Note: Descriptions are shown in the official language in which they were submitted.
i
2173904
A Procedure for Determining the Type and Quantity of a Substance
that can be Converted Electrochemically and that is
Contained in a Gas Sample
The present invention relates to a procedure for determining the
type and quantity of a substance that can be converted
electrochemically and that is contained in a gas sample, which
generates a physical process variable in a measuring cell, said
process variable changing over time, and rising from a base line
to a maximum value and then returning once again to the base
line.
An apparatus for measuring the concentration of alcohol as the
substance in the breath that is to be indicated is described in
U.S. 4.770.026. In this known apparatus, a gas sample with an
alcohol-vapor component acts on a fuel cell, and the physical
process variable i(t) that is obtained by electrochemical
conversion is passed to an analysis circuit that determines a
value that is proportional to the alcohol vapor concentration by
integrating the signal pattern of the physical process variable
over time. When alcohol vapor acts on the measuring cell, the
measured signal increases, starting from a base line, passes
through a maximum value I max and returns to the minimal value
that is close to the base line after the complete electro-
chemical conversion of the alcohol molecules. The area that is
enclosed between the function value of the measured signal and
1
2173904 ~ ~~
the base line represents the flowed electrical charge and is
proportional to the quantity and concentration of the alcohol
vapor in the gas sample.
In the known measuring cell, the shape of the curve of the
measured signal changes as the measuring cell ages, i.e., the
curve becomes flatter and wider during the period of use. A
similar change in the shape of~the curve of the measured signal
can occur after a number of short measurement cycles that follow
closely one after the others these changes to the signal are
reversible, at least in part, after a lengthy recuperation phase.
Since integration is carried out over almost the whole course of
the measured signal in order to determine the concentration
component of the alcohol vapor in the gas sample, changes to the
shape of the curve will affect the content of the area and will
thus have an effect on measurement precision, so that repeated
calibration cycles using a gas sample containing a known
concentration of alcohol have to be carried out. Such calibration
cycles make it more difficult to use the apparatus, particularly
if the measurement cell has to be gassed at frequent intervals.
In addition, in the known analysis process, the composition of
the substance that is to be indicated is known. Accompanying
substances, such as methanol or acetone, are not recognized
during this kind of analysis.
2
CA 02173904 1999-03-02
DE-43 44 196 refers to a determination procedure of this kind,
in which quantitative statements with respect to the quantity
and type of the substance that is to be indicated are meant to
be made with the help of integration across sections of the
curve for the process value.
All known analysis procedures suffer from
disadvantages, in particular the high computing costs for the
integration procedure in particular, and the way in Which the
shape of the curve depends on the age of the sensor, on
temperature and on marginal effects. No accompanying
substances are identified, and neither are deviant shapes of
the curve that may be attributable to faulty sensors or
defective equipment.
It is the task of the present invention to so
improve an analysis procedure for electrochemical measuring
cells that the type and quantity of a substance in the gas
sample, Which can be electrochemically converted, can be
determined rapidly and in a manner that can be replicated.
According to a broad aspect, the present invention
provides a method for determining the type and/or amount of an
electrochemically convertible substance in a gas sample, which
method comprises subjecting the substance in the sample to
electrochemical conversion in a measuring cell to produce a
currant whose intensity changes over time in that it rises
from a reference value to a maximum value and then falls back
to the reference value again, wherein at least one of the type
and amount of the substance is established from a linear
relationship between the currant intensity and the charge that
- 3 -
26541-102
CA 02173904 1999-03-02
has flo'ved in the time period after the maximum value of the
currant intensity has been reached.
An important advantage of the present invention is
that the linear connection bet'veen current strength and floraed
charge
- 3a -
26541-102
2173904
makes it very simple to determine the type and quantity of the
substance that is to be indicated. Furthermore, temperature and
aging effects are reduced during the determination, since only
the linear area of the measured curve in the time frame directly
after the maximal value is used as a basis for the current
strength.
Most surprisingly, it has now been found that the Coulometric
analysis procedure and its underlying theory, formerly used in
for liquid samples, can also be used in the gas phase. This
means, in particular, that current strength and flowed charge are
in a linear relationship; that the total flowed charge can be
determined from the condition of current strength equals zero
from the corresponding linear equation/lines: and that, finally,
the quantity/concentration of the substance in the gas sample can
be determined from the total flowed charge. A description of the
known theory can be found, for example, R. Greef et al.,
Instrumental Methods in Electrochemistry, 1985, pp. 44-47.
In particular, the determination of ethanol in gas samples has
been examined as a known area of application for the present
invention.
Figure 1 shows a typical measurement curve for the current
strength I (in uA) ("sensor current") as a function of the time t
4
2173964
(in s) for the determination of ethanol with an electrochemical
sensor.
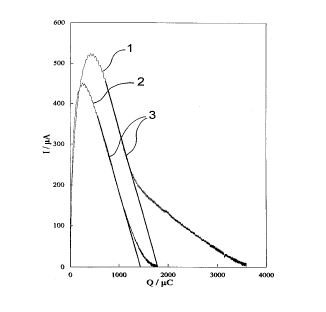
If one now measures current strength I (in uA) as a function of
the flowed charge Q (in uC), as is shown graphically in Figure 2
for a 2- and 3-electrode operation of an electrochemical
measuring cell (reference number 2:2 - electrode operation;
reference number l:3 - electrode operation), on linear matching
of the section of the curve behind the maximum one obtains the
total charge that has flowed because of the electrochemical
conversion of the ethanol. This follows from the theoretical
condition for potential-controlled Coulometry, to the effect that
in the case of a linear relation between current strength and
charge, the total flowed charge results from the condition
current strength equals zero on the charge axis. This applies in
to a specific measuring cell in each instance. In the example
that is shown in Figure 2, the slope of the linear section behind
the maximum is identical for both curves, which is obviously an
"indicator" for the identical measured substance. The regression
lines are numbered 3. Further on, the slope of the curve and
shape of the curve change, which could be an indication of
continuing or subsequent electrochemical reactions of the
ethanol, expressed for the three-electrode sensor (curve 1).
Taken all in all, the measurements corroborate the fact that the
linearly matched section of the curve behind the maximum is
5
2173904
substance specific, which is to say that the presence of
additional substances that affect the measurement method, e.g.,
methanol, results in a modified slope of the line, and can be
recognized thereby. Parallel lines result for a substance, e.g.,
ethanol, for different measuring cells. The total flowed charge
quantity is thus different, as can be seen from Figure 2. The
correlation coefficient for the regression lines 3 is an
indication of the quality of the sensors and can thus serve to
check their function.
In comparison to the prior art, the present procedure permits
considerably faster and more reliable evaluation of measurements,
since the line equation can be computed with fewer measurement
points and it is no longer necessary to go through the whole
evaluation.
In practical application, the evaluation of the measurement and
the computation of the concentration of the substance to be
examined that is ultimately desired can be effected with the help
of suitable electronic components, in particular by using a
microprocessor.
6