Note: Descriptions are shown in the official language in which they were submitted.
~175144
TITLE OF THE INVENTION:
Thermosetting Well Treatment Composition
NAME(S) OF INVENTOR(S):
Brian H. Tomlinson
FIELD OF THE INVENTION
This invention relates to acid soluble
magnesium oxysulphate and oxychloride cement
composition~ and their method of use.
P~XGROUND OF THE INVENTION
The use of magnesium oxysulphate or
oxychloride cements to plug underground formations
during oil and gas well drilling and production is
well known. These cements are introduced as slurries
into a well bore where they harden against the
formation and provide an impermeable block to fluid
migration into or from the formation. These cements
are also acid solu~le so that once they are no longer
needed, acid solutions may be applied to the well to
remove the cements.
For example, Canadian patent no. 1,053,892
of Barthel describes a magnesium oxysulphate cement
that is mixed with drilling fluid to form a hardenable
cement. The magnesium oxysulphate cement is made with
an additive comprising magnesium oxide, magnesium
sulphate and a filler comprising magnesium carbonate
or dolomite.
More .-ecently, United States patents
5,213,161; 5,220,960; and 5,298,069 of Halliburton
Company, Oklahoma, have disclosed improvements on
magnesium oxychloride cements. These improvements
include use of a foaming agent and foam stabilizer,
- 2175144
and use of a set retarder comprising a water soluble
borate and a sugar.
SU~ARY OF THE INVENTION
These prior art compositions, however, have
disadvantages. The use of slow setting compositions,
and in particular, the use of a retarder tends to
delay the setting time of the composition so that the
cement tends to invade deep cracks and pores in the
formation to such an extent that subsequent
application of acid cannot remove the cement from the
formation. This tends to cause loss of permeability,
which can be disastrous to subsequent production from
the formation.
Further, the inventor has found that it is
important that the hardening process, though initially
stimulated by the temperature of the cement, rapidly
becomes exothermic, and that the cementation process
should result in a rapidly setting firm composition.
In addition, the inventor has found that
selection of the components of the cement according to
the prior art teachings does not uniformly produce
satisfactory results. Thus, inappropriate selection of
the magnesium sulphate, or the magnesium oxide, used
in such a cement may result in lack of controllability
of the hardening process.
In one aspect of the invention, the inventor
therefore proposes the use of magnesium sulphate
trihydrate as th~ magnesium sulphate component in a
cement formed of a solids component and an aqueous
liquid. Magnesium sulphate trihydrate is unstable and
difficult to handle, and is not believed to be an
obvious candidate for use as a magnesium oxysulphate
or oxychloride cement component. Moreover, it has the
2175144
feature that the setting of the resulting magnesium
cement is thermally triggered and rapidly becomes
exothermic such that the cement has an exponential
setting curve. The solids component of the cement
composition is preferably 33 - 40% by weight magnesium
oxide; 35 - 43% by weight of magnesium sulphate
trihydrate; and 20 - 27% inert filler, typically
calcium carbonate or cement filler. The inert filler
must be acid soluble.
In a further aspect of the invention, for
low temperature applications, the magnesium oxide is
mined and may have impurities, while for high
temperature applications, the magnesium oxide is pure,
over 99% pure, and is calcined from brine. For high
temperature applications, the aqueous liquid is
preferably saturated magnesium chloride brine.
The resulting cement compositions, supplied
under the trademark THERMAX, are non-invasive non-
damaging thermosetting compounds, and are believed to
represent a technological advance in the control of
formation fluid invasion and lost circulation. The
thermosetting composition is applied in pill form to
the desired zone where the composition sets like
cement but with many added benefits. Applications for
the thermosetting composition of the invention
include: drilling applications, including lost
circulation, formation consolidation, zone isolation,
gas shut-off and horizontal drilling; production
applications, including zone protection, water
blocking, zone isolation. Other applications include
mining, seismic hole plugging and salt dome sealing.
The thermosetting composition of the present
invention hardens through hydration. Shrinkage is
limited to 0.5% or less. The thermosetting
2175144
composition of the present invention is 100% inorganic
and soluble in a 15% oilfield HCl solution. Due to
its non-invasive characteristics the thermosetting
composition of the present invention allows full
return permeability.
BRIEF DESCRIPTION OF THE DRAWINGS
There will now be described preferred
embodiments of the invention, with reference to the
drawings, by way of illustration, in which like
numerals denote like elements and in which:
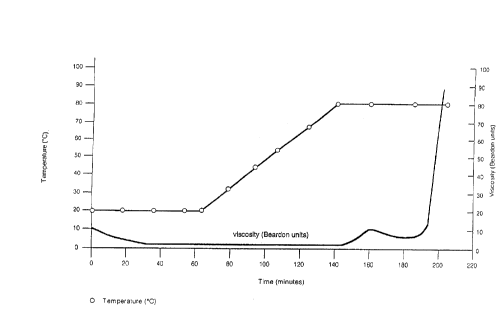
Fig. 1 is a graph showing setting time for
a high temperature thermosetting composition according
to the invention having, in 1 m3 of slurry, 793 kg of
a composition consisting of about 35.8% by weight
magnesium oxide, about 41.5% magnesium sulphate
trihydrate, about 22.7% inert filler (calcium
carbonate and dolomite), 637.5 litres saturated MgCl2
brine and 41.7 kg inhibitor (5% sodium pentaborate);
Fig. 2 is a graph showing return
permeability behaviour of berea cores (3" long at
150~F) after acidization of previously applied
thermosetting composition according to the invention;
Fig. 3 is a graph showing setting time for
a low temperature thermosetting composition according
to the invention with and without inhibitor;
Fig. 4 is a graph showing setting time for
a high temperature thermosetting composition according
to the invention with and without inhibitor;
Fig. 5 is a table showing magnesium chloride
solution requirements using 100% sacked MgCl.6H2O (for
requirements for one bbl, divide bbls of water and
required pounds of MgCl2 by 6.2; and
21751~ ~
Fig. 6 is a table showing sodium chloride
solution requirements using 100% sacked NaCl (for
requirements for one bbl, divide bbls of water and
required pounds of NaCl by 6.2.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The composition of the invention is an acid
soluble, water impermeable, inorganic, thermosetting
composition formed from a solids mixture and an
aqueous liquid in which the amount of aqueous liquid,
magnesium oxide, magnesium sulphate trihydrate and
inert filler are selected such that upon setting the
composition becomes exothermic and has an exponential
setting curve at the temperature T.
The preferred aqueous liquid is a brine
formed from calcium chloride, sodium chloride or
magnesium chloride. The amount of liquid is preferably
about 1 liter of liquid for each 1.25 kg of solids,
resulting in a volume increase to about 1.56 liters of
slurry. The magnesium chloride solution, particularly
for high temperature applications, is preferably
saturated MgCl2.6H2O brine. Sea water may be used.
For low temperature applications, namely
15~C - 70~C, the preferred solids composition is about
38% by weight magnesium oxide, about 38% magnesium
sulphate trihydrate, and about 24% inert filler, the
inert filler being selected from the group comprising
calcium carbonate and dolomite (5 micron size). The
temperature of calcination of the magnesium oxide may
be used to regulate the setting temperature of the
thermosetting composition.
For high temperature applications, namely
55~C - 100~C, the preferred solids composition is
about 35.8% by weight magnesium oxide, about 41.5%
217S144
magnesium sulphate trihydrate, and about 22.7% inert
filler, the inert filler being selected from the group
comprising calcium carbonate and dolomite (5 micron
size). In the high temperature application, the
composition preferably includes 5% sodium pentaborate
as an inhibitor to delay the setting time of the
thermosetting composition. For high and extra high
temperature applications, namely 80~C and higher, the
magnesium oxide is preferably calcined from pure
brine. For low temperature applications, the magnesium
oxide is preferably mined from a magnesite deposit and
calcined at between 750~C and 900~C. In this manner,
selection of the source and purity of the magnesium
oxide allows controllability of the setting time of
the composition.
The low temperature thermosetting
composition is normally inhibited above 30~C and can
be mixed in fresh water and compatible brines (not
zinc bromide for example). High temperature
thermosetting composition should only be mixed with
saturated magnesium chloride brine.
While reasonably precise compositions have
been disclosed that are known to work, it is believed
that other compositions in the range 33 - 40% by
weight magnesium oxide; 35 - 43% by weight of
magnesium sulphate trihydrate; and 20 - 27% inert
filler will also provide the exponential thermosetting
characteristics when mixed with an appropriate aqueous
solution.
The magnesium sulphate trihydrate peferably
has a bulk density of between 37.68 and 46.31 lbs per
cubic foot and a loss on ignition ratio of between 32
and 35~. It has been found that 6uch a magnesium
sulphate trihydrate gives greater controllability over
217S14~
the thermosetting process as compared with that
disclosed by Barthel.
The thermosetting characteristics of the
above described compositions are dictated only by the
temperature. They can be extremely rapid or may be
slowed down by the use of the inhibitor. Due to
hydration, shrinkage on setting is less than 0.5%.
The composition is unaffected by chlorides, and make-
up water may be of any salinity. For sealing salt
formations saturated NaCl brine is preferred. The
composition adheres to salt formations and due to the
availability of extremely short setting times, the
thermosetting composition provides excellent
characteristics for the prevention of microannular gas
migration or remediation of channelling.
The exponential hardening characteristics of
the thermosetting composition is triggered by the
external temperature of the medium in which they are
placed. This is normally the temperature of the
formation or by addition of special additives. Once
this temperature trigger has started the hardening
process, then it becomes exothermic and self-
generating. In a formation reservoir, the hardening
process is accelerated even faster once the
thermosetting composition enters the immediate
periphery of the wellbore. Although drillpipe,
tubing, etc. may still be pulled out of the slurry in
the wellbore, away from the wellbore, the set is
almost instantaneous with minimum penetration of the
formation matrix. Hence, the thermosetting
composition provides easy removability of the compound
by acidization with full return permeability being
possible. For water shut-off where fracture
penetration may be required, the use of inhibitors
2175144
will extend the setting time without any alteration of
the exponential properties. The use of a saturated
brine (MgCl2) in these circumstances will ensure
maximum structural integrity. Use of the brine also
ensures the slurry will not be diluted in the
placement operation. Figure 1 illustrates a typical
exponential setting curve for the invention. It will
be appreciated that in cracks in the formation, when
the appropriate thermosetting composition is used, the
hardening will be almost immediate. A typical return
permeability is shown in Fig. 2.
Setting time for low temperature and high
temperature compositions of the invention are shown in
Figs. 3 and 4 respectively.
The thermosetting composition is an off
white powder with a median particle size of 7 microns
and a viscosity of 18-30 cP, Slurry Density: 1.44-1.6
kg/l (11.9-13.37 ppg), depending on Mix Water. Bulk
Density is 3.0 gm/cc. Compressive Strength 24 hour
compressive strength for the low temperature
composition varies as follows: 650 psi mixed with
fresh water, 1500 psi mixed with saturated NaCl, 1950
psi mixed with saturated MgCl2. For the high
temperature composition, the 24 hour compressive
strength is 900 psi when mixed with saturated
magnesium chloride. The set composition, whether high
or low temperature composition, is 100% acid soluble
in 15% HCl and requires approximately seven m3 (43.4
bbls) of 15% HCl to remove one m3 (6.2 bbls) of the
set composition. A ratio of seven bbls to one bbls
allows for possible 100% return permeability.
In the method of the invention, a
thermosetting composition according to the invention
includes mixing and injecting the thermosetting
2175~44
composition into the well bore adjacent the zone
through tubing to fill the well bore adjacent the
zone; removing the tubing from adjacent the zone; and
allowing the composition to set at least adjacent the
zone. The mixing is preferably carried out on the fly.
In the preparation of the invention, the
following steps are carried out: Calculate the volume
of required slurry, calculate the final volume of
water or brine, calculate the kilograms of
thermosetting composition, use the attached graphs to
determine setting time, calculate the final slurry
density (kg/m3), determine the amount of Inhibitor, if
required, from the attached graphs (see Figs. 3 and
4). Steps: fill the tank with water as calculated in
step two above, add Inhibitor, if required, as
calculated above, and agitate for 10 to 15 minutes,
add MgCl2 or NaCl if required, add thermosetting
composition, with vigorous agitation, as calculated
above, displace the slurry into the well with a
pumping unit.
Requirements of less than two cubic metres
will be batch mixed. For larger volume applications,
the composition will be continuously mixed through
standard oilfield cementing equipment. Maintain the
slurry density at +/-10 kg/m3 of the density
calculated above. Maximum inhibitor concentration
should not exceed 5% by dry weight of the solids
composition (i.e. maximum 40 kg of inhibitor/m3 of
slurry). It is recommended that the composition be
mixed with magnesium chloride brine for maximum
compressive strength.
Example #1
In September, 1993, a well in Alberta,
Canada, had been shut in for some years. A
217~ 1 44
radioactive tracer had shown communication between the
Cardium Conglomerate above Cardium sandstone in
Alberta, Canada. Top of the production perforations
was only 4 feet from the conglomerate. To solve the
problem, a packer was set between sand and
conglomerate, with a retainer at top of conglomerate
(conglomerate interval perforated). Then tubing was
inserted into the retainer and the conglomerate
squeezed off with a low temperature thermosetting
composition described above. 1.0 m3 of the low
temperature thermosetting composition was batch mixed
into a pumper and squeezed over a perforation interval
of 6.4 m. Squeezing was to ~ust below fracture
gradient at 4 mPa. The tubing was pulled out of the
retainer. 24 hours later, the retainer and cement was
drilled out to the packer, then the well pressure
tested to 600 psi. After drilling out the packer and
re-perforating the Cardium sandstone, it was found
that the thermosetting composition successfully shut
off vertical communication outside the casing.
Example #2
In this well, the producing zone watered out
from migrating fluid below production zone. Log
report indicated water was channelling upward along
sides of the casing. A sleeved retainer was set 10 m
below production zone. Then a high density
perforating gun was used to make staggered radial
perforations at 150 intervals. The thermosetting
composition was displaced to the bottom of the tubing,
the annulus closed in and the composition squeeze to
seal water invasion. After squeezing, the tubing was
removed, the composition allowed to set for 12 hours
then drilled out. The well swabbed dry and stood full.
2175144
After perforating the production zone, the well surged
3 - 4 m3 of fluid with no the water invasion.
Example #3
An upper hole gas zone was encountered while
drilling 311 mm hole in the Colony formation plus
total lost circulation after drilling the Nisku
(fractured carbonate). The well was drilled ahead
blind to horizontal casing depth of 891 m. Over 6500
m3 of LCM pumped with no results (Nisku on vacuum).
Unable to pull out of the hole due to pack-off above
the bit which allowed circulation down the backside to
control the gas influx. To solve this, a thermosetting
composition plug was established from the top of the
Nisku at 723 m to total depth by displacement through
the drill bit. Procedure Jet mixed a total of 10 MT
Thermax Lt (12.5 m3) with cementing unit and displaced
through the bit. After pulling out, packing-off,
clearing the drill bit, waiting 8 hours, circulation
was re-established, with the thermosetting composition
plug sealing off the massive lost circulation in the
Nisku formation.
Example #4
A abandoned well leaked gas from casing.
Pressure and void space in casing was not known. A
low temperature thermosetting composition was used to
seal off the void in casing and eliminate gas flow at
surface. To do this, surface casing was exposed 2 m
below surface. Then, casing was hot-tapped and 100
psi initial pressure recorded that reduced immediately
thereafter. Then a lubricator was attached to a valve
on casing. 34 liters thermosetting composition was
mixed into a slurry @ 40O Celsius, then poured into
lubricator and squeezed with service rig pumps to 6500
kPa. The pressure held. Approximately 25 liters of
217~i144
composition was squeezed into casing, the lubricator
knocked off and flushed with water. Sample set up in
15 minutes. The thermosetting composition plug sealed
off the void in the casing, enabling the crews to cut-
off the cap with a cutting torch to put on the BOPs.
Example #5
While drilling at 1643 feet (507 m) the
Grosmount formation in Alberta, Canada, was
encountered and partial lost returns were experienced.
Drilling continued down to 1665 feet (514 m) where
total lost returns occurred. With the lost
circulation occurring, a possible gas kick from the
exposed Colony formation could take place. 307 sacks
of Bentonite, 373 sacks of Sawdust, 128 sacks of
Primaseal, 86 sacks of Cellophane were mixed in
different lost circulation plugs along with a 660 sack
cement plug, all of which were unsuccessful. A gas
kick was experienced from the Colony and due to a
sloughing formation below that created a bridge, the
zone was top killed by pumping weighted drilling fluid
down the annulus. The bridge would not allow the
drill collars to pull past, thus creating a seal for
the annulus.
The drill pipe was pulled to 1555 feet (480
m) and a 6.29 bbls (1.0 cubic meter) low temperature
thermosetting plug was spotted by displacing the drill
pipe with 12.58 bbls (2.0 m3) of water. The drill
pipe was pulled out of the plug to 972 feet (300 m).
5 hours was allowed for setting the plug before
circulation. To do this, 158.5 gal us (600 litres) of
fresh water was loaded onto an oil field cementer 32
sacks of low temperature thermosetting composition jet
mixed off the ground hopper at approximately 15
seconds per sack which yielded 6.29 bbls (1.0 m3).
217~144
The slurry was mixed and pumped down the drill pipe.
It was displaced with 12.58 bbls (2.0 m3) of fresh
water. 20 stands of drill pipe were pulled and 5
hours waited for setting. The drill pipe was run in to
450 meters where the top of the plug was found. Full
circulation was established and the drill pipe was
pulled out of the hole. The production casing was run
and cemented above the top of the plug.
Example #6
While drilling the last portion of the Leduc
formation (Alberta, Canada) at 5835 feet (1801 m)
total lost circulation was encountered. The
Glauconite formation kicked gas at approximately 4050
feet (1250 m). Flow from the Glauconite up the
annulus peaked at 8 mmcfd. The drill pipe was free to
move. Attempts to control the Glauconite were made by
top killing the gas zone. Lost circulation material
was mixed into 12,580 bbls (2,000 m3) of drilling mud
and pumped through the bit into the Leduc with a large
amount of pumping pressure and no success in bridging
the lost circulation zone. Explosives were utilized
to blow the bit and sub off the end of the drill
string to achieve higher pump rates. Following the
removal of the bit and sub 12,580 bbls (2,000 m3) of
LCM pills were pumped into the lost circulation zone
without success. A total of eight (8) rig days were
spent fighting the lost circulation.
12.0 m3 low temperature thermosetting
composition was mixed and pumped with an oilfield
cementer and spotted across the Leduc formation. To do
this, the drill pipe was landed at 5074 feet (1566 m).
The thermosetting composition was loaded in to a
cleaned bulk transport truck and brought to location.
Water was supplied by two 100 bbl (16 m3) tank trucks.
217~i144
14
A slurry of the thermosetting composition was
continuously mixed and pumped down the drill pipe and
displaced with 37.7 bbls (6.0 m3) of fresh water.
Waited 8 hours prior to attempting to establish
circulation which was successful. Upon retrieving the
bottom collar of the drill string it was discovered
that the bit and sub were still attached. The only
damage made to the drill collar from the explosives
was a slight bulge. The nozzles in the bit were blown
out by the explosion. The pipe was run back into the
well and the plug was drilled down to 5654 feet (1745
m). It was then logged and casing cemented into the
well.
Example #7
A well drilled and suspended four (4) years
previously experienced a pressure build up of 130 psi
(896 kPa) in the surface casing assumed to be a gas
zone below the casing shoe. The Regulatory Board
would not allow the resource company to cap and
abandon the well. Previous attempts were made with
cement. The procedure was as follows: perforating
the production casing and circulating the cement
slurry through the perforations into the annulus and
up into the surface casing annulus. Attempts to
squeeze cement into the perforation were unfavourable.
The solution proposed in accordance with the invention
was to perforate the casing using both shallow and
deep penetration charges, then mix 4.0 m3 of low
temperature thermosetting composition and squeeze the
product into the perforations. To do this, the day
before the production casing was perforated below the
surface casing shoe form 375.0 to 376.0 m. An
injection rate was established into the perforations
at 30 litres per minute at 28 mPa. On the day of
217~1~4
treatment 38.1 mm coil tubing (total length 3800 m)
were run in to 386 m. 4.0 m3 low temperature
thermosetting composition was continuously mixed and
pumped 3.2 tonnes down the coil tubing and across the
perforations. Once the plug was balanced on bottom
the coil was pulled above the plug. 3.0 m3 of the
composition was squeezed into the perforations at 80
litres per minute at 1450 psi (10 mPa). Coil tubing
was pulled from the well. Due to the well's remote
location, a 38.1 mm coil tubing unit was utilized for
its economic benefit versus moving in a service rig.
The day after the treatment a pressure recorder was
utilized to measure the build up pressure over a 24
hour period. In the first hour the pressure built up
to 24 kPa and stayed constant for 24 hours. The
pressure that built up was thought to be residual gas
from the well and would take a couple of weeks to come
to surface.
The setting time of the thermosetting
composition of the present invention can be precisely
determined through pilot testing of the waters to be
used. The thermosetting composition of the present
invention can be formulated with fresh water,
magnesium chloride brine or sodium chloride brine.
Salinity has no measurable effect upon the hardening
reaction. The thermosetting composition of the
present invention can be mixed through jet hoppers, in
batch mix tanks, pumped with cementing trucks, and
spotted open-ended or through the bit. A cement plug
made in accordance with the invention requires the
same mud conditioning to drill as is used when
drilling green cement. Slurries of the invention
require no spacers when spotted. Balanced plugs may
also be run where required. Once the thermosetting
2175144
composition of the present invention has been spotted
the drillpipe should be pulled up above the pill. The
thermosetting composition of the present invention has
exponential setting characteristic but do not generate
gel strengths, allowing minimum pump pressures
throughout the application, due to consistent
rheological properties right up to the setting point.
The exothermic and rheological properties of
the thermosetting composition of the present invention
compounds allow a wide variety of applications, and
complement the most up-to-date engineering concepts in
placement and workover techniques, including the
latest coiled tubing equipment. In horizontal
drilling, the thermosetting composition of the present
lS invention has the unique capability of providing the
facility of selective zone isolation. This together
with its compressive strength and extremely fast
drillability allows the utilization of the compound
across the critical build section of the well with the
assured capability of drilling out on track without
kicking off.
A particular advantage of the present
invention is that the thermosetting composition is
suitable for use at various depths. At lower depths,
hence lower temperatures, the lower hydrostatic
pressure means that the composition will not invade
deeply into the formation even though setting time
will be slower. In deeper wells, the higher
hydrostatic pressure as offset by the faster setting
time within the immediate well bore periphery.
A person skilled in the art could make
immaterial modifications to the invention described
and claimed in this patent without departing from the
essence of the invention.