Note: Descriptions are shown in the official language in which they were submitted.
2171755
WO 95/07615 - 1 - PCT/EP94/03007
Description
LE, ~ 1~1 Tlr ~ ;G~D
~ ~ L ,r~ f~i s~N
Synergistic pesticides
Amongst the group of the 4-amino- and 4-alkoxypyrimi-
dines, compounds are known which have an insecticidal and
acaricidal activity. These compounds are described in
P 42 08 254.4. Surprisingly, it has now been found that
the combination of these pyrimidines with known insec-
ticides and acaricides results in synergistic effects.
Synergism is to be understood a~ mean;ng the mutually
enh~ncing activity of two or more substances. In the
present case, the combined use of the active substances
allows the application rates to be reduced while still
achieving the same effect, or a higher acti~ity to be
achieved with the same application rates than the
activity to be expected when the active substances are
applied indi~idually (synergistic effect).
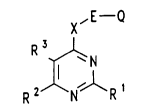
The present in~ention therefore relates to pesticides
comprising at least one compound of the formula (I) or a
salt thereof
x,E Q
Rj~N ( I )
2 ,1 R 1
in which
R1 is hydrogen or methyl,
R2 is methyl, ethyl, methoxy, ethoxy or methoxymethyl,
R3 is methyl, ethyl, methoxy, chlorine or bromine,
X is NH or oxygen,
E is a direct bond,
Q i~ a cycloalkyl group substituted in the 3- or
~ - 2 - ~l755
4-position, of the formula II
-
/~
-CH ( CH2 ) n ( I I )
in which
n is 4 or 5,
R4 is (C3-C5) -alkyl and R4 is preferably in the cis
5configuration relative to E,
-
in combination with at least one compound B selected from
the series consisting of phosphoric esters, carbamates,
carboxylic esters, formamidines, tin compounds, substan-
ces produced by microorganisms, oximes and diacylhydra-
zines.
Compounds which are of particular interest amongst the
combinations which are to be employed according to the
invention (type B compounds) are those which follow:
1. From the group of the phosphorous compounds:
acephate, azamethiphos, azinphosethyl, azinphosmethyl,
bromophos, bromophosethyl, chlorofenvinphos, chloro-
mephos, chloropyrifos, chloropyrifosmethyl, demeton,
demeton-S-methyl, demeton-S-methyl sulfone, dialifos,
diazinon, dichlorvos, dicrotophos, 0,0-1,2,2,2-tetra-
chloroethyl phosphorothioate (SD 208 304), dimethoate,
disulfoton, EPN, ethion, ethoprophos, etrimfos,
fA~r~r, fenamiphos, fenitrothion, fensulfothion,
fenthion, fonofos, formothion, heptenophos, isazophos,
isothioate, isoxathion, malathion, methacrifos,
methamidophos, methidathion, salithion, mevinphos,
monocrotophos, naled, omethoate, oxydemetonmethyl,
parathion, parathionmethyl, phenthoate, phorate,
phosalone, phosfolan, phosmet, phosphamidon, phoxim,
~ _ 3 _ 2171755
-
pirimiphosethyl, pirimiphosmethyl, profenofos, pro-
paphos, proetamphos, prothiofos, pyraclofos, pyrida-
penthion, quinalphos, sulprofos, temephos, terbufo6,
tetrachlorvinphos, thiometon, triazophos, trichloro-
phon, vamidothion;
2. From the group of the carbamates: aldicarb, 2-sec-
butylphenyl methylcarbamate (BPMC), carbaryl, car-
bofuran, carbosulfan, cloethocarb, benfuracarb,
ethiofencarb, furathiocarb, isoprocarb, methomyl,
5-methyl-m-cumenyl butyryl(methyl)carbamate, oxamyl,
pirimicarb, p,o~o~r, thiodicarb, thiofanox, ethyl
4,6,9-triaza-4-benzyl-6,10-dimethyl-8-oxa-7-oxo-5,11-
dithia-9-dodecenoate (OR 135), 1-methylthio(ethyli-
deneamino)-N-methyl-N-(morpholinothio)carbamate
(UC 51717);
3. From the group of the carboxylic esters: allethrin,
alphamethrin, 5-benzyl-3-furylmethyl (E)-(lR)cis-2,2-
dimethyl-3-(2-oxothiolan-3-ylidenemethyl)cyclopropane-
carboxylate, bioallethrin, bioallethrin ((S)-cyclo-
pentyl isomer), bioresmethrin, biphenate, (RS)-1-
cyano-1-(6-phenoYy-2-pyridyl)methyl-(lRS)-trans-3-(4-
tert-butylphenyl)-2,2-dimethylcyclopropanecarboxylate
(NCI 85193), cycloprothrin, cyhalothrin, cypermethrin,
cyphenothrin, deltamethrin, empenthrin, esfenvalerate,
fenfluthrin, fenpropathrin, fenvalerate, flucy-
thrinate, flumethrin, fluvalinate (D isomer), perme-
thrin, pheothrin ((R) isomer), d-prallethrin, pyre-
thrins (natural products), resmethrin, tefluthrin,
tetramethrin, tralomethrin;
4. From the group of the amidines: amitraz, chlor-
dimeform;
5. From the group of the tin compounds: cyhexatin,
fenbutatin oxide;
6. Other preferred components for mixtures with pyrimi-
2171~55
_ - 4 -
dines of the formula I are: abamectin, bacillus
thuringiensis, ben~ultap, binapacryl, bromopropylate,
buprofezin, camphechlor, cartap, chlorobenzilate,
chlorfluazuron, 2-(4-(chlorophenyl)-4,5-diphenyl-
thiophene (~3I-T 930), clofentezine, 2-naphthylmethyl
cyclopropanecarboxylate (Rol2-0470), cyromazine, ethyl
N-(3,5-dichloro-4-(1,1,2,3,3,3-hexafluoro-1-propy-
loxy)phenyl)carbamoyl)-2-chlorobenzocarboximidate,
DDT, dicofol, N-(N-(3,5-dichloro-4-(1,1,2,2-tetra-
fluoroethoxy)phenylamino)carbonyl)-2,6-difluoroben-
zamide (XRD 473), diflubenzuron, N-(2,3-dihydro-3-
methyl-1,3-thiazol-2-ylidene)-2,4-xylidine, dinobuton,
dinocap, endosulfan, ethofenprox, (4-ethoxyphenyl)-
(dimethyl)(3-(3-phenoxyphenyl)propyl)silane, (4-
ethoxyphenyl)(3-(4-fluoro-3-ph~noYyphenyl)propyl)di-
methylsilane, fenoxycarb, 2-fluoro-5-(4-(4-ethoxy-
phenyl)-4-methyl-1-pentyl)diphenyl ether (MTI 800),
granulosis and nuclear polyhedrosis viruses, fen-
thiocarb, flubenzimine, flucycloxuron, flef~noYnron,
gamma-HCH, hexythiazox, hydramethylnon (AC 217300),
ivermectin, 2-nitromethyl-4,5-dihydro-6H-thiazine
(SD 52618), 2-nitromethyl-3,4-dihydrothiazole
(SD 35651), 2-nitromethylene-1,2-thiazinan-3-ylcar-
bamaldehyde (WL 108477), propargite, teflubenzuron,
tetradifon, tetrasul, thiocyclam and triflumuron.
7. From the group of the oximes, the compound fenpyroxi-
mate (compound of the formula III)
C H 3
~ N~N
CH!I ~L CH~ ( I I I )
C H ~ --C --O --C ~ ~ N = C~
C H ~
8. From the group of the diacylhydrazines, the compound
- 21 717~5
_ 5 -
tebufenozide (compound of the formula IV)
IC H 3
H O CH3-C-CH3 CH3
C H 3 C ~ C --N --N --C ~ ( I V )
H H O CH3
The abovementioned active substances are described in Ch.
R. Worthing, R.Y. Hance, The Pesticide Manual, British
Crop.; 9th Ed., Protection Council (1991).
The abovementioned active substance fenpyroximate was
described by T. Ronno et al. (Proc. 1990 Brighton Crop
Prot. Conf. - Pests Dis., (The Pesticide Manual, British
Crop.; 9th Ed., Protection Council (1991)).
The abovementioned active substance tebufenozide
(RH 5992) is disclosed in the European Patent Application
EP 236 618 (Aller et al., Rohm and Haas Comp.)
The exploitation with such synergistic effects allows the
application rates of the components in the mixtures to be
reduced considerably, and it is possible to control a
broad range of pests. The reduced application rates apply
not only to the pyrimidines, but also to the components
in the mixtures with regard to their specific activity.
However, the use of mixtures which produce synergistic
effects does not only bring substantial economic
advantages, but also ecological advantages.
The compositions according to the invention are well
tolerated by plants and have a favorable toxicity to
warm-blooded species and are ~uitable for controlling
animal pests, in particular insects, arachnids and
nematodes, particularly preferably for controlling
insects and their development stages which occur in
agriculture, in forests, in the protection of stored
2171755
-- 6
products and of materials, and in the hygiene sector.
They are active against normally sensitive and resistant
species and against all or individual development stage6.
The abovementioned pests include:
From the order of the Acarina, for example, Acarus siro,
Argas spp., Ornithodoros spp., Dermanyssus gallinae,
Eriophyes ribis, Phyllocoptruta oleivora, Boophilus spp.,
Rhipicephalus spp., Amblyomma spp., Hyalomma spp., Ixodes
spp., Psoroptes spp., Chorioptes spp., Sarcoptes spp.,
Tarsonemus spp., Bryobia praetiosa, Panonychus spp.,
Tetranychus spp., Eotetranychus spp., Oligonychus spp.
and Eutetranychus spp..
From the order of the Isopoda, for example, Oniscus
asellus, Armadillidium vulgare and Porcellio scaber.
From the order of the Diplopoda, for example, Blaniulus
guttulatus.
From the order of the Chilopoda, for example, Geophilus
carpophagus and Scutigera spp..
From the order of the Symphyla, for example, Scutigerella
immaculata.
From the order of the Thysanura, for example, Lepisma
saccharina.
From the order of the Collembola, for example, Onychiurus
armatus.
From the order of the Orthoptera, for example, Blatta
orientalis and Periplaneta americana, Leucophaea maderae,
Blattella germanica, Acheta domesticus, Gryllotalpa spp.,
Locusta migratoria migratorioides, Melanoplus
differentialis and Schistocerca gregaria.
From the order of the Isoptera, for example,
2171 ~
_ - 7 -
Reticulitermes spp..
From the order of the Anoplura, for example, Phylloxera
vastatrix, Pemphigus spp., Pediculus humanus corporis,
Haematopinus spp. and LinognAthus spp..
From the order of the Mallophaga, for example,
Trichodectes spp. and Damalinea spp..
From the order of the Thysanoptera, for example, Hercino-
thrips femoralis and Thrips tabaci.
From the order of the Heteroptera, for example,
Eurygaster spp., Dysdercus intermedius, Piesma quadrata,
Cimex lectularius, Rhodnius prolixus and Triatoma spp..
From the order of the Homoptera, for example, Aleurodes
brassicae, Bemisia tabaci, Trialeurodes vaporariorum,
Aphis gossypii, Brevicoryne brassicae, Cryptomyzus ribis,
Doralis fabae, Doralis pomi, Eriosoma lanigerum,
Hyalopterus ar~n~;n;s, Macrosiphum avenae, Myzus spp.,
Phorodon humuli, Rhopalosiphum padi, Empoasca spp.,
Euscelus bilobatus, Nephotettix cincticeps, Lecanium
corni, Saissetia oleae, Laodelphax striatellus,
Nilaparvata lugens, Aonidiella aurantii, Aspidiotus
hederae, Pseudococcus spp. and Psylla spp..
From the order of the Lepidoptera, for example,
Pectinophora gossypiella, Bupalus piniarius, Cheimatobia
brumata, Lithocolletis blancardella, Hyponomeuta padella,
Plutella maculipennis, Malacosoma neustria, Euproctis
chryaorrhoea, Lymantria spp., Bucculatrix thurberiella,
Phyllocnistis citrella, Agrotis spp., Euxoa spp., Feltia
spp., Earias insulana, Heliothis spp., Laphygma exigua,
Mamestra brassicae, Panolis flammea, Prodenia litura,
Spodoptera spp., Trichoplusia ni, Carpocapsa ~ ~ella,
Pieris spp., Chilo spp., Pyrausta nubilalis, Ephestia
kuehniella, Galleria mellonella, Cacoecia podana, Capua
reticulana, Choristoneura fumiferana, Clysia ambiguella,
21 ~7 1755
_ 8 -
Homona magnan;ma and Tortrix viridana.
From the order of the Coleoptera, for example, Anobium
punctatum, Rhizopertha dominica, Bruchidius obtectus,
Acanthoscelides obtectus, Hylotrupes bajulus, Agelastica
alni, Leptinotarsa decemlineata, Phaedon cochleariae,
Diabrotica spp., Psylloides chrysocephala, Epilachna
varivestis, Atomaria spp., Oryzaephilus surinamensis,
Anthonomus spp., Sitophilus spp., Otiorrhynchus sulcatus,
Cosmopolites sordidus, Ceuthorrhynchus assimilis, Hypera
postica, Dermestes spp., ~ o~oderma spp., Anthrenus spp.,
Attagenus spp., Lyctus spp., Meligethes aeneus, Ptinus
spp., Niptus hololeucus, Gibbium psylloides, Tribolium
spp., Tenebrio molitor, Agriotes spp., Conoderus spp.,
Melolontha melolontha, Amphimallon solstitialis and
Costelytra zealandica.
From the order of the Hymenoptera, for example, Diprion
spp., Hoplocampa spp., Lasius spp., Monomorium pharaonis
and Vespa spp..
From the order of the Diptera, for example, Aedes spp.,
Anopheles spp., Culex spp., Drosophila melanogaster,
Musca spp., Fannia spp., Calliphora erythrocephala,
Lucilia spp., Chrysomyia spp., Cuterebra spp.,
Gastrophilus spp., Hypobosca spp., Stomoxys spp., Oestrus
spp., Hypoderma spp., TAhAn-~ spp., Tannia 8pp., Bibio
hortulanus, Oscinella frit, Phorbia spp., Pegomyia
hyoscyami, Ceratitis capitata, Dacus oleae and Tipula
paludosa.
From the order of the SiphonArtera, for example,
Xenopsylla cheopis and Ceratophyllus spp..
From the order of the Arachnida, for example, Scorpio
maurus and Latrodectus mactans.
From the class of the Helminthes, for example,
Haemonchus, Trichostrongulus, Ostertagia, Cooperia,
2171755
g
-
Chabertia, Strongyloides, Oesophagostomum, Hyostrongulus,
Ancylostoma, Ascaris and Heterakis, and also Fasciola and
phytopathogenic nematodes, for example those of the
genera Meloidogyne, Heterodera, Ditylenchus, Aphelencho-
ides, Radopholus, Globodera, Pratylenchus, ~ongidorus andXiphinema.
The invention also relates to insecticidal and acaricidal
compositions which contain suitable formulation auxilia-
ries in addition to active substances of types A and B.
The active substance content of the use forms prepared
from the commercially available formulations can vary
from 0.0001 up to 99% by weight, it is preferably between
2 and 95% by weight.
They are applied in a customary manner adapted to suit
the use forms.
They can be formulated in variou~ ways, dep~nA;ng on the
prevailing biological and/or chemico-physical parameters.
The following are therefore suitable possibilities of
formulations:
Wettable powders (WP), emulsifiable concentrates (EC),
a~ueous solutions (SC), emulsions, sprayable solutions,
oil- or water-based dispersions (SC), suspoemulsions
(SC), dusts (DP), seed-dressing products, granules in the
form of microgranules, spray granules, coated granules
and adsorption granules, water-dispersible granules (WG),
ULV formulations, microcapsules, waxes or baits.
These individual types of formulation~ are known in
principle and are described, for example, in Winnacker-
Ruchler, "Chemische Technologien, [Chemical Technology],
Volume 7, C. Hauser Verlag Mi;nc~en~ 4th Ed. 1986; van
Falkenberg, "Pesticides Formulations", Marcel Dekker
N.Y., 2nd Ed. 1972-73; R. Martens, "Spray Drying Hand-
bcok", 3rd Ed. 1979, G. Goodwin Ltd. T.o~Ao~.
- 2171755
- 10 -
The formulation auxiliaries required, such as inert
materials, surfactants, solvents and other additives are
also known and are described, for example, in: Watkins,
"Handbook of Insecticide Dust Diluents and Carriers", 2nd
Ed., Darland Books, Caldwell N.J.; H.v. Olphen,
"Introduction to Clay Colloid Chemistry", 2nd Ed.,
J. Wiley & Sons, N.Y.; Marschen, "Solvents Guide", 2nd
Ed., Interscience, N.Y. 1950; McCutcheon~s "Detergents
and Emulsifiers Annual", MC Publ. Corp., Ridgewood N.J.;
Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chem. Publ. Co. Inc., N.Y. 1964; Schonfeldt,
"Grenzflac~e~Aktive Athylenoxi~a~ te", [Surface-Active
Ethylene Oxide Adducts], Wiss. Verlagsgesell., Stuttgart
1976; Winnacker-Kuchler, "Chemische Technologie",
[Chemical Technology], Volume 7, C. Hauser Verlag Munich,
4th Ed. 1986.
Wettable powders are preparations which are uniformly
dispersible in water and which additionally contain,
besides the active substance, wetting agents, for example
polyoxethylated alkylphenols, polyoxethylated fatty
alcohols, alkyl- or alkylphenolsulfonates and disper-
sants, for example sodium lignosulfonate, sodium 2,2'-
dinaphthylmethane-6,6'-disulfonate, sodium dibutylnaph-
thalenesulfonate or else sodium or oleoylmethyltaurate,
in addition to a diluent or inert substance.
Emulsifiable concentrates are prepared by dissolving the
active substance in an organic solvent, for example
butanol, cyclohe~anone, dimethylformamide, xylene or else
higher-boiling aromatics or hydrocarbons, with an addi-
tion of one or more emulsifiers. Examples of emulsifierswhich can be used are: calcium alkylarylsulfonate salts
such as calcium dodecylbenzenesulfonate, or non-ionic
emulsifiers, such as fatty acid polyglycol esters,
alkylaryl polyglycol ethers, fatty alcohol polyglycol
ethers, propylene oxide/ethylene oxide condensation
products, alkyl polyethers, sorbitan fatty acid esters,
polyoxyethylene sorbitan fatty acid esters or polyoxy-
~l ~ i 7 5~
11
ethylene sorbitol esters.
Dusts are obtained by gr;n~;ng the active substance withfinely divided solid substances, for example talc,
natural clays, such as kaolin, bentonite, pyrophillite,
or diatomaceous earth. Granules can be prepared either by
spraying the active substance onto adsorptive, granulated
inert material or by applying active substance concentra-
tes to the surface of carriers, such as sand, kaolinites
or granulated inert material, by means of binders, for
example polyvinyl alcohol, sodium polyacrylate or else
mineral oils. Suitable active substances can also be
granulated in the manner customary for the preparation of
fertilizer granules, if desired in the form of a mixture
with fertilizers.
The active substance concentration in wettable powders
is, for example, approximately 10 to 90% by weight, the
remainder to 100% by weight being composed of customary
formulation components. In the case of emulsifiable
concentrates, the active substance concentration can be
approximately 5 to 80% by weight. Formulations in the
form of dusts usually contain 5 to 20% by weight of
active substance, sprayable solutions approximately 2 to
20% by weight. In the case of granules, the active
substance content partly depends on whether the active
compound is in the liquid or solid form and on which
granulation auxiliaries, fillers and the like are being
used.
In addition, the abovementioned formulations of active
substances contain, if appropriate, the a &esives,
wetting agents, dispersants, emulsifiers, penetrants,
solvents, fillers or carriers which are customary in each
case. For use, the concentrates, which are in commer-
cially available form, are, if appropriate, diluted in
the customary manner, for example using water in the case
of wettable powders, emulsifiable concentrates, disper-
sions and, in some cases, also in the case of micro-
- 12 - 21 7 i 755
-
granules. Preparations in the form of dust and granular
preparations and also sprayable solutions are convention-
ally not diluted any further with other inert substances
prior to use.
The application rate required varies with external
conditions such as, inter alia, temperature and humidity.
It can vary within wide limits, i.e. between 0.0001 and
10 kg/ha or more of active substance, but it is prefer-
ably between 0.0001 and 1 kg/ha.
The active substances according to the invention, in
their commercially available formulations and in the use
forms prepared with these formulations, can be in the
form of mixtures with other active substances, such as
insecticides, attractants, sterilants, acaricides,
nematicides, fungicides, growth-regulating substances or
herbicides.
The examples which follow are intended to illustrate the
invention:
When the biological examples were carried out, tank mixes
of the active substances were applied in the form of
suitable formulations. Compound A, which was applied in
the Examples, is particularly preferred and has the
formula I in which
Rl is hydrogen,
R2 is ethyl,
R3 is chlorine,
X is NH,
E is a direct bond and
Q is 4-tert-butylcyclohexyl in the cis configura-
tion relative to E.
~ 1 7 1 7 5 3
- 13 -
-
1. ~e of rho~rhoru8 compo~A~ a~ component~ in mi~-
tures
Test subject: Tetranychus urticae (greenhouse redspider mites)
Host plant: Phaseolus vulgaris (beans)
Application method: Plant is sprayed to beginning run
off stage
Test period: 7 days
Active substance
- concentration
Compound (ppm) % Nortality
A 2 40
Triazaphos (B1) 1 35
A + B1 2 + 1 100
Test subject: Aphis fabae (black bean aphid)
Host plant: Vicia faba (field bean)
Application method: See above
Test period: 3 days
Active substance
concentration
Compound (ppm) % Mortality
A 1 40
Triazaphos (B1) 4 0
20A + B1 1 + 4 90
A 1 40
Heptenophos (B2) 1 0
A + B2 1 + 1 80
2~7l7~5
Test subject: Agrotis segetum
Host plant: Test is carried out using synthetic
feed
Application method: The approximately 1 mm thick feed i6
sprayed with amounts correspo~; ng
to 600 l/ha
Test period: 5 days
Active substance
concentration
Compound (ppm) % Mortality
A 125 40
10Heptenophos (B2) 250 0
A + B2 125 + 250 90
2. Use of r~lo~ylic esters as components in the mi~-
tures
Test subject: Aphis fabae
Host plant: Vicia faba
Application method: see above
Test period: 3 days
Active substance
concentration
Compound (ppm) % Mortality
A 1 40
20Deltamethrin (B3) 0.1 0
A + B3 1 + 0.1 70
Test subject: Tetranychus urticae (greenhouse red
spider mites)
Host plant: Phaseolus vulgaris (beans)
Application method: Spray to beg;nning run off stage
Test period: 7 days
~ l 7 1 -/ ~5
- 15 -
-
Active substance
concentration
Compound (ppm) % Mortality
A 1 10
Deltamethrin (B3) 16 10
A + B3 1 + 16 60
5 Test subject: Agrotis segetum (common cutworm)
Host plant: Test is carried out using synthetic
feed
Application method: The approximately 1 mm thick feed is
sprayed with amounts correspon~; ng
to 600 l/ha
Test period: S days
Active substance
concentration
Compound (ppm) % Mortality
A 16 0
Deltamethrin (B3) 0.125 60
15A + B3 16 + 0.125 100
3. ~se of ~ndosulfan a~ comæonent in the sture
Te~t subject: Agrotis segetum
Host plant: Test is carried out using synthetic
feed
Application method: The approximately 1 mm thick feed is
sprayed with amounts corregpon~;ng
to 600 l/ha
Test period: 5 days
~171~5~
- 16 -
-
Active substance
concentration
Compound (ppm) % Mortality
A 63 20
Endosulfan (B4) 63 40
A + B4 63 + 63 90
4. ~se of f~ v~mate as c-vmponent in the mixture
Test subject: Tetranychus urticae (greenhouse red
spider mites)
Host plant: Phaseolus vulgaris (beans)
Application method: Spray to beginn;ng run off stage
10 Test period: 7 days
Active substance
concentration
Compound (ppm) % Mortality
A 2 35
Fenpyroximate 1 25
(B5)
A + B5 2 + 1 97
A 1 10
B5 0.5 10
A + B5 1 + O.S 50
Test subject: Agrotis segetum (L3 larvae)
20 Host plant: Test in Petra dish using synthetic
feed
Application method: The approximately 1 mm thick feed is
sprayed with amounts correspo~i ng
to 600 l/ha
25 Test period: 5 days
- 17 _ 2171755
-
Active substance
concentration
Compound (ppm) % Mortality
A 31 10
B5 31 10
A + B5 31 + 31 60
A 63 30
B5 63 20
A + B5 63 + 63 90
5. ~se of Tebufenocide a~ component in the mixture
Test subject: Diabrotica undecimpunctata (10 lar-
vae per batch)
Host plant: Test in Petra dish contA;n;n~ paperfilter; no feed
Application method: 1 ml of solution is pipetted onto
the paper filter
15 Test period: 2 days at 28C in the dark
Active substance
concentration
Compound (ppm) % Mortality
A 0.5 50
Tebufenocide (B6) 16 0
A + B6 0.5 + 16 70
A 1 80
B6 31 0
A + B6 1 + 31 100