Note: Descriptions are shown in the official language in which they were submitted.
woss/07722 2 ~ 71~ 6 3 pcTlAu94loos4s
-- 1 --
INJECTION DEVICE
Technical Field
The present invention relates to an injection device
having a locating means causing a needle to be inserted
into the skin of a subject at a predetermined angle and
depth. Whilst the invention is according to one
embodiment directed towards and primarily described with
reference to skin testing for allergy, it is considered
that the invention is suitable for use for injecting other
substances into or under skin.
Background
Skin testing for allergy is conventionally performed
by placing a small drop of a solution of the test material
(allergen) on the skin (usually forearm or back) of the
subject and passing a hypodermic needle or lancet through
the drop into the first layer of skin without drawing
blood. An inflammatory response in the form of a wheal
and flare reaction occurring within about 15 minutes
indicates allergy to the suspected allergen. This test,
commonly called the "skin prick test~ has been used for
many years chiefly by specialist allergists as it is
labour-intensive and takes a valuable consultation time in
a surgery. In addition, it is costly to provide this form
of testing, especially if only done occasionally, and
requires some technical skill. The method is, however,
the most reliable diagnostic procedure for allergies and
is sensitive, rapid, specific and relatively inexpensive.
Testing for delayed, or cell-mediated hypersensitive
reactions may also be performed by skin testing wherein
the needle is used to penetrate the skin to a greater
depth allowing the test material (antigen) greater access
to the tissues underlying the dermal layer. This skin
reaction, which is an index of the patient's ability to
mount a cellular immune response to the test antigen is
usually measured over hours or even days.
W095/07722 ~1 7 ~ 5 6 3 PCT/AUg~/005~9
Given the value and widespread use of skin tests in
diagnostic medicine, it is desirable to adapt and
standardise the method to make it more available, easy to
use and acceptable to both clinicians and patients.
Furthermore, it is desirable to improve the speed,
reliability and reproducibility of skin test responses.
The present methods are cumbersome and laborious for
allergy testing. Often large numbers of tests are carried
out at one time on the one patient as many allergens are
usually tested (for example an individual pollen, house
dust mite or fungal species). The clinician must purchase
a bottle of each extract for testing and multiple sampling
of these bottles increases the chances of cont~;n~tion.
After placing a drop of the extract on the patient's skin,
a needle or lancet is used to administer the sample into
the skin. A fresh needle or lancet is required for each
extract tested and often involves many extracts. Where 10
to 20 samples must be tested it would be desirable to
reduce the time and labour involved for this testing by
having an injection device that can administer the desired
substance in a controlled manner.
~isclosure of Invention
In recognising these difficulties with prior art
devices, the present invention seeks to provide an
injection device which readily allows the controlled and
accurate injection of substances into skin of patients and
in particular for the use in allergen and delayed-type
hypersensitivity testing.
The present invention consists in an injection device
comprising a housing defining a base surface, a syringe
slidable with respect to the housing and having a syringe
barrel defining a chamber, a hollow needle mounted at one
end of the barrel and being in fluid communication with
the chamber, a plunger slidingly disposed in the barrel
for urging fluid from the chamber through the hollow
.
Wo95/07722 ~ 3 PCT/AU94/00549
needle and, wherein in use, the needle of the syringe
projects from the base surface at a distance no greater
than the depth of skin to be injected and at a
predetermined angle with respect to the base surface to
ensure optimum injection into the skin.
The syringe is preferably slidable in a passage in
the housing to an extended position wherein the needle
projects from the base surface, the passage extending
through the base surface, and wherein the extended
position is determined by a stop located within the
passage of the housing which, in use, engages with a
locating portion of the syringe barrel.
More preferably the syringe is slidable between the
extended position and a retracted position wherein the
needle is positioned so as not to project from the base
surface. Even more preferably the locating portion of the
syringe barrel is in the form of an annular ridge of
enlarged diameter located on the outer surface of the
syringe barrel which is slidably received in an enlarged
diameter portion of the passage of the housing, the
enlarged diameter portion having proximal and distal end
stops which determine the retracted and extended positions
of the syringe respectively.
Preferably the injection device is further provided
with a spring bias means to urge the syringe into its
retracted position. More preferably, the spring bias
means is a coil spring located within the enlarged
diameter portion of the passage and acting between the
locating portion of the syringe barrel and the distal end
30 stop, and wherein, in use the coil spring and the distal
end stop determines the extended position of the syringe.
Preferably the syringe contains within the chamber a
substance to be injected and most preferably the substance
is in a fluid form. Optionally the injection device is
provided for use in a sterile container with the substance
W095/07722 PCT/AU94/00549
~ 1 7 ~
to be injected preloaded in the chamber.
The substance to be injected may comprise a
composition, an active ingredient, an allergen, an
antigen, a drug or any combination thereof.
In a preferred embodiment the chamber of the syringe
is divided into a distal and a proximal part by means of
the seal and, wherein, in use actuation of the plunger
causes the seal to be broken and causes the contents
within the distal and proximal parts to combine and be
urged out through the needle.
Preferably, the plunger has a pin extending from its
distal end and wherein in use the pin penetrates and
breaks the seal causing the contents of the distal and
proximal parts to combine. Most preferably, the distal
part of the chamber contains a substance to be injected
and the proximal part contains a diluent. Most preferably
the substance is in a non-liquid form.
The injection device is suitable for injecting
substances into skin and the angle of the needle with
respect to the base surface will be determined by the type
of injection to be made by the device. Preferably the
injection device of the present invention has the needle
defining an angle of less than about 45 degrees relative
to the base surface and more preferably the angle of the
needle is between 10-30 degrees. Most preferably, the
needle of the syringe defines an angle of about 20 degrees
relative to the base surface.
The distance that the needle projects from the base
surface is determined by the type of injection to be made
by the device. Preferably the needle projects from the
base surface so the end of the needle is 0.5 to 1.5 mm
from the base surface.
The injection device is suitable for injecting
micro-volumes (approximately 5 to 25 microlitres), small
volumes (25-100 microlitres) and larger volumes of up to
W095/07722 2 ~ 7 1~ 6 3 PCT/AU94/00549
-- 5 --
approximately 250 microlitres. The volume of substance to
be injected will be determined by the type of injection to
be made by the device.
It will be appreciated that the base surface can be
substantially flat, or can be curved so as to conform with
the curvature of the site of injection.
The injection device is suitable for injecting
substances into or within layers of the skin. Injections
may be made to the epidermis (ectodern), dermis (mesoderm)
or the subcutaneous fat (adipose tissue). The device may
be adapted for different types of injection by varying the
angle of the needle to the base surface, the distance that
the needle projects from the base and the volume to be
injected.
Brief Description of Drawings
The invention will now be described by non-limiting
examples with reference to the following drawings.
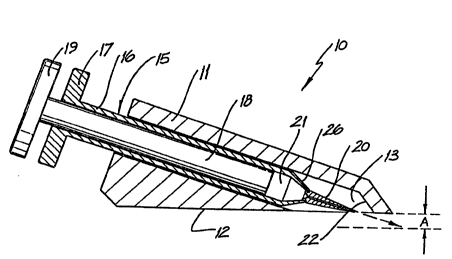
Figure 1 depicts a perspective view of an injection
device in accordance with the present invention with the
syringe in its extended position;
Figure 2 is a longitudinal-sectional view of an
injection device with the syringe in its retracted
position and showing one embodiment of the mounting of the
syringe on the housing;
Figure 3 is a longitudinal-sectional view of an
injection device showing embodiment of the mounting of the
syringe in the housing; and
Figure 4 is a longitudinal-sectional view of the
injection device similar to that shown in Figure 2 with an
alternative arrangement ~or containing the substance to be
injected.
Best Mode for Carrying out the Invention
An injection device in accordance with the present
invention is identified generally by the numeral 10 in
35 Figs 1-4. The injection device 10 includes a housing 11,
W095/07722 PCT/AUg4/00549
~171563
-- 6
a syringe 15 and a needle 20.
As shown in Figs 1-4, the housing 11 includes a base
surface 12 and a passage 13. A syringe 15 is slidable in
the passage 13 of the housing 11.
The syringe 15 includes a barrel 16 and a syringe
barrel head 17. Within the syringe barrel 16 is situated
a plunger 18 including a plunger head 19. The plunger 18
is slidingly disposed within, and is in sealing engagement
with the barrel 16 so as to form a chamber 21 towards the
distal end of barrel 16. A hollow needle 20 is mounted at
the distal end of the barrel 16 and is in fluid
communication with the chamber 21.
With reference to Fig 2, the syringe 15 is slidable
in the passage 13 of the housing 11 to an extended
position (as represented by the arrow), wherein the end of
the needle 20 is positioned a distance A from the base
surface 12. The extended position of the syringe 15 is
determined by a stop 22 located within the passage 13 of
the housing 11 so as to engage with a portion 26 of the
syringe barrel 16 when in use. Stop 22 ensures that
needle 20 projects from the base surface 12 at a distance
A which is no greater than the thickness of skin to be
injected. The positioning of the passage 13 in the
housing 11 determines the angle of the needle with respect
to the base surface 12 ensuring optimum entry and
subsequent injection into the skin.
In use, the base surface 12 of injection device 10 is
placed on the surface of the skin of a patient and syringe
15 is pushed via pressure on barrel head 17 and/or plunger
head 19 through the passage 13 until the barrel 16 is in
contact with the stop 22. In doing so the needle 20
penetrates the skin of the patient to the desired depth
A. Plunger head 19 is further pressed so as to move the
plunger 18 towards the needle 20 and move the contents in
chamber 21 through needle 20 into the site of injection.
WosS/07722 2 ~ 7 ~ 5 ~ ~: . PCTIAU94/00549
-- 7
To ensure that the contents of the syringe 15 is not
expelled from the needle prior to the insertion into the
skin, the resistance of the sliding movement of the
syringe 15 in the passage 13 is significantly less than
the resistance to the movement of the plunger 18 in the
barrel 16.
Another embodiment of the mounting of the syringe 15
in the housing 11 is shown in Fig 3 in which the injection
device 10 is arranged so that the syringe 15 is slidable
between a retracted position and an extended position. In
the retracted position the needle 20 is positioned so as
not to project from base surface 12 and the positioning of
the needle 20 is by a locating means 32 comprising an
annular ridge 27 of enlarged diameter located on the outer
sur:Eace of syringe barrel 16 adapted to be slidably
received in an enlarged diameter portion 30 of passage 13
of housing 11. The enlarged diameter portion 30 has end
stops 28, 29 which determi nq the extended and retracted
positions respectively of the syringe 15.
In order to ensure that needle 20 does not project
through the base surface 12 when the device is not in use
a coil spring 31 is provided within the enlarged diameter
portion 30 to urge syringe 16 into its retracted position.
In the embodiments shown in Figs 2 and 3, the
substance to be injected is preferably in a liquid form
and is contained in the chamber 21. In an alternate
embodiment shown in Fig 4, the chamber 21 is divided into
a proximal part 14 and distal part 25 by a seal 24.
Mounted on the distal end of the plunger 18 is a pin 23
extending towards the seal 24 such that, during actuation
of the plunger 18, the seal 24 is broken by the pin 23 and
the contents within the distal 25 and proximal 14 parts
combine and are urged out through the needle 20.
Other means for breaking the seal 24 are also
envisaged including pressure of the movement of the barrel
woss/o7722 2 ~ 7 ~ ~ ~ 3 8 - PCT/AU94/00549
18 towards the seal 24 and the like.
The needle 20 is positioned defining angle of less
than 45 degrees relative to base surface 12 to ensure that
needle 20 penetrates the skin at the required angle.
Preferably the angle is 10 to 30 degrees and most
preferably the angle is about 20 degrees relative to base
surface 12 to ensure optimum entry and injection into the
skin of the patient.
In order to ensure that the injection device provides
for injection of a substance into the skin, it is
preferred that the distance A that the end of the needle
20 is spaced from base surface 12 between 0.5 and 1.5 mm.
The injection device 10 may be supplied for use
preloaded with the substance to be injected and provided
in a suitable sterile cont~inPr. The injection device 10
is easy to use and may be disposable and suitable for the
use in skin testing of patients for immediate
hypersensitivity (allergic) or delayed hypersensitivity
(delayed or cell-mediated) immune reactions. The
injection device 10 may contain substances to be injected
in liquid form. The substance to be injected may contain
suitably formulated and preserved allergen solutions or
extracts, antigens, drugs or any combination thereof.
Alternatively, the device may be used with a labile or
unstable substances which are dissolved or mixed in a
liquid diluent in the device immediately before testing.
An embodiment as shown in Figure 4 having a chamber 21
divided into two parts enables this form of the invention
to be utilised.
The injection device 10 may be made of any suitable
material such as moulded plastic and needle 20 may be
plastic, metal or any other suitable material known to the
art.
The present invention is also suitable for use in
injecting any materials in the skin where a predetermined
~ 2 ~ 3
WOgS/07722 : PCT/AU94/00549
entry angle of a needle and depth is required for
administration of a desired substance.
The embodiments of the invention as described above
are given by way of example only as constituting preferred
forms of the invention defined broadly in the various
aspects.
It will be appreciated by persons skilled in the art
that various numerations and/or modifications may be made
to the invention as shown in the specific embodiments
without departing from the spirit or scope of the
invention as broadly described.