Note: Descriptions are shown in the official language in which they were submitted.
2 1 73282
TS 8542
PROCESS FOR REMOVING AROMATICS FROM HYDROCARBON
VAPOUR STREAMS
The present invention relates to a process for
removing aromatic hydrocarbons from hydrocarbon vapour
streams and in particular to a process for removing
monoaromatics, suitably benzene, from catalytic reformate
streams suitable as gasoline blending components.
Three general types of hydrocarbon components are
normally used to blend a finished gasoline. These
hydrocarbon components are paraffins, olefins and
aromatics, each having its own characteristics and
contribution to the properties of the final gasoline. The
paraffins include inter alia butane, isopentane,
alkylate, isomerate, straight-run naphtha and light
hydrocrackate and accordingly can be derived from a
number of different refinery processes, such as crude oil
distillation, isomerization, alkylation and
hydrocracking. The olefinic components include monomers,
oligomers and polymers and are usually obtained from
processes such as catalytic cracking, steam cracking and
polymerisation. The aromatic components, finally, are
usually obtained via catalytic reforming, which is a
I combination of various reactions (dehydrogenation,
isomerization, dehydrocyclization and hydrocracking) and
which has the aim of increasing the octane number of the
gasoline. A commonly used feedstock for catalytic
refoEming processes is heavy naphtha which contains a
relatively high amount of paraffinic hydrocarbons,
including straight-chain, branched and cyclic paraffins.
Catalytic reforming processes are well known in the
art. In general, the catalytic reforming results in the
boiling range of the reformer feed not being
2 1 732~2
-- 2
significantly changed, whereas the chemical composition
of the feed is significantly changed by the conversion of
paraffinic hydrocarbons into aromatic hydrocarbons with
hydrogen being formed. As is generally recognised, the
aromatics have a positive effect on the octane number and
therefore the products from the catalytic reformer are
very suitable gasoline blending components. Catalytic
reforming processes usually involve passing a naphtha
type feedstock over a suitable reforming catalyst under
reforming conditions, recovering the hydrogen formed and
separating the product into two or more reformates.
Usually, two or three reformates are formed by using a
two- or three-cut splitter, respectively. The composition
of each of the reformates obtained can be controlled to a
certain extent by the cutting temperature(s) and pressure
applied in the splitter. The top fraction or light
reformate recovered from the splitter usually comprises a
major amount of non-aromatic hydrocarbons and a minor
amount of monoaromatics, particularly benzene and to a
lesser extent toluene. The concentration in the light
reformate of monoaromatics in general and of benzene and
toluene in particular, can be regulated by variation of
the cutting temperature. Beside a light reformate one or
more heavier reformates are obtained, which comprise the
heavier aromatic hydrocarbons and which may suitably be
used as motor gasoline blending components. The light
reformate containing the benzene is also suitably used as
a blending component for gasoline. However, over the past
years the presence of benzene in motor gasoline has
become more and more undesired for environmental reasons
and it is expected that environmental limits on benzene
content of motor gasoline in the future will be below
current benzene levels in most motor gasolines.
Accordingly, there is an incentive to lower the benzene
concentration in motor gasolines.
2 1 73282
Benzene can be removed from a reformate stream by
ways known in the art. One way is fractional
distillation, another way is via an extraction process,
whereby use is made of an extracting solvent capable of
selectively dissolving monoaromatics and in particular
benzene. Ethylene glycols and N-substituted morpholines
are known to be suitable solvents for this purpose, but a
particular useful solvent is sulfolane. However, although
these methods remove benzene from the reformate, the
resulting benzene concentrate still contains substantial
amounts of other components beside benzene. Such
components typically include valuable raffinate
components which could suitably be used as gasoline
blending component. It would accordingly be advantageous
if these valuable raffinate components could be recovered
to add to the purified raffinate stream which is to be
used as gasoline blending component. Moreover, since
benzene is a valuable product, it would be advantageous
to provide a benzene concentrate having a higher benzene
concentration, thus making the concentrate an
economically attractive source for the production of
benzene.
In International Patent Application WO-A-94/19426 a
process for removing aromatics from a hydrocarbon vapour
stream is disclosed, which process involves contacting
the hydrocarbon vapour stream with a liquid absorbent
solvent which selectively absorbs the aromatics and
withdrawing at the upper portion of the absorption zone a
raffinate vapour stream which predominantly comprises
non-aromatic hydrocarbons, whilst withdrawing at the
bottom part of the absorption zone an aromatics-rich
liquid solvent. This aromatics-rich solvent is suitably
treated in a subsequent distillation step to produce a
distillate stream predominantly comprising aromatic
hydrocarbons and a bottom stream predominantly comprising
2 ~ 73282
-- 4
liquid absorbent solvent, which can be recycled to the
absorption zone. The process is particularly suitable for
the removal of benzene from gasoline blending stocks,
thereby producing a benzene concentrate having a
relatively high benzene concentration. However, this
concentrate still contains a significant amount of
components other than benzene, mainly non-aromatic
hydrocarbons which would be very useful components in
gasoline. Accordingly, a further separation treatment of
the benzene concentrate to produce benzene meeting the
specifications to be sold as such is absolutely required,
whilst in transporting the concentrate to benzene
manufacture facilities the volume of components other
than benzene increases the transport costs per volume
unit of benzene which has its impact on the cost price of
the final benzene product.
The present invention aims to provide a process not
having the disadvantages of the prior art processes. More
specifically, the present invention aims to provide a
more effective process for removing aromatic hydrocarbons
from hydrocarbon vapour streams, whereby the aromatics-
concentrate obtained contains a reduced amount of non-
aromatic hydrocarbons and has an increased aromatics
content. With respect to the removal of monoaromatics,
particularly benzene, from reformates suitable as
gasoline blending components, the present invention aims
to provide an economically attractive process wherein the
benzene concentrate obtained has a reduced content of
non-aromatic hydrocarbons and an increased benzene
content, thus making it very useful as a source for
benzene manufacture at reduced cost price, whilst at the
same time a raffinate is produced which is essentially
free of benzene, i.e. which contains less than 1% by
weight of benzene. As an ultimate object, the present
invention aims to provide a process wherein under optimum
2 ~ 73282
conditions benzene can be obtained directly in highly
pure form, i.e. at concentrations above 95% by weight and
even above 99% by weight, thus almost or entirely meeting
the benzene selling specifications.
These objects have been met by applying a specific
heating step in the lower portion of the absorption zone.
Accordingly, the present invention relates to a
process for the removal of aromatic hydrocarbons from a
hydrocarbon vapour stream containing such aromatic
hydrocarbons, which process comprises the steps of:
(1) contacting in an extractive distillation column the
hydrocarbon vapour stream with an extracting solvent
capable of selectively dissolving aromatic hydrocarbons,
(2) withdrawing at the upper portion of the extractive
distillation column a vaporous raffinate having a reduced
content of aromatic hydrocarbons,
(3) withdrawing from the bottom part of the extractive
distillation column a liquid solvent stream, which has
been enriched with aromatic hydrocarbons,
whereby at least one liquid comprising extracting solvent
and aromatic hydrocarbons is withdrawn from the lower
portion of the extractive distillation column and
subsequently at least part of the liquid withdrawn is
heated under such conditions that at least part of the
aromatic hydrocarbons present in said liquid evaporates
without substantial evaporation of the extracting
solvent, after which the heated liquid/vapour stream is
returned to the extractive distillation column.
In general, extractive distillation processes using
an extractive distillation column are known in the art.
Examples of such processes are e.g. disclosed in US-A-
3,723,256; EP-A-0,073,945 and EP-A-0,155,992. An
extractive distillation column generally comprises a tray
column containing sieve or valve trays or other
fractionating internals, and means for introducing
2 1 73282
-- 6
extracting solvent and feed as well as means for
withdrawing at least a vaporous top fraction and a liquid
bottom fraction. For the purpose of the present invention
the extractive distillation column additionally comprises
means for withdrawing, heating and reintroducing a heated
liquid/vapour stream in its lower zone. Such means
suitably consists of one or more reboilers.
The extracting solvent is introduced at a point in
the upper zone of the column and is allowed to flow
downwardly through the column. The vaporous feed is
introduced into the column at a point below the point
where said solvent is introduced and flows upwardly
through the column, thereby attaining an effective
contact between feed and solvent as they pass
countercurrently through the column. At the top of the
column a vapour fraction is recovered (the raffinate),
whilst at the bottom of the column a liquid solvent
stream enriched with aromatics is withdrawn. The number
of theoretical stages of the extractive distillation
column may vary within wide limits. It has, however, been
found advantageous, also taking into account technical
and economic considerations, that the number of
theoretical stages is in the range of from 2 to 25,
suitably 3 to 15. The distribution of the theoretical
stages above and below the feed inlet is also not
particularly critical. However, usually the number of
theoretical stages above the feed inlet will be equal to
or higher than the number of theoretical stages below the
feed inlet, as in the zone above the feed inlet the major
part of the extraction of aromatics from the feed takes
place. Accordingly, the ratio between the numbers of
theoretical stages above and below the feed inlet is
suitably at least 1, more suitably between 1.5 and 20 and
most suitably between 2 and 15.
~ 21 73282
-- 7
The hydrocarbon vapour stream used as the feed to the
extractive distillation column comprises both aromatic
and non-aromatic hydrocarbons, whereby the total content
of aromatic hydrocarbons is preferably less than 50~ by
weight. Suitably, however, the hydrocarbon vapour stream
comprises less than 35% by weight, more suitably less
than 25% by weight, of aromatic hydrocarbons. In
principle, the process of the present invention can be
applied to remove monoaromatic hydrocarbons as well as
aromatic hydrocarbons comprising two or more aromatic
rings, but the process is particularly suitable for the
removal of monoaromatic hydrocarbons from a hydrocarbon
vapour stream. Monoaromatic hydrocarbons which can be
suitably removed include benzene, toluene and xylenes.
For the purpose of the present invention, however, it is
preferred that at least 50% by weight, but preferably at
least 90% by weight, of the aromatic hydrocarbons present
is benzene. If at least 95% by weight, and more
preferably at least 99% by weight, of the aromatic
hydrocarbons present is benzene, the process according to
the present invention enables the recovery of benzene of
a very high purity. The hydrocarbon vapour fraction
should preferably have a boiling range between 50 and 200
C, more preferably between 60 and 175 C. Although
various mixed hydrocarbon vapour streams meeting one or
more of the above requirements as to the aromatics
content, benzene content and boiling range may be treated
in accordance with the present invention, it is preferred
to use a hydrocarbon vapour stream obtained as a light
reformate from a catalytic reforming process. Such light
reformate, namely, usually has a relatively low aromatics
content (usually below 25% by weight), whereby
essentially all the aromatics present are monoaromatic
hydrocarbons. A high percentage of these monoaromatics
(usually more than 90% by weight) is benzene, the exact
2 1 73282
-- 8
percentage of (mono)aromatics other than benzene
-particularly toluene- being determined by the cutting
temperature and pressure applied in the reformate
splitter. In order to directly obtain benzene in highly
pure form using the process of the present invention, the
cutting temperature at the given pressure applied in the
reformate splitter should be such that at least 99% by
weight of the aromatics present in the light reformate is
benzene. An additional advantage of using a light
reformate as the hydrocarbon vapour feed is that the
catalytic reforming process can be very well integrated
with the process of the present invention, as the light
reformate recovered from the reformate splitter can be
passed directly, i.e. without any intermediate
condensation and/or pressure release step, into the
extractive distillation column. It will be understood
that this is very attractive with respect to the
operating efficiency.
The conditions under which the extractive
distillation column is operated may vary within the
conventional operating windows for this type of devices.
Important operating parameters are temperature, pressure
and solvent to feed ratio. It will be understood that all
these parameters are closely correlated and it is within
the common skills of the person skilled in the art to
adjust the individual parameters to one another.
Accordingly, the temperature in the extractive
distillation column may range from a minimum of 30 C at
the top of the column to a maximum of 200 C at the
bottom of the column. A particularly suitable operating
window for the temperature of the extractive distillation
column is from 40 C (top) to 175 C (bottom). The
pressure in the extractive distillation column is
suitably within in the range of from 1 to 5 bar,
preferably 1 to 3 bar, and the solvent to feed weight
2 1 73282
g
ratio is suitably in the range of from 1 to 10,
preferably 1.5 to 5. The temperature of the feed upon
entry in the extractive distillation column should be
such that it is in the vapour phase at the pressure at
which it enters the column. Usually, this pressure will
be equal to or slightly higher than the pressure inside
the extractive distillation column and accordingly is in
the same order of magnitude as the pressure in the
column. The temperature of the extracting solvent upon
entry in the upper zone of the extractive distillation
column will suitably be in the range of from 30 to
120 C, more suitably 35 to 100 C.
As the extracting solvent any solvent which is
capable of selectively dissolving aromatic hydrocarbons
and in particular monoaromatic hydrocarbons and which has
a boiling point higher than those of the aromatics to be
dissolved in it, may be used. If the main incentive is to
remove benzene, the solvent accordingly should be capable
of dissolving benzene and should have a boiling point
higher than that of benzene. Such solvents are known in
the art and include N-substituted morpholines, such as N-
formyl-morpholin and several ethylene glycols. The
preferred extracting solvent, however, is sulfolane.
Sulfolane can be applied as such or in admixture with any
of the aforementioned solvents. Small amounts of water
may also be present.
In accordance with the present invention, at least
one liquid comprising extracting solvent and aromatic
hydrocarbons is withdrawn from the lower portion of the
extractive distillation column and subsequently at least
part of the liquid withdrawn is heated under such
conditions that at least part of the aromatic
hydrocarbons present in said liquid evaporates without
substantial evaporation of the extracting solvent, after
which the heated liquid/vapour stream is returned to the
21 73282
-- 10 --
extractive distillation column. This is suitably achieved
by using a reboiler. The use of reboilers is known in
separation technology and reboilers suitably applied for
the purpose of the present invention may in principle be
any reboiler commonly applied. In general, a reboiler can
be defined as a special type of heat exchanger for the
supply of heat to the bottom of a fractionating column,
in this case an extractive distillation column. The
liquid comprising extracting solvent and aromatics which
is withdrawn may be identical to the bottom fraction or
may have a different composition. In the latter case,
said liquid is withdrawn from the column at a point above
the point where the bottom fraction leaves the column.
After being withdrawn from the column, part of the liquid
may be routed to another destination and accordingly is
withdrawn as a bleedstream before being heated. In that
case, only a part of the liquid withdrawn from the column
is heated in the reboiler and reintroduced into the
column. Alternatively, the entire stream withdrawn from
the column is heated in the reboiler, and the resulting
liquid/vapour stream is led back into the column. It has
been found very advantageous to use at least one reboiler
in the lower portion of the extractive distillation
column. It enables the recovery of an aromatics
concentrate having an increased aromatics concentration
and in the case of a light reformate feed comprising a
minor amount of aromatics, whereby more than 99% by
weight of the aromatics is benzene, even enables the
recovery of benzene in a highly pure form.
The amount of heat to be supplied by the reboiler to
the bottom of the extractive distillation column,
conveniently referred to as the reboiler duty, is
determined by the operating conditions applied in the
extractive distillation column, the desired purity of the
2 1 73282
aromatics concentrate and the desired quality of the
raffinate used as the gasoline blending component.
The liquid, aromatics-rich solvent stream obtained in
step (3) of the process according to the present
invention is suitably subjected to a subsequent solvent
recovery step in order to separate the solvent from the
aromatics. Also some water is usually present which also
need to be separate from the solvent. The solvent
recovery can be attained by ways known in the art. A
particularly suitable solvent recovery step involves
passing the liquid solvent stream to a solvent recovery
column which is usually operated at subatmospheric
pressure and temperatures between 70 C (top of column)
and 200 C (bottom of column), withdrawing a vapour
fraction at the top of the solvent recovery column and a
liquid solvent fraction at the bottom of said column, and
condensing the top fraction to yield a water phase and a
hydrocarbon phase comprising the aromatic hydrocarbons.
Suitably, said hydrocarbon phase consists for at least
90% by weight, preferably at least 95% by weight, of
aromatic hydrocarbons, which preferably consist for more
than 90% by weight of benzene. In case an appropriate
light reformate feed is used, the hydrocarbon phase can
be highly pure benzene, i.e. it may consist for more than
99% by weight of benzene. In order to further increase
the yield and purity of the benzene, part of the
hydrocarbon phase may be reintroduced into the solvent
recovery column. The liquid solvent fraction withdrawn at
the bottom of the solvent recovery column is suitably
recycled -in total or in part- to the extractive
distillation column for reuse.
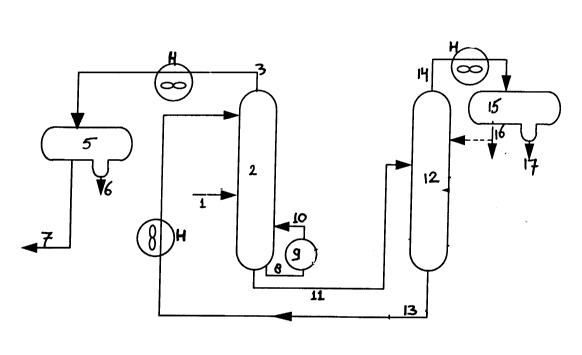
In Figure 1 a suitable embodiment of the present
invention is illustrated. Heat exchangers have been
indicated with H. Hydrocarbon vapour feed (1) enters
extractive distillation column (2) at point F, whilst the
2~ 73282
-
- 12 -
liquid extracting solvent enters the column at point S. A
vaporous raffinate (3) is withdrawn from the top of the
column at point R and is passed to condenser (5) via a
heat exchanger (H). Here, water (6) is separated from
aromatics-poor raffinate (7), which is used as a motor
gasoline blending component. Part of the aromatics-poor
raffinate (7) may be refluxed back to the extractive
distillation column (2) in order to improve the purity of
the stream (7) with respect to liquid extracting solvent
(not shown). A liquid aromatics-rich solvent (8) is
withdrawn from the lower portion of the extractive
distillation column (2) at point A and heated in reboiler
(9), after which the resulting liquid/vapour mixture (10)
re-enters the column at point B, which is higher in the
column than point A. A liquid bottom stream (11), which
is a further aromatics-enriched solvent, is withdrawn
from the bottom part of the column at point C and is
passed to solvent recovery column (12). Solvent (13)
leaves the solvent recovery column (12) at the bottom and
is passed to extractive distillation column (2) via a
heat exchanger H, where it enters said column (2) at
point S. The extract (14) leaves the solvent recovery
column (12) at the top and is passed to condenser (15)
via a heat exchanger H. Water (17) and aromatics
concentrate (16) are recovered from the condenser (15).
Part of the aromatics concentrate (16) may be refluxed
back to solvent recovery column (12) to enhance further
the efficiency of the solvent recovery.
The invention is further illustrated by the following
examples.
Example 1
A light reformate having a composition as listed in
Table I, is introduced into an extractive distillation
column having 10 theoretical stages and having a reboiler
attached to its lower portion. The reboiler duty is
2 ~ 73282
- 13 -
3472 kW. Operating pressure in the extractive
distillation column is 1.5 bar, the solvent to feed ratio
is 3.0 (on a weight basis) and the feed is introduced
into the extractive distillation column at a temperature
of 78 C, whilst the sulfolane is introduced into the top
of the column at a temperature of 75 C.
Table I Composition of light reformate
Component % by Component % by
weight weight
i-pentane 16.7 3-methylhexane 3.0
n-pentane 13.1 2,3-dimethylbutane 2.6
2-methylpentane 12.0 benzene 17.2
n-hexane 11.4 toluene 0.2
2-methylhexane 3.2 other non-aromatics 20.6
A vaporous fraction is withdrawn from the top of the
extractive distillation column and passed to a condenser,
where it is condensed and separated in a water phase and
a raffinate phase. The raffinate phase contains only 1.0%
by weight of benzene.
The liquid benzene-rich solvent is withdrawn from the
bottom of the extractive distillation column and passed
into a solvent recovery column which is operated at a
pressure of 0.4 bar and which has a top temperature of
94 C and a bottom temperature of 173 C. The vapour
fraction withdrawn from the top of the solvent recovery
column is passed to a condenser where it is condensed and
separated into a benzene-concentrate and a water phase.
The benzene-concentrate contains 97.7% by weight of
benzene and 0.9% by weight of toluene and total benzene
recovery, i.e. the weight percentage of benzene present
in the light reformate which is recovered, is found to be
95% by weight.
2 1 73282
- 14 -
Comparative Example 1
A light reformate as described in Example 1 is
introduced into an extractive distillation column having
no reboiler attached to its lower portion. The same
procedure as in Example 1 is followed. Total benzene
recovery is 94~ by weight, but the benzene-concentrate
contains only 66.3% by weight of benzene.
Example 2
A light reformate having essentially the same
composition as the one used in Example 1 except in that
it has a toluene content of 0.1~ by weight, is introduced
into an extractive distillation column having 10
theoretical stages and having a reboiler attached to its
lower portion. The reboiler duty is 4630 kW. Operating
pressure in the extractive distillation column is 1.05
bar, the solvent to feed ratio is 4.0 (on a weight basis)
and the feed is introduced into the extractive
distillation column at a temperature of 65 C, whilst the
sulfolane is introduced into the top of the column at a
temperature of 44 C. The same procedure as in example 1
is followed further.
Accordingly, a vaporous fraction is withdrawn from
the top of the extractive distillation column and passed
to a condenser, where it is condensed and separated in a
water phase and a raffinate phase. The raffinate phase is
found to contain less than 1.0~ by weight of benzene.
The liquid benzene-rich solvent is withdrawn from the
bottom of the extractive distillation column and passed
into a solvent recovery column which is operated at the
same conditions as in Example 1. The vapour fraction
withdrawn from the top of the solvent recovery column is
passed to a condenser where it is condensed and separated
into a benzene-concentrate and a water phase.
The benzene-concentrate obtained contains 99.8% by
weight of benzene and accordingly can be regarded as high
2 1 73282
- 15 -
purity benzene. Other components present are toluene
(0.1% by weight), water (0.1% by weight), sulphur (1 part
per million on a weight basis, ppmw) and sulfolane
(3 ppmw). Total benzene recovery is again 95~ by weight.