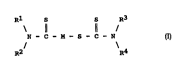Note: Descriptions are shown in the official language in which they were submitted.
~ WO 95115368 ~ ~ ~ 4 ~ 3 1 PCTIUS94~13767
-- 1 --
TTlR-~T~TTNr OIL
BAC OF TR~ INvl:!NTTflN
l. Field of the Invention
The present invention concerns a novel lubricating oil
'~ir~n ~md~ more particul~rly, it relateg to a lubricating oil
1-;r~n which i8 8uitable, for example, as engine oils (lubricAt-
ing oil for internal ;rn engines), gear oils, automatic trans-
mission fluids (ATF), power steering oils (PS oil), spindle oils,
hydraulic fluids ~nd inA---~r;A1 lubricating oils, excellent in wear
r~nlc~ n~.e~ ~Yhih~l inq a low friction ~ fEi~ nt~ capable of improv-
ing fuel conomy And with improved cop,oer corrosion property, ~a well
as a ~ rir~t;nq oil, t;nn having the ~ ,._ ;nnrA ~ aLa L~~
istics and capable of ~vh~h~1-;nq a low friction coefficient already
from an initial ~tAge of operation (cnnAit;nn;n~ drivlng).
2. DesCrintion of the Related Art
An increaE~e in the output of internal ~ i r~n engines such
a8 r il.~ engineg ha8 cau~ed individual partg of engines, for
ex~lmple valve tr~in ~ystems or cylinders, to bo exposed to high
c~Lu . . Mor-over, the number of cont~cts per unit time of metal
with each other hlls been increased, thus placing the internal combus-
tion engine under fsevere operating cnnA;t;~ . Lubricating oils for
internal ~ ;~n engines must function under severe cnnA;I ;nnn.
With a rc-A--~I ;nn of size and increase in the ~e~LI,~ l. e which results
in en increase in the number of revolutions ~md an increase in the
output, engine oils ~re re~uired to be versatile ~nd possess high
levels of ~ . L~
.
As ~ L reguired for the engine oils,
there can be ; nn~A~ reduction of friction, prevention of wear ~nd
~eizure, reduction of thermal and oxidative Ar~qr~A~ n~ d~:L~y_.~.y
and dispersancy, ;nh;hi~;rm of corrosion and cooling and sealing
WO 95115368 PCr/US94/13767 ~
3 ~
-- 2 --
functions, nnd use of vArious kLnds of ~dditives h~3 been studied for
~nti~fying ~uch functions.
For instAnce, ~8 A lubrlcnting oil _ itinn excellent,
particul~rly, in the reduction of ir:~1 friction loss for 4-cycle
~ngines, there hn~l been proposed a 1 rA- i nrJ oil : _ ' t i nn com-
prising n minernl oil ~nd/or synthetic oil hnving ~ kinematic
vi~cosity at 100C of 3 to 20 c8t nnd blended therewith, (n) 0.2 to 5#
by weight of ~ulfurized oxymolybdenum ~L~J~ LI _,I n 1:th~n~t-~ (here
imLfter referred to simply as NoDTP) and/or sulfurized oxymolybdenum
, 1th;1 ~ (hereinafter referred simply n8 MoDTC), (b) 0.l to 796
by weight of zinc ~-~thi ~ , (c) 0.l to 20# by weight of calcium
alkylbenzene sulfonate ~nd (d) l to l5# by weight of alkenyl
e--_r;nim~ nnd/or boron compound derivative of ~lkenyl ~ r~
(J~p~nese P~tent r 1 i rntinn No. 23595/l99l) . According to this
11.h~-~rnt~ oil _ ~inn/ the friction co~ffir~n~ in the
mixed/boundary r-s~ion c~n be r-duced to ~bout l/3 of th~t for the
conventional engine oil.
One of the important features of the lubric~ting oil i5 that
it does not attack a metnl within the engine during use. It is
cnni ~ that corrosion mny be c~used by free sulfur, sulfur com-
pound~ or ~cidic cr~ r--~. Since a copper plate i8 most sensitive
to thel:e L , the corrosion o~ n copper plate when exposed to
~r~t~Tg oil ig ev~luated A8 ~ meagure of the corrol3iveness of the
r..t:rg oil. It i8 A common practice to add niLL-,y_.._~,.. metal
de~ctiv~tors, such ns benzotri~zole, to the lubricating oil for the
purpose of reducing the ccpper corrosiveness. However, the ~-ddition
of these metal deactivators in a large ~imount resulto in hardening of
~eAling rubbers. Further, if MoDTP or MoDTC is added in a rel~tively
grent ~mount ns 0.2 to 59~ by weight ns disclosed in the ~bove-cited
patent r..h1irJIt~nn for reducing the friction, it results in a problem
of c~ul~ing n strong corrosive effective to copper.
Further, a lubric~ting oil it~nn hnving nn org~no-
molybdenum compound such ns MoDTP or MoDTC ndded thereto involves a
problem that n friction rn-~ff;r~nt nt ~n initinl stage (during
WO 95/15368 PCI~/US94J13767
~Si74~3~
-- 3 --
cQn~iitinn1n~ drivlng) i8 high. LubrLcating oLl addltLvea h~vo an
effect of reducing the boundary friction by i~dsorption to metal
surface to form boundary lubricating film but the organomolybdenum
compounds descr$bed ~bove require a relatively long period of time
until they are adsorbed on the metal surfAce to develop the friction
reduoing effect.
Accordingly, when a ~ r~r-fim7 oil f~nn having the
org~nomolybdenum compound added thereto Ls used as ~n engine oil, the
effect of reducing the frict$on develops after running a distAnce of
2, 000 to 3, 000 Icm, although thLs depends cn the running condLtion of
' i loc~. However, after the a~ Llbed running, the time for
the development of the effect of the friction often overlaps with the
time for the r rl r of the engine oil. In such a c~se, an
increase in the amount of ;~ddition of MoDTP or MoDTC does not lead to
the development of the effect of r-ducing the friction ~t ~n earlier
st~ge and rather incre~ses the copper corrosiveness.
cn~ rY OF m~TR INVENTION
The present invention has bRen mzlde under ~uch CiL.
with ~ view to provide a lubricz~ting oil i f i nn excellent Ln wear
r~ f~nre~ olrhihifiT~ low friction cce~ffiri~nf~ capable of improving
fuel economy, ~nd with improved copper oorrosiveness, 118 well aa a
lubricating oil ~ If i nn hAving the foregoing ~ L ~ f; r~l and,
in addition, capable of developing a low coefficient of friction from
the initiAl l~tage of nre~rnf i nn
The present inventors have made earnest studies for develop-
ing ~ lubricating oil ~ ' f i nn having desired properties as
~nrrih~l above, a8 ~ re8ult, hl~ve noted that oxymolybdenum mono-
glyceride or oxymolybdenum diethylate amide, not rnnf~ining sulfur
atoms in the molecule, does not increase the copper corroaivenes3 as
in the case of MoDTP or MoDTC And h~ve found that a 1 -hri rAf; nr~ oil
' f ~ nn of excellent wear re8igt~nce, ,.h i h i f i n.7 low cn~f f; r i onf
of friction and with reduced copper corrosiveness can be obtained by
combining the ~ compound with a met~l ~lifhi~ ~~ '
WO 95/15368 ~17 ~ ~ 31 PCT/US94/13767
"
-- 4 --
hnvLng n secondary Glkyl group ~md blonding them each at a predeter-
mined rGtLo to a lubricating oil ! '~ ' and, further, a lubricating
oil _ t~nn hGving the foregoing .I.aL--~ LeListics ~nd, in Gddition,
cGp~ble of reducing the frictlon coeffLcient Gt the initial stGge of
driving c~n be obt~ined by iurther blending an organic amide compound
at a yL. l 1_ . l I r~tio. The present invention has been
bGffed on such f indings .
Th~t is, the present invention provides a ll.h.-~rJ-~in~ oil
; ~ i nn compri-ing A lubricGting oil ~ - ` Gnd, based on the
oil composition,
(A) 0.01 to 10% by weight of at least one of organomolybdenum com-
pounds seleoted from oxymolybdenum monoglyceride and oxy-
molybdenum diethylate amide, Gnd
(B) 0.5 to 7% by weight of a metal rlithir~ ~ LG~L~ ` by the
general formula (1):
R1 S S R3
\ 11 11 /
N -- C -- M -- S -- C -- N (1)
R2 R4
where !5 L_~L~ - zinc, copper, nickel, iron, oGdmium, silver,
lead, antimony, tin or bismuth; Rl, R2, R3 Gnd R4 repreaent
~ r ` ~y an nlGnrhi 1 ir group of 1 to 30 carbon atoms in
which ~t le~st one of the four nl~nrh~l~r groups is a ~econd~ry
nl Gnrh ~1 i r group . Another: ' relates to a lubric~ting
oil _ r~inn comprising n lubricGting oil h~G~Gtnrl~ ~nd, bGsed
on the oil _ 11 ~nn, 0.01 to 10~6 by weight of the ingredient
(A) described above, 0.5 to 7~ by weight of the ingredient (E)
described Gbove, nnd (C) 0.01 to 5~ by weight of nn organic Gmide
compound.
~ WO 9S/15368 PCT~US94/13767
~17~g31
_ 5 _
DF!T1~TT.l;~n ~ -~lUN OF Tl~ INVENTIOII
There il no particular restriction for the ~- u~ed in
the lubricating oil _ ti~m according to the preaent invention ~nd
those proposed 80 far a~3 the ~~ for the 11~hriri~tin~ oil, for
example, mineral oils or synthetic oil~ can be used.
As the mineral oil, there c~n be ;~ -1, for ex~imple, 50
neutral oil, lOO neutral oil, lSO neutral oil, 300 neutral oil, 500
neutr~l oil ~nd bright stock obtained by :lolvent refining or hydro-
genation, which m~y be used alone or as a mixture of two or more of
them at ~n ~ppropriAte ratio.
As the rynthetic oLl, there can be 1 1, for example,
poly a-olefin oligomer, polybutene, alkylbenzene, alkyldiphenyl,
polyol eater, polyglycol ester, dibagic acid e~ter, phosphoric acid
este r ~nd silicone oil, which may be u~ed alone or ~ a mixture of two
or more of them at ~n Arrrr~pri At~ ratio . In addition, mlneral oi 1 ~md
the 3ynthetic oil hc~a above may be u8ed in admixture.
As the b~se o$1 used in the lubric~ting oil i ~ i rn
according to the present invention, tho~e having a kinematic viscosity
nt a L _ e of 100C within a range from 3 to 20 mm2/s, prefer-
ably 4 to lS mm2/s are preferred.
A!; the; n~re~ nt (A) in the . ; ~ n according to the
present invention, oxymolybdenum monoglyceride cr oxymolybdenum
diethyl~te ~mide (hereinafter referred to MoOxide type ccmpound~ ) i5
used. The oxymolybdenum monoglyceride is ~e~ Led by the general
formula (2):
O
Il
R - C - O -- CH2 - C}l - Cl H2
o o (2)
Mo
// \\
O O
WO 95115368 ~ PCTI~S94113767
-- 6 --
whlle oxymolybdenum diethylnte nmide i8 .~ .. Led by the general
formula (3):
O CH2 - CH2 - O O
Il / \ //
R - C - N No (3)
/ \\
CH2 - CH2 - O O
In the general formul~e (2) and (3), R is hydrogen atom,
alkyl group of l to 20 carbon Atoms, alkenyl group of 2 to 20 carbon
atoms, cyclo~lkyl group of 6 to 26 carbon atoms, aryl group of 6 to 26
carbon ~toms, alkyl~ryl or arylalkyl group of 7 to 26 carbon atoms or
I,.,....L~.. group rnntl~;n;ng ~ter bond, cther bond, ~lcohol group
or c~rboxyl group. Preferred R is alkyl group of 6 to 18 cnrbon
atom~, alkenyl group of 6 to 18 c~rbon iltoms, cyclo~lkyl group or 12
to 24 cnrbon atoms or Alkylnryl group of 12 to 24 carbon atoms. As
preferred specific xi~mple~ of them, there can be innorl alkyl or
~lkenyl group~ of 6 to 18 carbon ~toms such ~8 n-hexyl group, 2-
ethylhexyl group, n-octyl group, nonyl group, decyl group, lauryl
slroup, tridecyl grcup, oleyl group ~md linoleyl group, nnd alkylaryl
group h~ving An ~lkyl group of 3 to 18 carbon atoms such as nonyl-
phonyl group.
The MoOxide type compound for the ingredient (A) may be used
alone or two or more of them mny be combined for use. Further, it is
necossary th~t the blending nmount is selected within a range from
0.01 to l09~ by weight, preferably, 0.05 to 8~ by weight based on the
entire weight of the oil . _ it~nn. No 5~1ffir;ont friction reducing
effect cnn be obtnined if the blending amount is less than 0.0l1 by
weight wherea~ no ~ i ng; ~.. ' for the friction reducing
effect cnn be observed if Lt exceeds l091 by weight.
As the ingredient (B) in the l ~r~t;n~ oil ~ t;nn
nccording to the pre~ent invention, ~ metal ~l;thil ' '~ (herein-
after referred to N-DTCn) .~ ..Led by the general formuln (l) is
uued.
WO 95/15368 PCTIUS94113767
3 1
-- 7 --
Rl S S R3
\ 11 11 /
N -- C -- M -- S -- C -- N (1)
R2 \R4
In the above general formula (1), M is zinc, copper, nickel,
iron, cadmium, ~iilver, lead, antimony, tin or bilimuth. Each of R1,
R2, R3 and R4 i8 an ol~rrhllir group of 1 to 30 carbon atoms in which
at least one of them ls a secondary nlerrh;lir qroup, preferably, at
least two of them are seoondary Alerrh~l;r groupli and the residue
include3 primary nl~rrhilir group~i. Further preferred nlr--~rh;lir
group are thoLie in which _t l~_st t_ree of them are secondary A lkyl
group~. Rl, R2, R3 _nd R4 may be ldentical or different with each
other .
A~i the r~lerph;l;r group of 1 to 30 c~rbon atoms, there can be
' irno~l for Oxample, alkyl group of 1 to 30 carbon atoms, alkenyl
group o 2 to 30 carbon atoms, cycloalkyl group of 6 to 30 carbon
atoms, aryl group of 6 to 30 c~rbon atomEi, alkylaryl group of 7 to 30
c_rbon atoms, ArylAlkyl group of 7 to 30 cArbon atoms or l~ylL~ L~
group h~ving eLiter bond, ether bond, alcohol group or carboxyl group.
N-DTC Le~ ..L.zd by the general formula (1) in the oil
'~irn _ccording to the pregent invention having four nlenph;l;r
groups with the average number of oarbon _tomg between 1 and 5 are
desirably reActed with an oil soluble amine compound for ~ leYin~
L.~ in order to incre_~e the ~iolubility to the lubricating oil
` and improve the wear rel;istance. M-DTC having four
nlenrhilir group2s with an average number of carbon atoms of 1 (in
which all the nlerrhilir groups are methyl) cannot provide a homo-
geneouf~ hrirAl ;nq oil _ ~ir~ even if it ifl reacted with the oil
soluble _mine compound ~iince the solubility of the resultant complex
to the lubricAting oil b '~ poor. Since M-DTC exhibits it~
function by ~dsorption to metal ~urf_ce, no ~..ff;rienf effect c~n be
expected if it di~isolves excessively but it will be quite useless
unless it is soluble. The lower limit for the aver_ge number of
carbon atoms of the nl~orhilir group ig preferably two or more in view
WO 95115368 PCTIITS94/13767
~7~3~
-- 8 --
of both functions of the solubility and the wear rr~ni ,.1 Anr~ If the
~verage number of cnrbon atomD of the four nl~.nrh;lir groups i8 5 or
more, solubLlity to the lubricatLng oil hA~.~n~nrl~ is ~ ;nf~-tnry with
no lf~vinq ~ ' but it may be used after lf-Yinq if neces-
~ary.
In N-DTC, it i8 preferred that all the four nl~nrh~lir groups
~re identical l.~ ..LL.,I. groups, more guit~bly, the four nlenrh;l~r
grcups ~re ~lkyl groups of 3 to 6 carbon ~toms in view of e~sy produc-
tion nnd wear r~ e
Nore preferred r,l~-rhil;r group is alkyl group of 3 to 4
c~rbon ~toms. For the metal ~tom (M), zinc is preferred in view of
e~sy availability And wear r~ nr~.
H-DTC clm be produced by a known method. For 1nstAnce, &inc
diiYcpropyl ~I;l-h~- ' ' can be produced by reacting diiaopropyl-
amine, c~rbon disulf ide ~nd sodium hydroxide to prep~re sodium
diisopropyl ~ hif- - whioh is then reacted with zinc nitrate.
A~ the oil soluble ~mine compound uaed for 1 -Yi nq M-DTC,
there cAn be ; I, for example, ashless oetergent dispersant such
A8 polyi~lkenyl ~ rc;n;m;~- or ~lkylbenzylamine or ~lkylamine, alkyldi-
mine and alkylpoly~mine.
For previously forming a complex of M-DTC _nd the oil soluble
zlmine compound, it 18 preferred to employ a methcd of adding both of
them to a lubricatlng oil ~ at a ratio of providing a high
-Llon ~nd he~ting them. For instance, when a lubricating oil
'i-;nn rnnl ~in; nq 2 to 79~ by weight of M-DTC and 2 to 2591 by
weight of oil aoluble amine compound, ~t ~ ratio of 1 to 10 parts by
weight of the oil ~ioluble ~mine compound b~sed on 1 p~rt of weight of
M-DTC i~ Atirred at a _ ~Lu~ of 100 to 230C, more preferably,
150 to 200C, prefer~bly for 1 to 60 minutes, more prefer~bly, 1 to 30
minutes, both of the compounds form A complex which is uniformly
di~solved in the lubrionting oil hAn~ni-nrl~ As the heating temper~-
ture 18 hlgher, they form ~ complex ln ~I shorter period of time and
--W095/153C8 PCI'Il~S9U13767
~17~
g
_re dLssolved more uniformly. A I _ lubricating oil compo~L-
tion cnnr~inin~ both of the compounds at a desired ratio can be
obtA ined easily by diluting the resultant complex solution ~t high
..... ~_ I ~Al inr with the lubricnting oil I
In the ~ ' 'rAI ;ng oil i~inn according to the present
Lnvention, ~5-DTC as the; ~ n~ (B) may bo u~ied alone or U5 a
' ' r ~e i nn of two or more of them. It is _ y to blend l~-DTC by
0.5 ~o 79 by weight, preferably, 1 to 59i by weight baaed on the oil
itinn No sufficient wear resist_nt effect cAn be obtained if
the blending Amount i8 less thAn 0.59 by weight, whereas the solu-
bility tends to be lowered if it exceeds 79~i by weight.
sec~-DTc has _ function of _n extreme pressure ~gent, ~5 well
a5 A function as an Anl inY;~ a corrosion inhibitor or the like.
In the l~.hl-irAI i~ oil il ~nn according to the pre~ent
invention, the org~nic amide compound ~s the ingredient (C) can be
us~ed ~8 required. The org~nic ~mide compound is a compound repre-
ented by the general formula (4):
O R5
Il /
R7 - C - N (4)
R6
In the general formula (4), R5 and R6 each L~
hydrogen ~tom, alkyl group of 1 to 20 carbon atoms, alkenyl groups of
2 to 20 cArbon atoms, cycloalkyl group of 6 to 26 carbon ~toms, aryl
group of 6 to 26 cArbon atoms, alkylaryl group or _rylalkyl group of 7
to 26 carbon atoms or alkylene oxide group of 2 to 30 carbon atoms
which may be identic~l or different with etlch other. R7 ..,~ ..L~.
hydrogen atom, alkyl group of 1 to 20 carbon atoms, alkenyl group of 2
to 20 carbon Atoms, cycloalkyl group of 6 to 26 carbon ~toms, aryl
group of 6 to 26 carbon atoms, alkylaryl or arylalkyl group of 7 to 26
c~rbon atoms or 1,,~ .1 . group ~nn~inin~ ester bond, ether bond,
alcohol group or c~rboxyl group.
WO 9511S368 PCINS94/13767 ~¦1
31
-- 10 --
The alkylene oxide group ; l ~ herein is ~ group repre-
sented by general formula (5) or (6).
CH2CHO ~ H
(5)
R'
CHCH20 ~; H
(6)
R'
wherein R~ .. L8 hydrogen ~tom or methyl group and n is an
inteyer of 1 to 10.
Preferred R5 and R6 in the general formula (4) are hydrogen
Atom, alkyl group of 2 to 8 cArbon atoms, cyclo~lkyl group of 8 to 14
cArbon ~tom3, ~lkyl~ryl group of 8 to 14 carbon atoms, and alkylene
oxide group in which n i8 1 to 5. Preferred R7 can include alkyl
group of 6 to 18 cArbon atoms, alkenyl group of 6 to 18 cArbon atoms,
cyclo~llkyl group of 12 to 24 carbon atoms and alkylaryl group of 12 to
24 carbon ~toms. As specific ex~mples of the organic amide compound,
there can be i~ , for ex~mple, ole~mide or lnuramide, which mAy
be used alone or as a inJIl inn of two or more of them.
In the lubricating oil ~ i nn according to the present
invention, the org~nic ~imide compound 118 the i nqr~ i Pn~ (C) is blended
by 0.01 to 59~ by weight, prefer~bly, 0.05 to 29~ by weight b~sed on the
oil _ le~n Blending of the organic ~mide compound c~n reduce
the friction ~ ff~Hf.n~ ~lre~dy from the initial stage of driving
while ,, 'nq the copper corrosiveness. No sufficient effect for
reducing the friction coefficient from the initial stage of driviny
c~ln be ~ttained if the blending amount is less than 0.0196 by weight,
whereas no ~ ,..ding ~ _ ~,. for the effect can be recognLzed
if the ~imount exceeds 596 by welght.
In the lubricating oil _ i~rn according to the present
invention, there c~n be properly added various kinds of additives used
~ WO 95/15368 PCIIUS94113767
~17~9~1
-- 11 --
_ ~ly in the lubricating oil8, for example, other extreme
pressure _gents, ashless detergent fi~rfr~--nt~ Anl ~nY~tiAn~ metallic
detergent, metal deactivdtor, viscosity index improver, pour point
depressor, rust inhibitor, Ani-;' and corrosion inhibitor within
such a range as not f~-' frifrA~in~ the purpose of the present inven-
tion .
For other extreme .Ay.~ .: agent, there can be ~ inn~
fcr example, organomolybdenum compounds such as MoDTC or MoDq'P.
However, if the amount of the org_nomolybdenum compound used is
increa_ed, the copper corro~ion becomes fnn~r;r~ a. Ag the a3hless
detergent ~iiorfr~ , there cAn be ~ 1 i, for exAmple,
8--- f~n~m~fif., 8~f~-- fif., benzylAmine or egter type, as well as
L~ AininfJ _shless detergent dispersant can be used. ~rhey are
u~ed usually at a rAtic of 0.5 to 7% by weight.
Further, o8 the An1-~nvir~ there cAn be innfd, for
~x_mple, dmine-bA-ed ~ni-~nYi~nl such as alkylated dlphenylAmine,
phenyl a ' ' hylamine, alkyl~Lf=d ~ r..~l.Lhylamine, and phenolic
~nt~nY~fi--lt euch 48 2,6-di-terti~ry-butylphenol, and 4,4~-methylene-
bi~-(2,6-di-tertiAry-butylphenyl) And it ig usually u_ed At a ratio of
0 . 05 to 2 . 0% by weight .
As the metallic detergent, there can be ;nnf,fi, fcr
ex~nple, C~ 1 fn-~ f~ lly _ 1 fnn~e~ Ba-sulfcnate, C~ ' ' , Mg-
Phenate, BL r ' -, Ca-sOaliCylate, Ng-salicylate and Ba-salicylate
which is generally u_ed _t a r~tio of 0.1 to 5.0% by weight. As the
metal deactivator, there can be ~nnf~ for example, hfn7r~triA~nlf.
bcn_otri_zole derivative, bf~n7ni-h~ nlf~ bf-n7nfh;A-n]P deriv~tive,
tri_zole, tri_zole derivative, f~ilh~f ` ~ ~lh~
derivative, indazole And indazole derivative, which may be used
usually at ~ ratio of 0 . 005 to 0 . 3% by weight .
As the viscosity index improver, there can be I ~nnf~ for
exAmple, polymethacrylate, polyisobutylene, ethylene-propylene co-
polymer and ~Ly ~ if nf. I,y~ copolymer type improver,
which m_y be u_ed usua ly at d rA-tio of 0. 5 to 35% by weight.
WO 95/15368 PCTII~S9U13767
217~31
-- 12 --
Purther, ~8 the rust lnhibitor, there cAn be inn~rl~ for exAmple,
~lkenyl succinic acid or its parti~l egter and as the :~nl i-
there cAn be ;t 1, for example, dimethyl polysilox~ne and poly-
~cryl~te, which may be Added properly.
The lubricating oil 'tinn according to the preaent
invention c~m be prepared by blending each of the various hinds of
additives del~cribed above at ~ n~d amount with a h-
and uniformly mixing them. The lubricating oil ~ It;nn according
to the present invention c~n be used suitably, for example, to ~uto-
mobile engine oil, gear oil5~ ATF oils, PS oils, spindle oils,
hydrAulic fluids or industrial lubric~ting oils.
The present invention will now be described more in details
by way of preferred ex~mples but the invention is not .~ rl ..A at
all to such ex~mples.
1-9 and Com~llrative Exam~les 1-3
The pe~r~ of the lubricating oil itinn was
--` by the method shown below.
1. C oef f icient of Friction
The rneff~ri~nt of friction was measured by u3ing a
reciprocal vibration friction te~ter ISRV). In the S~IV tester, a
steel ball of l/2 inch diameter ~SUJ-2 specified in JIS G-4805) was
used as the upper test piece, and a steel disc (SUJ-2 specified in JIS
G-4805 ) was used as the lower test piece . A sample oil was dropped on
the lower test piece, a load was applied to the upper piece from the
top, and the upper test piece w~s vibrated parallel to the lower test
pi~ce with the upper test piece being passed against the lower test
piece. The lateral load applied to the lower test piece was measured
to c~lculate the rrJ~ff;r;~nt of friction (1~)
~ WO 95~1S368 PCI`/US9~1113767
~7~31
-- 13 --
The rroffir;ent of friction was me~sured twice, that i~, 5
minutes ~nd 20 mLnutes after the ~nit1Ai~rn of the vibr~tion of the
upper teit piece. Testing crn~ irn~ are as follows:
Lozld: lOON
- 130C
p , 8 Hz
r 1;- ' 4 mm
2. CoPDcr Corrosiveness
Corrosiveness was tested in a.. ,~,' wlth JIS X2513 "Copper
strip Corro-ion Test Method ror Petroleum Products" at a test temper~-
ture of 100C for a test period of 8 hourl~ by the test method, in
which the state of ~ ol rrAi- i rr~ of the copper strip was observed in
Accordance wLth "the StAnd~rd for Copper Corrosion~ and the corrosive-
ness was cv~luated by LiubdLvision symbols 1A-4C. The corrosiveness is
lower as the 'r~l value of the subdivisional mark ii smaller and
the corroLiiveness is i nrr~^ 1 in the il l r~ '~ei- i r~ 1 order .
Specific ev~luation ex~imples are as follow~:
1~: p_le orAnge color which is ~Inl ~ally identical color with
that of poll-hed copper pl~te upon finishing,
lb: deep orAnge color, and
3~: reddish brown p~ttern on bronze color.
r 1~-- 1--4, OrmnJ~rAtive r 1~ 1--3
Lubricating oil _ li- i rn~. were prepared by blending 496 by
weight o~ CA--~ nn~t~, 59~ by weight of ~ r1nlmi~ 0.59~ by weight of
alkylAted diphenylAmine, 0.396 by weight of 2,6-di-t-butylphenol and
0.029~ by weight of 5-methyl-benzotri~zole to ~ mineral oil (150
neutral mineral oil having kinematLc viscotity at 100UC of 5.1 mm2/ti)
batied on the entire weight of the oil , _ ' ~1 rn, ~nd blending each
WO 95/15368 PCT/US94113767
~17~31
-- 14 --
of the ;n7re~l~n~ shown in Table 1, ~nd the pe.C.,.~"."..,es were
evaluated. The result~ of ~ of properties are ~hown ln
Table 1.
Each of the in~r~ .nl ~ ~re ~8 shown below.
( 1 ) MoOxide compound
o
n -- C61113 -- C -- O -- C~2 -- CH -- CH2
O o
Mo
// \\
O O
(2) MoDTC (51lkur~--Lube0 _d by Asahi Denk~ Kogyo K.K. )
(2EII) 5 o s C 5 (2EEI)
11 11 / \ 11 11
N -- C -- S -- Mo Mo -- S -- C -- N
\ /
(2EH) S (2E~)
2EH: 2-ethylhcxyl group, here and hereinafter
(3) MoDTP (Molyvnn L0 ' by R.T. Yzmderbilt)
2EH - O 5 0 5 0 5 0 - ( 2E}I )
\11 11 / \ 11 11/
P -- S -- Mo Mo -- S -- P
\ /
(2EH) - o 5 o - (2EH)
( 4 ) ~ec-ZnDTC
Rl S S R3
\ 11 11 /
N - C - M -- S - C - N
R2 R4
wherein two groups of Rl-R4 are isopropyl groups and the re~ldue~
are n-propyl group).
. wo
95115368 PCTIUS94/13767
- 15 _ ~17~31
s ) Ca-~ulf onat~
~`~53
~)~ Ca
loH21 - 2
~6) S~ n;m~
o
H2N ~ ) 4 CH2CH2 _ ~ fH -- PiB
~2
P~B: pol~i-obatyl~
-
W095/15368 2~ 7~93~ PCT/US94/13767 ~
-- 16 --
rl I I o I I ~1 --I
"' I o I I ~ I I o o
O i ~i .-i 1 1 i O O _I
1~ 1 o o
o I .i ~ I I I o o _l ~
O I I I I i l O l O O , O
I I I I ~ I I I o C~ -
,~ i i o i o o _I C
~ i i i i i i I --I ~
I` I I I O I I I I _I O
É~ m o , I I _i I I I I O O
I I , I I j o ~ C~ o
~q i , i i i i O i O O ,, E
j o j j I j j j N o
,~ o j 0. j j j j j j No d p,
3 E .-1 N
_~ û ~, , ~, ~ ` ~ ol
wo 95/15368 ~ 7 4 9 3 ~ PCTII~S94~13767
-- 17 --
The present invention can provide a lubric~tlng oil composi-
tion excellent ln we~r reslstance, exhibLting a low cn~ffiri~nt of
friction, capablc of improving fuel economy and having improved copper
corro~iv2nes~, all wcll ~ a ll~h~ ;n~ oil _ ~ir.n having the
and, in addltlon, c~pable of attalning
~ low ~o~ffiri~nt of friction already from the initial ~itage of
drivlng.