Note: Descriptions are shown in the official language in which they were submitted.
217571~
1038-591 MIS 729 1996 05 02 D3
TITLE OF THE INVENTION
C(L ~O~lllONS AND METHODS FOR PROTECTION AGAINST
._O~ICALLY DIVERSE ISOLATES OF INFLUENZA VIRIJS
FrRT~n OF T~R INVENTIO~
The present invention is related to the field of
immunology and i8 particularly concerned with vaccines
against immunologically diverse isolates of influenza
virus .
BACKGROUND OF THE INVENTION
The development of improved vaccines against
influenza virus is an active area of research. Current
influenza (flu) vaccines are about 7096 effective at
preventing illness in healthy adults (ref. 1 - various
references are referred to in parenthesis to more fully
describe the state of the art to which this invention
pertains. Full bibliographic information for each
citation is found at the end of the specification,
immediately preceding the claims. The disclosure of
these ref erences are hereby incorporated by ref erence
into the present disclosure) and approximately 5096
effective at preventing hospitalization and rn~ in
people aged 65 and over (ref. 2) . Desired properties of
improved vaccines for influenza include the production of
increased titers of virus neutralizing antibodies (IgG
and secretory IgA) against influenza antigens including
HA in the serum and at mucosal surfaces to prevent
infection (refs. 3 and 4), and induction of virus-
specific cross-reactive T lymphocyte (CD8i and/or CD4')
and cytokines to enhance recovery from infection (refs.
4, 5, 6).
Various strategies are being investigated to try and
develop; ~_uved parenterally administered sub-unit
vaccines. Approaches include the use of alternative
delivery vehicles for ~nf~ n7a antigens, such as
liposomes (ref. 7) and oil-in-water emulsions (ref. 8),
new adjuvants (ref. 9) or; ;7at;on with DNA encoding
various viral proteins (ref. 10, 11). Each of these
~17'~7~9
approaches is attempting to induce protective immune
responses that are enhanced compared to current subunit
preparations .
Immunostimulating complexes (ISCOMs) are an
5 adjuvanted particulate vaccine system comprising
cholesterol, phospholipid, antigen and Quil A (ref. 12).
Studies in various animal species have demonstrated
Pnh~n~-ed humoral and cell mediated immune responses
against model antigens and microbial proteins when
10 formulated as ISCOMs (ref. 13).
Various properties oi ISCOMs contribute to the8e
enhanced immune responses. Compared with soluble
antigen, ISCOMs are more rapidly distributed from the
site of inj ection to the draining lymph nodes and spleen
15 where they persist for longer periods of time (ref. 14) .
Following subcutaneous; i7~;0n, antigens formulated
as ISCOMs are taken up by a subset of splenic macrophages
distinct from those that take up soluble antigens (ref.
15). Within the APC, intact ISCOMs associate with
20 intracellular lipid membranes and localize within
cytosolic and vesicular compartments (ref. 16), enabling
processing for both class I and class II MHC-restricted
T cell responses. ISCOMs have been shown to upregulate
the expression of MHC class II expression on monocytes
25 (ref. 16), enhance the production of GM-CSF, TNF-~, IL-1
and IL-6 from macrophages and Thl (IL-2, IFN-~r) and Th2
(IL-4) cytokines from antigen-specific T cells (ref. 17) .
Both IgG1 and IgG2A responses are induced in mice
following; In; 7~tion with antigens formulated as ISCOMs
30 (ref. 18).
Infection with influenza virus leads to serious
disease. It would be desirable to provide improved
immunogenic compositions including vaccines comprising
influenza virus antigens for vaccination against disea8e
3 5 caused by inf luenza virus and f or the generation of
diagnostic reagents.
2175719
SUMM~RY OF TH~ INVENTION
The present invention is directed towards the
provision of compositions and methods for the protection
again3t immunologically diverse isolates of influenza
virus.
In accordance with one aspect of the invention,
there is provided a method of protecting a host against
disease caused by infection with an influenza virus,
comprising administering to the host a complex of
solubilized influenza virus comprising haemagglutinin
(HA) or at least one fragment thereof and an immune
stimulating complex (ISCOM) and effective to produce in
the host cytotoxic T cells specific for HA of both H1 HA
and H2 HA subtypes of inf luenza virus .
The aolubilized influenza virus may be an H1 HA
subtype inf luenza virus strain . The H1 HA subtype
against which cytotoxic T cells are produced may be an
HlN1 subtype influenza virus strain, including
A/Taiwan/1/86 and the H2 HA subtype may be an H2N2
2 0 subtype inf luenza virus strain, including A/~Japan/3 0 5/57 .
In a particular aspect, the immunizing influenza virus is
purified substAnt;Ally free from non-viral proteins and
may be solubilized by, for example, detergent extraction
to provide solubilized influenza virus consisting
substantially of HA. One suitable detergent is 296 Mega-
10 .
Cytotoxic T-cells recognize a cytotoxic T-cell
epitope comprising amino acids HA 189 to 199, which is
conserved among H1 and H2 inf luenza viruses but not H3
influenza viruses.
In a particular aspect, the present invention
provides a method of protecting a host against disease
caused by infection with influenza virus, comprising
administering to the host substantially purified
haemagglutinin (HA) or fragment thereof retA;n;ng the
immunological properties of HA inco--orated into
217~7~9
immunostimulating complexes (ISCOMs) and effective to
produce in the host cytotoxic T-cells specific for HA of
both of H1 XA and X2 HA subtypes of inf luenza virus .
The complexes for ; ; 7~tion provided herein
contain an immunologically effective amount of
haemagglutinin, for example, between about O . 05 ~g and
about 10 ~g of haemagglutinin per dose.
The complexes for; In; 7ation provided herein may
be administered by a variety of routes and a variety of
; ; 7;n~ schedules may be used. The 1 ; 7ation may
comprise a single administration of the complex or a
plurality (including two) administrations of the complex
may be u3ed to produce the protective immune response.
Advantages of the invention include the ability to
generate a cytotoxic immune response that protects
against disease caused by infection with immunologically
distinct isolates of influenza virus.
BRIEF DESCRIPTION OF FIGURES
The present invention will be further understood
from the following General Description and specific
Examples with reference to the Figures in which:
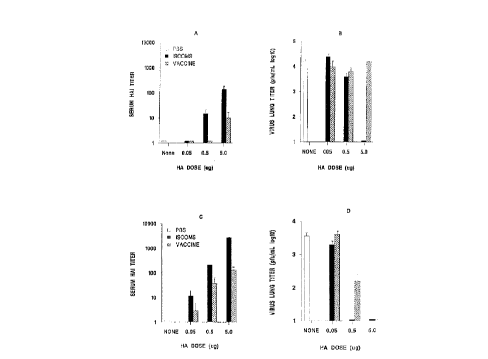
Figure 1, consisting of panels A, B, C and D, shows
HAI and virus lung titers in BABL/c mice immunized with
flu-ISCOMs or subvirion vaccine and challenged with live
homologous virus;
Figure 2, consisting of panels A and B, shows a
comparison between viral lung titers and weight 1088
following virus challenge of mice immunized with
flu-ISCOMs or subvirion vaccine;
Figure 3 shows the induction of influenza H1 and X2
subtype cross-reactive cytotoxic T-cells in mice
immunized with H1 XA flu-ISCOMs;
Figure 4, consisting of panels A, B, C and D, shows
the mortality and weight 1088 following heterologous (X2)
virus challenge of mice immunized with H1 flu-ISCOMs,
subvirion vaccine or live virus; and
' 4 2l757l~
Figure 5, consisting of panels A, B, C and D, shows
the mortality and weight 1088 following heterologous (H3)
virus challenge of mice immunized with Hl flu-ISCOMs,
3ubvirion vaccine or live virus.
(~.T~ T, DESCRIPTION OF THE INVENTION
As discussed above, the present invention is
concerned with the use of ISCOMs in combination with a
solubilized f raction of inf luenza virus, including
haemagglutinin, particularly derived from ~1 subtype
lo inf luenza virus, ( " f lu- ISCOMS " ) to stimulate a cross-
protective cytotoxic T-cell (CTL) response.
Immune stimulatory complexes or ISCOMs are a complex
composed of typically 0.5 wt96 Ouillaia saponins, 0.1 wt96
cholesterol, O .1 wt~ ph ~nsphnl; ~id and antigen in PBS .
Occasionally, surfactants are used to prepare ISCOMs
(such as, Mega 10) but are removed from the final
formulation before use. The ad]uvant-active components
of ISCOMs are derived by aqueous extraction of the bark
of Quillaja sa~onaria and are further purified by
chromatography. Quil A is a complex but purified mixture
of Ouillai a saponins which are glycosides of quillaic
acid and carbohydrates. ~urther chromatographic
purification provides components with high adjuvant
activity and ISCOM-forming properties.
In the data presented in the Examples below, we have
demonstrated the ability of flu-ISCOMs to protect mice
against challenge with both homologous and serologically
distinct type A influenza virus. The protective efficacy
of flu-ISCOMs was significantly better than that seen for
commercial subvirion vaccine comprising 10-fold higher
doses of HA. In addition, this increased efficacy of
flu-ISCOMs over vaccine was demonstrated when protection
was assessed in various way, including, virus load in the
lung, mortality, morbidity and rate of recovery from
infection. The immunological correlates associated with
protection and recovery from influenza virus infection
~ ~ 2I7~719
have previously been elucidated from the results of
studies performed in humans and animal models.
Pre-existing local secretory IgA and serum IgG specific
for HA correlate with preventi4n against infection by
5 homologous virus (refs. 3, 4) . Recovery from infection
with homotypic virus involves both serum IgG and class I
and class II MHC restricted cytotoxic T lymphocytes (CTL)
(ref. 19). Cytotoxic T cells also appear to enhance the
elimination of virus following heterotypic challenge,
10 thereby reducing morbidity and mortality (ref. 10). In
the present invention, the ability of flu-ISCOMs to give
improved protection in mice against inf ection with
homologous virus correlated with the induction of higher
serum HAI titers, ref lecting the presence of antibodies
15 specific for HA. At equivalent doses of HA, the serum
HAI titers induced by flu-ISCOMs were at least 10-fold
higher than those induced by vaccine . Similar f indings
have been shown for antigens from laboratory strains of
influenza and for other proteins formulated as ISCOMs
20 tested in various species (ref. 13). ISCoMs induce CD8'
class I MHC restricted T cell responses against
formulated soluble antigens derived from influenza virus
and HIV (gpl60) (ref. 20) and ovalbumin (ref. 21) . In an
earlier study, Jones and colleagues (ref. 22) reported
25 the induction of influenza specific CTL in mouse lungs
following intranasal inoculation of flu-ISCOMs.
The data presented herein is the first disclosure of
the antigen specificity and virus cross-reactivity of CTL
induced by flu-ISCOMs. Flu-ISCOMs comprising influenza
3 0 HA of the subtype H1 generated CTL that lysed target
cells infected with H1 and H2, but not infected with H3
strains of influenza virus. In contrast, CTL from mice
immunized with live H1 virus killed target cells infected
with either H2 or H3 subtype viruses. No CTL were
3 5 generated in mice immunized with the subunit vaccine .
This dif f erentiaI virus subtype recognition by CTL
. ~, 2175719
generated by flu-ISCOMs and live virus correlated with
their abilities to lyze target cells pulsed with known
MHC class I H-2Kd-restricted peptides from influenza NP
(NP 147 to 158) and- HA (HA 189 to 199), previously shown
to be recognized by CD81 CTI. generated after infection
with live ;nf1llPn7~ virus (refs. 23, 24). CT~ generated
by flu-ISCOMs recognised a synthetic peptide representing
amino acids XA 189 to 199 that is conserved amongst H1
and H2, but not H3 flu viruses. In contrast, the
; t~rl( ;n~nt responge induced by infection with live
virus was against NP 147 to 158, a sequence that is
conserved among all three virus subtypes (ref. 25).
Flu-ISCOMs failed to generate CTL against NP despite the
association of this protein with the ISCOM particles, as
~Pt~rTrinPd by polyacrylamide gel electrophoresis. There
may be a number of explanations for the inability of
flu-ISCOMs to induce CTL specific for NP, for example, NP
may not be present in sufficient amounts in the ISCOM
preparation. Alternatively, NP may not be associated
with ISCOMs in a manner that allows efficient cytoplasmic
processing of the protein for production of peptides that
bind to MHC class I molecules.
It has been demonstrated that, following
endocytosis, flu-ISCOMs accumulate intact, within the
endosomes of antigen presenting cells and that these
particles are exposed to the cell cytoplasm (ref. 16).
Viral membrane proteins, such as HA, that are able to
associate with phospholipid on the surface of the ISCOM
particle may be more accessible to cytoplasmic proteases
involved in processing for class I presentation. Weiss
et al., (ref. 26) reported that lipidated, but not
soluble NP, could be efficiently incorporated into
ISCOMs. ISCOMs c~nti~inins the lipidated NP preparation
did not induce CTl- in vivo.
In the data presented herein, we have shown that
there was a correlation between the ability of
, ~ 217~71~
flu(H1)-ISCOMs to induce cross-reactive CTL in mice
against H1 and H2 inf luenza viruses and protection
against mortality and morbidity due to infection with H1,
H2 but not H3 inf luenza viruses .
In eontrast to the cross-protection induced by
flu-ISCOMs described herein, mice immunized with live
HlN1 inf luenza virus were poorly protected against
mortality and morbidity following challenge with a H2N2
virus. This result was unexpected since live virus
induced CTL that were specific for a known
H-2Kd-restricted epitope within flu NP (NP 147 to 158)
that is conserved amongst H1 and H2 viruses. One
explanation for these observations is that the
cross-protection observed may be mediated by
15 immunological me~n;P-"q other ~ than CD8+ T cells.
Indeed, in this mouse model, there may be qualitative and
quantitative differences in the T lymphocyte subsets and
their secreted cytokines following i ; 7ation with
flu-ISCOMs compared with live virus that could contribute
20 to the enhanced clearance of virus after infection. For
example, in the absence of CD8+ T cells, 2-/- mice can
still clear virus after infection (ref. 27) and ean be
proteeted against death following lethal virus ehallenge
by; ; zation with vaeeinia expressing NP (ref . 6) . In
25 both studies, CD4+ eytotoxie T cells were implieated in
mediating the proteetion.
No signifieant differenees have been seen in the
quantity and type of eytokines produeed by virus and
flu-ISCOMs. Both induee Thl and Th2 cytokines (ref. 17).
30 Another explanation for the differences in
eross -proteetion observed between f lu virus and
flu-ISCOMs is that the funetional aetivities of CTL
indueed by flu-ISCOMs and live virus differ and may
depend on their speeifieities for the HA and NP proteins
35 respectively. Cytotoxic T-eells speeifie for HA have
been implieated in mediating eross-proteetion in miee
217~71~
given a recombinant HA2 fragment from influenza HA
expressed as a fusion protein. Immunization with the HA2
protein of the Hl strain protected BAIB/c mice against Hl
and H2, but not H3 strains of influenza virus (ref. 28).
5 Evidence for a protective role for CTL specific for NP
has been less conclusive and may depend on the form and
ability of the antigen to r-;nti~;n long term memory T
cell responses (ref. 29). For example, while
cross-protection has been observed in mice; ; ~7~1 with
10 cDNA encoding flu-NP (ref. 10), conflicting results have
been reported for soluble NP (refs. 30, 31) and
vaccinia-NP recombinants (refs. 32, 33) .
The flu-ISCOM preparations and vaccines provided
herein are administered in a manner compatible with the
15 dosage formulation, and in such amount as will be
therapeutically effective, protective and immunogenic.
The r1uantity to be administered depends on the subject to
be treated, including, for example, the capacity of the
individual ' 8 immune system to synthesize antibodies and
20 to produce a cell-mediated immune response. Precise
amounts of active ingredient required to be administered
depend on the judgement of the practitioner. However,
suitable dosage ranges are readily detf~rrn; nA~le by one
skilled in the art and may be of the order of micrograms
25 of the haemagglutinin. Suitable regimes for initial
administration and booster doses are also variable, but
may include an initial administration followed by
subse~uent administrations. The dosage may also depend
on the route of administration and will vary according to
30 the size o~ the host.
The concentration of HA protein in an immunogenic
composition according to the invention may vary widely
but may be in the range of micrograms. A vaccine which
rr,nt~inc antigenic material of only one pathogen is a
35 monovalent vaccine. Vaccines which contain antigenic
material of several pathogens are combined vaccines and
~, 217~719
also belong to the present invention. Such combined
vaccines contain, for example, material from various
pathogens or from various strains of the same pathogen,
or from combinations of various pathogens.
EXAMPLES
The above disclosure generally describe3 the present
invention. A more complete understanding can be obtained
by reference to the following specific Examples. These
Examples are described solely for purposes of
illustration and are not intended to limit the scope of
the invention. Changes in form and substitution of
equivalents are contemplated as circumstances may sugge3t
or render exPedient. Although specific terms have been
employed herein, such terms are ;nt,~nAf~l in a descriptive
sense and not for purposes of limitations.
ExamPle 1:
This Example describes influenza viruses and vaccine
preparations .
A/Taiwan/1/86 (HlN1) and A/Philippines/1/82 (H3N2)
as live influenza viruses in egg-derived allantoic fluid,
mouse-adapted A/Taiwan/1/86 and commercial A/Taiwan/1/86
monovalent subunit vaccine ('Fluzone') were obtained from
t~r7nn~ t I,aboratories Inc., Swiftwater, PA, USA. Mouse
adapted A/Japan/305/57 (H2N2) was generated by repeated
passage of allantoic fluid virus in mouse lungs. A/Hong
Kong/1/68 virus was obtained as allantoic f luid.
ExamPle 2:
This Example describes the preparation of complexes
comprising influenza antigens and immunost; l~t;nS
complexes (ISCOMs).
ISCOMs were prepared according to a dialysis
procedure (ref. 34). Briefly, influenza virus was grown
in embryonated eggs and purified from allantoic fluid by
sucrose density gradient centrifugation (20 to 6096
sucrose in PBS, 10, 000 x g for 60 min. ) . Purified virus
was solubilized with 2~ Mega-10 (Bachem, Bubendorf,
11 2175719
Switzerland) and the core pelleted through 30% (w/w)
sucrose .
To the supernatant was added 1 mg each of
phosphatidyl choline, cholesterol (Sigma, St ~ouis, MO)
5 and 5 mg Quil A (Spikoside, ISCOTEC Lulea, Sweden) per mg
of total viral protein as determined by the Bradford
assay (ref. 35). The mixture was incubated for 2 hours
at room temperature, dialysed against PBS and layered
onto a 10 to 5096 (w/w) sucrose gradient. After
centrifugion at 40, 000 rpm for 18 hours, fractions were
collected for characterization. Formation of ISCOMs was
verif ied by negative staining electron microscopy and
analytical sucrose density centrifugation. Cholesterol
incorporation was ~l~tf~rm; nf~d using 3H-cholesterol .
Fractions showing co-migration of formed ISCOM particles,
HA and cholesterol were pooled and dialyzed extensively
against PBS. The HA content of the flu-ISCOM fractions
and commercial sub-virion vaccine was determined by
single radial immunodiffusion (SRID) after solubilization
with 1~6 Zwittergent 314 non-ionic detergent (Calbiochem,
La Jolla, CA) . The assays were performed using
A/Taiwan/1/86 antigen and antiserum reference standards
(Centers for Biologics Evaluation and Research) . The
f inal Quil A content of the ISCOMs was estimated using
the orcinol procedure (ref. 36). The ratios by weight of
Quil A to HA in the final flu-ISCOM preparation was
estimated as 7 :1.
Example 3:
This Example describes the; ; 7i~t; on of mice .
3 o Female BALB/c mice between 6 to 8 weeks of age were
obtained from Charles River (Quebec, Canada). Mice were
immunized subcutaneously on days 0 and 21 with A/Taiwan
flu-ISCOMs or monovalent vaccine comprising 0.01 to 10 ~Lg
HA in 0.1 mL PsS. Control mice received either PBS alone
or 200 to 400 hemagglutination units (H~U) of live
A/Taiwan virus as allantoic f luid . Fourteen days later
217~7~
12
the mice were challenged intranasally while under
anaesthesia with 50 IlL of live mouse-adapted
A/Taiwan/1/86 (5 LDso) or A/Japan/305/57 (5 LDso) or a 1
in 10 dilution of A/HK/1/68 in allantoic fluid.
Protection was assessed by either monitoring mortality
daily and morbidity (weight changes in the mice) every 2
to 3 days up to 14 days post-challenge or by quantitation
of virus in mouse lungs as follows. Four days following
mouse challenge, lungs were dissected from individual
animals and homogenized in 2 mL PBS/0 . 59~ gelatin using a
Polytron homogenizer (Brinkman, Ontario). The lung
suspensions were centrifuged at 3, 000 rpm for 15 min.,
supernatants filtered through a 0.45 ,um membrane
(Millipore, Bedford, MA) and stored at -70 C until
assayed. Serial dilutions of lung homogenates (100 ~L)
were incubated in duplicate with washed confluent Madine
Darby canine kidney cells in 24 well flat bottomed tissue
culture plates for 1 hour at 37 C in 696 CO2. To each well
was added 1 mL of overlay medium comprising 29~ Noble agar
(DIFCO, Detroit, MI), 0.2g~ D(+) glucose (Sigma), 0.029
DEAE Dextran (Pharmacia, Quebec), 2.0~ BME vitamins (ICN,
Ontario) and 20 ~g/mL TPCK trypsin (Sigma) in 2 x DMEM
medium (ICN) . Following incubation for 3 days at 37 C
(5~ CO2), 1 mL of a 296 neutral red solution in PBS was
added to each well and, incubation r~nt; n~ overnight .
The stain was aspirated and the plaques counted and
expressed as the mean logl0 plaque forming units/mL. The
ability of mice sera to prevent influenza virus-mediated
hemagglutination was ~ t~ n~d by a hemagglutination
inhibition assay as follows. Six mice were bled at
random via the orbital sinus vein for pre- and
post-immunization bleeds. Sera samples were heated at
56 C for 30 min. to inactivate complement and pre-treated
with trypsin/periodate to destroy endogenous inhibitors
of hemagglutination. Serially diluted antisera were
tested for their ability to inhibit the agglutination of
13 21757~
196 chick red blood by 4 HA units of A/Taiwan virus in a
standard HAI assay (ref. 37) .
Exam~le 4:
This Example describes the determination of
5Cytotoxic T cell activity in; i z~d mice.
Peptides representing known H-2Kd-restricted
cytotoxic T cell ~CTL) epitopes within A/PR8/34 influenza
haemagglutinin, HA2 189 to 199 (I Y S T V A S S L V L)
(SEQ ID NO. 1) (ref. 23) and nuclear protein (NP), NP 147
10 to 158 (R-) (T Y Q R T R A L V T G) (SEQ ID NO. 2) (ref .
24) were synthesized on an automated ABI 430A peptide
synthe3izer using an optimized t-Boc chemistry according
to the manufacturer' 8 instructions . Synthetic peptides
were cleaved from the resin using hydrogen fluoride. The
15 purity of t~le peptides exceeded 85% as judged by
reverse-phase HPLC. Amino acid analyses, performed on a
Waters Pico-Tag 3ystem agreed with the theoretical amino
acid compositions.
For CTL studies, mice were given a single
20 immunization with either 1 ~Lg HA as A/Taiwan flu-ISCOMs
or 10 llg HA as monovalent subvirion vaccine in 0.1 ml PBS
via the subcutaneous route. Ten days later, spleens were
removed and restimulated with live virus. Spleen cells
(2.5 x 107) were incubated at 37 C, 69~ CO2 in an upright
25 flask in 20 mL RPMI/10% FCS with the same number of ~-
irradiated (3000 Rads) normal syngeneic spleen cells
previously infected for 1 hour at 37-C with A/Taiwan
virus in ;~ n~o; c fluid at 5, 000 HAU/8 x107 cells .
After int~llhAti~n for 5 to 6 days, the cells were
30 tested in a standard Slchromium release assay. Briefly,
washed effector cells were incubated with 10~ P815
mastocytoma target cells labelled with Slchromium at 50
to 100 ~LCi/106 cells in 96-well v-bottom tissue culture
plates for 4 to 6 hours at 37 C in 6~ CO~. Target cells
35 were either untreated, pulsed with 10 IlM synthetic
peptide or infected with live A/Taiwan/1/86 flu virus at
217571~
14
1000 HAU/106 cells, for 90 min. at 37 C. Plates were
centrifuged at 1, 000 rpm for 5 min. and O .1 m~ of
supernatant was removed for mea~u~ t of Slchromium
content in a gamma counter. Spontaneous and total
5 release of S1chromium were determined by incubating target
cells with either medium or 2 . 5g6 Triton-X100
respectively, in the absence of responder lymphocytes for
the duration of the assay. Percentage specif ic chromium
release was calculated as (counts - spontaneous
10 counts) / (total counts - spontaneous counts) x 100 . The
spontaneous release of S1chromium in the absence of
effector cells was between 5 to 15~ in these studies.
SUMMARY OF THE DISCI.OSURE
In summary of thi3 disclosure, the present invention
15 provides a novel immunization procedure for protecting
hosts against disease caused by inf luenza virus which
utili2es a complex of solubilized influenza virus and
ISCOMs to produce cytotoxic T-cells specific for ~ of
both H1 HA and H2 HA subtypes of influenza virus.
20 Modifications are possible within the scope of this
invention .
217S719
m s~ N ;~
' ~ r~ LD ~D ~ O -3 ~ c) n ~
~ ~ n ~ ~ ~ ~ ~ ~;
, ,, ~ . ~ o - J'
m ~ O ,~ a~
-~ e O ~ a ~:3
3 ~ ~ m 3
n O ~ O ~ o 3 E~ ,o
';J ~I d~ ~I N (~ N m ~ m ~ a
a
$ ~ N ~ r l LL~ H
)r r~ r~ ~ r (~ m ~,
) LOD N ~) ~OD 3 ~ ~~nr ~ r N ~ O
.~ , ~, E ,~ m
O ~ o ~ , ) $
~ 1 H ~> a O ) ~ ~ J~
r v ~ ~' ' ' ~ m
~1 -- ~ ~
c) - ,
217~;719
a
rD ~ ~ r
N ~ O ~ h
r~ ~ ~ m
~, ~ ~ ~ m
-rl
rQ ~I r l ~I ri ;
m , r~l r
~ H ~ r r
~ ' V ~ r~ O O ~
r-l O ~ ~ r
~ ~,
~ rQ ~ r~
r~
r~
~ ~ ro
1 rl
1~ ~ 'I ~ o ~ ~
r ~ , rD
H 3
~ J~ U ~ (a
- ~ ~ m m (a ~ ~
~ ~ H S-l O
r~
a) ~ rn
R ,~ ~ ,¢ m~
21757I9
17
K~ ;N~;S
1. Rubin F. ~. 1987. Prevention and control of
influenza: Role of vaccine. Am. J. Med. 82 (Suppl . 6A):
26-30 .
2. Gross, P.A., Hermogenes, A. W., Sacks, H. S., ~au, J.
and R. A. Levandowski. 1995. The efficacy of
influenza vaccine in elderly persons. A meta-analysis
of the literature. 1995. Annals of Internal Med.
123: 518-527.
3. Couch, R. B. and J. A. Kasel. 1983. Immunity to
influenza in man. Ann. Rev. Microbiol . 37: 529-549 .
4. Tomada, T., Morita, ~., Kurashige, T. and H. F.
Maassab. 1995. Prevention of influenza by the
intranasal administration of cold-recnmh; n~nt, live
attenuated inf luenza virus vaccine: importance of
interferon-y production and local IgA response.
Vaccine 13 :185-190 .
5. Yap, K. L., Ada, G. L. and I. S. C. McKenzie. 1978.
Transfer of specific cytotoxic T cells protects mice
inoculated with inf luenza viruses . Nature
273: 238 -239 .
6. Bender, B. S., Bell, W. E., Taylor, S. and P. A.
Small. 1994. Class I major historn~~t;h;l;ty
complex-restricted cytotoxic T lymphocytes are not
necessary for heterotypic immunity to influenza. J
Infect. Dis. 170 :1195-2000.
7. Guink, N. E., Kris, R. M. Goodman-Snitkoff, G., Small,
P. A. and R. J. Mannino. 1989. Intranasal
; 7~tion with proteoliposomes protects against
influenza in mice. Vaccine 7: 147-153.
8. Van Nest, G., Steimer, K. S., Haigwood, N. ~., Lyn
Burke, R. and G. Ott. 1992. Advanced adjuvant
formulations for use with recombinant subunit
vaccines. In Vaccines 92. F. Brown, R. M. Chanock
and R. A. ~erner editors. Cold Spring Harbor
Laboratory, Cold Spring Harbor, N.Y. 57-62.
9 . Tsu; imoto, M. Kotani, S., Okunaga, T., Kubo, T.,
Takada, H., Shiba, T., Kusumoto, S., T~k~h~h;, T.,
Goto, Y. and F. Kinoshita. 1989. ~nhi~nr t of
humoral immune responses against viral vaccines by a
non-pyrogenic 6-O-acylmuramyl peptide and synthetic
low toxicity analogues of lipid A. Vaccine 7:39-48.
10. Ulmer, B., Donnelly, J. J., Parker, S. E., Rhodes, G.
H., Feigner, P. I.., Dwarki, V. J., Grmokowski, S. H.,
~ 217~719
18
Deck, R. R., DeWitt, C. M., Friedman, A., Hawe, L. A.,
Leander, K. R., Martinez, D., Perry, H. C. Shriver, J.
W., Montgomery, D. L., and M.A. Liu. 1993.
Heterologous protection against influenza by injection
of DNA encoding a viral protein. Science.
259: 1745-1749 .
11. Robinson, H. L., Hunt, L. A. and R. G. Webster. 1993.
Protection against a lethal inf luenza virus challenge
by immunization with a hemagglutinin-expressing
plasmid DNA. Vaccine 11: 957-961.
12. Morein, B., Sundcluist, B., Hoglund, S., Dalsgaard, K.
and A. Osterhaus. 1984. Iscom. A novel structure for
antigenic presentation of membrane proteins from
enveloped viruses. Nature 308:457-460.
13. Hoglund, S., K. Dalsgaard, K. Lovgren, B. SundcIuist,
A. Osterhaus, and B. Morein. 1989. ISCOMs and
immunostimulation with viral antigens. In Subcellular
Biochemistry . J. R . Harris, editor. Plenum Publishing
Corporation . 3 9 - 68 .
14. Watson, D. L., Lovgren, K., Watson, N. A., Fossum, C.,
Morein, B. and S. Hoglund. 1989 . Infl i ~t~ry
response and antigen localization following
;7~tion with influenza ISCOMs. Infli ~lr;~n
13 :641-649.
15. Claassen, I. J. T. M., Osterhaus, A. D. M. E. and E.
Claassen. 1995. Antigen detection in vivo after
immunization with different presentation forms of
rabies virus antigen: involvement of marginal
metallophilic macrophages in the uptake of
immune-stimulating complexes. Eur. J. Immunol.
25: 1446-1452 .
16. Watson, D. L., Watson, N. A., Fossum, C., Lovgren, K.
and B. Morein. 1992. Interactions between
immune-stimulating complexes (ISCOMs) and peritoneal
r~c r~n1l~ l ear leukocytes. Microbiol. Immunol .
36: 199-203 .
17. Morein, B., Lovgren, K., Ronnberg, B., Anders, S. and
Villacres-Eriksson, M. 1995. Immunostimulating
complexes. Clinical potential in vaccine development.
Clin. Immunother. 3: 461-475.
18. Villacres-Eriksson, M., Bergstrom-Mollaoglu, M.,
Kaberg, H. and B. Morein. 1992. Involvement of
Interleukin-2 and interferon-gamma in the immune
response induced by influenza virus ISCOMs. Scand. J.
Immunol. 36:421-426.
~ ~ 21 7~719
19. Bender B. S. and P. A. Small. 1992. Influenza
pathogénesis and defence r~ hAni ~ Seminars in
respiratory Infections 7:38-45.
20. TAkAhAch;, H., Takeshita, T., Morein, B., Putney, S.,
Germain, R. N. and J. A. Berzofsky. 1990. Induction
of CD8+ cytotoxic T cells by immunization with
purified HIV-1 envelope protein in ISCOMs. Nature
344: 873-875.
21. Mowat, A. MCI., Donachie, A. M., Reid, G. and O.
Jarrett. 1991. Immune-stimulating complexes
c~ntA;n;ng Qull A and protein antigen prime class I
MHC-restricted T lymphocytes in vivo and are
immunogenic by the oral route. T ~nt~ gy 72: 317-322.
22. Jones, P. D., Hla, R., Morein, B., Lovgren, K. and G.
L. Ada. 1988 . ~ l Ar immune responses to local
immunization in the murine lung with influenza A virus
glycoproteins in micelles and immunostimulatory
complexes (Iscoms). Scand. J. Immunol. 27: 645-652.
23. Bodmer, H. C. Pemberton, R. M., Rothbard, J. B. and B.
A. Askonas. 1987. Enhanced recognition of a modified
peptide antigen by cytotoxic T cells specific for
influenza nuclear protein. Cell 52: 253-258.
24. Braciale, T. ~., Braciale, V. L., Winkler, M.,
Stroynowski, Hood, L., Sambrook and M. J. Gething.
1987. On the role of the trAn, ' dne anchor
sequence of inf luenza hemagglutinin in target cell
recognition by class I MHC-restricted
hemagglutinin-specific cytolytic T lymphocytes. J.
Exp. Med. 166: 678-692.
25. Buckler-White, A. J. and B. R. Murphy. 1986.
Nucleotide sequence analysis of the nucleoprotein gene
of an avian and human inf luenza virus strain
identifies two classes of nuclear proteins. Virology
155: 345-355.
26. Weiss, H. P., Stitz, L. and H. Becht. 1990.
Immunogenic properties of ISCOM prepared with
inf luenza virus nucleoprotein . Arch . Virol .
114: 109-120 .
27 . Eichelberger, M., Allan, W ., Zilj lstra, M ., Jaenisch,
R. and P. C. Doherty. 1991: 174: 875-880. Clearance of
inf luenza virus respiratory tract inf ection in mice
lacking class I major histocompatibility
complex-restricted CD8+ T cells. J. Exp. Med. 174:
875-880 .
217~719
28. Kuwono, K., Scott, M., Young, J. F. and F. Ennis.
1989 . Active ;r~-ln; 7 ition against virus infections
due to antigenic drif t by induction of crossreactive
T lymphocytes. J. Exp. Med. 169: 1361-1371.
29. Rimmelzwaan, G. F. and A. D. M. E. Osterhaus. 1995.
Cytotoxic T cell memory: role in cross-protective
immunity against influenza? Vaccine 13: 703-706.
30. Wraith, D. C., Vessey, A. E. and B. A. Askonas. 1987.
Purified influenza nucleoprotein protects mice from
lethal infection. J. Gen. Virol. 68: 433-440.
31. Stitz, L. , Schmitz, C. , Binder, D. , 7;nk~rnA~el, R. ,
Paoletti, E. and H. Becht. 1990. Characterization and
immunological properties of inflilf~n7a A virus
nucleoprotein (NP): cell associated NP isolated from
infected cells or viral NP expreYsed by vaccinia
recombinant virus do not confer protection. J. Gen.
Virol. 71: 1169-1179.
32. Webster, R. G., ~awaoka, Y., Taylor, J., Weinberg, R.
and E. Paoletti. 1991. Efficacy of nucleoprotein and
haemagglutinin antigens expressed in fowl pox virus as
vaccine for 1nflliPn7a in chickens. Vaccine 9: 303-308.
33. Endo, A., Itamura, S., Iinuma, H., Flln;ihri~ih;, S.,
Shida, H., r~oide, F., ~erome, K. and A. Oya. 1991.
Homotypic and heterotypic protection against influenza
virus infection in mice by recombinant vaccinia virus
expressing the _aemagglutinin or nucleoprotein gene of
influenza virus. J. Gen. Virol. 72: 699-703.
34. Lovgren, K. and B. Morein. 1988. The requirement of
phospholipid3 for the iormation of immunoYtimulating
complexes (iscoms). Biotechnol. Appl. Biochem. 10:
1 61 - 172 .
35. Bradford, M. M. 1976. A rapid and sensitive method ~or
the quantitation of microgram quantities of protein
utilizing the principle of protein-dye binding. Anal.
Biochem. 72: 248-254.
36. Lee, Y. C., McKelvy, J. F. and D. Lang. 1969. Rapid
automatic analysls of sugar components of
glycoproteinq. Anal. Biochem. 27: 567-574.
37. Palmer, D. F., Coleman, M. T., Dowdle, W. R. and G. C.
Schild. 1975. Advanced laboratory techniques for
influenza ri~ ign~ . In Immunology series no. 6, ~S
Dept. Health, Education and Welfare. Washington DC.
51-52 .