Note: Descriptions are shown in the official language in which they were submitted.
21 7 1 575
A COMPOSITE MOLDED PRODUCT
Background of the Invention
~ield of the Invention
The present invention relates to a composite molded
product in which a molded crosslinked rubber is integrally
laminated with a molded thermoplastic polymer and to a
production method thereof.
Description of the ~elated Art
Conventionally, a crosslinked rubber product with a
simple shape has been produced by an extrusion crosslinking
method. However, a molded product with a complicated shape
cannot be produced by the extrusion crosslinking method alone.
For this reason, methods are known wherein the portion having a
simple shape is first molded by an extrusion crosslinking
method, and the portion having a complicated shape is produced
by injection molding of a crosslinkable rub~er and at the same
time curing the crosslinkable rubber, or alternatively by
injection molding a thermoplastic resin instead of the
crosslinkable rubber. For example, it is described in Japanese
Patent Laid-Open No.6-47816 that a thermoplastic resin powder is
first adhered on a surface of the crosslinked rubber to be
joined with a thermoplastic resin, then the thermoplastic resin
is bonded to the crosslinked rubber by injection molding.
However, since the adhesion between injected
crosslinkable rubber or uncrosslinked thermoplastic resin and
the crosslinked rubber is insufficient when using such an
injection molding method, it is proposed that the joint surface
of the crosslinked rubber should be subjected to a blast
treatment with sandpaper, before adhering an adhesive and a
thermoplastic resin powder thereon it and then carrying out
injection molding. Since such a complicated step as a blast
treatment is required, this is not an efficient process.
It is also proposed that a primer treatment be carried
-1-
21 7 1 575
on the joint surface of the crosslinked rubber. But even by this
process, the manual labor can not be sufficiently simplified
enough, and problems in terms of the product cost are not
alleviated.
Summary of the Invention
Object of the Invention
An object of the present invention is to provide a
composite molded product in which a molded crosslinked rubber is
firmly bonded and laminated with a molded thermoplastic polymer
by heat fusing and an injection molding method by which even a
composite injection molded product having a high bond strenght,
a complicated shape and a relatively large size can be produced.
Brief Summary of the Invention
According to one aspect of the present invention,
there is provided a composite molded product in which a molded
crosslinked rubber (I) is integrally bonded with a molded
product (II~, the base material thereof comprising (a) a
thermoplastic polymer containing a polar group and/or (b) a
thermoplastic polymer containing an inorganic filler.
According to another aspect of the present invention,
there is provided a method of producing a composite molded
product, comprising placing a molded crosslinked rubber (I) in
an injection molding die, injecting (a) a thermoplastic polymer
containing a polar group, and/or (b) a thermoplastic polymer
containing an inorganic filler into the molding die, thereby
producing a molded product (II), and integrally bonding the
product (Il) onto the molded crosslinked rubber (I).
lIl Raw materials
(1) Molded crosslinked rubber
<1> Rubber
Examples of rubber used as a raw material for
producing a molded crosslinked rubber include natural rubber
(NR), derivatives thereof, synthesized rubbers and the like.
-2-
21 7 1 575
Examples of the above-mentioned synthesized rubber
include diene type rubbers such as butadiene rubber (BR),
styrene-butadiene rubber (SBR), nitrile rubber (acrylonitrile-
butadiene rubber, NBR), isoprene rubber (IR), and chloroprene
rubber (CR); and olefin type rubbers such as butyl rubber
(isobutylene-isoprene copolymer, IIR), ethylene-propylene rubber
(EPM), ethylene-propylene-nonconjugated diene rubber (EPDM),
ethylene-butene rubber (EBM), ethylene-propylene-butene
copolymer rubber, chlorosulfonated polyethylene (CSM), and
chlorinated polyethylene (CPE).
These rubbers can be used alone or in an admixture of
two or more components.
These rubber can be blended with a thermoplastic resin
such as polypropylene and polyethylene if necessary. If
necessary, these rubbers can be also blended with a filler such
as talc and calcium carbonate, a plasticizer such as paraffin
oil and liquid polybutene, and other various additives including
a vulcanizing accelerator, peroxide, crosslinking assistant,
mastication accelerator, antiscorching agent, foaming agent,
antioxidant, thermal stabilizer, light stabilizer, UV absorbing
agent, neutralization agent, slip additive, lubricant, anti-
-fogging agent, anti-blocking agent, dispersing agent, coloring
agent, antibacterial agent, and fluorescent whitener.
<2> Molded crosslinked rubber
A molded crosslinked rubber can be obtained by adding
0.1 to 10% by weight, preferably 0.1 to 5% by weight of a
crosslinking agent to the above-mentioned rubber, followed by
heating and molding.
Examples of a crosslinking agent to crosslink the
above-mentioned rubber include sulfur and aromatic or aliphatic
peroxides such as 2,5-dimethyl-2,5-di(tert-butylperoxy)hexane,
2,5-dimethyl-2,5-di(tert-butylperoxy)hexane-3,tert-butylperoxy
benzoate, dicumyl peroxide, 2,5-dimethyl-2,5-
di(benzoylperoxy)hexane, tert-butylcumyl peroxide, diisopropyl
-3-
` 21 71 575
benzohydroperoxide, benzoylperoxide, and di-tert-butylperoxide,
the above can be used alone or in admixtures.
Among these crosslinking agents, sulfur is preferably
employed.
The molded crosslinked rubber can be produced by
blending the above crosslinkable rubber material with sulfur or
a peroxide, molding the blend into a desired shape by such
molding methods as extrusion molding, injection molding or
vacuum molding, followed by heat treatment at 100 to 200 ~ for 1
to 10 minutes so that the crosslinkable rubber material is
crosslinked or cured by the sulfur or the peroxide contained
therein. Among the molded crosslinked rubber products, those
produced by the extrusion molding method are particularly
preferred.
In one method of laminating the molded crosslinked
rubber with the below-mentioned thermoplastic polymer, the
molded crosslinked rubber may be first produced in an injection
molding die which the thermoplastic polymer is then injected and
molded to provide a composite injection molded product in the
die, or alternatively, the molded crosslinked rubber may be
previously produced in a separate place, and then inserted into
the die and the thermoplastic polymer is injected in the die.
Degree of crosslinking
The crosslinked rubber has little or no flowability,
and has a melt flow rate (MFR:230 ~, ~ kg load) according to
JIS-K7210 (ASTM D1238~ of 0 to 0.01 g110 min. As it does not
have flowability, the crosslinked rubber is distinguished from
the below-mentioned thermoplastic polymer having flowability.
(2) Thermoplastic polymer
<1> Thermoplastic polymers
Appropriate examples of thermoplastic polymers as a
raw material for the thermoplastic polymer used for the
injection molding according to the present invention include
those having a melt flow rate (MFR:230 ~, 2.16 kg load)
-4-
21 7 1 575
according to ASTM D1238 of 0.01 to 1000 g/10 min, preferably0.01 to 300 g/10 minutes, more preferably 0.1 to 100 g/10 min,
and even more preferably 0.1 to 50 g/10 min from the view point
of flowability which makes the injection molding possible.
When the below-mentioned inorganic fillers are
included, it is appropriate to use a thermoplastic polymer
having a high melt flow rate.
When it is necessary that the thermoplastic polymer to
have the same level of flexibility and rubber elasticity as
those of a crosslinked rubber, a thermoplastic polymer having a
JIS-A hardness according to a JIS-K6301 lASTM D2240 (Type A)] of
98 or less is generally used, but a thermoplastic polymer having
the hardness of 95 or less is preferably used and a
thermoplastic polymer having the hardness of 90 to 20 is most
preferably used.
The above-mentioned thermoplastic polymers can be
thermoplastic resins or thermoplastic elastomers.
Examples of the thermoplastic resin include olefin-
type thermoplastic resins such as polyethylene, polypropylene,
polybutene-1, ethylene-vinyl acetate copolymer, ethylene-acrylic
acid copolymer, ethylene-methyl acrylate copolymer, ethylene-
ethyl acrylate copolymer; soft vinyl chloride polymer, styrene
type resin, polyester type resin and polyamide-type resin and
the like.
Examples of the thermoplastic elastomer include a
copolymer of 15 to 90% by weight of ethylene and 85 to 10% by
weight of an alkene having 3 or more carbon atoms, preferably a
copolymer of ethylene and an alkene having 3 to 10 carbon atoms.
Illustrative examples of the thermoplastic elastomer
include an olefin-type elastomer such as ethylene-propylene
copolymer rubber (EPM), ethylene-butene copolymer rubber (EBM),
ethylene-hexene copolymer, ethylene-octene copolymer and
ethylene-propylene-nonconjugated diene copolymer rubber (EPDM)
or ethylene-propylene-butene copolymer and the like wherein 5-
-5-
21715~5
ethylidenenorbornene, 5-methylnorbornene, 5-vinylnorbornene,
dicyclopentadiene and butene and the like are used as the third
component; styrene-type elastomer; polyester-type elastomer;
polyamide-type elastomer and the like.
These thermoplastic polymers can be used alone or in
admixtures of two or more components.
Among them, preferably used are olefin type
elastomers, and a mixture of an olefin type thermoplastic resin
and an olefin type elastomer which are mixed at the ratio of
10:90 to 90:10 are preferably used. A ratio of 20:80 to 80:20
(~ by weight) is particularly preferable used since the defining
process can be omitted.
The term resin refers to a thermoplastic polymer
having a crystallinity index by an x-ray diffraction method of
30 to 70%, and the term elastomer refers to a noncrystalline
thermoplastic polymer or a thermoplastic polymer having a
crystallinity index of less than 30%.
As the above-mentioned olefin elastomers, those having
a Mooney viscosity at 100 ~ (MLl I 4 (100 ~) ) of 10 to 400
according to JIS K6300, particularly those having a Mooney
viscosity of 15 to 350 are preferable. Elastomers having a
Mooney viscosity exceeding the above-mentioned range tend to
have an inferior appearance when they are molded, and those
having a Mooney viscosity below that range tend to exhibit an
inferior rubber elasticity.
The production methods and the forms of such olefin
elastomers are not particularly limited. They can be produced
by, for example, mixing an olefin type elastomer and an olefin
type thermoplastic resin, and heating the resulting mixture in
the presence of an organic peroxide to carry out partial or
complete crosslinking mainly by radicals. The product produced
by such a crosslinking treatment is different from the a~ove-
mentioned crosslinked rubber (1) since it has flowability.
For use as the above-mentioned olefin-type elastomers
-6-
21 71 575
are the commercially available "JSR EP" and "JSR EBM" produced
by Japan Synthetic Rubber Co., Ltd., "MITSUI EPT" and "TAFMER"
produced by Mitsui Petrochemical Industries, Ltd., "ESPRENE"
produced by Sumitomo Chemical Company, Ltd., "ENGAGE" produced
by Dow Chemical Japan ~td.
Examples of the partially or completely crosslinked
products include "Thermorun" produced by Mitsubishi Chemical
Corporation, "MILASTOMER" produced by Mitsui Petrochemical
Industries, Ltd., "Sumitomo TPE" produced by Sumitomo Chemical
Company, Ltd., and "Santoprene" produced by Advanced Elastomer
System, L.P..
<2> Other components
The above-mentioned thermoplastic polymer may be
blended with a polymer component including a resin other than
the above-mentioned resins and an elastomer, as well as other
additives including a plasticizer such as paraffin oil and
liquid polybutene; an antioxidant, light stabilizer, UV
absorbing agent, antiblocking agent such as silicone oil, a
neutralizing agent, lubricant, dispersing agent, antibacterial
agent, coloring agent, flame retardant, and fluorescent whitener
in amounts that do not remarkably detract from the effects of
the present invention.
(3) Thermoplastic polymer having a polar group
<1> Polar groups
According to the present invention, a thermoplastic
polymer described in the above-mentioned paragraph ~2) may have
a polar group such as a hydroxyl group, carboxyl group, epoxy
group, amino group, carboxylic acid anhydride group, thiol
group, and silanol group. Among these, the hydroxyl group,
carboxyl group, carboxylic acid anhydride group are preferable
from the view point of adhesion with the molded crosslinked
rubber.
<2> Process for introducing a polar group (modification of a
thermoplastic polymer)
-7-
2 1 7 1 57 5
Methods for introducing a polar group into a
thermoplastic polymer are basically divided into two groups,
i.e. (i) a method in which the above-mentioned thermoplastic
polymer is blended with a copolymer wherein a polar group has
already-been introduced (the copolymer may be the same as the
above-mentioned thermoplastic polymer) to carry out
modification, or (ii) a method in which the above-mentioned
thermoplastic polymer and a compound having a polar group are
directly subjected to graft reaction to carry out modification.
(i) Method for blending a copolymer wherein a polar group has
been introduced
(i-1) A copolymer wherein a polar group has been introduced
Preferable examples of the copolymer wherein a polar
group has already been introduced include the following
compounds.
(a) A diene polymer having a terminal hydroxyl group
or a hydrogenated product thereof.
Among the diene polymers having a terminal hydroxyl
group and the hydrogenated product thereof, an illustrative
example of the diene polymer having a terminal hydroxyl group is
polyhydroxy polybutadiene.
Illustrative examples of the diene polymer include a
polymer which has at least one terminal hydroxyl group, whose
molecular weight is in the range of 200 to 100,000, preferably
500 to 50,000, more preferably 800 to 10,000, and which is
liquid, semi-solid or solid at normal temperatures. The average
number of hydroxyl groups per molecule of such a diene polymer
is generally in the range of 1 to 10, preferably 1.5 to 5, and
those having a hydroxyl value generally in the range of 15 to
250, preferably 25 to 125 (KOHmg/g) are particularly preferable.
Illustrative examples of the diene polymer include
polyhydroxy polybutadiene.
The diene polymer having a terminal hydroxyl group can
be produced from 1,3-diene by a ~nown method such as a radical
-8-
2171575
polymerization process or an anion polymerization process. An
illustrative example of such a method is described in Japanese
Patent Laid-Open No.51-71391, wherein a compound such as a
functional hydrocarbon polymer containing an aromatic ring is
catalytically hydrogenated by using molecular hydrogen.
It can also be produced by carrying out reaction
between a monoepoxy compound, formaldehyde, acetoaldehyde,
acetone, halogenoalkylene oxide or polyepoxide and a living
polymer having an al~ali metal bonded to at least one of its
terminals, which is produced by anionic polymerization of a
conjugated diene monomer according to a known method, using an
anionic polymerization catalyst such as an al~ali metal or an
organic alkali metal compound.
As the raw material monomer for producing these
polymers, at least one kind of conjugated diene monomer is used.
Examples of the conjugated diene monomer include 1,3-butadiene,
1,3-pentadiene, isoprene, chloroprene, 2,3-dimethyl-1,3-
butadiene, 1-phenyl-1,3-butadiene and the like.
Next, a hydrogenated product of the diene polymer
having a terminal hydroxyl group can be produced by
hydrogenating the above-mentioned diene polymer having the
terminal hydroxyl group by an ordinary method such as a method
described in Japanese Patent Laid-Open No. 51-71391 .
As for the degree of hydrogenation, the double bonds
contained in a polymer may be completely or partially
hydrogenated. A polymer normally having an iodine value of
generally 0 to 20 (g/100 g) is preferable; more preferable is a
polymer having an iodine value of O to S (g/100 g).
The diene polymer having a terminal hydroxyl group and
the hydrogenated product thereof can be used alone or in
admixtures of two or more compounds.
Among the diene polymer having a terminal hydroxyl
group, and the hydrogenated product thereof, the hydrogenated
product is preferable since it gives further improved weather-
_g_
21 7 l 51 5
resistance and adhesion.
Such a hydrogenated product is available form
Mitsubishi Chemical Corpration as "Polytail H"(trade name).
(b) Copolymer of ethylene and an unsaturated compound
containing carboxyl group (including a carboxylic acid anhydride
group).
An illustrative example of the copolymer of ethylene
and an unsaturated compound containing carboxyl group (including
a carboxylic acid anhydride group) is a polymer wherein, for
example, ethylene and acrylic acid are copolymerized randomly or
at certain set intervals. Here, from the structural point of
view, this refers to all polymers having a structure wherein an
unsaturated compound containing carboxyl group, that is an
unsaturated carboxylic acid compound or its anhydride is
copolymerized randomly or regularly in a branched or linear
carbon chain.
Examples of such a copolymer include a polymer in the
form of a liquid, semi-solid or solid at normal temperatures,
wherein the content of the unsaturated carboxylic acid compound
or the anhydride thereof is 0.1 to 40% by weight, preferably 0.5
to 35 % by weight, more preferably 1 to 30% by weight, and the
melt flow rate measured according to ASTM D1238 (190 ~ and 2.16
kg load) is 0.1 to 1,000 g/10 min, preferably 0.5 to 700 g/10
min, particularly preferably 1 to 500 g/10 min.
The copolymer of ethylene and an unsaturated
carboxylic acid compound or an anhydride thereof may be produced
from ethylene and the unsaturated carboxylic acid compound or
anhydride thereof, by a known method such as a high pressure
radical polymerization process. In the case of the high pressure
radical polymerization process, ethylene, an unsaturated
carboxylic acid or an anhydride thereof and a radical reaction
initiator are continuously inserted at the ethylene-to
unsaturated carboxylic acid compound or its anhydride ratio of
10,000:1 to 100:2, into a reaction zone which is kept under such
-10-
~l 7 1 $7~
conditions as, for example, a pressure of 1,000 to 3,000 atm,
and a temperature of 90 to 300 ~. Thereby 3 to 20% of the
ethylene is changed to a copolymer and the copolymer is
continuously taken out from the reaction zone.
Examples of such an unsaturated carboxylic acid
compound and the anhydride thereof include acrylic acid,
methacrylic acid, crotonic acid, maleic acid, fumaric acid,
itaconic acid, citraconic acid, tetrahydrophthalic acid,
norbornene-5,6-dicarboxylic acid and an anhydride thereof.
They can be also used in the form of a terpolymer or a
multi component copolymer wherein in addition to ethylene and an
unsaturated carboxylic acid compound component, an unsaturated
carboxylate such as methyl acrylate, ethyl acrylate, butyl
acrylate, methyl methacrylate; a vinyl aromatic compound such as
styrene, a-methylstyrene and vinyl toluene; a nitrile compound
such as acrylonitrile and methacrylonitrile; a vinylpyridine
such as 2-vinylpyridine and 4-vinylpyridine; a vinyl ether such
as methyl vinyl ether, 2-chloroethyl vinyl ether; a vinyl halide
such as vinyl chloride and vinyl bromide; a vinyl ester such as
vinyl acetate; or acryl amide are used as a third copolymer
component.
These copolymers can be used alone or in admixture of
two or more compounds.
One of the copolymers is available form Mitsubishi
Petrochemical Co. LTD.,as "YUKALON EAA A500W".
(c) Copolymer of ethylene and an unsaturated compound
containing hydroxyl group
An illustrative example of the copolymer of ethylene
and an unsaturated compound containing hydroxyl group is a
polymer wherein, for example, ethylene and 2-
hydroxyethylmethacrylate are copolymerized randomly or at
certain set intervals. Here, from the structural point of view,
this refers to all polymers having a structure wherein an
unsaturated compound containing hydroxyl group is copolymerized
-11-
21 7 1 575
.
randomly or regularly in a branched or linear carbon chain.
Examples of such a copolymer include a polymer in theform of a liquid, semi-solid or solid at normal temperatures,
having a content of the unsaturated compound containing hydroxyl
group of 0.1 to 50 % by weight, preferably 0.5 to 45 % by
weight, more preferably 1 to 40 % by weight, and a molecular
- weight of 200 to 200,000, preferably 500 to 150,000, and more
preferably 800 to 100,000.
The copolymer of ethylene and an unsaturated compound
containing hydroxyl group may be produced from ethylene and the
unsaturated compound containing hydroxyl group, by a known
method such as a high pressure radical polymerization process.
In the case of a high pressure radical polymerization process,
ethylene, an unsaturated compound containing hydroxyl group and
a radical reaction initiator are continuously inserted at the
ethylene to unsaturated compound containing hydroxyl group ratio
of 1:0.0001 to 1:0.02, into a reaction zone which is kept under
such conditions as, for example, a pressure of 1,000 to 3,000
atm, and a temperature of 90 to 280 ~. Thereby 3 to 20% of the
ethylene is changed to a copolymer and the copolymer is
continuously taken out from the reaction zone.
Examples of such an unsaturated compound containing
hydroxyl group include 2-hydroxyethyl methacrylate, 2-
hydroxyethyl acrylate, 2-hydroxylpropyl methacrylate,
poly(ethyleneglycol)monomethacrylate and the like.
They can be also used in the form of a terpolymer or a
multi component copolymer wherein in addition to ethylene and a
hydroxyl group containing unsaturated compound component, an
unsaturated carboxylate such as methyl acrylate, ethyl acrylate,
butyl acrylate and methyl methacrylate; a vinyl aromatic
compound such as styrene, a-methylstyrene and vinyl toluene; a
nitrile compound such as acrylonitrile and methacrylonitrile; a
vinylpyridine such as 2-vinylpyridine and 4-vinylpyridine; a
vinyl ether such as methyl vinyl ether and 2-chloroethyl vinyl
-12-
21 71 575
-
ether; a vinyl halide such as vinyl chloride and vinyl bromide;
a vinyl ester such as vinyl acetate; and acryl amide or the like
are used as a third copolymer component.
These copolymers can be used alone or in admixtures of
two or more compounds.
(i-2) Amount of the polymer blended into the above-mentioned
thermoplastic polymer
The polymer wherein a polar group has already been
introduced is blended in an amount of 0.01 to 10 parts by
weight, preferably 0.05 to 9 parts by weight, more preferably
0.1 to 8 parts by weight with 100 parts by weight of the above-
mentioned thermoplastic polymer. When the amount is below the
above-mentioned range, the resulting adhesion becomes inferior
and when the amount exceeds the above-mentioned range, the
resulting molded product provides inferior mold releasing
properties.
(ii) Graft modified polymer
Modified polymers obtained by subjecting the following
various polymers and compounds containing a polar group to graft
reaction can also be used.
(ii-1) Polymers to be modified
The polymers to be modified include a main chain a
propylene-type polymer such as propylene homopolymer, propylene-
ethylene block copolymer, propylene-ethylene random copolymer;
an ethylene-type polymer such as a low density polyethylene
(branched ethylene polymer), medium density, high density
polyethylene (linear ethylene polymer), a polyolefin resin such
as a copolymer of ethylene and an unsaturated compound such as
unsaturated carboxylic acid; ethylene-propylene rubber (EPM),
ethylene-propylene-nonconjugated diene rubber (EPDM) wherein 5-
ethylidenenorbornene, 5-methylnorbornene, 5-vinylnorbornene and
dicyclopentadiene and the like are used as a nonconjugated
diene; an olefin type rubber such as ethylene-butene rubber
(EBM) and ethylene-propylene-butene copolymer rubber;
-13-
21 7 1 575
-
hydrogenated product of styrene butadiene rubber (SBR); a
compound having a hydrogenated product of styrene-conjugated
diene block copolymer such as a styrene-ethylene-butylene-
styrene copolymer (SEBS), styrene-ethylene-propylene styrene
copolymer (SEPS), a hydrogenated product of a styrene-isoprene-
butylene-styrene block copolymer and the like.
The preferable number average molecular weight of such
a polymer is generally 50,000 or less, but polymers having an
number average molecular weight of 30,000 or less, particularly
those having the number average molecular weight of 1,000 to
30,000 are preferable.
The number average molecular weight corresponds to a
value calculated from the number average molecular weight of
polystyrene or polypropylene measured by a gel permeation
chromatography method (GPC3 under the conditions shown below:
Apparatus: 150C ALC/GPC (Millipore Corp.)
Column: AD80M/S (Showa Denko K.K.)
Solvent: o-dichlorobenzene
Temperature: 140 ~
Flow rate: lml/min.
Chaged amount: 200~1
Concentration: 2 mg/ml
(0.2 wt% of 2,6-di-t-butylphenol was added
as an antioxidant and the detection of
concentration was carried out with a wave
length of 3.42 ~m by an infrared
spectrophotometer "MIRAN 1A"(FOXBORO
COMPANY))
(ii-2) Compound containing a polar group
A compound containing a polar group employed according
to the present invention is one or more compounds selected from
an unsaturated carboxylic acid or a derivative thereof, and
examples of such a compound include unsaturated carboxylic acids
such as acrylic acid, methacrylic acid, 3-butenoic acid,
-14-
`` 2171575
crotonic acid, pentenoic acid, heptenoic acid, octenoic acid,
nonenoic acid, decenoic acid, undecenoic acid, maleic acid,
itaconic acid, citraconic acid, tetrahydrophthalic acid,
norbornene-5,6-dicarboxylic acid, or a derivative thereof such
as an anhydride, ester, amide, imide and metal salt. Among
these, acrylic acid, methacrylic acid, maleic acid or an
anhydride thereof are preferable. Particularly preferable is the
use of maleic anhydride.
(ii-3) Graft reaction
Graft reaction can be carried out, speci~ically by the
use of 100 parts by weight of the above-mentioned polymer, and
0.01 to 10 parts by weight, preferably O.OS to 9 parts by
weight, more preferably 0.1 to 8 parts by weight of at least one
compound selected from either the unsaturated carboxylic acid or
the derivative thereof, together with an organic peroxide in the
presence of an appropriate catalyst or under heating and melting
conditions.
Examples of the organic peroxide to be used include
dialkyl peroxides such as di-t-butyl peroxide, dicumyl peroxide,
t-butylcumyl peroxide, 2,5-dimethyl-2,5-bis(t-
butylperoxy)hexane; diacylperoxides such as acetyl peroxide,
isobutyl peroxide, octanoyl peroxide, decanoyl peroxide, lauroyl
peroxide, benzoyl peroxide, and 2,4-dichlorobenzoyl peroxide;
peroxy esters such as t-butyloxy acetate, t-butylperoxy
butylate, t-butylperoxy-2-ethyl hexanote, t-butylperoxy
laurylate, t-butylperoxy benzoate, di-t-butylperoxy
isophthalate, 2,5-dimethyl-2,5-di(benzoylperoxy)hexane, t-
butylperoxy maleic acid, t-butylperoxy isopropyl carbonate, and
cumylperoxy octate; peroxy ketals such as 1,1-bis(t-
butylperoxy)-3,5,5-trimethylcyclohexane, 1,1-bis(t-
butylperoxy)cyclohexane, 2,2-bis(t-butylperoxy)octane, and 2,2-
bis(t-butylperoxy)butane; and hydroperoxides such as t-butyl
hydroperoxide, cumene hydroperoxide, diisopropylbenzene
hydroperoxide, 2,5-dimethyl-2,5-dihydroperoxide. Among these,
-15-
21 71 575
diacyl peroxides and peroxy esters are preferable, and
particularly preferable are diacyl peroxides.
The organic peroxide is used in an amount of 0.01 to
20 parts by weight, preferably 0.05 to 15 parts by weight, most
preferably 0.1 to 10 parts by weight for 100 parts by weight of
the polymer.
The graft modified polymer is available, for example,
from Sanyo Chemical Industries, Ltd. as "YOUMEX 1210"(trade
name).
(4) Thermoplastic polymer containing an inorganic filler
<1> Anorganic filler
Examples of an inorganic filler of the present
invention to be blended with the above-mentioned thermoplastic
polymer for the injection molding described in paragraph (2)
include talc, mica, glass fiber, whisker, carbon fiber, calcium
carbonate, titanium oxide, carbon black, glass balloon and the
like.
Among these, calcium carbonate and plate-shaped
inorganic fillers such as talc and mica are preferable from the
point of view of heat fusion properties.
Particularly preferable is talc which has an average
particle size of 1 to 20 microns, more preferably it has a
substantial length of 1 to 15 microns, and a particularly
preferable average aspect ratio is 5 to 10.
"Substantial" length of the talc means that most of
the talc particles have a length within that range.
The above-mentioned talc is produced by, for example,
grinding a raw talc ore by an impact grinder or a micro-type
grinder, followed by pulverization by a micron mill and a jet
type pulverizer and classification by a cyclone or micron
separator.
The average particle size is the value measured by a
particle-size distribution analyzer by using a laser beam
diffraction method, and as a measuring apparatus, for example,
-16-
- 2171575
the LA-500 type apparatus produced by Horiba, Ltd. is desirable
due to its high measurement precision. The diameter, length and
aspect ratio are the values measured by a microscope etc.
As a preferable calcium carbonate, that having a
specific surface area of 40,000 cm2/g or less is generally used,
that having a specific surface area of 30,000 cm2/g or less,
particularly of 5,000 to 30,000-cm2/g is more preferably used
with that having an average particle size of 0.5 to 20.0 ~m
being generally used, and that having an average particle size
of 1.0 to 2.5 microns is preferably used.
These inorganic fillers are used generally in an
amount of 1 to 40 parts by weight, preferably 3 to 3~ parts by
weight, and more preferably 5 to 30 parts by weight for 100
parts by weight of the above-mentioned thermoplastic polymer.
When the amount of the filler blended is below the
above-mentioned range, the resulting adhesive strength and
dimensional stability (molding shrinkage) tend to be poor, and
when it exceeds the above-mentioned range, the resulting
moldability tends to be inferior.
The thermoplastic polymer described in the above-
mentioned paragraph (2) can be used as a raw material for the
thermoplastic polymer containing an inorganic filler according
to the present invention. However, in order to improve adhesion
with the molded crosslinked rubber, which is an effect of the
present invention, it is preferable to replace a part or all of
the thermoplastic polymer described in the above-mentioned
paragraph (3) with a thermoplastic polymer wherein a polar group
has been introduced.
From the view point of improving adhesion, it is
preferable to replace all the thermoplastic polymers by the
thermoplastic polymer wherein a polar group has been introduced,
however, from the view point of mold releasing properties, the
amount should be in the range of 0.01 to 20% by weight,
preferably 0.05 to 10~ by weight, more preferably 0.05 to 8%,
-17-
21 71 575
even more preferably 0.1 to 7% by weight.
The inorganic filler can be incorporated into the
composition in an amount of 1 to 40 parts by weight based on 100
parts by weight of the thermoplastic polymer.
<2~ Production of a thermoplastic polymer containing an
inorganic filler
A thermoplastic polymer containing an inorganic filler
to be used according to the production of the composite
injection molded body of the present invention is produced by
mixing and blending the above-mentioned thermoplastic polymer
with an inorganic filler in the above-mentioned ratio by a
kneader currently used for mixing thermoplastic polymers such as
a Banbury mixer, kneader, single-screw extruder, and double-
screw extruder.
(5) Others
In order to further improve the adhesion of a molded
crosslinked rubber with a thermoplastic polymer, it is
preferable to coat the following primer on the portions of the
surface of the molded crosslinked rubber that are in contact
with the injected thermoplastic soft polymer, in a thickness of
0.01 to 100 ~m, preferably 0.1 to 80 ~m, by a spray, brush,
coater or printing machine.
Preferable examples of such a primer include a
hydrogenated styrene-butadiene-styrene block copolymer (SEBS)
which is graft modified by maleic anhydride, similarly modified
hydrogenated styrene-isoprene-styrene block copolymer (SEPS),
similarly modified ethylene-propylene copolymer and similarly
modified chlorinated polypropylene dissolved in a solvent such
as toluene, xylene and methyl ethyl ketone.
[II3 Production of a composite injection molded product
A composite injection molded product of the present
invention can be produced by inserting and fixing a molded
crosslinked rubber in a cavity of an injection molding die, and
injecting a molten thermoplastic polymer onto the molded
-18-
21 71 575
crosslinked rubber so that a composite resin molded product isformed wherein the injected thermoplastic polymer having a polar
group is adhered on the surface of the molded crosslinked
rubber. Also by inserting and fixing a molded crosslinked rubber
in a cavity of an injection molding die, and injecting a molten
thermoplastic polymer onto the molded crosslinked rubber, a
composite resin molded product wherein the injected
thermoplastic polymer containing an inorganic filler is adhered -
and coated on the surface of the molded crosslinked rubber can
be produced.
As for the conditions for the injection molding, the
resin temperature is generally in the range of 180 to 280 ~,
preferably 200 to 280 ~, the injection molding die temperature
is generally in the range of 30 to 80 ~, preferably 40 to 80 ~,
the injection molding pressure is generally in the range of 300
to 800 kgf/cm2, preferably 400 to 800 kgf/cm2, the holding
pressure is generally in the range of 300 to 800 kgf/cm2,
preferably 400 to 800 kgf/cm2, and the retention time is
generally in the range of 15 to 60 seconds, preferably 20 to ~0
seconds.
According to the present invention, a composite
injection molded product having a complex shape and a relatively
large size can be produced, thus the composite injection molded
product of the present invention is useful for a wind shield
gasket, or for weatherstrip of an automoble, or for various
gaskets used as building materials.
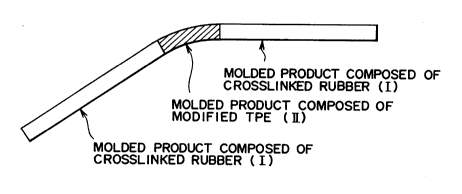
Brief Description of the Drawings
Fig.1 is a plan view of a part of an automobile
weatherstrip.
Fig.2 is a plan view of an automobile weatherstrip.
Description of the Preferred Embodiments
The method for producing the composite injection
-19-
217i575
molded product of the present invention will be illustrated by
the following Examples and Comparative Examples.
The raw materials and the evaluation method employed
in the Examples and Comparative Examples are as follows.
Example group A
(Corresponding to "containing a polar group" of the present
invention)
[I] ~aw materials
(1) Thermoplastic polymer
<1> Thermoplastic elastomer component
TPE-1:
"Thermorun 3801B" produced by Mitsubishi Chemical
Corporation (partially crosslin~ed olefin type thermoplastic
elastomer, ASTM D2240(Type A) hardness: 87)
TPE-2:
"Thermorun 2920B" produced by Mitsubishi Chemical
Corporation (uncrosslinked olefin type thermoplastic elastomer,
ASTM D2240(Type A) hardness: 96)
TPE-3:
A mixture of "Thermorun 3551B" produced by Mitsubishi
Chemical Corporation (partially crosslinked olefin type
thermoplastic elastomer, ASTM D2240(Type A): 55) and "RABALON
T3314B" produced by Mitsubishi Chemicai Corporation (styrene
type thermoplastic elastomer, ASTM D2240(Type A) hardness: 30)
blended at 7:3 ASTM D2240 (Type A) hardness: 43).
<2> Modification method
Method 1 (M1):
A Propylene-ethylene random copolymer containing 3~ by
weight of ethylene was subjected to thermal oxidation at 300 to
350 ~, and maleic anhydride groups were grafted to both ends of
the main chain as well as into the main chain of the polymer in
an amount of 10% by weight of the whole polymer using 1,1'-
azobis(cyclohexane-1-carbonitrile) which is an azo type free-
-20-
2171575
radical initiator. The maleic anhydride groups were then
neutralized with ethanol amine to give a modified propylene
polymer. The modified propylene polymer having a MFR (230 t,
2.16 kg load) of 50 g/10 min, a number average molecular weight
in terms of polypropylene of 7,000, was used.
Method 2 (M2):
Into an autoclave having a capacity of 500 ml, were
added 100 g of 1,3-butadiene, 70 g of isopropyl alcohol and 10 g
of 60~ aqueous hydrogen peroxide solution, and polymerization
was carried out in argon atmosphere at 90 ~ for 5 hours. After
the reaction was finished, unreacted monomers were removed and
the resulting butadiene polymer was dried. The hydroxyl value
of the resulting polymer was about 44.5 KOHmg/g.
Into an autoclave having a capacity of 200 ml, were
added 50 g of thus obtained butadiene polymer, 50 g of
cyclohexane and 5 g of 5% by weight ruthenium catalyst supported
on carbon and the system was purged with argon gas. Then
hydrogen gas was introduced until the pressure of the hydrogen
reached 50 kg/cm2. The system was heated to 100 ~, and reaction
was carried out for 10 hours while the hydrogen gas was supplied
so that the total pressure was kept at 50 kg/cm2. After the
reaction was finished, hydrogen was removed and the catalyst was
removed by filtration and the resulting hydrogenated product was
precipitated in methanol, filtered out and dried. The
hydrogenated butadiene polymer product thus obtained was used,
i.e., a waxy polyolefin polyol having hydroxyl groups at both
ends (with an iodine value of 1.0 g/100 g, a hydroxyl value of
44 KOHmg/g, and a number average molecular weight in terms of
polystyrene of 5,400) was used.
Method 3 (M3):
An ethylene acrylic acid copolymer having an acrylic
acid content of 20 % by weight, an MFR (190 ~, 2.16 kg load) of
300 g/10 min, and a number average molecular weight in terms of
polystyrene of 25,000, was used.
-21-
i 2171 575
Method 4 (M4):
Maleic anhydride in an amount of 40 g and benzoyl
peroxide in an amount of 40 g were dry blended with 5 kg of TPE-
1 and melted and kneaded to carry out graft modification in a
double-screw extruder which was set at the preset temperature of
160 to 200 ~, the rotation number of 300 rpm, and the discharge
rate of 10 kg/h, to give a modified TPE-1 wherein 0.6% by weight
of maleic anhydride was added.
(2) Molded crosslinked rubber
EP-1:
A sheet made of crosslinked EPDM (crosslinked by
sulfur, ASTM D2240 (Type A) hardness of 74, carbon concentration
of 40~ and crosslinking degree of 95%) was used.
Example A1
A crosslinked rubber sheet EP-1 ~thickness: 3 mm) was
cut to a width of 30 mm and a length of 100 mm to produce an
insert molded product. Then the surface of the insert molded
product to be joined with an injection molding material was
wiped with isopropyl alcohol to remove dust and dirt.
Then, the insert molded product was placed and fixed
into the movable half of the cavity of a die for forming a sheet
of 100 mm x 100 mm x 3 mm, and a thermoplastic polymer
comprising 100 parts by weight of TPE-1 and 5 parts by weight of
the modified propylene polymer obtained in the modification
method 1 (M1) was injected to produce an injection molded,
double layer test piece.
As for the conditions of the injection molding, an
inline screw type injection molding machine (a small injection
molding machine produced by Toshiba Machine Co., Ltd. : IS9OB)
was used, the resin temperature was 240 ~, the die temperature
was 40 ~, the injection pressure was 600 kgf/cm2, the retaining
pressure was 600 kgf/cm2, and the pressure retaining time was 30
seconds.
-22-
2171575
Three sample chips (75 mm x 2~ mm x 3 mm) for
evaluation were cut out from the double layer test piece and the
sample chips for evaluation were sub~ected to measurement of
adhesion strength using a tension testing machine (tension speed
500 mm/min). The average values are given in Table A1.
Examples A2-A7, Comparative Examples A1-A3
Double layer test pieces were obtained in a manner
similar to that of Example Al except that the thermoplastic
polymer laminated by injection molding was changed to the
compositions given in Table A1.
The adhesive strength between the two layers is given
in the Table.
Table Al
- Example No. Comparative
Example No.
Al A2 A3 A4 A5 A6 A7Al A2 A3
Thermoplastic
polymer
Elastomer TPE-l TPE-l TPE-l TPE-l TPEl TPE-2 TPE-3 TPE-l TPE-2 TPE-3
Amount of
elastomer(pbw) 100 100 100 100 100 100 100 100 100 100
Modification
method Ml M2 M3 M4 Ml Ml Ml
Amount of
modified
elastomer(pbw) 5 5 5 5 3 5 5
Molded product of
crosslinked rubber EP-l EP-l EP-l EP-l EP-l EP-l EP-l EP-l EP-l EP-l
Adhesion strength
~kg/cm2) 45 40 42 40 38 48 30 30 35 20
Example group B
(Corresponding to "containing an inorganic filler" of the
-23-
` 2171575
present invention)
lI] Raw materials
(1) Thermoplastic polymer
<1> Thermoplastic elastomer component
TPE-4;
50% by weight of ethylene-propylene-nonconjugated
diene rubber (EPDM, wherein 5-ethylidenenorbornene was used as a
nonconjugated diene, obtained from Japan Synthetic Rubber Co.,
Ltd. under the trade name of "EP 98"), 10% by weight of paraffin
oil (process oil "PW 380" produced by Idemitsu Kosan Co., Ltd.)
and 40% by weight of polypropylene ("BC2" produced by Mitsubishi
Chemical Corporation) were put in a Banbury mixer having a
capacity of 4 liters and kneaded for about 5 minutes at 170~ and
60 rpm, it was then made into sheeting using rollers, and the
sheeting was subjected to a sheet cutter to produce pellets.
100 parts by weight of the pellets produced, 1.0 part
by weight of l1,3-bis(tert-butylperoxyisopropyl)]benzene
obtained from KAYAKU AKZO COPPOPATION under the trade name of
"Perkadox 14/40", 0.4 parts by weight of divinyl benzene, 0.8
parts by weight of carbon black, 0.1 parts by weight of Irganox
1010 produced by Ciba-Geigy AG, and 0.1 parts by weight of Sanol
770 produced by Sanyo Company Limited were blended together and
mixed by a Henschel mixer at a normal temperature for 1 minute,
then subjected to dynamic crosslinking at 200 ~ and 210 rpm by a
double-screw extruder (PCM 45 produced by Ikegai Corporation) to
obtain a partially crosslinked olefin type thermoplastic
elastomer (ASTM D2400 (Type A) hardness: 85).
TPE-5:
40% by weight of ethylene-propylene-nonconjugated
diene rubber (EPDM, wherein 5-ethylidene norbornene was used as
a nonconjugated diene, obtained from Japan Synthetic Rubber Co.,
Ltd. under the trade name of "EP 98"), 10% by weight of paraffin
oil (process oil "PW 380" produced by Idemitsu Kosan Co., Ltd.)
and 50% by weight of polypropylene ("BC2" produced by Mitsubishi
-24-
`` 2171575
Chemical Corporation) were put in a Banbury mixer having acapacity of 4 liters and kneaded for about 5 minutes at 170 ~
and 60 rpm. Then it was made into sheeting by rollers, and the
sheeting was subjected to a sheet cutter to produce pellets.
100 parts by weight of the pellets produced, 0.8 parts
by weight of carbon black, 0.1 parts by weight of Irganox 1010,
and 0.1 parts by weight of Sanol 770 were blended together and
mixed by a Henschel mixer at room temperature for 1 minute, then
subjected to kneading at 200 ~ and 210 rpm by a double-screw
extruder (PCM 45 produced by Ikegai Corporation) to obtain a
noncrosslinked olefin type thermoplastic elastomer ASTM D2400
(Type A) hardness: 95).
TPE-6:
100 parts by weight of a mixture comprising 70% by
weight of TPE-1, 15% by weight of styrene-ethylene-butylene-
styrene copolymer (SEBS, "Kraton G1651" produced by Shell Japan
Ltd.), and 15% by weight of paraffin oil (process oil "PW 380"
produced by Idemitsu Kosan Co., Ltd.), 0.8 parts by weight of
carbon black, 0.1 parts by weight of Irganox 1010, and 0.1 parts
by weight of Sanol 770, were blended together and mixed by a
Henschel mixer at normal temperature for 1 minute, and then
subjected to kneading at 200 ~ and 210 rpm by a double-screw
extruder (PCM 45 produced by Ikegai Corporation) to obtain a
thermoplastic elastomer (ASTM D2240 (Type A) hardness: 45).
<2> Inorganic fillers
Filler 1 (F1):
Talc having a specific surface area of 38,000 cm2/g,
an average particle size of 2.8 ~m, and an average aspect ratio
of 6.
Filler 2 (F2~:
Calcium carbonate having a specific surface area of
11,000 cm2/g, and an average particle size of 3.5 ~m.
<3> Modification method
Method 5 (M5):
-25-
2 1 7 1 5 /5
A propylene-ethylene random copolymer containing 3% by
weight of ethylene, having an MFR (230 ~, 2.16 kg load) of 50
g/10 min, and a number average molecular weight in terms of
polypropylene of 7,000 was subjected to thermal oxidation, and
maleic anhydride was grafted to both ends of the main chain as
well as into the main chain of the polymer in an amount of 10
by weight of the whole polymer by using azoisobutyronitrile
which was an azo type free-radical initiator. The maleic
anhydride was then neutralized with ethanol amine to give a
modified propylene polymer. The resulting modified propylene
polymer was used.
Method 6 (M6):
A waxy polyolefin polyol having a terminal hydroxyl
group (wherein the iodine value was 1.0 g/100 g, the hydroxyl
value was 44 KOHmg/g, and the number average molecular weight in
terms of polystyrene was 5,400) was used.
Method 7 (M7):
An ethylene-acrylic acid copolymer having an acrylic
acid content of 20% by weight, a MFR (190 ~, 2.16 kg load) of
300 g/10 min, and a number average molecular weight in terms of
polystyrene of 25,000 was used.
Method 8 (M8):
Maleic anhydride in an amount of 40 g and benzoyl
peroxide in an amount of 40 g were dry-blended with 5 kg of TPE-
4 and melted and kneaded to carry out graft modification in a
double-screw extruder which was set at the preset temperature of
160 to 200 ~, a rotation of 300 rpm, and a discharge rate of 10
kg/h, to give a modified TPE-4 wherein 0.6% by weight of maleic
anhydride was added.
(2) Molded crosslinked rubber
EP-2:
A sheet made of crosslinked EPDM (crosslinked by
sulfur, ASTM D2240 (Type A) hardness of 74, a carbon
concentration of 40% and a crosslinking degree of 95% or more)
-~6-
2171575
was used.
[II] Experiment examples
Examples B1-B2
A crosslinked rubber sheet ~thickness: 3 mm) obtained
by crosslinking EPDM with sulfur was precisely cut to a width of
30 mm and a length of 100 mm to produce an insert molded
product.
The surface of the insert molded product to be joined
with an injection molding material was then wiped with isopropyl
alcohol to remove dust and dirt.
Next, the insert molded product was placed and fixed
in the movable half of a cavity of a die for forming a sheet
(internal dimensions: 100 mm x 100 mm x 3 mm) and a
thermoplastic polymer comprising 100 parts by weight of TPE-4
and 30 parts by weight of filler-1 as shown in Table B1 was
injected to produce an injection molded, double layer test piece
having a thickness of 3 mm.
As for the conditions of the injection molding, an
inline screw type injection molding machine (a small injection
molding machine produced by Toshiba Machine Co., Ltd.:IS 90 B)
was used, the resin temperature was 240 ~, the die temperature
was 40 ~, the injection pressure was 600 kgf/cm2, the retaining
pressure was 600 kgf/cm2, and the pressure retaining time was 30
seconds.
Three sample chips for evaluation [length: 75 mm,
width: 25 mm, and thickness: 3 mm, comprising an injection
molded part (length: 45 mm, width: 25 mm and thickness: 3 mm)
and a crosslinked rubber part (length: 30 mm, width: 25 mm,
thickness: 3 mm) bonded by a joint surface of 25 mm x 3 mml were
cut out of each of the double layer test pieces (length: 100 mm,
width: 100 mm, and thickness: 3 mm) and these sample chips for
evaluation were subjected to measurement of adhesion strength
and a degree of material breakdown using a tension testing
machine (tension speed 500 mm/min). The average values are
-27-
- 2 l 7 1 57 5
given in Table B1.
Examples B3
The crosslinked rubber sheet (thickness of 3 mm) used
in Example B1 was precisely cut to a width of 30 mm and a
length of 100 mm to produce an insert molded product.
The surface of the insert molded product to be joined
with an injection molding material was then wiped with isopropyl
alcohol to remove dust and dirt.
Next, the insert molded product was placed and fixed
in the movable half of a cavity of a die for forming a sheet
(100 mm x 100 mm x 3 mm) and a thermoplastic polymer comprising
100 parts by weight of TPE-4, 5 parts ~y weight of the modified
propylene polymer obtained in modification method 1 and 30 parts
by weight of filler 1, as shown in Table B1, was injected to
produce an injection molded, double layer test piece.
As for conditions of the injection molding, an inline
screw type injection molding machine (a small injection molding
machine produced by Toshiba Machine Co., Ltd.:IS 90 B) was used,
the resin temperature was 240 ~, the injection molding die
temperature was 40 ~, the injection pressure was 600 kgf/cm2,
the retaining pressure was 600 kgf/cm2, and the pressure
retaining time was 30 seconds.
Three sample chips for evaluation llength: 75 mm,
width: 25 mm, and thickness: 3 mm, comprising an injection
molded part (length: 45 mm, width: 25 mm and thickness: 3 mm)
and a crosslinked ru~ber part (length: 30 mm, width: 25 mm,
thickness: 3 mm) ~onded with a joint surface of 25 mm x 3 mm]
were cut out of one double layer test piece shown in Fig.1
(length: 100 mm, width: 100 mm, and thickness: 3 mm) and
su~jected to measurement of adhesion strength using a tension
testing machine (tension speed 500 mm/minutes). The average
values are given in Ta~le B1.
-28-
21 7 1 575
Examples B4-B12 and Comparative Examples Bl-B3
Double layer test pieces were obtained in a manner
similar to that of Example Bl or B3 except that the
thermoplastic polymer to be by injected and molded was changed
to the compositions given in Table B1.
The adhesive strength between the two layers is shown
in Table Bl.
.
Table Bl
Example No.
Bl B2 B3 B4 B5 B6 B7 B8
Thermoplastic polymer
Elastomer TPE-4 TPE-4 TPE-4 TPE-4 TPE-4 TPE-4 TPE-4 TPE-5
Amount of
elastomer(pbw) 100 100 100 100 100 100 100 100
Filler Fl F2 Fl Fl Fl Fl Fl Fl
Amount of
filler (pbw) 30 30 30 30 30 30 30 30
Modification method - - M5 M6 M7 M8 M5
Amount of modified
elastomer (pbw)
- - 5 5 . 5 5 3
Molded product of
crosslinked rubberEP-2 EP-2 EP-2 EP-2 EP-2 EP-2 EP-2 EP-2
Adhesion strength
(kg/cm2) 39 38 46 42 43 43 42 43
Degree of material
breakdown (~) 70-80 50-60 290 290 290 290 290 70-80
-29-
2171575
Table B1 (continued)
Example No. Comparative Ex. No
B9 B10 Bl 1 B12 Bl B2 B3
Thermoplastic polymer
Elastomer TPE-5 TPE-6 TPE-6 TPE-6 TPE-4 TPE-5 TPE-6
Amount of
elastomer(pbw) 100 100 100 100 100 100 100
Filler Fl Fl Fl Fl
Amount of
filler (pbw) 30 30 30 15 - _ _
Modification method M5 - M5 M5
Amount of modified
elastomer (pbw)
- 5 5
Molded product of
crosslinked rubberEP-2 EP-2 EP-2 EP-2 EP-2 EP-2 EP-2
Adhesion strength
(kg/cm2) 50 26 32 40 30 35 20
Degree of material
breakdown(X) - ~90 70-80 290 50-60 20-30 20-30 20-30
-30-