Note: Descriptions are shown in the official language in which they were submitted.
\!ge 1 of 20
~ ~a2~54
NOVEL ANTI:MICROBIAL INDOLE DERIVATIVES
SPECIFICATION
The present invention relates to the novel antibiotics, nematophin and its
derivatives having
antimicrobial activity, and salts thereof. The present invention also provides
methods for the
production of nematophin and its derivatives, comprising the step of
cultivating the microorganism X.
rtematophilus or the organic synthetic methods. The present invention further
provides novel
~~ntimicrobial compositions comprising nematophin and its derivatives or their
salts thereof, and
methods of using the inventive compounds as antibacterial and antimycotic
agents.
:Background
Protection of humans, agricultural crops, stored foods, gardens, ornamental
plants, trees and
wood products, and animals from bacterial and firngal diseases is extremely
important. Unfortunately,
bacteria and fungi continue to be problematic pathogens for humans because of
the increasing
ge2of20
occurrence of strains which are resistant to commonly used antibiotics. Such
resistant strains lead to a
constant need for new antibacterial and antifungal substances.
Although there are a limited number of publications on Xenorhabdrrs and
Photorhabdus, it
has been recognized that active, antibacterial and antifungal substances are
produced by Xenorhabdu.s
species and Photorhabdrrs species. Sorne of these specific compounds have been
isolated, identified
and their structures elucidated (Li et al., Antimicrobial metabolites from a
bacterial symbiont J. Nat.
prod Vol. 58, 1081-1086 (1995); Paul e1 al., "Antibiotics in Microbial
Ecology: Isolation and
Structure Assignment of Several New Antibacterial Compounds from the Insect-
Symbiotic Bacteria
Xenorhabdus spp." J. Cheer. Ecol. Vol. 7, pp. 589-597 ( 1981 ); Richardson et
al., "Identification of
~~n Anthraquinone Pigment and a Hydroxystilbene Antibiotic from Xeuorhahdos
~Photor-habdarsJ"
.~lpp. Enviror~. Microbiol. vol. 54, pp. 1602-1605 ( 1988);. McInerney et al.
Biologically Active
Metabolites from Xenorhahdars spp., Part 1. Dithiolopyrrolone Derivatives with
Antibiotic Activity"
.I. Nat. Prod. Vol. 54, pp. 774-784 ( 1991a); McInerney et crl. "Biologically
Active Metabolites from
_Xenorhabdus spp., Part 2. Benzopyran-I-one Derivatives with Gastroprotective
Activity" J. Nat.
Prod. Vol. 54, pp. 785-795 ( 1991b). Recently the cell free culture broths of
Xerrorhahdrr.s species and
P. lumirrescens, bacterial symbionts carried by nematodes of the genus
Steirrernema, and
Heterorhahditis were found to be active against many fungi of agricultural and
medicinal importance
(Chen et al., Antifungal activity of two Xerrorhabdrrs species and P.
lrrnrinescerrs, bacteria associated
with the nematodes Steiraer°rtema species and Heterorhcrbditi.s
nregidis. Biological Control. Vol. 4,
157-162 (1994)). We discovered the novel group of antibiotics, 3-indoleethyl 2-
oxo-alkanamides and
3-indoleethyl 2-oxo-alkanoates with or without substitutes) on indole ring,
and the importance of
these specific purified metabolites as extremely potent antibacterial and
antifungal agents(Webster et
CA 02172854 2002-04-10
al., 1996, US patent 5,569,668), that are the subjects of this invention.
Prior art references have
not shown use of these specific compounds or any operable aspects as
antibacterial and
antifungal agents.
The microorganisms
X. nematophilus and its nematode symbiont Steinernema jeltiae used in this
study were
collected from soil in British Columbia, Canada and maintained in culture in
this laboratory
(Maxwell et al. 1994). Briefly, last instar larvae of the greater wax moth,
Galleria mellonella, were
infected with infective juvenile (IJ) nematodes, carrying the X. nematophilus
BC 1 strain, at a rate of
25 IJs/larvae. After 24 to 48 h the dead insect larvae were surface
disinfected by dipping them into
95% EtOH and igniting them. The cadavers were aseptically dissected,
haemolymph was streaked
onto an NBTA medium (nutrient agar supplemented with 0.025 grams of
bromothymol blue and
0.04 gram of 2,3,5-triphenyltetrazolium chloride per liter) and incubated in
the dark at room
temperature. The resulting primary form of X. nematophilus was maintained and
subcultured at 14 d
intervals. Other sources and depositories of Xenorhabdus species and strains
are noted in Akhurst
and Boemare "A numerical taxonomic study of the genus Xenorhabdus
(Enterobactereacea) and
proposed elevation of the subspecies of X. nematophilus to species" J. Gen.
Microbiol. Yol 134,
pp.1835-1845 (1988). Putz et al. "Development and application of
oligonucleotide probes for
molecular identification ofXenorhabdus species" Appl. Environ. Microbiol. Vol.
56, pp. 181186
(1990) notes additional sources and depositories, including the American Type
Culture Collection,
Rockville, MD. Candidate bacterial and fungal pathogens used in bioassays are
readily available
from many sources, including the American Type Culture Collection, Rockville,
MD. For
consistency, 14% sucrose lyophilized powder of bacteria stored at -20°C
was frequently used as
starting material for cultures.
Replacement sheet 3
ge 4 of 20
Preparation of nematophin and its derivatives
Cultivation of the microorganism X. r~ematophiln.s BC 1 strain yields the
novel antimicrobial
~,ubstance nematophin (+ stereoisomer). Nematophin may be formed as a
metabolite thereof.
To prepare nematophin, X. r~ematophiln.s BC 1 strain may be cultivated
(fermented), for example, at about 2_S°C under submerged aerobic
conditions in an adueous nutrient
medium containing assimilable carbon {carbohydrate) and nitrogen sources until
antibiotic activity due to
nematophin is imparted to the medium. The fermentation may be carried out for
a time period such as
approximately 48 to 96 hours, at the end of which time the antibiotic
nematophin has been formed, and
may be isolated from the fermentation medium and purified.
After the fermentation has been completed, the fermented broth may be filtered
or centrifuged and
the pH of the filtrate adjusted to about 7.0 by the addition of hydrochloric
acid or kept as it was. The
E'rltrate may then be extracted with a water immiscible organic solvent, for
example, with ethyl acetate or
.chloroform. The combined organic laryers (e.g. pooled ethyl acetate or
chloroform extracts) may be
concentrated in vacuum (e.g. at about 30°C.) to an oily residue
("syrup"). The oil may be mixed with a
small amount of organic solvent and chromatographed on a silica gel column (EM
Science, Darmstadt,
Germany). After introduction of the sample, chloroform or other organic
solvent may be applied to elute
the bioactive fraction out.
The relative simplicity of the organic molecules of the present instance lend
themselves to organic
synthetic methods, in addition to microbial production methods. Such standard
synthetic processes are
described in many parts of the chemical literature(Vogel, 1989) and provide a
practical way for large
scale production. The reaction of tryptamine and 2-oxo-3-methylbutanoic
chloride, or acid, or anhydride
4
CA 02172854 2002-04-10
yields nematophin, while the reaction of substitutod tryptamine and 2-oxo-
allcanoic acid or its
derivative gives the amide derivatives of nematophin, 3-indoleethyl 2-oxo-
alkanamides, and the
reaction of substituted tryptol and 2-oxo-alkanoic acid or its derivative
gives the ester derivatives of
nematophin, 3-indoleethyl 2-oxo-alkanoates.
The antibiotic and use thereof
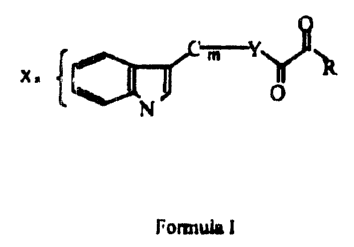
Nematophin and its derivatives possess antibacterial and antimycotic
properties, and have
been found to have the characteristics shown in the Formula and in Examples
herein.
The compounds of the present invention include nematophin and its derivatives,
and salts
thereof. The compounds of the present invention form salts with acids when a
basic amino funcion,
i.e. carboxyl, is present. All such salts are useful as acceptable salts with
both acids and bases.
Suitable acids include, for example, hydrochloric, sulfuric, nitric,
benzeenesulfonic, acetic, malefic,
tartaric and the like. Basic salts for use are the NA, K, Ca, and Mg salts,
and the like.
It is preferred that the inventive compounds have a degree of purity such that
they are
suitable for use as antibiotic agents. A particularly preferred embodiment of
the instant invention
provides nematophin or a derivative, or a salt thereof in a substantially pure
state. The substantially
pure compounds are preferably employed in the compositions and methods
described following.
The inventive compounds are useful as antimicrobial agents, useful in
inhibiting the growth
of microorganisms, particularly as an antibiotic drug, useful in treating
bacterial infection caused by
antibiotic resistant bacteria such as gram positive bacteria, for example,
bacteria of the genera
Bacillus and Staphylococcus. Inhibition of the growth of a bacterium may be
achieved by contacting
the bacterium with a compound of the present invention in an amount effective
therefore.
Thus, the compounds of the present invention may be employed in utilities
Replacement sheet
age 6 of 20
~;uitable for antibacterial and antimycotic agents.
The inventive compounds may, for example, be used in treating a host infected
with a bacterium
and fungus, comprising the step of administering to the host nematophin or a
physiologically tolerated
salt thereof in an amount effective for the treatment. Treatment of such
infections according to the instant
invention includes both mitigation as well as elimination thereof.
Hosts treatable according to the method of the present invention include
plants and animals,
particularly mammals such as dogs, cats and other domestic animals and,
especially, humans. The dosage
i:orm and mode of administration, as well as the dosage amount, may be
selected by the skilled artisan.
'The dosage amount will vary with the severity of the infection, and with the
size and species of the host.
Exemplary daily dosages for an adult human are those within the range of from
about 2.5 mg
~.o about 2,000 mg/day. Administration to a mammalian host, may, for example,
be oral, parenteral, or
'topical. Administration to a plant host may be accomplished, for example, by
application to seed, foliage
~~r other plant part, or to the soil.
Compositions are also provided by the present invention which comprise
nematophin or a
physiologically tolerated salt thereof in an amount effective for the
treatment of infection by a
microorganism, and a physiologically tolerated vehicle or diluent. The term
"physiologically tolerated" is
equivalent to the term "pharmaceutically acceptable" when used in reference to
the treatment of a
mammalian host. The appropriate solid or liquid vehicle or diluent may be
selected, and the compositions
prepared, by methods known to the skilled artisan. Treatment of simultaneous
infections by more than
one bacterium is, or course, contemplated.
The inventive compounds may be employed also as antibacterial and antimycotic
agents useful in
inhibiting the growth of microorganisms present on a surface or in a medium
outside a living host. The
age 7 of 20
present invention therefore provides a method for inhibiting the growth of at
least one rrricroorganism
present on a surface or in a medium, comprising the step of contacting the
surface or medium with
nematophin or a derivative, or a salt thereof in an amount effective for the
inhibition. Thus, the inventive
compounds may be employed, for example, as disinfectants for a variety of
solid and liquid media
susceptible to microbial growth. Suitable amounts of the inventive compounds
may be determined by
methods known to the skilled artisan. Compositions comprising nematophin or a
salt thereof in an amount
affective for inhibiting the growth of at least one bacterium, and a vehicle
or diluent, are also provided by
the present invention.
For agricultural application, the bactericidal and fungicidal compositions may
be formed using
~~ne of the active ingredients in an inert carrier. If' formulated as a solid,
the ingredients may be mixed
with typical carriers such as Fuller's earth, kaolin clays, silicas or other
wettable inorganic diluents.
Free-flowing dust formulations may also be utilized by combining the dry
active ingredient with finely
divided solids such as talc, kieselguhr, pyrophyllite, clays, diatomaceous
earth and the like.
The powders may also be applied as a suspension or solution, depending on the
solubility in
the liquid carrier. Pressurized sprays, typically aerosols with the active
ingredient dispersed in a low
boiling dispersant solvent carrier, may be used. Percentages of weight may
vary according to the
manner in which the composition is to be applied, and formulation used. In
general, the active
ingredient will comprise 0.005% to ~)5% of the active ingredient by weight in
the bactericidal and
fungicidal composition. The bactericidal and fungicidal composition may be
applied with other
ingredients, including growth regulators, insecticides, fertilizers, and the
like Formulation of the
active ingredients to assist applicability, ease handling, maintain chemical
stability and increase
effectiveness may require addition of various materials. Solvents may be
chosen on the basis of
age 8 of 20
affecting the volubility of the active ingredient, fire hazard and flash
point, emulsifiability, specific
l;ravity and economic considerations. Adjuvants may be added to enhance the
active ingredients, and
can include surfactants which are anionic, cationic or nonionic. Stabilizers
and antifreeze compounds
will prolong storage. Additionally, synergists, stickers, spreaders and
deodorant compounds can be
added to improve the handling characteristics of the commercial formulation.
Alternatively, the active
ingredient can be combined with an inert carrier, such as calcium carbonate,
and formed into a pill or
other consumable delivery device, including controlled release devices
intended to deliver metered
doses of the active ingredient.
The following examples are provided to further illustrate the invention, and
are not intended to in
;any way limit the scope of the instant claims.
EXAMPLE 1.
Preparation of nematophin
A. Isolation of the nematophin from the BC 1 strain of .X f~ernatophilrr.s
Cultures were shaken at 120 rpm on an Eberbach gyrorotary shaker for 24 h at
:ZS°C.
Bacterial fermentation was initiated by adding 100 ml of this bacterial
culture to 900 ml of tryptic soy
broth (TSB) in a 2,000 ml flask. The flask was incubated in the dark at
25°C on a gyrorotary shaker.
After 96 h, the culture was immediately centrifuged (12,000 g, 20 minutes,
4°C) to separate the
bacterial cells. The cell-free broth (41) was then extracted with ethyl
acetate 4 times. The combined
extracts were dried with anhydrous sodium sulfate and then filtered through
filter paper. The filtrate
was concentrated on a rotary evaporator below 30°C under vacuum to
yield a brown oil.
approximately 2.1 g of the oil was obtained. The crude extracts were then
loaded onto a silica gel
8
age 9 of 20
(200g silica gel 60, 40 em x 5 cm, EM Science, Darmstadt, Germany)
chromatographic column. The
bioactive component (NID)(nematophin, + stereoisomer) was eluted out with 100%
chloroform.
13. Identification of the active component (NID) from BC 1 strain of X.
nematophihis
NMR spectra were recorded on a Broker WM400 spectrometer in CDC13, using
residual
CHC13 07.25) as internal standard. Low resolution mass spectra were obtained
on a Hewlett-
~Packard 5985B GC/MS system operating at 70 eV using a direct probe. High
resolution MS spectra
were recorded on a Kratos MS80 instrument. IR spectra were recorded as neat
film on NaCI using a
:Perkin-Elmer 599B spectrometer. (Abbreviations used as follows: EI = Electron
Impact, M
=Molecular ion, t = triplet, J = coupling constant, Hz = Hertz, d == doublet,
m = multiplet, sext =
;sextet, dd = doublet doublet, q = quartet, bs = broad singlet, hept =-
heptet).
NID: [a,]2S D= +1.0 (c 0.58, CHCl3); EIMS: 273(M+1, 24), 272(M, 24), 144(:30),
143(89),
131(11), 130(100), 115(6), 103(5), 77(7), 57(8); HRMS: 272.1528 (Calc. for
C16H21N202:
272.1525, 22), 143.0727 (Calc. for CI OH9N: 143.0735, 100), and 130.0654
(Calc. for C9H8N:
130.0657, 95); IR(KBr): 3369, 2969, 2933, 2874, 1683, 1619, 1533, 1458, 1380,
1339, 1227, 1168,
1099, 138crn-I; 1HNMR (CDCl3) 8: 8.03 (1H, bs,-NH), 7.61 (IH, dd, J=7.9Hz,
J=0.6Hz), 7.38 (1H,
d, J=8.2Hz), 7.22 ( 1 H, td, J=8.1 Hz, 1 2Hz), 7.1 3 ( l H, td, J=7.9Hz, J== l
.OHz) 7.05(2H, bd, J=2.4Hz),
3.64(2H, q, J=6.8Hz, CH2), 3.49( 1 H, sext, J=6.7Hz, CH), 3 .02(2H, t,
J=6.9Hz, CH2), 1.70 ( 1 H, m),
1.39 (1H, m), 1.08(3H, d, J=6.7Hz, CH3), 0.88(3H, t, J=7.4Hz); 13CNMR (CDC13)
8: 202.4(s, CO),
160.1(s, CONH-), 136.5(s), 127.2(s), 122.3(d), 122.0(d), 119.6(d), 118.7(d),
112.6(s), 111.3(d),
40.4(d, CH), 39.6(t, CH2), 25.5(t, C"H2), 25.2(t, CH2), 15.2(q, CH3), 11.5(q,
CH3).
9
.ige 10 of 20
(:. Synthesis of racemic 3-indoleethyl (3-methyl-2-oxo)pentanamide (SID)
(nematophin, racemic
mixture).
Sodium 3-methyl-2-oxopentanoate (Aldrich chemical company, Milwaukee, WI, USA)
was
treated with 10% HCI. The acidic solution was extracted with ethyl ether 3
times. The combined
ether extracts were then washed with saturated NaCI solution twice, dried over
anhydrous Na2S04
and concentrated under vacuum under 25°C. Thionyl chloride was then
added at room temperature
~:0 3-methyl-2-oxopentanoic acid, obtained from above process, under stirring.
After stirring for 2 h
;~t about SO°C, the excess of thionyl chloride was evaporated. The
solution of tryptamine (Aldrich) in
~~yridine in excess was added to this crude 3-methyl-2-oxopentanoyl chloride
at 0°C. After the
reaction was continued under stirring at room temperature for over 2 h, it was
quenched with water.
The reaction mixture was then extracted with ether 3 times. The combined ether
extracts were then
washed with saturated NH4C',l 3 timE;s, 5% NaOH twice, water twice, saturated
NaCI twice, dried
over anhydrous Na2S04 and concentrated under vacuum under 30°C. The
crude product was then
subjected to silica gel chromatography with ethyl acetate : hexane (1:2) as
eluent to yield racemic 3-
indoleethyl (3-methyl-2-oxo)pentanamide (SID) which had the same Rfvalues as
those of NID on
thin layer chromatographic plates developed with different solvents.
The MS, IR, and 1HMNR spectra of'the product SID were identical to those of
the isolated
compound NID.
EXAMPLE 2.
Nematophin as an antibiotic
The following experiments were conducted, demonstrating the antibiotic
properties of nematophin.
to
ge 11 of 2U
To determine minimum inhibitory concentration (MIC) of the nematophin, the
standard
procedures (The National Committee for Clinical Laboratory Standards and
Motheds for Evaluating
Pesticides for Control of Plant Pathogens of the Ammerican Pytopathological
Society) for testing
antibiotics was followed. Briefly, test chemicals were dissolved in dimethyl
sulphoxide (DMSO), filter
sterilized and diluted into TSB or potato dextrose broth (PDB) or distilled
water resulting a final DMSO
~~oncentration < 0.4%(v/v) at a chemical stock concentration of 1,000~rg/ml.
The active compounds were
serially diluted by twofold {or mixed with equal amount of media /agar) to
produce culture media
~~ontaining the compound from 100~rg/ml to 0. llrg/ml (i.e.100, 50, 25, 12.5,
6.3, 3.2, 1.6, 0.8, 0.4, 0.2,
0.1) for the determination of MICs. Test bacteria were grown on nutrient agar
for 24h (36°C), then were
scraped from the plate by flooding the plate with 0.8% saline and diluted with
the saline to make inocula
.;containing 2.5-2.8x10 CFU/ml). B. oir~er~ea was grown on potato dextrose
agar for 7 d (25°C) before
vhe conidia were harvested by flooding the plate with sterile, distilled water
and diluted to make the final
inocula(2.5-3.0x106 conidia/ml). 'The inoculated test media were incubated at
35°C IB. cir~enea 24°C)
;end the MICs were visually determined after 24 h incubation (2 d for B.
cir~erea). The MIC was defined
;~s the lowest chemical concentration which prevents the growth of the test
organism at the above
~~onditions. The tests on Phytophlhor~a iyrfe.sta~zs were done on rye agar
(Caten & Jinks, l 967). Chemicals
were diluted with distilled water, mixed with equal amount of agar, mycelium
plug (0.5 cm in diameter)
were placed in the center of each plate, incubated at room temperature, MICs
determined 4 d after
incubation.
Etesults: It was found that similar results were obtained from both liquid and
agar culture methods.
'Table 1 shows the MICs determined for the compounds against each bacterial
organism. In
a ,~ t
ge 12 of 20
conclusion, it is shown that 3-indoleethyl 2-oxo-pentanoate, isolated from
Xenorhabdus and racemic
mixture synthesized chemically show potent properties, in particularly against
antibiotic resistant
:> taphylococcus .
'CABLE 1: Minimum Inhibitory Concentrations (MIC) of nematophin, isolated from
Xenorhabdus
nematophilrrs or synthesized, on candidate microbial pathogens.
MICs(~tg/ml
j
Organisms NID SID
Bacilhr.s srrblilis 12 12
Botrylis cirrerea 12 12
Escherichia coli ATCC25922 >100 > 100
Phytophlhora ir~festarrs 25
Staphylococcus auretr.s ATCC292130.7 I .5
S. arrrerrs 0012* 0.7 I .5
S'. arrrerrs 0017 * 0. 7 I . 5
* clinical isolates of multi-antibiotic-resistant isolates, provided by S.
Farmer of the Canadian
Bacterial Diseases Network, Vancouver, British Columbia, Canada.
1JXAMPLE 3.
Preparation of the derivatives of nematophin
,~. Synthesis of 3-indoleethyl (2-oxo)propanamide.
Thionyl chloride was added to 2-oxopropanoic acid (Aldrich chemical company,
Milwaukee,
'JVI, USA) under stirring at room temperature. After stirring for 2 h at about
SOC, the excess of
thionyl chloride was evaporated. The solution of tryptamine (Aldrich) in
pyridine in excess was added
12
~ge 13 of 20
to this erode 2-oxopropanoyl chloride at 0°C. After the reaction was
continued under stirring at room
temperature for over 2 h, it was quenched with water. The reaction mixture was
then extracted with
ether 3 times. The combined ether extracts were then washed with saturated
NH4C1 3 times, 5%
NaOH twice, water twice, saturated NaCI twice, dried over anhydrous Na2S04 and
concentrated
under vacuum under 30°C. The crude product was then subjected to silica
gel chromatography with
ethyl acetate : hexane (1:1) as eluent to yield racemic 3-indoleethyl (2-
oxo)propanamide (PAMIDE).
PAMIDE: EIMS: 230(M, 2S), 144(15), 143(51), 131(11), 130(100), 115(5), 103(5),
77(8);
1HMNR (CDC13) 8: 8.03 (1f1, bs,-NH), 7.60 (III, dd, J=7.9Hz, J=0.6Hz), 7.38
(1H, d, J=8.2Hz),
7.22 (1H, td, J=8. lHz, 1.2Hz), 7.13( 1 H, td, J=7.9Hz, l .OHz) 7.05(2H, bd,
J=2.4Hz), 3.64(2H, q,
.I=6.8Hz, CH2), 3.02(2H, t, J=6.9Hz, CH2), 2.4(~(s, CH3).
B. Synthesis of other 3-indoleethyl (?-oxo)alkanarnides
The same process was used in the synthesis of 3-indoleethyl (2-oxo)butanamide
(BAMIDE).
3-indoleethyl (3-methyl-2-oxo)propanamide (MAMIDE).
BAMIDE: EIMS: 244(M, 24), 144(21), 143(62), 131(1 I), 130(100); IHMNR (CDCl3)
b:
.3.03 (1H, bs,-NH), 7.61 (IH, dd, J=7.9Hz, J=0.6Hz), 7.38 (1H, d, J=8.2Hz),
7.22 (1H, td, J=8.lHz,
l.2Hz), 7.13(1H, td, J=7.9Hz, J=I.OHz) 7.05(2H, bd, J=2.4Hz), 3.64(2H, q,
J=6.8Hz, CH2),
3.02(2H, t, J=6.9Hz, CH2), 2.94(2H., q, J=7.2Hz, C,'H2), 1.08(3H, t, J=7.2Hz,
CH3).
MAMIDE: 1HMNIZ (CDC13) b: 8.03 (1H, bs,-NH), 7.61 (IH, dd, J=7.9Hz, J=0.6Hz),
7.38
(1H, d, J=8.2Hz), 7.22 (1H, td, J=8. lHz, l.2Hz), 7.13(1H, td, J=7 9Hz,
J=I.OHz) 7.05(2H, bd,
t3
~ge 14 of 20
.1=2.4Hz), 3.63(2H, q, J=6.6Hz, CHp), 3.62( 1 H, sext, J=6.9, CH), 3.02(2H, t,
J=6.6Hz., CH2),
1.11(6H, d, J=6.9Hz, CH3).
~~. Synthesis of substituted 3-indoleethyl (3-methyl-2-oxo)pentanamides.
The same process as that discussed above was used in the synthesis of 5-methyl-
3-indoleethyl
~;2-oxo)pentanamide (5-MeIN), 5-methoxyl-3-indoleethyl (2-oxo)pentanamide {5-
MeOIN) and 6-
iluoro-3-indoleethyl (2-oxo)pentanamide (6-FIN) ) except that 5-methyl-
tryptamine and 6-fluoro-
1=ryptamine were used respectively instead of tryptamine.
5-MeIN: 1HMNR (C'.DCl3) b: 7.91 (1H, bs,-NH), 7.37 (IH, q, J=0.8Hz), 7.26
I;1H, d,
:f=8.2Hz), 7.03 (2H, m), 3.63(2H, q, J=6.8Hz, CH2), 3.49(1H, sext, J=6.7Hz,
CH), 2.99(2H, t,
:f=6.9Hz, CH2), 2.46(3H, s, CH3), 1.72 (1H, m), 1.39 (1H, m), 1.08(3H, d,
J=6.7Hz, CH3),
0.88(3H, t, J=7.4Hz).
5-MeOIN: 1HMNR (CDC13) 8: 7.92 (1H, bs,-NH), 7.27 (IH, dd, J=8.9Hz, J=O.SHz),
7.02
(2H, m), 6.87(1H, dd, J=8.lHz, l.2Hz), 3.86(3H, s, OCH3), 3.63(2H, q, J=6.8Hz,
CH2), 3.49(1H,
~;ext, J=6.7Hz, CH), 2.99(2H, t, J=6.9Hz, CH 2), 1. 70 ( 1 H, m), 1.39 ( 1 H,
m), 1.08(3H, d, J=6.7Hz,
(JH3), 0.88(3H, t, J=7.4Hz).
6-FIN: 1HMNR (CDC13) 8: 8.03 (1H, bs,-NH), 7.50 (IH, dd, J=8.7Hz, 5.3Hz). 7.05
(1H,
dd, J=9.6, 2.3Hz), 7.02 (1H, d, J=2.2Hz), 6.90(1H, td, J=9.lHz, J=2.3Hz),
3.62(2H, q, J=6.8Hz,
(lH2), 3.49( 1 H, sext, J=6.7Hz, CH), 3 00(2H, t, J=6.9Hz, CH2), 1.70 ( 1 H,
m), 1.39 ( 1H, m),
1.08(3H, d, J=6.7Hz, CH3), 0.88(3H, t, J=7.4Hz).
D. Synthesis of 3-indoleethyl (3-methyl-2-oxo)pentanoate (01N)
to
ige 1~ of 20
The same process as that for SlN was used in the synthesis of 3-indoleethyl (3-
methyl-2-
~~xo)pentanoate except that tryptol was used instead of tryptamine.
OIN: EIMS: 237(M, 0.6), 245(5), 144(19), 143(100), 131(6), 130(64), 115(7),
103(5),
77(7); 1HMNR (CDCl3) 8: 7.99 (1H, bs,-NH), 7.65 (IH, dd, J=7.8Hz, J=0.6Hz),
7.36 (1H, dd,
.I=8.0, 0.8Hz), 7.19 (1H, td, J=8.lHz, l.lHz), 7.15(1H, td, J=8.lHz, J=l.lHz)
7.05(2H, bd,
.I=2.4Hz), 4.36(2H, t, J=7.2Hz, CH2), 3.11(2H, t, J=6.9Hz, CH2), 2.37 (1H,
sext, J=6.9)1.66 (1H,
m), 1.46 (1H, m), 1.13(3H, d, J=6.71-lz, CH3), 0.88(3H, t, J=7.4Hz).
EXAMPLE 4.
The derivatives of nematophin as antibiotics
Similar bioassays as those described for nematophin were conducted,
demonstrating the
;antibiotic properties of the derivatives of nematophin.
'TABLE 2: Minimum Inhibitory Concentrations (MIC) of the derivatives of
nematophin on candidate
bacterial pathogens.
Chemicals MICs(yg/ml)
Sta~hylococ2~s c:rnr~cms ATCC29213
PAMIDE 100
BAMIDE 100
MAMIDE 3
5-MeOIN
5-MeIN
6-FIN 0. 7
OIN 25
1>
age 16 of 20
While our above description contains many specificities, these should not be
construed as
limitations on the scope of the invention, but rather as examples of preferred
embodiments.
.Accordingly, the scope of the invention should not be determined by the
embodiments presented, but
by the appended claims and their legal equivalents.
l6
CA 02172854 2001-03-29
References Cited
US Patent document:
Webster, John M., Jianxiong Li and Genhui Chen. Antibacterial And Antifungal
Properties Of Novel Indole Derivatives. US patent 5,569,668.
Publications:
1. Akhurst, R. J.and N. E. Boemare "A numerical Taxonomic Study of the Genus
Xenorhabdus (Enterobactereacea) and Proposed Elevation of the Subspecies of X.
nematophilus to Species" J. Gen. Microbial. Vol 134, pp. 1835-1845 (1988).
2. American Phytopathological Society. Methods for Evaluating Pesticides for
Control of Plant Pathogens. St. Paul, Ma, (1986).
3. Caten, C. E. and J. L. Jinks. Spontaneous Variability of Single Isolates of
Phytophthora infestans. I. Cultural Variation. Can. J. Bot. 46:329(1967).
4. Chen, G., G. B., Dunphy, and J.M., Webster. "Antifungal Activity of Two
Xenorhabdus Species and Photorhabdus luminescens, Bacteria Associated with
the Nematodes Steinernema Species and Heterorhabdus megidis. Biol. Control,
Vol. 4, pp 157- ( 1994).
5. Li et al. Antimicrobial Metabolites from a Bacterial Symbiont J. Nat. Prod
Vol.
58, 1081-1085 (1995).
6. Maxwell et al. Stability and Activities of Antibiotics Produced during
Infection
of the Insect Galleria mellonella by Two Isolates ofXenorhabdus nematophilus
Appl. Environ. Microbiol. Vol. 60, pp. 715-721 (1994).
Replacement sheet
17
CA 02172854 2001-03-29
7. McInerney et al. "Biologically Active Metabolites from Xenorhabdus spp.,
Part
1. Dithiolopyrrolone Derivatives with Antibiotic Activity" J. Nat. Prod. Vol.
54,
pp. 774-784 (1991a).
8. McInerney et al. "Biologically Active Metabolites from Xenorhabdus spp.,
Part
2. Benzopyran-1-one Derivatives with Gastroprotective Activity" J. Nat. Prod.
Vol. 54, pp. 785-795 (1991b).
9. National Committee for Clinical Laboratory Standards. Methods for Dilution
of
Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically.
Approved
standards M-7A2. National Committee for clinical Laboratory Standards,
Villaniova, Pa. ( 1990).
10. Paul et al., "Antibiotics in Microbial Ecology: Isolation and Structure
Assignment
of Several New antibacterial Compounds from the Insect-Symbiotic Bacteria
Xenorhabdus spp." J. Chem. Ecol. Vol. 7, pp. 589-597 (1981).
11. Putz et al. "Development and Application of Oligonucleotide Probes for
Molecular Identification of Xenorhabdus Species" Appl. Environ. Microbial.
Vol.
56, pp. 181-186 (1990).
12. Richardson et al., "Identification of an Anthraquinone pigment and a
Hydroxystilbene Antibiotic from Xenorhabdus ~PhotorhabdusJ"App. Environ.
Microbiol. Vol. 54, pp. 1602-1605 (1988)
13. Vogel, A. L, Vogels Textbook of Practical Organic Chemistry. 5th Ed.
Longman,
London ( 1989).
Replacement sheet
1s