Note: Descriptions are shown in the official language in which they were submitted.
WO 95/07908 2171 ~ ~ ~ pCT~94/00221
-1-
HETEROARYLAMINO AND HETEROARYLSULFONAMIDO SUBSTITUTED
3-BENZYLAMINOMETHYL PIPERIDINES AND RELATED COMPOUNDS
Backaround of the Invention
The present invention relates to novel heteroarylamino
and heteroarylsulfonamido substituted 3-benzylaminomethyl
piperidines substituted benzylamino nitrogen containing non
aromatic heterocycles, pharmaceutical compositions
comprising such compounds and the use of such compounds in
the treatment and prevention of inflammatory and central
nervous system disorders, as well as several other
disorders. The pharmaceutically active compounds of this
invention are substance P receptor antagonists. This
invention also relates to novel intermediates used in the
synthesis of such substance P receptor antagonists.
Substance P is a naturally occurring undecapeptide
belonging to the tachykinin family of peptides, the latter
being named because of their prompt stimulatory action on
smooth muscle tissue. More specifically, substance P is a
pharmacologically active neuropeptide that is produced in
mammals and possesses a characteristic amino acid sequence
that is illustrated by D. F. Veber et al. in U.S. Patent No.
4,680,283. The wide involvement of substance P and other
tachykinins in the pathophysiology of numerous diseases has
been amply demonstrated in the art. For instance, substance
P has been shown to be involved in the transmission of pain
or migraine (see B.E.B. Sandberg et al., Journal of
Medicinal Chemistry, 25, 1009 (1982)), as well as in central
nervous system disorders such as anxiety and schizophrenia,
in respiratory and inflammatory diseases such as asthma and
rheumatoid arthritis, respectively, in rheumatic diseases
such as fibrositis, and in gastrointestinal disorders and
diseases of the GI tract such as ulcerative colitis and
Crohn's disease, etc. (see D. Regoli in "Trends in Cluster
Headache," edited by F. Sicuteri et al., Elsevier Scientific
Publishers, Amsterdam, pp. 85-95 (1987)).
Quinuclidine, piperidine, and azanorbornane derivatives
and related compounds that exhibit activity as substance P
21~ 1g7~
- 2 -
receptor antagonists are referred to in United States Patent
No. 5,162,339, United States Patent No. 5,232,929, United
States Patent No. 5,422,354, United States Patent No.
5,373,003, United States Patent No. 5,773,450, United States
Patent No. 5,807,867, United States Patent No. 5,604,252,
United States Patent No. 5,521,220, United States Patent No.
5,716,965, United States Patent No. 5,569,662 and United
States Patent No. 5,721,255.
Summary of the Invention
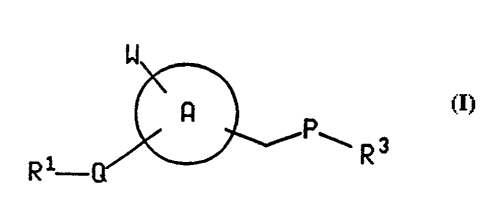
The present invention relates to compounds of the
formula
W
A I
PwR3
R1 Q
Wherein ring A is an aryl group selected from phenyl,
naphthyl, thienyl, dihydroquinolinyl and indolinyl, and
wherein the -CH2PR3 sidechain a.s attached to a carbon atom of
ring Af
P is NR2, O, S, SO or 5021
Q is 502, NH-N(C1-C6)alkyl or (C1-C6)alkyl-N-S02-
wherein the point of attachment of said (C1-C6)alkyl-N S02- to
ring A is the nitrogen atom and the point of attachment to R1
is the sulfur atoms
;..,
'_64680-872
WO 95!07908 PCT/IB94100221
2171972
-3-
W is hydrogen, (C,-C6) alkyl, S- (C,-C3) alkyl, halo or (C1-
C6)alkoxy optionally substituted with from one to three
fluorine atoms;
R' is a four to six membered heterocyclic ring
containing from one to three heteroatoms selected from
sulfur, nitrogen and oxygen (e. g., thiazolyl, pyrrolyl,
thienyl, triazolyl, oxazolyl, oxadiazolyl, thiadiazolyl or
imidazolyl), wherein said heterocyclic ring may optionally
be substituted with from one to three substituents,
preferably with from zero to two substituents, independently
selected from phenyl, (C,-C6)alkyl optionally substituted
with from one to three fluorine atoms, (C1-C6)alkoxy
optionally substituted with from one to three fluorine atoms
and halo;
R2 is hydrogen or -COZ (C,-C,o) alkyl;
R3 is selected from
25
35
WO 95/07908 PCT/IB94100221
2171972
-4-
(CH2:
R8
2
N~ R9
II III
10 R10
IV V
Rio
R15 \ 3
R10 X 2 R12
~N~
~ R13
Ri~ ~CH~~
~16
VI VII
R15 R11
(CHZ)x
3
(CH2>z
R12 3
(CH2)Y' Ot" (CH )~ 2
R13 N R19
R1~--CCH
~16
VIII IX
WO 95107908 PCT/IB94/00221
-5-
wherein R6 and R'° are independently selected from furyl,
thienyl, pyridyl, indolyl, biphenyl and phenyl, wherein said
phenyl may optionally be substituted with one or two
substituents independently selected from halo, (C1-Clo)alkyl
optionally substituted with from one to three fluorine
atoms, (C,-Clo) alkoxy optionally substituted with from one to
three fluorine atoms, carboxy, benzyloxycarbonyl and (CI-C3)
alkoxy-carbonyl;
R' is selected from (C3-C4) branched alkyl, (CS-C6)
branched alkenyl, (CS-C~) cycloalkyl, and the radicals named
in the definition of R6;
R~ is hydrogen or (C,-C6) alkyl;
R~ and R'9 are independently selected from phenyl,
biphenyl, naphthyl, pyridyl, benzhydryl, thienyl or furyl,
and R9 and R'9 may optionally be substituted with from one to
three substituents independently selected from halo, (C1-Cio)
alkyl optionally substituted with from one to three fluorine
atoms and (C,-C,°) alkoxy optionally substituted with from one
to three fluorine atoms;
Y is (CHZ), wherein 1 is an integer from one to three,
or Y is a group of the formula
(J>
Z is oxygen, sulfur, amino, (C1-C3) alkylamino or (CHZ) o
wherein n is zero, one or two;
x is zero, one or two;
y is zero, one or two;
z is three, four or five;
o is two or three,
p is zero or one;
r is one, two or three;
the ring containing (CHZ) Z may contain from zero to three
double bonds, and one of the carbon atoms of (CHZ)Z may
optionally be replaced by oxygen, sulfur or nitrogen;
WO 95/07908 PCT/IB9~/00221
2171912
-6-
R" is thienyl, biphenyl or phenyl optionally
substituted with one or two substituents independently
selected from halo, (C,-C,o) alkyl optionally substituted with
from one to three fluorine atoms and (C,-C,o) alkoxy
optionally substituted with from one to three fluorine
atoms;
X is (CHZ) q wherein q is an integer from 1 to 6, and
wherein any one of the carbon-carbon single bonds in said
(CHz)q may optionally be replaced by a carbon-carbon double
bond, and wherein any one of the carbon atoms of said (CH2)q
may optionally be substituted with R'°, and wherein any one
of the carbon atoms of said (CHz)q may optionally be
substituted with R'S;
m is an integer from 0 to 8, and any one of the
carbon-carbon single bonds of (CHz)m, wherein both carbon
atoms of such bond are bonded to each other and to another
carbon atom of the (CHZ)m chain, may optionally be replaced
by a carbon-carbon double bond or a carbon-carbon triple
bond, and any one of the carbon atoms of said (CHz)m may
optionally be substituted with RI';
R'2 is a radical selected from hydrogen, (C,-C6) straight
or branched alkyl, (C3-C~) cycloalkyl wherein one of the
carbon atoms may optionally be replaced by nitrogen, oxygen
or sulfur; aryl selected from biphenyl, phenyl, indanyl and
naphthyl; heteroaryl selected from thienyl, furyl, pyridyl,
thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, triazolyl,
tetrazolyl and quinolyl; phenyl-(Cz-C6) alkyl, benzhydryl and
benzyl, wherein the point of attachment on R'z is a carbon
atom unless R'z is hydrogen, and wherein each of said aryl
and heteroaryl groups and the phenyl moieties of said
benzyl, phenyl-(C~-C6) alkyl and benzhydryl may optionally be
substituted with one or more substituents independently
selected from halo, nitro, (C,-C,o) alkyl optionally
substituted with from one to three fluorine atoms, (C,-C,o)
alkoxy optionally substituted with from one to three
fluorine atoms, amino, hydroxy-(C,-C6)alkyl,
WO 95107908 PCT/IB94/00221
(C~-C6) alkoxy- (C,-C6) alkyl, (C,-C6) -alkylamino,
O 0
(C,-C6) alkyl-O-C-, (C1-C6) alkyl-O-C- (C,-C6) alkyl,
O O
(C,-C6) alkyl-C-O-, (C,-C6) alkyl-C-(C,-C6) alkyl-O-,
O O
(C,-C6) alkyl-C-, (C,-C6) alkyl-C-(C1-C6) alkyl-,
O
I)
di-(C,-C6)alkylamino, -CNH-(C,-C6)alkyl,
O O O
(C,-Ch) -alkyl-C-NH- (C,-C6) alkyl, -NHCH and -NHC-(C,-C6) alkyl;
and wherein one of the phenyl moieties of said benzhydryl
may optionally be replaced by naphthyl, thienyl, furyl or
pyridyl;
R'3 is hydrogen, phenyl or (C1-C6) alkyl;
or R'Z and R'3, together with the carbon to which they
are attached, form a saturated carbocyclic ring having from
3 to 7 carbon atoms wherein one of said carbon atoms that is
neither the point of attachment of the spiro ring nor
adjacent to such point of attachment may optionally be
replaced by oxygen, nitrogen or sulfur;
R'° and R'S are each ~ independently selected from
hydrogen, hydroxy, halo, amino, oxo (=O) , cyano, hydroxy-(C1-
C6) alkyl, (C,-C6) alkoxy- (C,-C6) alkyl, (C,-C6) alkylamino,
O
di-(C,-C6) alkylamino, (C,-C6) alkoxy, -C-OH,
O O
(C,-Ch) alkyl-O-C-, (C~-C6) alkyl-O-C- (C,-C6) alkyl,
O O
(C~-Ch) alkyl-C-O-, (C~-C6) alkyl-C-(C,-C6) alkyl-0-,
WO 95/07908 PCT/IB94l00221
~ 1~ I ~~2
_8_
O O
ii ii
( , 6) alkyl-C-, (C,-Cb) alkyl-C- (C,-C6) alkyl-, and the radicals
set forth in the definition of R'z;
O
R'6 is NHCR'8, NHCHzR'g, SOzR'8, COzH or one of the
radicals set forth in any of the definitions of R'z, R'4 and
R~s .
R" is oximino (=NOH) or one of the radicals set forth
in any of the def initions of R'z, R'4 and R's; and
R'R is lC,-C~l alkyl _ hvArnncn .,Y,e.,..~ .,~.. ...~.,..~..., ...
C6) alkyl;
with the proviso that (a) when m is 0, one of R'6 and R"
is absent and the other is hydrogen, (b) when R3 is a group
of the formula VIII, R" and R's cannot be attached to the
same carbon atom, (c) when R" and R's are attached to the
same carbon atom, then either each of R'4 and R's is
independently selected from hydrogen, fluoro, (C1-C6)alkyl,
hydroxy-(C,-Cb) alkyl and (C,-C6) alkoxy-(C,-C6) alkyl, or R'° and
R's, together with the carbon to which they are attached,
form a (C3-C6) saturated carbocyclic ring that forms a spiro
compound with the nitrogen-containing ring to which they are
attached; (d) when R' is amino, (C~-C6)alkylamino, di-(C~-
O
C6) alkylamino or NHC (C~-C6) alkyl, R3 is a group of the formula
II, III, IV, V or VI, and (e) when R" or R's is attached to
a carbon atom of X or (CHz)y that is adjacent to the ring
nitrogen, then R'4 or R's, respectively, must be a substituent
wherein the point of attachment is a carbon atom.
The present invention also relates to the
pharmaceutically acceptable acid addition and base salts of
compounds of the formula I. The acids which are used to
prepare the pharmaceutically acceptable acid addition salts
of the aforementioned base compounds of this invention are
those which form non-toxic acid addition salts, i.e., salts
WO 95/07908 PCT/IB94/00221
-g-
containing pharmacologically acceptable anions, such as the
hydrochloride, hydrobromide, hydroiodide, nitrate, sulfate,
bisulfate, phosphate, acid phosphate, acetate, lactate,
citrate, acid citrate, tartrate, bitartrate, succinate,
maleate, fumarate, gluconate, saccharate, benzoate,
methanesulfonate, ethanesulfonate, benzenesulfonate,
p-toluenesulfonate and pamoate [i.e., 1,1'-methylene-
bis-(2-hydroxy-3-naphthoate)]salts. The chemical bases
which are used as reagents to prepare the pharmaceutically
acceptable base salts of this invention are those which form
non-toxic base salts with the acidic compounds of formula I.
Such non-toxic base salts include those derived from such
pharmacologically acceptable cations as sodium, potassium
calcium and magnesium, etc.
The term "halo", as used herein, unless otherwise
indicated, includes chloro, fluoro, bromo and iodo.
The term "alkyl", as used herein, unless otherwise
indicated, includes saturated monovalent hydrocarbon
radicals having straight, branched or cyclic moieties or
combinations thereof.
The term "alkoxy", as used herein, includes 0-alkyl
groups wherein "alkyl" is defined as above.
The term "one or more substituents," as used herein,
includes from one to the maximum number of substituents
possible based on the number of available bonding sites.
Preferred compounds of the formula I include those
wherein the substituents at positions "2" and "3" of the
nitrogen containing ring of R3 are in a cis configuration.
When R3 is a group of the formula VII or VIII, "a cis
configuration," as used herein, means that the non-hydrogen
substituent at position "3" is cis to R~Z.
Other preferred compounds of the formula I are those
wherein Rj is a group of the formula II, III, VII or IX; RZ
is hydrogen; ring A is phenyl; W is (C,-C3)alkoxy optionally
substituted with from one to five fluorine atoms; Q is
WO 95/07908 PCT/IB94/00221
-10-
-SOZ-N- (C,-C6) alkyl and R' is 5-thiazolyl.
More preferred compounds of the formula I are the
foregoing preferred compounds wherein: (a) R3 is a group of
the formula III and R9 is benzhydryl; (b) R3 is a group of
the formula VII, R'Z is phenyl, each of R'3, R'°, R's and R'6 is
hydrogen, m is zero and X is -(CHZ)3-; or (c) R3 is a group of
the formula IX, r is two and R'9 is benzhydryl.
Other more preferred compounds of the formula I are
those wherein: (a) R3 is a group of the formula III wherein
the substituents at positions "2" and "3" of the nitrogen
containing ring are in the cis configuration, R9 is
benzhydryl and ring A is phenyl; (b) R3 is a group of the
formula VII wherein R'2 and the substituent at position "3"
of the nitrogen containing ring are in the cis
configuration, ring A is phenyl, R'2 is phenyl, each of RZ,
R'3, R'4, R'S and R'6 is hydrogen, m is zero, W is methoxy,
trifluoromethoxy or isopropoxy, X is -(CHZ)3-, Q is
CH3-N-SOz- or [ (CH3)zCH]-N-SOZ- and R' is 2,4-dimethyl-5-
thiazolyl; or (c) R3 is a group of the formula IX wherein the
substituents at positions "2" and "3" of the nitrogen
containing ring are in the cis configuration, R'9 is
benzhydryl, r is two and ring A is phenyl.
Especially preferred compounds of this invention are
those wherein R3 is a group of the formula VII, R'Z is phenyl,
each of R'3, R'4, R'S and R'6 is hydrogen, m is zero, X is
-(CH,)3-, ring A is phenyl, W is selected from OCF3, OCH3,
isopropoxy, OCHF, and OCHzCF3, Q is CH3-N-SOz- and R' is 2, 4-
dimethyl-5-thiazolyl.
Specific preferred compounds of the formula I include
the following:
2,4-dimethylthiazole-5-sulfonic acid [4-methoxy-3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]-
methylamide;
WO 95!07908 PCTlIB94/00221
21197
-11-
N-(4,5-dimethylthiazol-2-yl)-N-[4-methoxy-3-((2S,3S)-2-
phenylpiperidin-3-yl-aminomethy!)phenyl]-methanesulfonamide;
~5-[(4,5-dimethylthiazol-2-yl)methylamino]-2-
methoxybenzyl}-((2S,3S)-2-phenylpiperidin-3-yl)amine;
~5-(4,5-dimethylthiazol-2-ylamino)-2-methoxybenzyl}-
((2S,3S)-2-phenylpiperidin-3-ylamine;
4,5-dimethylthiazole-2-sulfonic acid methyl-[3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)-4-
trifluoromethoxyphenyl]-amide;
2,4-dimethylthiazole-5-sulfonic acid [4-isopropoxy-3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]-
methylamide;
2,4-dimethylthiazole-5-sulfonic acid [4-isopropoxy-3
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]
isopropylamide;
2,4-dimethylthiazole-5-sulfonic acid [4-methoxy-3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]-
isopropylamide;
2,4-dimethylthiazole-5-sulfonic acid [4-methoxy-3
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]
isobutylamide; and
2,4-dimethylthiazole-5-sulfonic acid [4-isopropoxy-3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]-
isobutylamide.
Examples of other compounds of the formula I are:
2-trifluoromethylthiazole-5-sulfonic acid {4-methoxy-
3[((2S,3S)-2-phenylpiperidin-3-ylamino)methyl]phenyl}-
methylamide;
2,4-bis-trifluoromethylthiazole-5-sulfonic acid {4-
methoxy-3-[((2S,3S)-2-phenylpiperidin-3-
ylamino)methyl]phenyl}-methylamide;
oxazole-5-sulfonic acid {4-methoxy-3-[((2S,3S)-2-
phenylpiperidin-3-ylamino)methyl]phenyl}-methylamide;
2,5-dimethylthiazole-4-sulfonic acid ~4-methoxy-3
[((2S,3S)-2-phenylpip.eridin-3-ylamino)methyl]phenyl}
methylamide;
WO 95/07908 PCT/IB94100221
2171972
-12-
4,5-dimethylthiazole-2-sulfonic acid {4-methoxy-3-
[((2S,3S)-2-phenylpiperidin-3-ylamino)methyl]phenyl}-
methylamide;
thiazole-5-sulfonic acid {4-methoxy-3-[((2S,3S)-2-
phenylpiperidin-3-ylamino)methyl]phenyl}-methylamide;
2,5-dimethylthiazole-4-sulfonic acid {4-
trifluoromethoxy-3-[((2S,3S)-2-phenylpiperidin-3-
ylamino)methyl]phenyl}-methylamide;
2,5-dimethylthiazole-4-sulfonic acid {4-isopropoxy-3-
[((2S,3S)-2-phenylpiperidin-3-ylamino)methyl]phenyl}-
methylamide;
2,5-dimethylthiazole-4-sulfonic acid{3-[(2-benzhydryl-
1-azabicyclo[2.2.2]oct-3-ylamino)methyl]-4-methoxyphenyl}-
methylamide;
2,5-dimethylthiazole-4-sulfonic acid {3-[(-2-
benzhydryl-1-azabicyclo[2.2.1]hept-3-ylamino)methyl]-4-
methoxyphenyl}-methylamide;
thiophene-2-sulfonic acid {4-methoxy-3-[((2S,3S)-2-
phenylpiperidin-3-ylamino)methyl]phenyl}-methylamide;
[1,3,4]thiadiazole-2-sulfonic acid {4-methoxy-3-
[((2S,3S)-2-phenylpiperidin-3-ylamino)methyl]phenyl}-
methylamide;
thiophene-2-sulfonic acid {4-methoxy-3-[((2S,3S)-2-
phenylpiperidin-3-ylamino)methyl]phenyl}-amide;
thiophene-2-sulfonic acid {4-isopropoxy-3-[((2S,3S)-2-
phenylpiperidin-3-ylamino)methyl]phenyl}-methylamide;
[5-(2,4-dimethylthiazole-5-sulfonyl)-2-methoxybenzyl]-
[(2S,3S)-2-phenylpiperidin-3-yl]-amine;
[5-(2,4-dimethylthiazole-5-sulfonyl)-2-
isopropoxybenzyl]-[(2S,3S)-2-phenylpiperidin-3-yl]-amine;
[5-(2,4-dimethylthiazole-5-sulfonyl)-2-
trifluoromethoxybenzyl]-[(2S,3S)-2-phenylpiperidin-3-yl]-
amine;
[2-methoxy-5-([1,2,3]thiadiazole-5-sulfonyl)benzyl]-
[(2S,3S)-2-phenylpiperidin-3-yl]-amine;
[2-methoxy-5-(pyridine-2-sulfonyl)benzyl]-[(2S,3S)-2-
phenylpiperidin-3-yl]-amine;
WO 95/07908 PCT/IB94100221
2171972
-13-
[2-methoxy-5-(pyridine-3-sulfonyl)benzyl]-[(2S,3S)-2-
phenylpiperidin-3-yl]-amine;
[2-methoxy-5-(pyrimidine-2-sulfonyl)benzyl]-[(2S,3S)-2-
phenylpiperidin-3-yl]-amine; and
[2-methoxy-5-(thiophene-2-sulfonyl)benzyl]-[(2S,3S)-2-
phenylpiperidin-3-yl]-amine.
The present invention also relates to compounds of the
formulae
0
i .~~~~ ''G
R -0
X
H
a
R ~l~
R1-a ~R3
XI
and
0
a
/H
A ~N
~R3
R 1-0
XII
wherein ring A, Q, R', R3 and W are defined as above and G is
hydrogen. These compounds may be used as intermediates in
the synthesis of compounds of the formula I.
The present invention also relates to a pharmaceutical
composition for treating or preventing a condition selected
from the group consisting of inflammatory 3iseases (e. g.,
arthritis, psoriasis, asthma and inflammatory bowel
disease), anxiety, depression or dysthymic disorders,
urinary incontinence, gastrointestinal disorders such as
WO 95/07908 PCTIIB94100221
z~~~~~2
-14-
emesis and colitis, psychosis, pain, allergies such as
eczema and rhinitis, chronic obstructive airways disease,
hypersensitivity disorders such as poison ivy, vasospastic
diseases such as angina, migraine and Reynaud's disease,
fibrosing and collagen diseases such as scleroderma and
eosinophilic fascioliasis, reflex sympathetic dystrophy such
as shoulder/hand syndrome, addiction disorders such as
alcoholism, stress related somatic disorders, peripheral
neuropathy, neuralgia, neuropathological disorders such as
Alzheimer's disease, AIDS related dementia, diabetic
neuropathy and multiple sclerosis, disorders related to
immune enhancement or suppression such as systemic lupus
erythematosus, and rheumatic diseases such as fibrositis in
a mammal, including a human, comprising an amount of a
compound of the formula I, or a pharmaceutically acceptable
salt thereof, effective in treating or preventing such
condition, and a pharmaceutically acceptable carrier.
The present invention also relates to a method of
treating or preventing a condition selected from the group
consisting of inflammatory diseases (e. g., arthritis,
psoriasis, asthma and inflammatory bowel disease), anxiety,
depression or dysthymic disorders, urinary incontinence,
gastrointestinal disorders such as emesis and colitis,
psychosis, pain, allergies such as eczema and rhinitis,
chronic obstructive airways disease, hypersensitivity
disorders such as poison ivy, vasospastic diseases such as
angina, migraine and Reynaud's disease, fibrosing and
collagen diseases such as scleroderma and eosinophilic
fascioliasis, reflex sympathetic dystrophy such as
shoulder/hand syndrome, addiction disorders such as
alcoholism, stress related somatic disorders, peripheral
neuropathy, neuralgia, neuropathological disorders such as
Alzheimer's disease, AIDS related dementia, diabetic
neuropathy and multiple sclerosis, disorders related to
immune enhancement or suppression such as systemic lupus
erythematosus, and rheumatic diseases such as fibrositis in
a mammal, including a human, comprising administering to
WO 95/07908 PCT/IB94/00221
211972
-15-
said mammal an amount of a compound of the formula I, or a
pharmaceutically acceptable salt thereof, effective in
treating or preventing such condition.
The present invention also relates to a pharmaceutical
composition for antagonizing the effects of substance P in
a mammal, including a human, comprising a substance P
antagonizing amount of a compound of the formula I, or a
pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable carrier.
The present invention also relates to a method of
antagonizing the effects of substance P in a mammal,
including a human, comprising administering to said mammal
a substance P antagonizing amount of a compound of the
formula I, or a pharmaceutically acceptable salt thereof.
The present invention also relates to a pharmaceutical
composition for treating or preventing a disorder in a
mammal, including a human, resulting from an excess of
substance P, comprising a substance P antagonizing amount of
a compound of the formula I, or a pharmaceutically
acceptable salt thereof, and a pharmaceutically acceptable
carrier.
The present invention also relates to a method of
treating or preventing a disorder in a mammal, including a
human, resulting from an excess of substance P, comprising
administering to said mammal a substance P antagonizing
amount of a compound of the formula I, or a pharmaceutically
acceptable salt thereof.
The present invention also relates to a pharmaceutical
composition for treating or preventing a condition selected
from the group consisting of inflammatory diseases (e. g.,
arthritis, psoriasis, asthma and inflammatory bowel
disease), anxiety, depression or dysthymic disorders,
urinary incontinence, gastrointestinal disorders such as
emesis and colitis, psychosis, pain, allergies such as
eczema and rhinitis, chronic obstructive airways disease,
hypersensitivity disorders such as poison ivy, vasospastic
diseases such as angina, migraine and Reynaud's disease,
WO 95/07908 PCT/IB94/00221
2 ~ ~' 1972
-16-
fibrosing and collagen diseases such as scleroderma and
eosinophilic fascioliasis, reflex sympathetic dystrophy such
as shoulder/hand syndrome, addiction disorders such as
alcoholism, stress related somatic disorders, peripheral
neuropathy, neuralgia, neuropathological disorders such as
Alzheimer's disease, AIDS related dementia, diabetic
neuropathy and multiple sclerosis, disorders related to
immune enhancement or suppression such as systemic lupus
erythematosus, and rheumatic diseases such as fibrositis in
l0 a mammal, including a human, comprising an amount of a
compound of the formula I, or a pharmaceutically acceptable
salt thereof, effective in antagonizing the effect of
substance P at its receptor site, and a pharmaceutically
acceptable carrier.
The present invention also relates to a method of
treating or preventing a condition selected from the group
consisting of inflammatory diseases (e. g., arthritis,
psoriasis, asthma and inflammatory bowel disease), anxiety,
depression or dysthymic disorders, urinary incontinence,
gastrointestinal disorders such as emesis and colitis,
psychosis, pain, allergies such as eczema and rhinitis,
chronic obstructive airways disease, hypersensitivity
disorders such as poison ivy, vasospastic diseases such as
angina, migraine and Reynaud's disease, fibrosing and
collagen diseases such as scleroderma and eosinophilic
fascioliasis, reflex sympathetic dystrophy such as
shoulder/hand syndrome, addiction disorders such as
alcoholism, stress related somatic disorders, peripheral
neuropathy, neuralgia, neuropathological disorders such as
Alzheimer's disease, AIDS related dementia, diabetic
neuropathy and multiple sclerosis, disorders related to
immune enhancement or suppression such as systemic lupus
erythematosus, and rheumatic diseases such as fibrositis in
a mammal, including a human, comprising administering to
said mammal an amount of a compound of the formula I, or a
pharmaceutically acceptable salt thereof, effective in
antagonizing the effect of substance P at its receptor site.
WO 95/07908 PCT/IB94/00221
-17-
The present invention also relates to a pharmaceutical
composition for treating or preventing a disorder in a
mammal, including a human, the treatment or prevention of
which is effected or facilitated by a decrease in substance
P mediated neu~ ransmission, comprising an amount of a
compound of the formula I, or a pharmaceutically acceptable
salt thereof, effective in antagonizing the effect of
substance P at its receptor site, and a pharmaceutically
acceptable carrier.
The present invention also relates to a method of
treating or preventing a disorder in mammal, including a
human, the treatment or prevention of which is effected or
facilitated by a decrease in substance P mediated
neurotransmission, comprising administering to said mammal
an amount of a compound of the formula I, or a
pharmaceutically acceptable salt thereof, effective in
antagonizing the effect of substance P at its receptor site.
The present invention also relates to a pharmaceutical
composition for treating or preventing a disorder in a
mammal, including a human, the treatment or prevention of
which is effected or facilitated by a decrease in substance
P mediated neurotransmission, comprising an amount of a
compound of the formula I, or a pharmaceutically acceptable
salt thereof, effective in treating or preventing such
disorder, and a pharmaceutically acceptable carrier.
The present invention also relates to a method of
treating or preventing a disorder in mammal, including a
human, the treatment or prevention of which is effected or
facilitated by a decrease in substance P mediated
neurotransmission, comprising administering to said mammal
an amount of a compound of the formula I, or a
pharmaceutically acceptable salt thereof, effective in
treating or preventing such disorder.
The compounds of the formula I have chiral centers and
therefore exist in different enantiomeric forms. This
invention relates to all optical isomers and all
WO 95107908 PCT/IB94/00221
X171972
-18-
stereoisomers of compounds of the formula I, and mixtures
thereof .
Detailed Description of the Invention
The compounds of the formula I may be prepared as
described in the following reaction schemes and discussion.
Unless otherwise indicated, ring A, P, Q, W, R1, Rz, R3, R6,
R~ ~ Rs ~ R9 ~ Rio ~ Rn ~ R~z ~ R13 ~ R~4 ~ Ris ~ R~6 ~ Rl~ ~ Rl8 ~ Rly ~ X
m, n, o, p, q, r, x, y, and z, and structural formulas I,
II, III, IV, V, VI, VII, VIII, IX, X, XI and XII in the
reaction schemes and discussion that follow are defined as
above.
WO 95!07908 PCT/IB94/00221
2~7~9~z
-19
Scheme 1
0
W
+ NH2R3
R 1- Q
0= ~=OH, CI, Br,O-alkyl
W H W 0
R \N\ 3 A NsR3
R1 Q R R1-Q
XI XII
W N ~H
A ~R3
2o R1-Q
I C R2=H >
1
~R
A N ~R 3
R 1- Q
I (R2 not - H)
SUBSTITUTE SN~ET (RULE 26)
WO 95107908 PCT/IB94/00221
2'171912
-20
Scheme 2
II
C G
1_
to
XIII X
Scheme 3
W W
~ R 0 ~ R --~ A
i , ~ i
R -0 C R -0 CH~OH Ri-0 CHO
G
XIV X
i0 = SO~, (0 = SO2,
G=cCl-C3)alkoxy> G=H)
WO 95!07908 21 l 19 ~ 2 pCT~94/00221
-21
Scheme 4
21 + R2~-R3
R1-Q
XV XVI
R3
2o P/
R1-Q
CP - oxygen or sulfur)
WO 95/07908 PCT/IB94/00221
2~ 7~ 972
-22-
z
0
E
~O
N rl X
v Z-=-~
U X
r' X
O
N
O
r~
C'~ CL
N r1
Z -_ -~ J
U X
X
O
U
N
N
Z ,.., i
Y
v c-~ c c-~
d' Q'
M ~ ~ E (''7 CL
N .~ N H
Z -_ -Q' 7 Z -2 -d' X
.. U X X v X
X
O
,n O
'~ v W
Q'
rl r1 N N
SUBSTITUTE SHEET (RULE 26)
WO 95107908 PCT/IB94/00221
-23-
10
Scheme 5
(continued)
XX
Ria
0
X
R15 2 R12 Q_R1
Ri3
(CH2)m
Ri6
C P=0 , R3=V I I >
WO 95/07908 PCT/IB94/00221
217 r 972
-24-
Scheme 1 illustrates the preparation of compounds of
the formula I wherein P is NRZ from starting materials of the
formula X wherein G is hydrogen, hydroxy, chloro, bromo or
(C1-C6) alkoxy.
Referring to scheme 1, a compound of the formula X
wherein G is hydrogen may be converted directly into the
corresponding compound of the formula I by reacting it with
a compound of the formula NHzR3 in the presence of a reducing
agent. Reducing agents that may be used include sodium
cyanoborohydride, sodium triacetoxyborohydride, sodium
borohydride, hydrogen and a metal catalyst, zinc and
hydrochloric acid, and formic acid. This reaction is
typically conducted in a reaction inert solvent at a
temperature from about 0°C to about 150°C. Suitable
reaction inert solvents include lower alcohols (e. g.,
methanol, ethanol and isopropanol), 1,2-dichloroethane,
acetic acid and tetrahydrofuran (THF). Preferably, the
solvent is acetic acid, the temperature is about 25°C, the
reducing agent is sodium triacetoxyborohydride, and the
reaction is conducted in the presence of a dehydrating agent
such as molecular sieves.
Alternatively, the reaction of a compound of the
formula X with a compound of the formula NHzR3 may be carried
out in the presence of a dehydrating agent or using an
apparatus designed to remove azeotropically the water
generated, to produce an imine of the formula
a
~R3
R1_0
XI
which is then reacted with a reducing agent as described
above, preferably with sodium triacetoxyborohydride in an
acetic acid or 1,2-dichloroethane solvent at about room
temperature. The preparation of the imine is generally
WO 95/07908 PCT/IB94/00221
-25-
carried out in a reaction inert solvent such as benzene,
xylene or toluene, preferably toluene, at a temperature from
about 2 5 ° C to about 110 ° C, preferably at about the ref lux
temperature of the solvent. Suitable dehydrating
agents/solvent systems include titanium
tetrachloride/dichloromethane titanium
isopropoxide/dichloromethane and molecular sieves/THF.
Titanium tetrachloride/dichloromethane is preferred.
Compounds of the formula X wherein G is hydroxy,
chloro, bromo or (C,-C6)alkoxy may be converted into the
corresponding compounds of formula XII having the desired R3
group by reacting them with the appropriate compound of the
formula NHZR3 under conditions that will be obvious to those
skilled in the art, and then reducing the resulting amides
to yield the desired compounds having formula I wherein RZ is
hydrogen. When G is hydroxy, the compound of formula X is
reacted with NHzR3 in the presence of an activating agent.
Appropriate activating agents include carbonyldiimidazole,
diethylphosphoryl cyanide and dicyclohexylcarbodiimide.
Carbonyldiimidazole is preferred. This reaction is
generally conducted at a temperature from about 0°C to about
50°C, preferably at about 25°C, in an inert solvent such as
chloroform, diethyl ether, THF or dimethylformamide (DMF).
When G is chloro or bromo, the reaction of the compound
of formula X with the appropriate compound of formula NHZR3
is typically carried out in the presence of an acid
scavenger in an aprotic solvent at a temperature from about
0°C to about 100°C. Suitable acid scavengers include
triethylamine (TEA), pyridine and inorganic salts such as
sodium and potassium carbonate. Suitable solvents include
methylene chloride (CHZCIz) , chloroform (CHC13) , benzene,
toluene and tetrahydrofuran (THF). Preferably, the reaction
is conducted in CHzCl2 at room temperature using TEA as the
acid scavenger.
When G is O-(C,-C6)alkyl, the reaction of the compound
of formula NHZR3 is usually conducted in an aprotic solvent
such as benzene, toluene, chlorobenzene or xylenes, at a
x,17 1972
- 26 -
temperature from about 25°C to about 100°C, preferably at
about the reflux temperature of the solvent.
Reduction of the compound of formula XII so formed
yields the corresponding compound of the formula I wherein R2
is hydrogen. This is generally accomplished using a reducing
agent such as lithium aluminum hydride, borane dimethylsulfide
complex or diborane, in an aprotic solvent such as THF,
dioxane or diethyl ether, at a temperature from about 0°C to
about 70°C. Preferably, the reducing agent is borane
dimethylsulfide complex and the reaction is carried out at
about room temperature in an ethereal solvent such as THF.
Compounds of the formula I wherein R2 is hydrogen
may be converted into the corresponding compounds wherein R2
is -C02(C1-C10)alkyl by reacting them With a (C1-C10)alkyl
halo carbonate such as methyl or ethyl chloroformate in the
presence of an acid scavenger. Typically, this reaction is
conducted in a polar solvent such as chloroform, methylene
chloride, water or a water/acetone mixture, at a temperature
from about 0°C to about 100°C, preferably at about room
temperature. Suitable acid scavengers include triethylamine,
pyridine and potassium and sodium carbonate or bicarbonate.
When R3 is a group of the formula II, the starting
materials of the formula NH2R3 may be prepared as described in
United States Patent No. 5,162,339.
When R3 is a group of the formula III, the starting
materials of the formula NH2R3 may be prepared as described in
United States Patent No. 5,451,586 and United States Patent
No. 5,422,354.
64680-872
217 X972
- 27 -
When R3 is a group of the formula IV, V or VI, trhe
starting materials of the formula NH2R3 may be prepared as
described in United States Patent No. 5,422,354.
When R3 is'a group of the formula VII, the starting
materials of the formula NH2R3 may be prepared as described in
United States Patent No. 5,232,929, United States Patent No.
5,364,973 and United States Patent No. 5,686,615.
When R3 is a group of the formula VIII, the starting
materials of the formula NH2R3 may be prepared as described in
United States Patent No. 5,364,973 and United States Patent
No. 5,686,615.
When R3 is a group of the formula I8, the starting
materials of the formula NH2R3 may be prepared as descra,bed in
International Application No. PCT/US92/04697 filed on June 11,
1992 and published as International Publication No. WO 93/00330
on January 7, 1993.
Scheme 2 illustrates the preparation of the starting
materials of formula X wherein G is hydrogen and Q is other
than 502. Once formed, these compounds can be converted into
the corresponding compounds of the formula I or RI according
to the procedures described above.
Referring to scheme 2, a compound of the formula
gIII wherein Q is other than S02 is reacted with titanium
tetrachloride (TiCl4) and dichloromethyl methyl ether (CHC12-
O-CH3) at a temperature from about 0°C to about room
temperature in a methylene chloride solvent to yield the
corresponding aldehyde of formula g wherein G is hydrogen.
...
64680-872
,.
- 27a -
Alternatively, the compound of the formula XIII may be reacted
with hexamethylene tetramine and trifluoroacetic acid at about
70°C to yield the same product.
64680-872
WO 95107908 PCT/IB94/00221
-28-
Those compounds of the formula X wherein Q is SOZ may be
obtained from their deoxygenated counterparts of the formula
X wherein Q is -S- by reacting them with an oxidizing agent.
For example, such oxidation may be carried out using
metachloroperbenzoic acid in methylene chloride at about
room temperature. It may also be carried out using
peroxyphthalic acid magnesium hydrate in aqueous ethanol at
a temperature from about 70°C to about 100°C. The foregoing
oxidation reactions can produce mixtures of the oxy and
dioxy products (-SO- and -SOz-) which can be separated by
ordinary means.
Scheme 3 illustrates an alternate preparation of the
starting materials of the formula X wherein G is hydrogen
and Q is SO2. Referring to scheme 3, a compound of formula
X wherein Q is SOZ and G is (C,-C3) alkoxy is reacted with a
reducing agent in a reaction inert solvent, for example
lithium borohydride (LiBH4) in tetrahydrofuran (THF). The
reduction, which yields an alcohol of the formula XIV, is
usually conducted at a temperature from about 0°C to about
100°C, preferably by heating the reaction mixture to the
reflux temperature of the solvent. The alcohol of formula
XIV may then be oxidized using methods known to those
skilled in the art. For example, treatment of a solution of
such alcohol in a solvent such as methylene chloride with an
oxidizing agent such as pyridinium dichromate at a
temperature from about 0°C to about 50°C, preferably at room
temperature, will yield the corresponding compounds of
formula X wherein G is hydrogen and Q is SO2. Other
oxidizing agents/solvent systems such as manganese
dioxide/acetone and chromium trioxide/acetic
anhydride/acetic acid are also capable of producing this
conversion.
Compounds of the formula I wherein P is O or S may be
prepared as described below and illustrated in Scheme 4.
Referring to Scheme 4, a compound of the formula XV is
reacted with a compound of the formula RZ°-R3 in the presence
of a base to form the corresponding compound of formula I.
. WO 95/07908 ' ~ PGT/IB94/00221
~1 ~ 1 97 2
-29-
One of R2° and RZ' is PH, wherein P is O or S, and the other
is a suitable leaving group such as chlorine, bromine,
iodine, mesylate or tosylate. This reaction is generally
conducted in a reaction inert solvent, such as an ether
(e. g., diethyl ether, tetrahydrofuran, dimethoxyethane,
dioxane), dialkyl amide (e.g., dimethylformamide or
dimethylacetamide) or dimethylsulfoxide, at a temperature
range from about -5°C to about 100°C. The reaction may be
performed at from one to about three atmospheres of
pressure, although it is normally done at atmospheric
pressure. Suitable bases include alkali metal amides or
hydrides, such as sodium amide, potassium
bis(trimethylsilyl)amide or potassium hydride, or alkali
metal alkoxides such as sodium methoxide. Preferably, the
reaction is carried out in dimethoxyethane in the presence
of potassium bis(trimethylsilyl)amide at about 25°C.
Compounds of the formula XV wherein R21 is a leaving
group may be prepared using procedures familiar to those .
skilled in the art. For example, such a compound wherein R2i
is mesylate may be prepared by reacting a compound of the
formula XIV, as depicted in Scheme 3, with methanesulfonyl
chloride in methylene chloride in the presence of
triethylamine at about 0°C.
Compounds of the formula Rzo_P3 wherein RZ° is OH may be
prepared from the corresponding ketones via reduction, using
any of a variety of reducing agents such as sodium
borohydride in methanol or lithium aluminum hydride in a
suitable . inert solvent such as diethyl ether or
tetrahydrofuran. The corresponding cis and trans isomeric
alcohols may be prepared using selective reducing agents to
obtain the desired isomer, or by selective oxidation of the
racemic alcohol followed by isolation of the desired isomer.
Such procedures are described in European Patent Application
EP 0 499 313 A1, which was published on August 19, 1992.
,..d:~..i~»:,.~ao
~,,__
-W O 95/07908 ~ ~ ~ PGT/IB94/00221 ' - .
_- 21 7 ~ 9 7 2
-30-
The ketone intermediates used in the foregoing process
may be prepared by methods known in the art from
commercially available starting materials or by minor
variations of such methods that will be obvious to those
skilled in the art. Such ketone intermediates wherein R3 is
a group of the formula IV, V or VI may be prepared as
described in World Patent Application WO 92/01688, which was
published on February 6, 1992.
Such
ketone intermediates wherein R3 is a group of the formula II,
III or IX may be prepared as described in United States
Patent 5,162,339, which issued on November 10, 1992.
Compounds of the formula RZ°-R3 wherein RZ° is OH may also
be prepared from the corresponding compounds of the formula
O
R3-C-O-CH3. (The latter compounds may be prepared, for
example, when R3 is a group of the formula VII or VIII, as
described by Desai et al. in J. Med. Chem. , 35, 4911-4913
(1992) and in United States Patent 5,232,929, which issued
on August 3, 1993) .
First, the ring nitrogen of the compound_.of formula
O
R3-C-O-CH3 is blocked with a suitable protecting group, e.g.,
carbonylbenzyloxy (CBZ), as described in U.S. Patent
5,232,929. The resulting compound is then hydrolysed using
10% potassium hydroxide or sodium hydroxide in
methanol/water, or 1M lithium hydroperoxide in
tetrahydrofuran (THF)/water, at a temperature from about
room temperature to about 100°C.
The above reaction produces a carboxylic acid of the
formula R3COOH. The acid is then heated in benzene at about
~",~., ..
W O 95/07908 PCTIIB94/00221
2171972
-31-
the reflux temperature in the presence of lead tetraacetate
and cupric acetate, to produce a compound of the formula
O
R3-O-C-CH3. Variations of this reaction, which involves the
conversion of a carboxylic acid to an acetate, are described
in Corey et al. , J. Amer. Chem. Soc. , 85, 165-169 (1963) .
Hydrolysis of the resulting compound of the formula
O
R3-O-C-CHI according to the procedure described above for the
O
hydrolysis of compounds of the formula R3-CI-O-CH3 yields the
desired intermediate of the formula RZ°-R3 wherein R2~' is
hydroxy.
The protecting group (e.g., CBZ) can be removed at a
later stage in the synthesis using methods described in the
literature, e.g., hydrogenation in the presence of a
palladium on carbon catalyst in ethanol or ethyl acetate at
a pressure of from about one to three atmospheres and a
temperature from about 25°C to about 50°C.
The foregoing method for preparing compounds of the
formula Rz°-R3 is preferred for such compounds wherein R3 is
a group of the formula VII or VIII.
Compounds of the formula RZ°-R3 wherein RZ° is SH may be
prepared from the corresponding compounds wherein Rz° is OH
by treating the latter with phosphorus pentasulfide or
Lawesson's reagent[2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4
diphosphetane-2,4-disulfide] in a suitable inert solvent
such as pyridine, at a temperature between about room
temperature and the 120°C.
Alternatively, alcohols of the formula HO-R3 may be
converted to the corresponding thiol esters, which may be
subsequently converted to thiols of the formula R3SH
according to the procedure of R. P. Volante, Tetrahedron
Letters, 22 (33), 3119-3122 (1981).
WO 95!07908 PCT/IB94/00221
~~r19~7~
-32-
Compounds of the formula I wherein R3 is a group of the
formula VII or VIII and P is oxygen may also be prepared by
the procedure depicted in scheme 5 and described below.
Referring to scheme 5, a compound of the formula XVII,
wherein X' is defined as X in formula I except that it has
one less carbon atom in the (CHZ)q chain, is converted into
the corresponding 3-oxo compound of formula XVIII. This can
be accomplished, as exemplified in Example il, by reacting
the compound of formula XVIII with an alkali metal alkoxide
such as potassium tert-butoxide in an inert solvent such as
dichloromethane/methanol at about room temperature, cooling
the reaction mixture to about -78°C, treating the mixture
with ozone for approximately one hour, and then bubbling
nitrogen gas through the mixture to remove the excess ozone.
Reduction of the resulting compound of formula XVIII
with sodium borohydride in methanol yields the corresponding
hydroxy derivative of formula XIX. Appropriate reducing
agents include potassium borohydride and sodium borohydride.
The reaction is usually carried out in a methanol or ethanol
solvent at a temperature from about -5°C to about 100°C,
preferably at about 25°C. Alternatively, the ketone of
formula XVIII can be converted into the corresponding
alcohol of formula XIX using an aluminum alkoxide,
preferably aluminum isopropoxide, in an alcohol solvent,
preferably isopropanol, at a temperature from about 20°C to
about 125°C, preferably at the boiling point of the solvent.
Compounds of the formula XX are then formed by reacting
the corresponding compounds of the formula XIX with a
compound of the formula XV, as depicted in scheme 4 and
wherein RZ' is a suitable leaving group such as chlorine,
bromine, iodine, mesylate or tosylate. The reaction is
carried out as described above for preparing compounds of
the formula I wherein P is oxygen or sulfur from compounds
of the formula XV.
Reduction of the oxo group of the resulting compounds
of formula XX yields the corresponding compounds of formula
I wherein R3 is a group of the formula VII or VIII. Examples
WO 95/07908 PCT/IB94100221
-33-
of suitable reducing agents are lithium aluminum hydride,
borane dimethylsulfide in THF, borane in THF and sodium
borohydride-titanium (IV) chloride. Best results are
obtained using borane dimethylsulfide in THF. The reaction
may be carried out at temperatures from about room
temperature to about 150°C, and is preferably carried out at
the reflux temperature of the solvent.
Compounds of the formula I where P is SO or SOZ may be
prepared from the compounds of formula I where P is S by
methods well known to those skilled in the art using
oxidizing reagents such as metachloroperbenzoic acid or
potassium peroxymonosulfate, as described in the literature.
The preparation of other compounds of the formula I not
specifically described in the foregoing experimental section
can be accomplished using combinations of the reactions
described above that will be apparent to those skilled in
the art.
In each of the reactions discussed or illustrated in
schemes 1 to 5 above, pressure is not critical unless
otherwise indicated. Pressures from about 0.5 atmospheres
to about 5 atmospheres are generally acceptable, and ambient
pressure, i.e. about 1 atmosphere, is preferred as a matter
of convenience.
The novel compounds of the formula I and the
pharmaceutically acceptable salts thereof are useful as
substance P antagonists, i.e., they possess the ability to
antagonize the effects of substance P at its receptor site
in mammals, and therefore they are able to function as
therapeutic agents in the treatment of the aforementioned
disorders and diseases in an afflicted mammal.
The compounds of the formula I which are basic in
nature are capable of forming a wide variety of different
salts with various inorganic and organic acids. Although
such salts must be pharmaceutically acceptable for
administration to animals, it is often desirable in practice
to initially isolate a compound of the Formula I from the
reaction mixture as a pharmaceutically unacceptable salt and
WO 95107908 PCT/IB94/00221
217972
-34-
then simply convert the latter back to the free base
compound by treatment with an alkaline reagent and
subsequently convert the latter free base to a
pharmaceutically acceptable acid addition salt. The acid
addition salts of the base compounds of this invention are
readily prepared by treating the base compound with a
substantially equivalent amount of the chosen mineral or
organic acid in an aqueous solvent medium or in a suitable
organic solvent, such as methanol or ethanol. Upon careful
evaporation of the solvent, the desired solid salt is
readily obtained.
Those compounds of the formula I which are also acidic
in nature, e.g. , where R6 or R'° is carboxyphenyl, are capable
of forming base salts with various pharmacologically
acceptable cations. Examples of such salts include the
alkali metal or alkaline-earth metal salts and particularly,
the sodium and potassium salts. These salts are all
prepared by conventional techniques. The chemical bases
which are used as reagents to prepare the pharmaceutically
acceptable base salts of this invention are those which form
non-toxic base salts with the acidic compounds of formula I.
Such non-toxic base salts include those derived from such
pharmacologically acceptable cations as sodium, potassium,
calcium and magnesium, etc. These salts can easily be
prepared by treating the corresponding acidic compounds with
an aqueous solution containing the desired pharmacologically
acceptable cations, and then evaporating the resulting
solution to dryness, preferably under reduced pressure.
Alternatively, they may also be prepared by mixing lower
alkanolic solutions of the acidic compounds and the desired
alkali metal alkoxide together, and then evaporating the
resulting solution to dryness in the same manner as before.
In either case, stoichiometric quantities of reagents are
preferably employed in order to ensure completeness of
reaction and maximum yields of the desired final product.
The compounds of formula I and their pharmaceutically
acceptable salts exhibit substance P receptor-binding
WO 95107908 PCT/IB94100221
2111972
-35-
activity and therefore are of value in the treatment and
prevention of a wide variety of clinical conditions the
treatment or prevention of which are effected or facilitated
by a decrease in substance P mediated neurotransmission.
Such conditions include inflammatory diseases (e. g.,
arthritis, psoriasis, asthma and inflammatory bowel
disease), anxiety, depression or dysthymic disorders,
urinary incontinence, gastrointestinal disorders such as
emesis and colitis, psychosis, pain, allergies such as
l0 eczema and rhinitis, chronic obstructive airways disease,
hypersensitivity disorders such as poison ivy, vasospastic
diseases such as angina, migraine and Reynaud's disease,
fibrosing and collagen diseases such as scleroderma and
eosinophilic fascioliasis, reflex sympathetic dystrophy such
as shoulder/hand syndrome, addiction disorders such as
alcoholism, stress related somatic disorder , peripheral
neuropathy, neuralgia, neuropat~-: ogical disorders such as
Alzheimer's disease, AIDS re_ated dementia, diabetic
neuropathy and multiple sclerosis, disorders related to
immune enhancement or suppression such as systemic lupus
erythematosus, and rheumatic diseases such as fibrositis.
Hence, these compounds are readily adapted to therapeutic
use as substance P antagonists for the control and/or
treatment of any of the aforesaid clinical conditions in
mammals, including humans.
The compounds of the formula I and the pharmaceutically
acceptable salts thereof can be administered via either the
oral, parenteral or topical routes. In general, these
compounds are most desirably administered in dosages ranging
from about 5.0 mg up to about 1500 mg per day, although
variations will necessarily occur depending upon the weight
and condition of the subject being treated and the
particular route of administration chosen. However, a
dosage level that is in the range of about 0.07 mg to about
21 mg per kg of body weight per day is most desirably
employed. Variations may nevertheless occur depending upon
the species of animal being treated and its individual
WO 95/07908 PCT/IB94/00221
-36-
response to said medicament, as well as on the type of
pharmaceutical formulation chosen and the time period and
interval at which such administration is carried out. In
some instances, dosage levels below the lower limit of the
aforesaid range may be more than adequate, while in other
cases still larger doses may be employed without causing any
harmful side effect, provided that such larger doses are
first divided into several small doses for administration
throughout the day.
The compounds of the formula I and their
pharmaceutically acceptable salts ("the therapeutic
compounds") may be administered alone or in combination with
pharmaceutically acceptable carriers or diluents by either
of the three routes previously indicated, and such
administration may be carried out in single or multiple
doses. More particularly, the novel therapeutic agents of
this invention can be administered in a wide variety of
different dosage forms, i.e., they may be combined with
various pharmaceutically acceptable inert carriers in the
form of tablets, capsules, lozenges, troches, hard candies,
powders, sprays, creams, salves, suppositories, jellies,
gels, pastes, lotions, ointments, aqueous suspensions,
injectable solutions, elixirs, syrups, and the like. Such
carriers include solid diluents or fillers, sterile aqueous
media and various non-toxic organic solvents, etc.
Moreover, oral pharmaceutical compositions can be suitably
sweetened and/or flavored. In general, the
therapeutically-effective compounds of this invention are
present in such dosage forms at concentration levels ranging
from about 5.0% to about 70% by weight.
For oral administration, tablets containing various
excipients such as microcrystalline cellulose, sodium
citrate, calcium carbonate, dicalcium phosphate and glycine
may be employed along with various disintegrants such as
starch (and preferably corn, potato or tapioca starch),
alginic acid and certain complex silicates, together with
granulation binders like polyvinylpyrrolidone, sucrose,
WO 95/07908 PCT/IB94/00221
2~~1912
-37-
gelatin and acacia. Additionally, lubricating agents such
as magnesium stearate, sodium lauryl sulfate and talc are
often very useful for tabletting purposes. Solid
compositions of a similar type may also be employed as
fillers in gelatin capsules; preferred materials in this
connection also include lactose or milk sugar as well as
high molecular weight polyethylene glycols. When aqueous
suspensions and/or elixirs are desired for oral
administration, the active ingredient may be combined with
various sweetening or flavoring agents, coloring matter or
dyes, and, if so desired, emulsifying and/or suspending
agents as well, together with such diluents as water,
ethanol, propylene glycol, glycerin and various like
combinations thereof.
For parenteral administration, sc tions of a
therapeutic compound of the present inve:..ion in either
sesame or peanut oil or in aqueous propylene glycol may be
employed. The aqueous solutions should be suitably buffered
if necessary and the liquid diluent first rendered isotonic.
These aqueous solutions are suitable for intravenous
injection purposes. The oily solutions are suitable for
intraarticular, intramuscular and subcutaneous injection
purposes. The preparation of all these solutions under
sterile conditions is readily accomplished by standard
pharmaceutical techniques well known to those skilled in the
art.
Additionally, it is also possible to administer the
compounds of the present invention topically when treating
inflammatory conditions of the skin and this may preferably
be done by way of creams, jellies, gels, pastes, ointments
and the like, in accordance with standard pharmaceutical
practice.
The activity of the therapeutic compounds of the
present invention as substance P receptor antagonists may be
determined by their ability to inhibit the binding of
substance P at its receptor sites in bovine caudate tissue,
employing radioactive ligands to visualize the tachykinin
WO 95/07908 PCTlIB94100221
~1~1972
-38-
receptors by means of autoradiography. The substance P
antagonizing activity of the herein described compounds may
be evaluated by using the standard assay procedure described
by M. A. Cascieri et al., as reported in the Journal of
Bioloaical Chemistry, Vol. 258, p. 5158 (1983). This method
essentially involves determining the concentration of the
individual compound required to reduce by 50% the amount of
radiolabelled substance P ligands at their receptor sites in
said isolated cow tissues, thereby affording characteristic
ICSO values for each compound tested.
In this procedure, bovine caudate tissue is removed
from a -70°C freezer and homogenized in 50 volumes (w./v.)
of an ice-cold 50 mM Tris (i.e., trimethamine which is
2-amino-2-hydroxymethyl-1,3-propanediol) hydrochloride
buffer having a pH of 7.7. The homogenate is centrifuged at
30,000 x G for a period of 20 minutes. The pellet is
resuspended in 50 volumes of Tris buffer, rehomogenized and
then recentrifuged at 30,000 x G for another twenty- minute
period. The pellet is then resuspended in 40 volumes of
ice-cold 50 mM Tris buffer (pH 7.7) containing 2 mM of
calcium chloride, 2 mM of magnesium chloride, 4 ug/ml of
bacitracin, 4~,g/ml of leupeptin, 2~g of chymostatin and 200
g/ml of bovine serum albumin. This step completes the
production of the tissue preparation.
The radioligand binding procedure is then carried out
in the following manner, viz., by initiating the reaction
via the addition of 100 ~,1 of the test compound made up to
a concentration of 1 ~,M, followed by the addition of
100 ~,1 of radioactive ligand made up to a final
concentration 0.5 mM and then finally by the addition of 800
~,1 of the tissue preparation produced as described above.
The final volume is thus 1.0 ml, and the reaction mixture is
next vortexed and incubated at room temperature (ca. 20°C)
for a period of 20 minutes. The tubes are then filtered
using a cell harvester, and the glass fiber filters (Whatman
GF/B) are washed four times with 50 mM of Tris buffer (pH
7.7), with the filters having previously been presoaked for
WO 95/07908 ~ PCT/IB94~002?'
2171972
-39-
a period of two hours prior to the filtering procedure.
Radioactivity is then determined in a Beta counter at 53%
counting efficiency, and the ICS values are calculated by
using standard statistical methods.
The ability of the therapeutic compounds of this
invention to inhibit substance P induced effects in vivo may
be determined by the following procedures "a" through "d".
(Procedures "a'! through "c" are described in Nagahisa et
al., European Journal of Pharmacoloav, 217, 191-5 (1992).
a. Plasma extravasation in the skin
Plasma extravasation is induced by intradermal
administration of substance P (50 ~C1, 0.01% BSA-saline
solution) in dorsal skin of pentobarbital (25 mg/kg i.p.)
anesthetized male Hartley guinea pigs weighing 450-500 g.
The compound to be tested is dissolved in 0.1% methyl
cellulose-water (MC) and dosed p.o. 1 hour before substance
P challenge (3 pmol/site). Evans blue dye (30 mg/kg) is
administered intravenously 5 minutes before challenge.
After 10 minutes, the animals are sacrificed, the dorsal
skin is removed, and the blue spots are punched out using a
cork borer (11.5 mm oral dose (o.d.)). Tissue dye content
is quantitated after overnight formamide extraction at 600
nm absorbance.
b. Capsaicin-induced plasma extravasation
Plasma extravasation is induced by intraperitoneal
injection of capsaicin (10 ml of 30 ~cM solution in 0.1%
BSA/saline) into pentobarbital anesthetized (25 mg/kg i.p.)
guinea pigs. The compound to be tested is dissolved in 0.1%
MC and dosed p.o. 1 hour before capsaicin challenge. Evans
blue dye (30 mg/kg) is administered i.v. 5 minutes before
challenge. After 10 minutes, the animals are sacrificed,
and both right and left ureters are removed. Tissue dye
content is quantitated as in "a" above.
c. Acetic acid-induced abdominal stretchincr
Male ddY mice (SLC, Japan), weighing 14-18 g, were
fasted overnight. The compound to be tested is dissolved in
,'~'r
_.. -.-_
WO 95/07908 21719 l 2 PCT~94/00221
-40-
0.1% MC and dosed p.o. 0.5 hour before acetic acid (AA)
injection (0.7%, 0.16 ml/10 g body weight). The animals are
placed in clear beakers (1 per beaker) and the stretching
response is counted 10 to 20 minutes after the AA injection
(10 minute interval).
d. Substance P-induced hynerlocomotor paradictm
The anti-psychotic activity of the therapeutic
compounds of the present invention as neuroleptic agents for
the control of various psychotic disorders may be determined
by a study of their ability to suppress substance P-induced
or substance P agonist induced hypermotility in guinea pigs.
This study is carried out by first dosing the guinea pigs
with a control compound or with an appropriate test compound
of the present invention, then injecting the guinea pigs
with substance P or a substance P agonist by intracerebral
administration via canula and thereafter measuring their
individual locomotor response to said stimulus.
The present invention is illustrated by the following
examples. It will be understood, however, that the invention
is not limited to the specific details of these examples.
PREPARATION 1
2-Methoxy-5-[N-methyl-N-j2,4-dimethyl-5-thiazole-
sulfonyl)amino)benzaldehyde
A. N-(4-Methoxyphenyl~~-N,2,4-trimethylthiazole-5-
sulfonamide
Under nitrogen in a flame-dried round-bottomed flask
fitted with a dropping funnel, stir bar and condensor, was
added N-methyl-p-anisidine (1.0 grams, 7.29 mmol) in 30 mL
of anhydrous tetrahydrofuran (THF). To this was added
triethylamine (1.01 mL, 7.29 mmol) and the flask was cooled
to 0°C with an ice bath. Next, 2,4-dimethyl-5-
thiazolesulfonyl chloride (1.54 grams, 7.29 mmol, Maybridge
Chem. Co.) in 20 mL of THF was added dropwise and the
reaction allowed to stir at 25°C overnight. The reaction
was quenched by pouring it slowly into 200 mL of saturated
aqueous sodium bicarbonate and extracting the crude product
with dichloromethane (CHZC12). After drying the organic
WO 95/07908 PCTIIB9:1/00221
2171972
-41-
extracts over magnesium sulfate (MgS04), the solvent was
removed in vacuo to a dark brown oil. Chromatography on
silica gel, eluting with hexanes:ethyl acetate (EtOAc)
(4:1), gave 380 mg of pale brown oil.
Mass spectrum (%): m/e 312 (12, M+), 136 (100).
1H NMR (CDC13) 8 2.2 (s, 3H), 2.7 (s, 3H), 3.3 (s, 3H),
3.8 (s, 3H), 6.8 (m, 2H), 7.1 (m, 2H).
B. 2-Methoxy-5-jN-methyl-N-x(2.4-dimethyl-5-
thiazolesulfonyl)amino]benzalde~vde
To a flame-dried round-bottomed flask with a nitrogen
inlet and stir bar was added the preceding intermediate from
step "A" ( 0 . 2 0 g, 0 . 7 mmol ) and 2 0 mL of anhydrous CHZC12.
After cooling to 0°C, titanium chloride (0.33 mL, 3 mmol)
was added dropwise and the reaction was stirred at 0°C for
30 min. Dichloromethyl methyl ether (0.14 mL, 1.56 mmol)
was then added via syringe and the reaction stirred at 0°C
for another 3 hours, then at room temperature for a further
18 hours, at which time thin-layer chromatography (tlc)
showed no starting material remaining. The reaction was
quenched by pouring it into 200 mL of saturated aqueous
sodium bicarbonate (NaHC03), stirring for 30 min and
extracting with CHZCIz. The extracts were dried (MgS04) and
concentrated in vacuo to an oil. Chromatography on silica
gel eluting with hexanes:EtOAc (3:2) gave the title product
as a yellow oil, 70 mg (29%).
Mass spectrum (%) m/e 340 (10, M+), 164 (100).
'H NMR (CDC13) S 2.1 (s, 3H), 2.5 (s, 3H), 3.1 (s, 3H),
3.9 (s, 3H), 7.0 (d, 1H), 7.5 (m, 1H), 7.6 (m, 1H), 10.4 (s,
1H) .
The following intermediate aldehydes of the general
formula X were prepared by a procedure similar to that
described in Preparation 1.
PREPARATION 2
2-Methoxy-5-jN-(4.5-dimethyl-2-thiazolyl)-N-
methanesulfonyl)amino]benzaldehyde
Waxy solid, 39% yield.
MS: m/e 340 (M+, 20%), 261 (65%).
WO 95/07908 PCTIIB94/00221
2171972
-42-
1H NMR (CDC13) d 2.3 (d, 6H), 3.4 (s, 3H), 4.0 (s, 3H),
7.0 (d, 1H), 7.7 (q, 1H), 10.5 (s, 1H).
PREPARATION 3
2-Methoxv-5-fN-(4 5-dimethyl-2-thiazolvl )
N
meth vllaminobenzaldehyde
Oil, 7% yield.
MS: m/e 277 (M+1, 20%), 276 (100%), 126 (30).
1H NMR (CDC13) d 2. 1 (d, 6H) , 3.4 (s, 3H) , 4. 3H)
0 (s, ,
7.0 (d, 1H), 7.6 (q, 1H), 7.8 (d, 1H), 10.5 (s, 1H).
PREPARATION 4
2-Methoxv-5-fN-f4 5-dimethyl-2-thiazo ~1)lamino
benzaldehyde
Mp 137-139C, 20% yield.
MS: m/e 262 (M+, 100%).
1H NMR (CDC13) d 2.15 (s, 3H), 2.25 (s, 3H), 3.9 (s,
3H), 7.0 (d, 1H), 7.6 (dd, 1H), 7.7 (dd, 1H), 10.5 (s, iH).
PREPARATION 5
2-Isot~ropoxv-5-fN-methyl-N-(2 4-dimeth~l 5
thiazolesulfonvl)aminolbenzaldehyde
Oil, 34% yield.
1H NMR (CDC13) S 1.4 (d, 6H), 2.20 (s, 3H), 2.6 3H),
(s,
3.25 (s, 3H), 4.70 (m, 1H), 6.95 (d, 1H), 7.45 (d, 1H), 7.55
(dd, 1H), 10.4 (s, 1H).
PREPARATION 6
2-Isoprot~oxy-5-[N-isobutvl-N-(2 4-dimethyl 5
thiazolesulfonvl) amino benzaldehyde
Oil, 54% yield.
MS: m/e 411 (M+1, 100) , 412 (M+2, 30) 236.
,
'H NMR (CDC13) d 0.9 (d, 6H), 1.45 (d, 6H), 1.6 (m, 1H),
2.2 (s, 3H), 2.7 (s, 3H), 3.4 (d, 2H), 4.7 (m, 1H), 7.0 (d,
1H), 7.5 (m, 2H), 10.4 (s, 1H).
PREPARATION 7
2-Methoxv-5-fN-isobutyl-N- (2 4-dimeth~l 5
thiazolesulfonyl)amino]benzaldehyde
Oil, 96% yield.
MS: m/e 383 (M+1, 100) , 384 (M+', 30) 208.
,
PCTIIB94/00221
WO 95/07908 2 i ~ ~ 9 7 ~.
-43-
1H NMR (CDC13) 1.0 (d, 6H), 1.6 (m, 1H), 2.2 (s, 3H),
~
2.6 (s, 3H), 3.4 (d, 2H), 3.9 (s, 3H), 7.0 (d, 1H),7.5 (m,
2H), 10.4 (s, 1H).
PREPARATION 8
2-Methoxy-5-f N-isoprogvl-N-(2,4-dimethyl-5-
thiazolesulfonyl~,~minolbenzaldehyde
Oil, 48% yie_d.
MS: m/e 369 (M+1 , 100), 194 (30).
1H NMR (CDC13) 1.1 (d, 6H), 2.35 (s, 3H), 2.65(s,
6
3H), 3.9 (s, 3H), 4.5 (m, 1H), 7.0 (d, 1H), 7.4 (m, 1H),7.5
(d, 1H) , 10.4 (s, .
1H)
PREPARATION 9
2-Iopropoxv-5-fN-isonropvl-N-(2.4-dimethvl-5-
thiazolesulfonyl)amino]benzaldehyde
Oil, 52% yield.
MS: m/e 397 (M+1, 100),, 398 (M+2, 30) , 222 (45) .
1H NMR (CDC13) d 1.1 (d, 6H), 1.4 (d, 6H), 1.4 (d, 6H),
2.4 (m, 3H), 2.6 (m, 3H), 4.5 (m, 1H), 4.7 (m, 1H), 7.0 (d,
1H), 7.4 (q, 1H), 7.5 (d, 1H), 10.4 (s, 1H).
PREPARATION 10
2-Trifluoromethoxy-5-LN-(4.5-dimethyl-2-thiazolyl)-N-
methanesulfonyl)amino]benzaldehyde
Oil.
1H NMR (CDC13) d 2.3 (s, 3H), 2.7 (s, 3H), 3.4 (s, 3H),
7.4 (d, 1H), 7.6 (d, 1H), 7.8 (dd, 1H), 10.3 (s, iH).
EXAMPLE 1
2.4-Dimethylthiazole-5-sulfonic acid ~4-methoxy-3-
((2S,3S)-2-phenvlpiperidin-3-ylaminomethvllphenyl]-
methylamide dihydrochloride hemihydrate
To a flame-dried round-bottomed flask fitted with a
Dean-Stark trap, condensor, nitrogen inlet and a s-ir bar,
the title compound of Preparation 1 (70 mg, 0.21 mmol) in 5
mL of anhydrous toluene was added to (+)-(2S,3S)-3-amino-2-
phenylpiperidine (36 mg, 0.21 mmol). The mixture was
refluxed for approximately 3 hours, the solvent was removed
in vacuo and the residue was dissolved in 5 mL of 1,2-
dichloroethane. Sodium triacetoxyborohydride (62 mg, 0.29
WO 95/07908 PCT/IB94/00221
217192
-44-
mmol) was added and the reaction stirred at 25°C overnight.
The solvent was next removed in vacuo, the residue was
treated with 10 mL of water and extracted 4 x 2 0 mL with
CHzCl2. The organic extracts were dried (MgS04) and
concentrated in vacuo to a yellow oil. Chromatography on
silica gel eluting with CHZC12: CH30H: concentrated NH40H
(97:2:1) gave the free base as a clear oil, 37 mg. This was
converted to the hydrochloride salt in the usual manner
(dissolved the free base in ethyl ether (Et20) and treated
with hydrogen chloride gas, concentrated in vacuo, and
recrystallized the crude salt from CH30H:Et20) to give a
white salt, 31 mg (24%), m.p. 260-264°C.
Anal. calc'd for Cz5H32N4~3s~2HC1~1/2H20: C, 51.54; H,
6.06; N, 9.62. Found: C, 51.31; H, 5.79; N, 9.76.
1H NMR (CDC13, free base) a 1. 3-2. 0 (m, 5H) , 2.05 (d,
1H), 2.15 (s, 3H), 2.7 (s, 3H), 2.8 (m, 2H), 3.15 (s, 3H),
3.25 (d, 1H), 3.35 (d, 1H), 3.45 (s, 3H), 3.6 (d, 1H), 3.85
(d, 1H), 6.55 (d, 1H), 6.8 (d, 1H), 6.95 (dd, 1H), 7.25 (m,
5H).
The title compounds of Examples 2-l0 were prepared by
a procedure similar to that of Example 1.
EXAMPLE 2
N- ( 4 , 5-Dimethylthiazol-2 yl) -N-[4-methoxy-3- ( ~~25 3S) -2
phenylpiperidin-3-vlaminomethyl)phenyll-methanesulfonamide
dihvdrochloride hemihydrate
40% yield, mp 247-249°C.
MS: m/e 501 (M+1), 421, 381, 247 (100%).
1H NMR (CDC13, free base) 6 1.4 (d, 1H) , 1.6 (t, 1H) ,
1. 75 (m, 2H) , 1. 9 (m, iH) , 2. 15 (d, 1H) , 2. 3 (m, 6H) , 2.85
(m, 2H), 3.25 (d, 1H), 3.35 (d+s, 4H), 3.55 (s, 3H), 3.7 (d,
1H), 3.9 (d, 1H), 6.7 (d, 1H), 7.15 (d, 1H), 7.25 (m, 6H).
Anal. calc'd for CZSH3zNa03Sz~2HC1~1/2Hz0: C, 51.54; H,
6.06; N, 9.62. Found: C, 51.87; H, 5.81; N, 9.55.
EXAMPLE 3
~5-f14,5-Dimethylthiazol-2-yl)methylamino]-2-
methoxybenzyll-((2S 3S)-2-phenyl~iperidin-3-yl)amine
trihydrochloride hydrate
WO 95!07908 PCT/IB94/00221
-45-
26% yield, mp 220-225°C.
MS: m/e 436 (M+, 16%), 317 (45%), 262 (100%).
1H NMR (CDC13, free base) , d 1.5 (m, 1H) , 1. 6 (m, 1H) ,
1.9 (m, 1H), 2.1 (S, 3H), 2.2 (S, 3H), 2.8 (m, 2H), 3.2 (m,
1H), 3.3 (s, 3H), 3.4 (d, 1H), 3.5 (s, 3H), 3.6 (d, 1H), 3.9
(d, 1H), 6.4 (d, 1H), 6.9 (d, 1H), 7.1 (q, 1H), 7.4 (m, 5H).
Anal. calc'd for C~H32N40S~3HC1~3/2H20: C, 52.40; H,
6.68; N, 9.78. Found: C, 52.12; H, 6.64; N, 9.55.
EXAMPLE 4
~5-(4,5-Dimethylthiazol-2-ylamino)-2-methoxybenzyl~-
l2S.3S)-2-phenylpiperidin-3-ylamine trihydrochloride
28% yield, mp 272-275°C.
MS: m/e 422 (M+, 40%), 303 (54%), 248 (100%).
1H NMR (CDC13, free base) d 1.35-2.15 (m, 7H), 2.18 (s,
3H), 2.23 (s, 3H), 2.8 (m, 2H), 3.28 (d, 1H), 3.4 (d, 1H),
3.5 (s, 3H), 3.65 (d, 1H), 3.9 (d, 1H), 6.65 (d, 1H), 6.75
(d, 1H), 7.15 (dd, 1H), 7.3 (m, 5H).
Anal. calc'd for Cz4H3oN40S~3HC1: C, 54.19; H, 6.25; N,
10.53. Found: C, 53.91; H, 6.39; N, 10.27.
EXAMPLE 5
4L5-Dimethylthiazole-2-sulfonic acid methyl-[3-
l ( 2 S . 3S~ -2-p eny~pi~eridin-3-ylaminomethyll -4-
trifluoromethoxyphenyl~-amide trihvdrochloride hydrate
12% yield, mp 239-240°C (dec.).
MS: m/e 555 (M+1), 380.
1H NMR (CDC13, free base) d 1.5 (m, 1H) , 1.7 (m, 1H) ,
1.9 (m, 4H), 2.1 (m, 1H), 2.2 (s, 3H), 2.7 (s, 3H), 2.8 (m,
2H), 3.2 (s, 3H), 3.3 (m, 1H), 3.5 (q, 2H), 3.9 (d, 1H), 7.0
(m, 3H), 7.2 (m, 5H).
Anal. calc'd for CZSHz9F3N4O3S2~3HC1~HzO: C, 44.09; H, 4.88;
N, 8.23. Found: C, 44.36; H, 4.95; N, 8.51.
EXAMPLE 6
2.4-Dimethylthiazole-5-sulfonic acid [4-iso~ropoxy-3-
((2S,3S)-2-phenylpiperidin-3-ylaminomethyllphenyll-
methYlamide dihydrochloride
10% yield, mp 227-230°C.
MS: m/e 529 (M+', 100) , 354.
WO 95/07908 PCT/IB94/00221
2171972
-46-
'H NMR (CDC13, free base) 8 1.05 (dd, 6H), 1.35-2.10 (m,
6H), 2.15 (s, 3H), 2.70 (s, 3H), 2.85 (m, 2H), 3.15 (s, 3H),
3.30 (d, 1H), 3.45 (m, 2H), 3.85 (d, 1H), 4.30 (m, 1H), 6.65
(d, 1H), 6.83 (d, 1H), 6.95 (dd, 1H), 7.3 (m, 5H).
Anal. calc'd for C27H36N4O3S2~2HC1: C, 53.90; H, 6.37; N,
9.31. Found: C, 54.55; H, 6.29; N, 9.33.
EXAMPLE 7
2,4-Dimethylthiazole-5-sulfonic acid [4-isoprot~oxy-3-
( (2S 3S)-2-t~henvlpiperidin-3-vlaminomethyl~~~henvll
isopropvlamide dihydrochloride
24% yield, mp 250-254°C.
MS: m/e 557 (M+~, 100), 398, 382 (100).
1H NMR (CDC13, free base) 8 1. 0-1. 15 (m, 12H) , 1. 4 (d,
1H), 1.5-1.95 (m, 4H), 2.05 (d, 1H), 2.30 (s, 3H), 2.65 (s,
3H), 2.8 (m, 2H), 3.25 (m, 2H), 3.55 (d, 1H), 3.85 (d, 1H),
4.3 (m, 1H), 4.6 (m, 1H), 6.6 (d, iH), 6.8 (d, 1H), 6.85
(dd, 1H), 7.25 (m, 5H).
Anal. calc'd for CZ9H4~N4~3S2~2HC1: C, 55.31; H, 6.72; N,
8.90. Found: C, 55.55; H, 6.51; N, 8.64.
EXAMPLE 8
2,4-Dimethvlthiazole-5-sulfonic acid j4-methoxy-3-
((2S 3S)-2-phenylpiperidin-3-ylaminomethyl)phenyl]
isopropvlamide dihydrochloride
15% yield, mp 240-242°C.
MS: m/e 530 (M+2, 100), 371, 355 (100).
IH NMR (CDC13, free base) d 1. 05 (d, 6H) , 1. 4 (d, 1H) ,
1.55-1.95 (m, 4H), 2.05 (d, 1H), 2.35 (s, 3H), 2.70 (s, 3H),
2.80 (m, 2H), 3.25 (m+d, 2H), 3.45 (s, 3H), 3.65 (d, 1H),
3.85 (d, 1H), 4.6 (m, 1H), 6.6 (d, 1H), 6.8 (d, 1H), 6.9
(dd, 1H), 7.25 (m, 5H).
Anal. calc'd for CZ~H36N4O3S2~2HC1: C, 53.90; H, 6.37; N,
8.32. Found: C, 53.73; H, 6.30; N, 8.44.
EXAMPLE 9
2,4-Dimethylthiazole-5-sulfonic acid [4-methoxy-3-
((2S,3S)-2-phenvlpiperidin-3-ylaminomethyl)phenyl~-
isobutvlamide dihydrochloride hydrate
16% yield, mp 225-230°C.
WO 95/07908 PCT/IB94/00221
21 X1972.
-47-
MS: m/e 544 (M+2, 70), 385, 369 (100).
1H NMR (CDC13, free base) d 0. 9 (d, 6H) , 1.4 (d, 1H) ,
1.5-1.95 (m, 6H), 2.05 (d, 1H), 2.15 (s, 3H), 2.70 (s, 3H),
2.8 (m, 1H), 3.25 (m, 3H), 3.35 (d, 1H), 3.45 (s, 3H), 3.6
(d, 1H), 3.85 (d, 1H), 6.6 (d, 1H), 6.8 (d, 1H), 6.9 (dd,
1H), 7.25 (m, 5H).
Anal. calc'd for CzaH3gN4O3S2~2HC1~H20: C, 53.07; H, 6.68;
N, 8.84. Found: C, 52.88; H, 6.38; N, 8.85.
EXAMPLE 10
2.4-Dimethylthiazole-5-sulfonic acid [4-isopropoxy-3-
( 12S, 3S) -2-phenylpiperidin-3 _ylaminomethyl~"phenyll,-
isobutylamide dihydrochloride
5% yield, mp 150-160°C.
MS: m/e 572 (M+Z, 100), 570 (M+), 397.
'H NMR (CDC13, free base) d 0. 9 (d, 6H) , 1. 02 (d, 3H) ,
1.12 (d, 3H), 1.35-2.10 (m+s, 8H), 2.7 (s, 3H), 2.8 (m, 2H),
3.2-3.55 (m+d, 5H), 3.85 (d, 1H), 4.3 (m, 1H), 6.6 (d, 1H),
6.8 (d, 1H), 6.9 (dd, 1H), 7.25 (m, 5H).
Anal. calc'd for C3QH42N4~3S2~2HC1~Et20: C, 57.05; H, 7.32;
N, 7.83. Found: C, 57.41; H, 6.89; N, 8.25.
EXAMPLE 11
6-Phenylpiperidine-2,5-dione
To a stirred solution of 5-nitro-2-oxo-6-
phenylpiperidine (20 g, 90.8 mmol) in 320 mL of
dichloromethane and 320 mL of methanol, potassium tert-
butoxide (10.19 g, 90.8 mmol) was added in portions over one
minute. After stirring for 15 minutes at 25 °C, the
solution was cooled to -78°C and treated with ozone for
approximately one hour to produce a blue solution. The
solution was then treated with nitrogen gas for 15 minutes
to remove excess ozone. Dimethylsulfide (12 mL, 163 mmol)
was added and the reaction was allowed to warm to room
temperature. Removal of the solvent in vacuo provided a
yellow residue which was filtered, washed with
WO 95107908 PCT/IB94/00221
2111972
-48-
dichloromethane and diethyl ether and dried to a white
solid, 12.1 g (70%).
1H NMR (CDC13): d 2.6-2.8 (m, 4H), 5.0 (s, 1H), 6.7 (bs,
1H), 7.4 (s, 5H).