Note: Descriptions are shown in the official language in which they were submitted.
- 2172~7
.
2 ', 5 ' OLIGOADENYLATE 2 ', 3 ' -CYCLOPHOSPHATE
The present invention concerns 2',5' oligoadenylates having
a cyclophosphate group at the 3' end and a free OH group at
the 5' end, a procedure for producing these compounds, a
pharmaceutical preparation containing them and the use of
these compounds in treating papilloma-induced medical
conditions.
The present invention concerns new chemical compounds,
namely 2',5' oligoadenylate 2',3'-cyclophosphates having
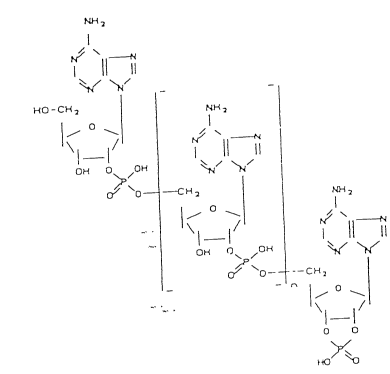
the general formula:
- I
~O - C~
1- ~
0~ 0 /O
0~o~
o~ /o~
~P o~ C~ 2
O
\1 1/
O O
~Y~o
wherein o c n 5 10, particularly 2 0 to 10, preferably 1 or
2.
In particular, compounds where n = 1 or 2 can be
advantageously used for medical purposes, namely for
2172~7
.
topical treatment of skin and epithelial lesions caused by
papilloma viruses.
Papillomatoses caused by papilloma viruses of the family
papovaviridae are widely occurring infections in human and
animal populations. More than 60 varieties of human
papilloma viruses are currently known.
All of these viruses are similarly structured. The genome
has a double-stranded covalent closed ring-form DNA with
8000 base pairs, which codes the virion proteins and other
proteins necessary for the intercellular development of the
virus. The genome of the papillomaviruses is replicated in
the infected cell in the form of episomes (dozens of copies
per cell) over the course of many generations. Formation
of mature virions does not occur within cells until the
final step of differentiation.
In the persistent intracellular state, only the early genes
of the papilloma virus genome are expressed. These cause
a change in the cell's phenotype and lead to the formation
of papillomatoses. Depending on the type of virus and
other factors, the virus genome may become integrated into
the genome of the infected cells. This in turn may trigger
malignant transformation. It is known that a significant
number of tumors in humans are the result of malignant
transformation by papillomaviruses. They thus represent a
result of persistent latent viral infections.
Sexually transmitted anogenital papillomatoses are among
the most likely to undergo malignant transformation (see M.
Spitzer, Obest. Gynecol, 1989 vol 73, N3, 303-307; H. zur
Hausen, A. Schneider, "The Role of Papillomaviruses in
Human Anogenital Cancer" in The Papovaviridae. (ed N.P.
Alzman), 1987, vol 2, 245-263; H. zur Hausen,
"Papillomaviruses as Carcinomaviruses" in Adv. in Virus
Oneology, (ed G. Klein), 1989, vol 8, 1-26.
21721~7
Since papillomatoses are easily diagnosed pre-cancerous
illnesses, the development of many tumors can be prevented
by treatment of benign papillomatoses, ie before
metamorphosis to a malignant state has occurred in the
infected cells.
Currently, the most important methods for treating
papillomatoses are surgical removal of the papillomas as
well as electro-, cryo-, or laser cauterizing therapy (see
"Virus infections: etiology, epidemiology, clinics,
pathogenesis and diagnosis", Rep . Col . of Sci en t . Publ i c .,
Sverdlovsk, 1985 (in Russian)). Liquid oxygen, acids and
their mixtures (saltpeter, oxal and lactic acid, etc) are
used for this purpose, but they cause necrosis of
surrounding healthy tissue and lead to formation of scars
on the site of application, and often lead to recurrence as
well as appearance of new papillomas near the site of
removal (see S.A. Bashi "Cryotherapy versus podophyllin in
the treatment of genital warts", Int. ~. Dermatol, 1985,
vol 24, N8, 535-536).
The effectiveness of medical methods in treating
papillomatoses using podophyllotoxin and interferon is low
and is furthermore accompanied by significant side effects
and sequelae, even if therapeutic doses are administered.
The biological activity of podophyllin may be explained by
its antimitotic effect, which is comparable to colchicine.
Its use often provokes local reactions (inflammation,
allergic contact dermatosis, occasionally erosion of the
skin, etc) as well as undesirable after effects such as
peripheral neuropathy, tachypnea, hematuria and abortus
spontaneus. (see K.R. Beutner "Podophyllotoxin in the
Treatment of Genital Human Papillomavirus Infections",
S~n1in~rs in Dermatology, 1987, vol 6, N1, 10-18).
The use of interferon in treating papillomatoses has only
2172~ 47
limited effectiveness and doses used for this treatment can
lead to suppression of the immune system as well as
triggering auto-immune diseases, etc. (see F.G. Bruins,
A.J.C. von den Brule, R. Mulinik, G.M.M. Walboomers, C.J.
Meijer, R. Willemze, J. Invest. Dermatol., 1989, vol 93,
N4, 544-545; M. Foldvan, A. Moreland, M. Nezei, ibid, 550;
G. Gross, Roussaki, ibid, 553; M. Niimura, ibid, 567.)
Synthetic analogs of 2',5' oligoadenylates (2.5 A) are
known to display immuno-suppressory activity and have
previously been recommended for surgical transplants. It
has been stated that oligoadenylates can mediate the
effects of interferon with less toxicity and greater
effectiveness both specifically and generally (see A.
Kimchi et al, US Patent 4378352(1983)).
The same effect is also achieved with a terminal synthetic
2.5 A containing a morpholine group (see R. Torrence et al,
US Patent 4515781 (1985)).
2.5 A analogs with at least three adenosine fragments are
known to be active inhibitors or virus protein synthesis in
vitro (see Jan M. Kerr et al. US Patent 4,21,P,746 (1980)).
Some synthetic analogs of 2.5 A oligo 3' deoxyadenylates
and their derivatives inhibit particularly the infection
and transformation of animal cells with herpes simplex and
Epstein-Barr-Viruses, but are inactive in the case of
already infected or transformed cells (see R.I. Suhadolnik
et al, US Patent 4464359 (1984); R.I. Suhadolnik et al, US
Patent 4539313 (1985); R.I. Suhadolnik et al, US Patent
4708935 (1987).
It is also possible to use 2.5 A to treat infectious
diseases caused by cytomegalovirus, hepatitis B virus and
varicella zoster viruses (see EP-B-121 635 (Au. No. J. Dk.
Fi. Fr. Es.) 1984).
2172~47
5
A task of research is to make available an effecti~e
medicine with selective effect for treating skin and
epithelial lesions caused by papillomaviruses.
The purpose of the invention is achieved by producing 2',5'
oligoadenylate 2~,3'cyclophosphates having formula I:
N~
I
~ ~ ~
W ~ - I
~O - C~
0~5 0 ~OH
0~0 {~ 2
O
2 0 0~ (~ ~G ~/
o~P ~o-- - - C ~ 2
O
\l 1/
~ ` O O
~0 ~
where 0 5 n 5 10, particularly 2 0 to 10, preferably 1 or
2.
The object of the invention is also the procedure for
producing the new compounds, as described in detail below,
beginning with poly(A).
These compounds may be produced in a recognizable manner
beginning with poly(A~ with irregular 2',5' and 3',5'
internucleotide bonds, using a familiar procedure (see A.M.
2172~47
t
Michelson in The Chemistry of the Nucleosides and
Nucleotides, Academic Press, 1963, 418 and 419) by chemical
polymerization of 2'(3') adenosine monophosphate. The
subsequent split of the 3',5' bonds in this polymer by
ribonuclease from B intermedius (EC.3.1.4.23) leads to a
monomer and 2',5' oligoadenylates of varying lengths with
a mixture containing a terminal 2',3' cyclophosphate group.
Another two-step procedure may be used to obtain the same
result:
1. Division of poly(A) with ribonuclease T2 (or similar
ribonucleases) which leads to a series of 2',5'
oligoadenylates in which every oligomer represents a
mixture of 2',3' cyclophosphate and 3' monophosphate; and,
2. Processing of this mixture with a lOOx excess of BrCN in
a buffered aqueous solution, which leads to a
transformation of the terminal 3' phosphate group into the
2',3' cyclophosphate group.
These two methods of synthesis may be represented
schematically as follows:
poly(A) with irregular 3',5' and
2',5' internucleotide bonds
Binase / \ RNAse T2
A>p + A2'p5'(A2'p5' )n A>p Br~ A3'p + A2'p5'(A2'p5' )n A3'p
The resulting mixtures of 2',5' oligoadenylates are
analysed and the desired oligonucleotides are purified
using HPLC according to formula 1.
2',5' oligoadenylates with a terminal 2',3' cyclophosphate
21721 ~7
7
group as isolated compounds have not been previously
described. Both the 2'(3')phosphate groups and the 2',3'
cyclophosphate groups in the 2',5' oligoadenylates
effectively prevent hydrolysis of these compounds by
cellular enzymes. The effectiveness of the 2',3'
cyclophosphate analogs as compared to the natural 2',5'
oligoadenylates containing the 5' triphosphate group may be
explained by the greater potential for cell permeation
because of a lower charge and the resistance to hydrolysis
with cell enzymes, such as phosphodiesterases.
The 2',5' oligoadenylates to be used in the inventlon can
be dissolved in water or aqueous solutions of neutral salts
and applied to skin lesions once or twice daily for 2 weeks
(usually flat warts and sole warts, condylomas etc). The
concentration of the active ingredient is 10-4 to 10-7 M.
This treatment of the condylomas stops their growth. There
was no new formation and, in most cases, the old condylomas
regress within 4 to 6 weeks.
In the case of ordinary warts, the papules generally
flatten out towards the end of the first week and disappear
completely 2 to 4 weeks after the beginning of treatment.
Patients with flat warts were completely cured 1 month
after the beginning of treatment.
In the course of treatment of the papillomas, new
formations shrunk after 1.5 to 2 weeks while smaller
papillomas disappeared. Complete reduction of skin lesions
occurred within 1 month. There was not a single instance
of scarring. The healing was also not accompanied by
toxic-allergic reactions or other side effects.
The concentration of active oligoadenylates in the
treatment solutions is two orders of magnitude greater than
for interferon induced cells, but the total quantity of
2172~47
8
solution applied generally falls under 1.0 ml. Even if all
the oligoadenylates are absorbed, the resulting average
concentration in cells and body fluids (blood, urine, etc)
is far below that in non-induced cells. The concentration
of compounds used will be higher only temporarily
(immediately after application) than that of their natural
analogs in normal cells, and that only in tissue
immediately surrounding the area of application.
The 2',5' oligoadenylates are easily degraded by nucleases
and phosphatases inside and outside of cells in the
organism. The end products include adenyl acid and
adenosine, which represent ordinary cell metabolites whose
average concentration in cells is under 10-3 M.
Both oligoadenylates corresponding to ormula I and
mixtures containing them may be used in treating skin and
epithelial lesions induced by papillomaviruses.
Topical application of low dosages of 2',5' oligoadenylates
based on this invention to treat papillomatoses was widely
ef~ective, and no negative skin reactions or systemic side
effects were observed. The result of treatment of the
lesions was complete reduction in more than 80~ of
patients. At the end of a 3-month observation period there
was no evidence of recurrence.
The preparation based on the invention can be administered
in various forms appropriate for topical application.
These are produced by careful mixing or dissolving of the
individual compounds or their mixtures with a
pharmaceutically compatible carrier using standard
techniques. Depending on the desired form of preparation
for the application (external skin, mucous membrane tissue,
etc), the form of the carrier may vary greatly. The most
diverse pharmaceutical media can be used to produce
preparations, for example liquid carriers such as water,
2~72~47
.
dimethyl sulfoxide with or without alcohols, glycols etc or
pomades, ointments and plasters. For convenience, it is
especially useful to arrange these preparations in single-
dose units. The expression "single-dose unit" refers to
physically discrete units of which each one contains a
specific quantity of the active ingredient calculated to
achieve the desired therapeutic effect when combined with
the required pharmaceutical carrier. The corresponding
dosage for a single application in treating papillomatoses
should be 10-9-10-8 M of active ingredient (n = 1 and/or 2)
per papula (3-5 mm in diameter). The total duration of
treatment is up to 30 days with daily application.
The following examples illustrate the production of
oligonucleotide mixtures and individual 2',5'
oligoadenylates.
Chemical-enzymatic synthesis, purification and analysis of
2',5~-oligoadenylates
All reactions are carried out at room temperature.
a) Synthesis of poly(A):
0.9 g (2.5 mMol) of adenosine 2'(3') monophosphate in H+
form and 1.2 ml (2.6 mMol) of tri-n-octylamine are
dissolved in 30 ml of methanol/ethanol (1:1) and the
solution is stirred for several hours. Then the
undissolved portions are filtered out using a glass filter,
and the filtrate is evaporated using a rotary evaporator
until dry. The resulting solid is then dissolved in 10 ml
abs. dioxane and evaporated until dry using a rotary
evaporator. Both procedures are repeated twice. Then the
resulting solid is dissolved in 5 ml abs. dioxane and 0.77
ml (3.75 mMol) of diphenylphosphorylchloride is dripped
onto it while the mixture i~ stirred with a magnetic
stirring device. Then 3.3. ml (7.5 mMol) tri-n-octylamine
21721~7
are added. The resulting mixture is stirred for an hour
after which 0.77 ml of diphenylphosphorylchloride and 3.2
ml tri-n-octylamine are added, and the mixture is stirred
for 4 more hours. Then the reaction mixture is poured into
5 volumes of hexane/ether (4:6) while being stirred. The
sediment is then filtered off using a glass filter, washed
with the same mixture and then with ether and dried in a
vacuum. The result is a white powder of polyadenylate with
irregular 2',5' and 3',5'-internucleotide bonds. The
general formula of the mixture is (A2'(3')p-)~A>p, where
n=10.
The dry polymer mixture is then dissolved in 12 ml of water
and the pH is adjusted to 9.3 by adding a conc. ammonia
solution. Then 3 volumes of ethanol are added and the pH
is adjusted to 3.0 by adding 5 M HCl. The sediment which
forms when the mixture is stored at -18C is centrifuged
off, washed with 75~ ethanol and dried in an air-stream.
A considerable portion of the monomer remains in the
supernatant.
b) Selective splitting of the 3',5' internucleotide bonds
by splitting with binase:
The precipitate resulting from a) is dissolved in 40 ml H2O
and the pH is adjusted to 7.0 with a 3 M solution of tris-
base, after which 2 ml of a solution of Bacillus
intermedius RNAse (binase, E.C.3.1.4.23) (150 E/mg) is
added and then stirred for 10 to 12 hours. The enzyme is
then extracted using the same volume of chloroform/isoamyl
alcohol (24:1). The resulting aqueous phase contains a
mixture of 2',3' cyclophosphates of adenosine and 2',5'
oligoadenylates.
c) Isolation of the individual compounds:
The procedure for separating the individual components is
21721 ~7
11
based on ion exchange chromatography using DEAE spheron
1000 in HCO3- form (16 x 600 mm, 120 ml). The enzymatic
division product of poly(A) is deproteinated and then
concentrated in a rotary evaporator 8 to 10 times. 10
volumes of ethanol are added and the mixture is left at -
18OC for 12 hours. A~ter centrifuging (10 to 20 min at 3000
rpm) the sediment is dissolved in water and loaded onto the
column (optical thickness 25,000 to 50,000 E at 260 nm).
After the column is washed with water and 0.05 M triethyl
ammonium bicarbonate (pH 8.0) the products are eluted at a
linear gradient of this salt (0.1 to 0.8 M, total volume 1
L). The fractions containing the individual compounds thus
separated are evaporated until dry in a rotary evaporator
and lyophilated to completely remove the buffer components.
HP~C is used for the final cleaning of the individual
compounds as explained below (a coating of ca. 2000 E
optical thickness on the semi-preparative columns).
Desalting the solution is achieved by adsorption of a 10 to
20x diluted solution on small DEAE columns, washing with
water, elution with a small quantity of 1 M triethyl
ammonium bicarbonate and lyophilation.
d) Cleaninq and analysis of the 2',5' oliqoadenylate with
HPLC chromatoqraphy:
Ion exchange chromatography is performed with a NH3 diasorb
column (8 ~m, 4 x 150 mm for the analysis and 10 ~m, 9.2 x
250 mm for the final preparative cleaning); linear
gradient; elutrient A - 20~ MeOH; B - 2M AmAc, 20~ MeOH, 2~
B per mixture; fluidity (flow-through time) - 0.7 ml/min
for the analytic column and 4 ml/min for the preparative
(fig. 1 - 3). Detection is with a W monitoring device at
260 nm.
On the basis of the optical thickness of 260 nm the
mixtures contain 40 to 50~ monomer, ca. 20~ to 30~
217~1~7
.
12
diadenylate, ca. 5~ to 15~ triadenylate, 2~ to 8~
tetraadenylate and 2~ to 7~ of higher oligoadenylates. The
average molar extinction coefficient was 15 x 103 per
adenosine residue, 36.8 x 103 for the trimer and 45.8 x 103
for the tetramer.
e) Determining the comPOsitiOn of individual compounds:
Each compound was kept at pH 1.0 (1 hour at 370C) to open
the cyclophosphate group, dephosphorylated with bacterial
alkaline phosphatases and subjected to alkaline hydrolysis
of the internucleotide bonds (0.3 M NaOH, 48 hours at
200C). The relation of the end products (adenosine to
adenosine 2'(3') phosphate was achieved by HPLC. The
actual results agree closely with those given above:
Ado:AMP = 1:1 for the dimer, 1:2 for the trimer and 1:3 for
the tetramer. The standard deviation was less than 5~.
On the basis of their HPLC mobility and NMR spectra (31p and
lH), the compounds produced by the acid opening of the 2',3'
cyclophosphate group were identical to familiar 2'(3')
phosphates of the corresponding oligoadenylates. On the
other hand, the resulting 2'(3') phosphates were
quantatively converted into 2',3' cyclophosphates of the
corresponding oligoadenylates in an aqueous solution at
room temperature due to the effect of the BrCN (lOOx molar
excess).
f) Determining the internucleotide bond type:
The treatment of the 2',3' cyclophosphates of the
individual 2',5' oligoadenylates with fresh binase operates
under conditions which lead to complete cleavage of 3',5'
polyadenylic acid cyclophosphate produces no change in the
HPLC separation profile and in the 31p NMR spectra.
Only peaks corresponding to the terminal cyclophosphate
217~1~7
13
group (20 to 21 ppm) and the 2',5'-internucleotide
phosphate (0.02 to 0.4 ppm) were determined in the 31p NMR
spectra, at a relation of 1:1 for the dimer, 1:2 for the
trimer and 1:3 for the tetramer. The standard deviation
was less than 2~. The 3',5' bond (less than 1~
internucleotide phosphate) has been demonstrated only for
the tetramer.
Oligoadenylates are colorless compounds readily soluble in
water (up to 10-2M), dimethylsulfoxide, aqueous ethanol or
glycerol. They remain dissolved in a neutral aqueous
solution at 40C for several months and indefinitely when
frozen in solution (-200C) or in a lyophilized state.
Pharmacoloqical ExamPles
Clinical research of the pharmaceutical preparation has
been conducted on papillomatose patients. Treatment was
with Na- or triethyl ammonium salt of the corresponding
compound, which had been diluted to the required
concentration with 0.1 ml NaCl. The concentration of the
effective compound in the final solution achieved using its
optical thickness at 260 nm following the molar extinction
coefficient procedure described above. The results are
described below. The examples serve to illustrate the
invention, but do not limit it.
Example 1
Clinical test of 2',5' triadenylate 2',3' cyclophosphate
(n=l, AIII).
Diagnosis of female patient M. (1958): 4 sharp-edged
condylomas located in the perineal region (d=l to 2 mm, h
= 2 to 8 mm), skin-colored, raw, elastic and sometimes
painful. Accompanying illness: colpitis.
21721 ~7
14
Daily application of ca. 50 ~m AIII(10-4M) per condyloma. On
the fourth day all condylomas showed a wrinkled surface; by
the eighth day they had completely disappeared. No
recurrence was observed after 8 weeks.
Diagnosis of female patient P. (1950): 14 condylomas
located in the perineal region (d=2 to 8 mm, h = 2 to 5
mm), elastic, skin-colored and often painful.
Daily application of 50 ~m AIII(10-4M) per condyloma for two
weeks. By the sixth day all condylomas had shrunk by one-
half to one-third. By the 10th day 8 condylomas had
completely disappeared without leaving any trace on the
skin. The remaining 6 condylomas were still present and 3
to 4 weeks after treatment regained their original size.
Treatment was not continued further. No changes were
observed 10 weeks after the end of treatment (no
recurrences, no formation of new condylomas).
Diagnosis of female patient C. (1958): a sole wart on the
underside of the big toe (d=5 mm, h 3 mm), compact, same
color as surrounding tissue, with rough outer surface and
extremely painful when walking.
Daily application of 50 ~m AIII(10-4M) per wart. On the
sixth day the wart had grown softer and the surface of the
wart was rubbed away mechanically. By the tenth day the
wart had completely disappeared and walking did not cause
any pain. At a checkup after 12 weeks no recurrence was
observed. The affected skin surface area was
indistinguishable from the surrounding tissue.
Diagnosis of female patient C. (1926): 7 papillomas were
found on the front and right side of the throat, 2 large
(d=3 and 2 mm, h = 2.5 mm, dark brown) and 5 smaller (d =
0.8 to 1.5 mm, h = 0.8 to 1.5 mm, skin-colored), The
papillomas had appeared in 1982 during menopause.
2172~7
Daily application of ca. 50 ~m AIII(10-4M) per papilloma for
two weeks. On the fifth day the smaller papillomas had
turned pale, shrunk and flattened out. No further changes
were ob~erved. Two to 3 weeks after the end of treatment
the papillomas had regained their original form and size.
Example 2
Clinical test of 2',5' tetraadenylate 2',3' cyclophosphate
(n=2, AIV).
Diagnosis of female patient S, 27 years old: ordinary warts
on the stomach, 3 cm underneath the navel, grey in color,
protruding, diameter 2 to 3 mm). The warts were treated
daily with 1 to 3 drops of AIV(10-5M). On the 7th to 10th
day after beginning of treatment all the warts had shrunk
and flattened out. Three weeks after the end of treatment
one of the warts had completely disappeared and four weeks
after the end of treatment all the others. No scar-like
changes in the skin.
Seven other patients with numerous warts, condylomas (six
patient~), ordinary warts (three patients) and unidentified
warts (four patients) were treated daily for two weeks with
an aqueous solution (10-sM) of AIV. The condylomas were
found on the external genitalia and on the breast, the
warts on the wrist, body and legs.
In most cases the condylomas and warts (especially the
former) shrank after 6 to 9 day treatment. 1 to 2 weeks
after the end of treatment or during treatment three
patients were already completely free of condylomas and two
showed no more ordinary warts. One patient was completely
free of all six condylomas under the armpit about 6 weeks
after the end of treatment. In two cases, small condylomas
(d < 3 mm, total 11) on the external genitalia disappeared
during or after treatment while larger ones in the same
2172~7
16
area (d > 5 mm, two in the first case, three in the second)
regained their original size and form 2 to 6 weeks after
treatment. Two of the six unidentified warts disappeared
2 to 3 weeks after treatment, but the others showed no
reaction. Negative reactions, traces on the skin or
recurrences were not observed in a single case during
treatment or 8 to 12 weeks afterwards.
Example 3
Clinical test of mixture A (mixture of 2',5' oligoadenylate
2',3' cyclophosphate (n>0). Two patients were tested, one
with three ordinary warts (3 to 4 mm) on the neckand the
other with two condylomata acuminata on the inner side of
the leg. Each wart was moistened daily with 1 to 2 drops
of 10-4M aqueous solution of mixture A. On the 9th day of
treatment all the ordinary warts had disappeared. One
small condyloma disappeared after 7 days of treatment,
another three weeks after the end of treatment, ie five
weeks after the beginning of treatment.
The results achieved show clearly the high effectiveness of
the compounds under research as well as the procedure for
treating skin and epithelial lesions caused by human
papillomaviruses. Treatment of the papillomaviruses with
2',5' oligoadenylate was not accompanied in a single case
by painful or inflammatory reaction nor by a subjective or
objective worsening of condition.
The high clinical effectiveness of 2',5' oligoadenylate at
very low dosages and the absence of local reactions and
systemic side effects as well as lack of recidivism
demonstrate the advantages of the compounds under
consideration for treating papillomatoses.
Similar results in treating papillomatoses were also
achieved using already familiar compounds, ie 2',5'
217~147
.
17
oligoadenylates with natural adenosine residues and 2'(3')
phosphate groups (series B) or free 2' and 3' hydroxyl
groups (series C) on a 3-terminal adenosine residue. In
these cases both isolated compounds, for example trimers
and tetramers or their mixtures with other oligomers,
(trimers B and C, tetramers B and C, mixtures B and C)
proved to be widely effective in the treatment of external
papillomaviruses.
Description of the figures
Fig. 1:
HPLC analysis of mixture A (2',5' oligoadenylate 2',3',
cyclophosphate. Column: diasorb NH2, 4 x 150 mm. Mobile
phase: A - 20~ CH30H; B - 2M Ac0NH4 in 20~ CH30H; Fluidity:
0.7 ml/min; 0'-2' - 1~ B, 2'-14' - 0.5~ B/min, 14'-50' - 2
B/min. Detection at 260 nm. Composition of the mixture
corresponding to absorption at 260 nm: 23~ monomer (8.7
min), 22.5~ dimer (17.5 min), 14~ trimer (23 min), 6.8
tetramer (26.8 min).
Fig. 2:
HPLC analysi~ of the purified 2',5' trimer (A) and tetramer
(B) 2',3' cyclophosphate. Column, composition of the mobile
phase and fluidity as in fig. 1, gradient - 2~ B/min.