Note: Descriptions are shown in the official language in which they were submitted.
WO 95!09667 PCTlUS94/10961
MEDICAL DEVICE BALLOONS CONTAINING
THERMOPLASTIC ELASTOMERS
BACKGROUND OF THE INVENTION
The present invention relates to catheters that can be
placed in bodily conduits. The invention particularly
relates to dilatation balloons and catheters using such
balloons for administering treatments to widen constricted
passages in, for example, angioplasty, valvuloplasty, or
~ urological procedures.
One example of such a procedure, angioplasty, is used
to treat a stenosis, i.e. to restore adequate blood flow to
a region of a blood vessel which has been narrowed to such a
degree that blood flow is restricted. Frequently the
stenosis can be expanded so that the vessel will permit an
acceptable blood flow rate. Coronary angioplasty, for
example, includes the insertion of a balloon catheter
through a patient's coronary artery to an arterial stenosis
and injecting a suitable fluid into the balloon to inflate
it, hence expanding the stenosis radially outwardly.
Angioplasty has proven to be a successful alternative to
coronary arterial bypass surgery.
Typically, balloon catheters have a balloon fastened at
at least one end around the exterior of a hollow catheter
shaft. The hollow interior of the balloon is in fluid flow
relation with the hollow interior of the shaft. The shaft
then may be used to provide a fluid supply for inflating the
balloon.
Presently used catheter balloons may be classified as
compliant or non-compliant balloons. Compliant balloons
expand and stretch with increasing pressure within the
balloon, and are made from such materials as polyethylene or
polyolefin copolymers. Non-compliant balloons, made from
such materials as polyethylene terephthalate (PET) or
polyamides, remain at a preselected diameter as the internal
CA 02173139 2005-09-02
60412-3241
2
balloon pressure increases beyond that required to fully
inflate the balloon.
Compliant balloon materials provide a degree of
softness to the balloon which aids its passage through,
e.g., blood vessels with minimal trauma. Known compliant
balloon materials also can display good abrasion and
puncture resistance at thicknesses typically used for
medical device balloons. However, as mentioned above, they
do not remain at the desired diameter with increasing
pressure. Such compliant balloons also lack sufficient hoop
strength to achieve high dilating forces.
A non-compliant balloon, that is one remaining at
a preselected diameter regardless of increasing pressure, is
often desirable. Typical non-compliant balloon materials do
not exhibit the same degrees of softness and abrasion
resistance as the compliant balloons.
It would be desirable, for many treatment
conditions, to have a dilatation balloon exhibiting the
combined characteristics of softness, abrasion and puncture
resistance, hoop strength, and the ability to maintain a
preselected diameter as the internal pressure within the
balloon is increased. The balloon described herein was
developed to address that need.
SUMMARY OF THE INVENTION
In one embodiment, the invention provides a
dilatation balloon for use in a medical catheter device,
said dilatation balloon comprising a first layer comprising
a thermoplastic elastomer in a blend with a first non-
compliant structural polymeric material, and a second layer
that is coextruded with the first layer.
CA 02173139 2005-09-02
60412-3241
3
The preferred thermoplastic elastomer includes an
engineering thermoplastic elastomer, for example a polyether
glycol/polybutylene terephthalate block copolymer. The
thermoplastic elastomer may be combined with the non-
compliant structural polymeric material as an outer
elastomeric layer disposed upon an inner structural layer of
the non-compliant structural polymeric material, as both an
inner elastomeric layer and an outer elastomeric layer
disposed upon an intermediate structural layer of the non-
compliant structural polymeric material, or as a blend of
the thermoplastic elastomer and the non-compliant structural
polymeric material.
In another embodiment, the invention provides a
catheter for insertion into a bodily conduit, said catheter
comprising: a shaft defining a lumen for delivery of fluid
inflation media; and a dilatation balloon bonded to said
shaft and defining a chamber, said chamber being in fluid
communication with said lumen to permit inflation of said
chamber, wherein said dilatation balloon comprises a first
layer comprising a thermoplastic elastomer in a blend with a
first non-compliant structural polymeric material, and a
second layer that is coextruded with the first layer.
In yet another embodiment, the invention provides
a method for fabricating a dilatation balloon for use in a
medical catheter device, said method comprising the steps
of: producing a generally cylindrical balloon blank
comprising a first layer comprising a thermoplastic
elastomer in a blend with a first non-compliant structural
polymeric material, and a second layer that is coextruded
with the first layer; and shaping said balloon blank to
produce said dilatation balloon.
CA 02173139 2005-09-02
60412-3241
3a
In another embodiment, the invention provides a
dilatation balloon for use in a medical device, the balloon
having a structural layer which is a blend consisting
essentially of polyethylene terephthalate as its major
component and as a minor component a randomized block
copolymer of polyether glycol and polybutylene
terephthalate.
In another embodiment, the invention provides a
dilatation balloon for use in a medical device, the balloon
comprising a structural layer which is a blend consisting
essentially of polyethylene terephthalate as its major
component and as a minor component a randomized block
copolymer of polyether glycol and polybutylene
terephthalate, the balloon further comprising an elastomeric
layer outside of the structural layer, said balloon being
made by a process which comprises coextruding the
elastomeric layer and the structural layer.
In another embodiment, the invention provides a
dilatation balloon for use in a medical device, the balloon
having a structural layer which is a blend comprising at
least two components: a) polyethylene terephthalate as its
major component; and b) as a minor component a randomized
block copolymer of polyether glycol and polybutylene
terephthalate, said blend components not being covalently
bound to other molecules.
The balloon blank may be produced by disposing an
elastomeric layer including the thermoplastic elastomer upon
a structural layer including the non-compliant structural
polymeric material to produce a layered, generally
cylindrical balloon blank. The thermoplastic elastomer and
CA 02173139 2005-09-02
60412-3241
3b
the non-compliant structural polymeric material may be
coextruded to produce the balloon blank. Alternatively, the
balloon blank may be produced by preparing a blend of the
thermoplastic elastomer and the non-compliant structural
polymeric material. A generally cylindrical balloon blank
if formed from the blend, and the balloon blank is then
shaped to produce the dilatation balloon. The balloon blank
may be shaped to have a generally cylindrical central
portion and generally conical end portions.
WO 95/09667 PCT/US94/10961~
4
IEF DESCRIPTION OF THE DRAWINGS
For a better understanding of the present invention,
together with other objects, advantages, and capabilities
thereof, reference is made to the following Description and
appended Claims, together with the Drawings in which:
Figure 1 is an elevation view of a medical balloon
catheter, partly in section, in accordance with one
embodiment of the present. invention;
Figure 2a is a cross-sectional view of the balloon of
Figure 1, taken along line 2a-2a, showing the balloon
layers;
Figures 2b, 2c, and 2d are cross-sectional views
similar to that shown in Figure 2a (omitting the shaft
distal end) illustrating balloons in accordance with
alternate embodiments of the invention.
DEmATLED DESCRIPTION OF THE PREFERRED EMBODIMENTS
An exemplary embodiment of the balloon and catheter in
accordance with the invention is described herein. The
angioplasty catheter includes a balloon mounted at the
distal end of a shaft including at least one lumen for
inflation of the balloon. The balloon is a generally
tubular body fabricated from a combination of a non-
compliant structural polymeric material and a thermoplastic
elastomer (TPE). The combination may be in the form of
coextensive coextruded layers, otherwise disposed layers,
blends, or blended layers of these materials. Once the
catheter is in position within the patient's artery, a fluid
inflation medium may be introduced via the lumen to inflate
the balloon to the preselected desired diameter.
The term "structural polymer" or "structural polymeric
material", as used herein, is intended to mean any polymeric
material suitable for use in medical balloons and compatible
with the TPE selected. As mentioned above, the term '°non-
compliant", as used herein, is intended to mean remaining at
a preselected diameter as the internal pressure in the
CA 02173139 2004-11-30
60412-3241
balloon is increased above that required to fully inflate
the balloon. The structural layer of the balloon must be
self supporting and capable of supporting at least one TPE
layer thereon. Suitable non-compliant structural polymeric
5 materials include, for example, modified polyesters,
polyethylene terephthalate (PET), modified polybutylenes,
polyvinyl chlorides, polyamides (e.g. Nylon), etc., or a
combination thereof. Preferred are biaxially oriented non-
compliant structural materials; most preferred is biaxially
oriented PET.
The term ~~thermoplastic elastomer" or ~~TPE", as
used herein, is intended to mean a polymeric material that
combines the mechanical properties of a thermoset rubber,
i.e. resiliency, softness, and toughness, with the
production economics of a thermoplastic polymer. The TPEs
include styrenic block copolymers, polyolefin blends (TPOs),
elastomeric alloys, thermoplastic polyurethanes (TPUs),
thermoplastic copolyesters, and thermoplastic polyamides.
These materials have varying patterns of hard and soft
segments included in the polymer chain or compound. The
hard segments melt or soften at processing temperatures,
producing a melt processable material for ease of
fabrication. In block copolymer TPEs, the hard and soft
regions are in the same polymer chain. Descriptions of
various types of TPEs may be found in Modern Plastics
Encyclopedia 1988, Vol. 64, No. 10A, pp. 93 - 100 (October
1987), and in Modern Plastics Encyclopedia 1990, Vol. 66,
No. 11, pp. 122 - 131 (Mid-October 1989).
The preferred TPEs for the balloon described
herein are engineering thermoplastic elastomers (ETEs),
which are randomized block copolymers having polyester
crystalline hard segments and amorphous glycol soft
segments. ETEs possess flexibility over a useful range of
' CA 02173139 2004-11-30
60412-3241
5a
strain, and are quite extensible when operating within their
elastic limit. Another advantage of ETEs for medical
devices is their
WO 95/09667 PCTlUS94/10961~
6
resistance to most radiation, permitting sterilization by
such means, although they must be protected from W
radiation.
The more preferred ETEs for use in the medical devices
described herein are randomized block copolymers of
polyether glycol and polybutylene terephthalate (PBT).
These combine crystalline PBT hard segments with melt stable
glycol soft segments, and come in a wide range of stiffness
grades. Most preferred are those having a flexural modulus
of about 21,000 - 440,000 psi (as measured in accordance
with ASTM D790, Method 1), for example Hytrel~ polymers
(available from E.I. DuPont de Nemours and Company,
Wilmington, DE).
As mentioned above, the combination of a TPE and a non-
compliant structural polymer may be in the form of blends,
coextensive coextruded layers, otherwise disposed layers, or
layers of blends of these materials. Suitable blends
include homogeneous and near-homogeneous blends, which may
be prepared by such conventional means as stirring, mixing,
compounding, etc.
In a layered embodiment of the balloon, one or more
base structural polymer layers are formed, for example by
extrusion, from a non-compliant structural polymer, as
described above. Alternatively, the base non-compliant
structural layer is formed from a blend of two or more
structural polymers, a blend of a structural polymer with a
minor amount of another polymeric material, or a blend of a
structural polymer with a minor amount of a TPE. As used
herein, the term ''minor amount°° is intended to mean an
amount selected to make the additive no more than a
secondary component, for example less than 50 weight %, of
the blend. The material of the structural layer, however,
must still contribute to the balloon the properties
described above for the structural material. This base
structural layer (or layers) is typically at least about
WO 95/09667 PCT/US94/10961
7
0.2 - 1.5 mil thick, and gives the balloon its tensile
' strength so that the balloon wall is self supporting.
At least one additional, elastomeric outer layer about
0.2 - 0.5 mil thick is coextruded with or otherwise disposed
on the base layer and, typically, generally coextensive
therewith. Normally, the elastomeric layer is significantly
thinner than the structural layer. The material of this
outer layer is based on a thermoplastic elastomer (TPE)
which, in some embodiments, may be combined in a blend with
other polymers known to be suitable for medical balloons.
The amount of these other polymers, however, should be
within limits which would permit such a blend to contribute
to the balloon the properties described herein for such an
elastomeric layer. Especially preferred for this outer
elastomeric layer is a blend of an ETE with a small amount
of a non-compliant structural polymer, e.g. a blend of about
1 - 10 weight ~ PET, remainder Hytrel elastomer.
In some of the above-described layered balloons, it may
be advantageous to dispose or coextrude an adhesive or other
polymer layer between two or more of the layers. In one
embodiment, an adhesive layer may be included to improve
adhesion between coextensive balloon layers and, if desired,
may be applied for adhesion of the medical device balloon to
a catheter shaft. In another embodiment, an additional
polymer layer may be included to contribute other desirable
properties to the balloon, for example to contribute further
to the softness and/or foldability of the balloon. In other
embodiments, the adhesive or other polymer may be blended
with a structural and/or elastomeric layer to contribute its
properties to the balloon. For example, in a three layer
balloon an adhesive polymer may be blended with a structural
polymer layer to improve adhesion of inner and outer ETE
layers to the structural layer. The amount of adhesive or
other polymer in such a blend is selected to provide the
desired enhancement of properties while permitting the blend
to possess the properties described herein for such a layer.
WO 95/09667 PCT/US94/1096~
8
Examples of adhesive materials for forming this layer or
blend are Bynel~ adhesive resin (E.I. DuPont de Nemours and
Company, Wilmington, DE) or Plexar~ adhesive resin (Quantum
Chemical Corp., Cincinnati, OH). Selar~ modified PET resin r
(E.I. DuPont de Nemours and Company, Wilmington, DE) is a
suitable polymer intermediate layer or blend additive for
improving softness and foldability of the balloon. Bynel
and Plexar resins can also serve to improve the abrasion
resistance and puncture resistance of the balloon, and
provide it with a softer feel.
In another embodiment of the balloon, a single layer
balloon wall is fabricated from a blend of a non-compliant
structural polymer and a TPE. The TPE, preferably the
above-described polyether glycol/PBT block copolymer, is
blended with the structural polymer in a TPE-to-structural
polymer ratio selected to provide the desired degree of
softness and abrasion resistance to the balloon without
unduly compromising the hoop strength or the desired
inflated diameter. As mentioned above, such blends may be
homogeneous or near-homogeneous, and may be blended in any
of several ways known in the art. Typical polymer ratios
for such a single layer balloon are about 40:60 to 60:40,
TPE:structural polymer.
In other embodiments, the TPE/structural polymer blend
used in the above-described single layer balloon may be used
as a structural layer in combination with other layers, or
may be blended to be used as an elastomeric layer in a
layered balloon. The polymer ratio for a blended structural
layer of such a balloon is typically about 40:60 to 60:40,
TPE:structural polymer; that for elastomeric inner or outer
layers is typically about 30:70 to 60:40, TPE:structural
polymer. The exact ratios within these ranges to produce
specific balloon characteristics are empirically determined
with minimal experimentation. These blended layers may be
used with or without an adhesive or softening component or
layer as described above
WO 95/09667 PCT/US94/10961
9
The use of thermoplastic elastomers in medical device
balloons results in a superior balance of balloon properties
when used as one or more outer layers over a structural
layer of currently used balloon materials or other suitable
structural polymers, or as outer and inner layers
surrounding such a structural layer. Alternatively, this
superior balance of balloon properties may be achieved by
using TPEs as a blend with currently used balloon materials
or other suitable structural polymers. By varying the
fabrication method and/or layer materials and/or blend
materials and ratios, as described herein, the structural
and surface properties of the ETE containing balloon may be
precisely tailored for a desired procedure.
The description below of various illustrative
embodiments shown in the Drawings refers to engineering
thermoplastic elastomers (ETEs). However, it is not
intended to limit the scope of the present invention, but
merely to be illustrative and representative thereof.
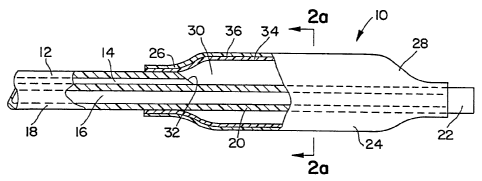
Referring now to Figure 1, catheter 10 in accordance
with one embodiment of the present invention includes shaft
12 having lumens 14 and 16 extending therethrough, and
having a proximal end 18 and a distal end 20. Distal end 20
extends to catheter tip 22. Dilatation balloon 24, shown in
Figure 1 in its inflated state, surrounds shaft distal
end 20. Balloon proximal end 26 is bonded to shaft distal
end 20 at a point spaced from tip 22, and balloon distal
end 28 is bonded to shaft distal end 20 near tip 22, each,
e.g., by a suitable adhesive (not shown). Balloon 24
defines balloon chamber 30 which is in fluid communication
with lumen 14 via aperture 32. Thus, balloon 24 may be
inflated by passing a fluid inflation medium through lumen
14 and aperture 32 into chamber 30. Lumen 16 may be used,
for example, to contain a guidewire or other device.
As shown in Figures 1 and 2a, dilatation balloon 24
surrounding shaft distal end 20 is made up of two layers, 34
and 36, of differing polymeric materials. Inner layer 34 is
WO 95/09667 PCT/US94/1096~
a structural layer of, e.g., PET approximately 0.2-1.0 mil
thick. Outer layer 36 has been co-extruded to be co-
extensive with layer 34, and is a layer of ETE, e.g. Hytrel .
copolymer, about 0.2 - 0.5 mil thick. '
5 Figures 2b, 2c, and 2d each illustrate alternate
embodiments of the balloon of the invention in cross-
section, similarly to Figure 2a. For simplicity, however,
shaft distal end 20, although actually present in the same
position as shown in Figure 2a, is not depicted in the view
10 shown in Figures 2b-2d.
Figure 2b illustrates in cross-section dilatation
balloon 24a, fabricated from single layer 38 of a blend of a
structural polymer, e.g. polyethylene terephthalate, with an
ETE, for example Hytrel copolymer.
Figure 2c shows balloon 24b fabricated from, e.g.,
coextruded triple layers, 34a, 36a, and 40. Structural
layer 34a and ETE outer layer 36a are similar to layers 34
and 36 of Figures 1 and 2a. In the embodiment illustrated
in Figure 2c, however, an additional ETE layer, innermost
layer 40, has been coextruded to be coextensive with layers
34a and 36a and internal thereto. Innermost layer 40
provides additional tear resistance to protect the balloon
wall from damage from internal pressure. Layer 40 also
provides for a softer, more foldable balloon.
Figure 2d illustrates balloon 24c, fabricated in a
similar manner to balloon 24 of Figures 1 and 2a, and having
inner structural layer 34b and ETE outer layer 36b. Thin
intermediate adhesive layer 42 of, e.g., Bynel resin is
coextruded with and between layers 34b and 36b to be
coextensive therewith, acting to bond together more securely
layers 34b and 36b.
In other alternate embodiments, one or more of layers
34, 34a, and 34b may be a blend of a structural polymer with
an ETE. Also alternatively, one or more of layers 36, 36a,
36b, or 40 may be a blend of ETE with a structural polymeric
material. In the embodiment of Figure 2c, a sufficient
WO 95/09667 PCT/US94/10961
11
amount of a polymeric adhesive to improve bonding of the
layers may be blended into layer 34a. Alternatively, layer
34a may be , e.g., a Selar resin balloon softening layer.
Also alternatively, the adhesive or other polymeric additive
may be blended into, e.g., layer 34, 36, 36a, 40, etc., as
described above. In other alternate embodiments, not shown,
the balloon may have more than one innermost and/or
outermost ETE layer. For example, a balloon may be similar
to that shown in Figure 2a but have an additional ETE layer
between layers 34 and 36, or may be similar to that shown in
Figure 2c but have an additional ETE layer between layers
34a and one or both of layers 36a and 40.
In operation, the catheter device including the novel
dilatation balloon is inserted into the vasculature of a
patient, and is manipulated into position by torquing,
pushing, and pulling. Positioning of the catheter is aided
by the softness of the balloon provided by the TPE component
of the balloon. Once the catheter is in position, the
balloon is inflated to the preselected diameter, then
deflated via the central lumen of the shaft. The inclusion
of a non-compliant structural polymer in the balloon makes
possible such preselection of the diameter. Upon completion
of the dilation procedure and deflation of the balloon, the
catheter is removed from the patient. Removal of the
catheter is also aided by the softness contributed to the
balloon by the TPE component.
The invention described herein presents~to the art
novel, improved catheters and composite medical device
balloons including thermoplastic elastomers as (a) one or
3o more layers in addition to one or more layers of currently
used balloon structural materials or other suitable
structural polymers, or (b) as a blend with such materials.
The inclusion of TPE results in a superior balance of
balloon properties. For example, softer feel; superior
abrasion and puncture resistance; lower required insertion,
placement, and withdrawal forces; lower balloon resistance
WO 95/09667
PCT/US94/1096
12
to inflation and deflation pressure; superior refoldability,
with fold memory; and the ability to maintain a preselected
diameter are all achievable in a single balloon fabricated
as described herein. Thus, the balloon described herein can
provide a non-compliant balloon with the softness of a
compliant balloon, as well as a soft balloon with ranges of
burst strength and hoop strength equivalent to those of
harder balloons. The use of the adhesives and other layers
or layer additives described herein, especially the Bynel
to and Plexar adhesives and Selar additive described, can offer
advantageous adhesive and/or softening properties. By
varying the fabrication method and/or layer or blend
materials and ratios as described herein, the balance of
structural and surface properties of the TPE containing
15 balloon may be precisely tailored for a specific procedure.
While there has been shown and described what are at
present considered the preferred embodiments of the
invention, it will be apparent to those skilled in the art
that modifications and changes can be made therein without
20 departing from the scope of the present invention as defined
by the appended Claims.