Note: Descriptions are shown in the official language in which they were submitted.
~173~64
Method and Apparatus for Determ;n;n~ Dissolved Substances
The invention relates to a method for operating an open
measuring probe with mechanical self-cleaning and a three-
electrode arrangement in accordance with claim 1 and a
corresponding apparatus therefor in accordance with claim 5.
The measurement of dissolved oxygen is performed in
accordance with DIN 38408, for examplç, wherein either the
idiometric titration of Winkler (DIN 38408-G21) is employed, or
the dissolved oxygen is determined by means of measurement with a
diaphragm-covered oxygen probe (DIN 38408-G22). Among the
diaphragm-covered oxygen probes are, for example, Clark sensors,
Makereth sensors and the sensors of Connery, Taylor and Muly.
They essentially differ by the construction of the sensor or the
type of electrode material employed.
The measuring principle on which these oxygen probes are
based is identical and is described in what follows: a portion of
the dissolved oxygen corresponding to the total concentration is
electro-chemically converted at one of the electrodes. The
current flowing in the process and registered as the primary
measurement signal functionally depends on the oxygen
concentration. The electrode potential required for conversion is
generated either by polarization by means of an external voltage
source or by suitable electrode reactions in the system itself.
A device of the last mentioned type is known from EP
144,325. In accordance with this patent an arrangement with two
electrodes is described, which essentially consist of different
materials, wherein both electrodes are completely embedded in an
insulating material with the exception of their effective free
ends. A movable, driven grinding element is provided for cleaning
~173464
these electrode end faces, wherein the shape, the size and the
mutual distance of the effective electrode surfaces remain
unchanged during continuing grinding of the electrodes and the
insulating material. The effect of a current flowing between an
amalgam electrode (cathode) and an iron or zinc electrode (anode),
whose value is a function of the actual oxygen concentration, is
used for generating the measuring signal. Thus, polarization
takes place only by means of the potentials being formed at the
anode. In part these are only partially defined and are subject
to various effects, so that the oxygen signal, for example, is
subject to cross interference. A result of this are instabilities
of the oxygen signal and non-linearities caused by cross effects,
such as occur for example in the presence of tensides from laundry
and cleaning materials in the measured solution. Also
disadvantageous is the use of only a few possible electrode
materials, which limits the use of the probe to defined analysis
media.
It is the object of the instant invention to propose a
method for determining electro-active substances in solution, in
particular oxygen, and a suitable apparatus, based on an open,
i.e. diaphragm-free measuring probe with mechanical self-cleaning,
which by means of a three-electrode arrangement is essentially
free of cross effects, can always be optimally adapted to the
measuring problem, and by means of which linearity can be `
improved, along with simultaneously increased stability of the
neutral point.
This object is attained in accordance with the invention
with a method according to the wording of claim 1, and with an
apparatus according to the wording of claim 5. The invention will
be described in detail below by means of the drawings. Shown are
ln:
~ 7346~
Fig. 1, the principle of the three-electrode arrangement in
a schematic representation with a reference electrode under
current load,
Fig. 2, an exemplary embodiment of an oxygen probe with a
three-electrode arrangement,
Fig. 3, an exemplary embodiment of an oxygen probe with a
three-electrode arrangement in a top view,
Fig. 4, an oxygen signal with a three-electrode arrangement
in comparison with previous ones,
Fig. 5, an oxygen signal with a three-electrode arrangement
in a sulfide-containing solution,
Fig. 6, an oxygen signal with a three-electrode arrangement
in a tenside-containing solution.
Fig. 1 shows the principle of the three-electrode
arrangement in a schematic representation. A work electrode 4, a
backplate electrode 5 and a reference electrode 6 are in the
container 1 which contains a solution of dissolved substances 2.
The dissolved substances are preferably those which are
susceptible to ampero-metric determination, which are therefore
electro-chemically active at the predetermined polarization
voltage, such as oxygen, chlorine and other disinfectants and
heavy metals. The work electrode 4 is made, for example, of noble
metal, noble metal alloys, steel, graphite materials, glassy
carbons or conducting polymers. The backplate electrode is mostly
made of noble metal, steel, pure metals, graphite materials or
glassy carbons. The reference electrode 6 is made of iron, zinc,
silver, copper or alloys. The reference electrode 6 is in the
near vicinity of the work electrode 4 in order to obtain the
smallest possible ohmic voltage drop.
A modified potentiostat 7 is provided for operating the
measuring arrangement, which connects the backplate electrode 5 or
the reference electrode 6 via lines 8 or 9. The work electrode 4
- 2173464
is connected to the ground of the potentiostat 7 via the line 10.
The potentiostat 7 essentially contains a controllable regulator
11, whose output provides a selectable voltage at the output 12,
which is defined in reference to the potential of the reference
electrode. The modified potentiostat 7 furthermore contains a
constant current source 13, which is switched in such a way that
in a branch circuit the reference electrode is continuously under
a load of constant current density. The main circuit of the
measuring arrangement is led from the potentiostat 7 via the line
8, the backplate electrode S, the solution with the dissolved
substances 2, the work electrode 4 and the line 10. The
instrument 14 is used for measuring the current in the line 8.
The solution with the dissolved substances 2 is an electrolyte
whose conductivity is a function of the type of the dissolved
substances and can vary over a wide range.
By means of the employment of such a three-electrode
arrangement it is possible to predetermine or set arbitrary
polarizing voltages in a defined manner and to minimize cross
effects in this way, improve linearity and stabilize the neutral
points.
Considerably greater flexibility and combination
possibilities of the electrode materials surprisingly result, in
addition to the advantages regarding the elimination of cross
effects, improvement of linearity and stability described ùp to
now. These advantages not only resulted with the use of the
previously described amalgam electrode, but also with a multitude
of electrodes which are mostly safe for food, such as those made
of noble metals, steel, graphite materials, glassy carbon or
conducting polymers, such as polypyrroles. Another of the past
disadvantages has been removed because of the possibility of the
specific selection of the electrode materials. Other materials
than those used up to now (iron/zinc) are also possible for the
~173464
backplate electrode, wherein chemically resistant ones, such as
special steel, noble metals or glassy carbon are preferably
employed.
Due to the fact that this is an open probe, i.e. not
covered with a diaphragm, conventional reference electrode systems
are no longer suitable. A completely new path is taken here. In
the process, action is taken on the mixed potential which is
formed on a metal electrode (for example made of iron). For
example, in order to utilize an iron electrode as the reference
electrode for the potentiostatic oxygen determination, its
potential must be independent of the oxygen content in the
solution and of accompanying substances. This is achieved by
using an anodic current flowing over the reference electrode which
suppresses the cathodic partial current during the mixed potential
formation. It is possible to reduce the oxygen dependency of such
an electrode, through which current flows, to an unexpected low
value of maximally i 10 mV by a chronologically constant current
load of a defined value. Furthermore, the potential surprisingly
displays only low sensitivity to accompanying substances, in
particular sulfide and iron. The good potential constancy assures
a potentiostatic operation of an open three-electrode arrangement
with mechanical self-cleaning. Besides iron, other pure metals
such as zinc, silver and copper as well as alloys can be used as
electrode materials.
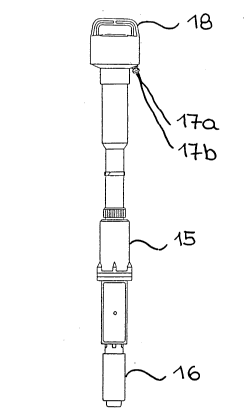
Fig. 2 shows an exemplary embodiment of such a measuring
probe with a three-electrode arrangement which, as an immersion
probe, is provided with a handle 18. All three electrodes are
placed on one level inside a probe cup 16. The required
electronic elements (potentiostat and measured value processing)
are components of the probe and housed in the housing of the drive
motor 15. This has the advantage that only two wires 17a and 17b
- - ~17346~
are required for signal transmission and that transmission
distances of several hundreds of meters are possible.
Fig. 3 shows the exemplary embodiment of such a measuring
probe with a three-electrode arrangement in a top view. The work
electrode 4, the reference electrode 6 and the backplate electrode
5 are made concentric, however, other geometric arrangements are
also possible. The surface of the reference electrode 6 is very
small compared with the surface of the work electrode 4 which, in
turn, is less than that of the backplate electrode 5. All three
electrodes are continuously cleaned by the grinding device 19.
Fig. 4 shows oxygen signals with the three-electrode
arrangement in comparison with previously achieved oxygen signals,
such as have been achieved with an oxygen probe (S12) manufactured
in accordance with Patent EP 144,325. The oxygen signals (S12-3E)
determined by means of the invention are linear over the entire
possible measuring range from O to approximately 50 mg/l. In
comparison with this, the signal of the previously described
oxygen probe (S12) is bent off the straight line starting
approximately at 15 mg/l. Because of good linearity a simple
calibration is also achieved in the upper measuring range.
Fig. 5 shows oxygen signals with a three-electrode
arrangement measured in a sulfide-containing solution. In the
process, the oxygen concentration was increased from 0.5 to 8 mg/l
in a constant sulfide concentration of 50 mg/l. The electrode
poisoning of the previously described oxygen electrode resulted in
that the measured signal (S12) had no relationship with the actual
oxygen content of the solutisn. In contrast and surprisingly, the
probe signal (S12-E3) of the three-electrode arrangement is
practically not affected at all.
Fig. 6 shows oxygen signals with a three-electrode
arrangement measured in a tenside-containing solution. The
anionic tenside, sodium dodecylbenzene sulfonate, is added to a
2173~64
constant oxygen content of approximately 8 mg/l. With the
previously described oxygen probe the effect of the tenside led to
a reduction of the signal (S12). In the process, a deviation of
approximately 30~ results at 50 mg tenside/l. Unaffected by the
tenside content, the probe of the invention with a three-electrode
arrangement shows an unexpected constant measured signal (S12-3E).
The method and apparatus of the type described are used for
the determination of dissolved substances in technical processing
installations, in particular in sewage and potable water
processing systems, in food technology, in pharmaceutical
technology and in biotechnology, as well as in chemical-technical
processes.
It is important for the invention that the attainment in
accordance with the invention of the object is distinguished by:
- an open, diaphragm-less system with a potentiostatic
three-electrode arrangement with mechanical self-cleaning,
- a novel possibility for generating the required reference
potential by means of a metal electrode under current load,
- the possibility of the employment of the most diverse
electrode materials,
- the integration of the potentiostat into the measuring
probe,
- the possibility to determine various analysis media,
- and the removal of cross-sensitivities.