability is determined. The results are
reported in Table I in ~nits of cc/m2-atm-day.
(ii) Atom % of the elements present in the oxide coating:
A Surface Science Model SSx-100 X-ray photoelectron
spectromenter (ESCA) was used to determine the atom % of the
23
2170~9
P-3032
elements present in the oxide coatings. Film samples were placed
inside the spectrometer and the elementral composition was determined
of approximately lOOA into the surface. The surface was then argon
ion etched as follows: 5000V and 9-10 mA agrgon ion beam was
s directed at the sample surface. After 5 seconds the ESCA spectra was
taken and this procedure was repeated for a total of 5 times. The etch
time was then increased to 20 seconds followed by ESCA and this
process was repeated for a total of ten times. Finally, the etch time
was inreased to 40 second and the ESCA spectra was obtained until
o the bulk acrylate or the polymer substrate was reached. The oxide
layer was clearly indicated by the presence of silicon in the ESCA
spectra between the etch times of about 0 through about 1.3 minutes.
(iii) Analysis of the Data in Table 1 and Figures 8 and 9:
Figures 8 and 9 illustrate atom % verses etch time for oxide
samples deposited on polypropylene with acrylate primer coating and
without acrylate coating. The data in Table 1 demonstrates for these
plasma deposition processes the improved barrier properties correlate
20 well with improved gas-barrier performance as a result of using the
multi-layer barrier coating. The SiOx deposition on an acrylate treated
surface occurs with less surface degradation and thele~ore resulting in a
more dense barrier structure. Since the acrylate coating itself provides
no detectable improvement in barrier, data demonstrates that the
25 acrylate-oxide coating method greatly improves the barrier properties
of plastic substrates.
24
~170809
P-3032
TABLE 1
Sample Acrylate sioX CoatingOygen Transmissio
Coating Method (cc/m2-atm-da
PP film, control no no 1500
PP plaque, 75 milno yes 37
PP plaque, 75 milTPGDA yes 3.5
PPplaque, 75 mil no yes 65
PPtube, control no no 70
PP tube IBA:DA no 70
PPtube no yes 46-60
PP tube IBA:DA yes 4.3-6.4
IBA:DA = iso-norbornyl: epoxydiacrylate (60:40), W cured
TPGDA = tripropylene glycol diacrylate E-beam cured
Oxide Coatings = 1000 - 3000 Angstroms (as measured by Sc~nnin~ Electron M;croscope)
PP = polypropylene
plaque= 75 mil thickness
film = 2 mil thickness
tubes = nominal wall thickness of 40 mil