Note: Descriptions are shown in the official language in which they were submitted.
WO 95113136 PCTlUS94I13045
i " 217b343
-1-
METHOD OF PREPARING ISOMERIZATION CATALYST
The present invention relates to a method of preparing an
isomerization catalyst comprising an alkali metal on a support. The
isomeriza,tion catalyst so prepared is useful in isomerizing 5-vinyl-2-
norbornene ("VNB") to 5-ethylidene-2-norbornene ('BNB"). ENB is used
commercially in the production of elastomeric polymers and synthetic rubber.
An isomerization catalyst prepared by contacting an alkali metal with
a high surface area support material such as alumina is described in
to US-A-3,405,196. The contacting is accomplished by agitation under an
atmosphere of inert gas. The agitation to disperse alkali metal on the
support material described by the patent is stirring. A fluidized bed is used
to
treat that catalyst with oxygen. US-A-3,793,382 describes dissolving an alkali
metal in a solvent, for example a liquid ammonia, and spraying the
solvent/aIkali metal onto a carrier, for example alumina. The prior art does
not describe dispersion of alkali metal onto supports utilizing a ffuidized
bed.
US-A-2,818,350 teaches a method to prepare a high surface alkali
metal by using high velocity and turbulent flow, so that the alkali metal
particles impinge themselves onto the solid particles of the carrier, such as
2o alumina, which results in coating the alumina particles with a thin layer
of the
metal (column 1, lines 31-35). US-A-2,818,350 accomplishes impingement of
particles by using the sodium in either the molten or solid state, in a
slurry,
wherein the carrier liquid is heated, and then vaporized such that a high
velocity turbulence is developed in the slurry. High velocities in excess of
25
z5 feet per second, and preferably greater than 1()D feet per second are
necessary (column 2, lines 17-22) to cause the metal impingement. If a gas
carrier is used "enough gas volume to provide the necessary high velocity and
turbulence to cause the solid sodium to rub off onto the alumina carrier
particles" is required (column 4, lines 6-9).
3o Most importantly, US-A-2,818,350 teaches that the alumina particles
may also disintegrate into a finer size and by starting with coarse materials,
such as coarser than 200 mesh, will disintegrate to 10 microns or less (column
2, lines 29-36). Further, this reference teaches that the particles are
generally
reduced considerably in size during the process and the final sodium coated
35 particles may average as little as 10 microns (column 3, lines 12-16). This
is
desirable for US-A-2,818,350's purpose since it provides an extremely large
sodium surface for chemical reactions (column 3, lines 16-19), such as the
w0 95113136 ,- ~ 7 7 6 3 4 3 p~~S94113045
-2-
reactions mentioned at column 1, lines 14-20. This is in distinct contrast to
the objectives of the current invention, which is to provide a catalyst
suitable
for liquid slurry isomerization reactions, where it is desirable to have the
sodium metal applied to the carrier without the breakdown of the support '
particles which accompanies US-A-2,818,350's method of harsh, abrasive, and
turbulent approach of applying the sodium to the carrier. '
In general, the prior art methods of mechanically mixing alkali metal
with supports and mixing using liquid solvents and/or slurries suffer from
several disadvantages, including relatively long mixing times, uneven
to distribution of alkali metal on the support, breakage of the alumina
particles
(attrition), batches of catalyst that are relatively small and an inability to
convert to a continuous process. These and other disadvantages of the prior
art are overcome by the present invention.
is SUMMARY OF THE INVENTION
The present invention relates to a method of preparing an
isomerization catalyst by dispersing a molten alkali metal on a support within
a fluidized bed environment. The fluidized bed can be established by
2o introducing support particles to a vessel having an inlet and outlet
through
which an inert gas is respectively introduced and withdrawn in a manner that
suspends the support particles in the inert gas. Alkali metal may be
introduced to the fluidized bed and the temperature of the fluidized bed
maintained above the melting point of the alkali metal so that the movement
25 of the support particles that occurs in the fluidized bed disperses the
alkali
metal onto the support particles. Optimizing the operating conditions in the
fluidized bed provides for more uniform and quicker dispersion of alkali
metal, resulting in a superior isomerization catalyst when compared to
catalysts prepared by prior art methods.
3o In one aspect the invention comprises a method of preparing an
isomerization catalyst comprising introducing an alkali metal into a fluidized
bed of support particles wherein said fluidized bed is maintained at a
temperature above the melting point of the alkali metal and the support
particles are maintained in a fluidized state for a time sufficient to evenly
35 disperse the alkali metal on the support particles. In another aspect the
invention comprises a method of preparing an isomerizadon catalyst which
comprises: (a) introducing support particles into a ffuidization zone,
CA 02176343 2002-04-09
-3-
(b) introducing a flow of fluidizing gas to said flu'idization zone at ~a rate
sufficient to suspend _ said- support particles as a dense fluidized bed
maintained in said fluidization zone, (c) maintaining the temperature in the
fluidization zone at a premixing temperature, (d) introducing an amount of
alkali metal into said fluidization zone at a rate of 0.01 to 0:45 1b alkali
metal
per hourper 1b of support particles, (e) maintaining said flow of fluidizing
gas
for a mixing period to disperse said alkali metal on said support particles
to,
form an isomerization catalyst, and (f) withdrawing said isomerization
catalyst from said fluidization zone.
-io Another aspect of this invention comprises isomerizing VNB to ENB
using an isomerization catalyst prepared in accordance with the methods set
forth herein. Contacting VNB with the isomerizatidn catalyst under
isomerization conditions will produce ENB.
Another aspect of this invention comprises a method of isomerizing an
i5 olefin which comprises contacting a stream comprising the olefin with an
activated
catalyst, wherein the activated catalyst is prepared by a method comprising
introducing an amount of alkali metal into a fluidized bed of support
particles
wherein said fluidized bed of support particles is maintained at a temperature
above
the melting point of the alkali metal and in a fluidized bed state for a time
sufficient
2o to evenly disperse the alkali metal on the support particles without
substantial
breakdown of the support particles, wherein said fluidized bed of support
particles'is~
maintained -in a fluidized state utilizing a fluidizing gas flowing at a
superficial
velocity of from 2 to 20 linear feet per minute, and wherein the diameter of
the
support particles is in the range from 10 to 500 microns.
Another aspect of this invention comprises a method isomerizing a
stream comprising an olefin which comprises contacting the stream comprising
the
olefin with an activated catalyst, wherein the activated catalyst is prepared
by a
method which comprises: introducing alumina particles having a particle
diameter in
the range of 10 to S00 microns into a vertical fluidization zone; introducing
an
upward flow of fluidizing gas to said fluidization zone at a rate sufficient
to suspend
said alumina in a fluidized bed maintained in said fluidization zone;
maintaining the
temperature in the fluidization zone at a premixing temperature within the
range of
100° to 400°C; introducing an amount of alkali metal selected
from the group
CA 02176343 2002-04-09
-3A-
consisting of sodium, potassium, rubidium and cesium into said fluidization
zone at
a rate of 0.01 to 0.45 1b alkali metal/hr per 1b alumina while maintaining the
temperature in the fluidization zone within the range of 100° to
200°C; maintaining
said flow of fluidizing gas for a mixing period of at least 30 minutes after
said
amount of alkali metal has been introduced to said fluidization zone to
disperse said
alkali metal on said alumina to form an isomerization catalyst; and
withdrawing said
isomerization catalyst from said fluidization zone.
A feature of this invention is that the isomerization catalyst produced
in accordance with this method shows improved properties in comparison to
l o prior art isomerization catalysts. Another feature of this invention is
the
improved uniformity of dispersion of the alkali metal on the support. These
and other features of the invention will be apparent from the following
detailed description of the invention wherein reference is made to the Figure
in the accompanying drawings.
l s BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic drawing of one embodiment of a fluidized bed
apparatus that can be used to practice the process of preparing an Y
isomerization catalyst in accordance with this invention.
20 THE INVENTION
This invention relates to an isomerization catalyst that is prepared by
contacting an alkali metal with a particulate support material. In a preferred
but optional embodiment, the isomerization catalyst is contacted with oxygen
after the alkali metal has been dispersed upon the support particles. The
2$ support particles useful in preparing the isomerization catalyst according
to
this invention include aiumina, silica, aluminosilicates (including zeolites
and
molecular sieves), hydrotalcites; carbon (including charcoal and graphite)
oxides of metals of Groups ?A., 3B and 4B of the Periodic Table of Elements,
WO 95113136 PCTIU894II3045
clays and combinations thereof. References to the Periodic Table in this
application are to CRC Handbook of Chemistry and Physic, CRC Press,
Cleveland, OH, 53rd Edition, 1972-1973. A preferred support particle is
alumina including alpha, eta, chi, theta, kappa, gamma, delta, aluminas.
Gamma, alumina is particularly preferred. Descriptions of alumina
characteristics in this specification also apply to support particles in
general.
The water content of alumina that may be used as a support
material will depend partly on the type of alumina and will generally be in
the . range of 0 to 10% by weight (water/alumina + water). The water
to content of alumina referred to here is the total amount of water contained
by
the alumina, including adsorbed water. Optionally the alumina may be dried,
as discussed further herein, to reduce the water content to a preferable
range.
The surface area of the alumina, measured by the B.E.T. Nitrogen
Adsorption method will generally range from 5 to 300 m2/g, and more
preferably from 140 to 300 m2/g. Presently, gamma alumina having a surface
area of 140 to 180 m2/g is most preferred. Although not critical to the
invention, the pore volume of the alumina, measured by the B.E.T. Nitrogen
Adsorption method, is generally in the range of 0.1 to 1.0 ml/g.
Suitable alumina is available commercially from a number of
2o suppliers. Alumina is generally available in various shapes and particle
sizes.
The shape of alumina particles useful in this imrendon is not critical, but
spherical shaped particles are preferred because attrition losses are smaller.
The size of the alumina particles should be selected to permit fluidization of
the alumina particles in the fluidized bed apparatus. An apparatus having a
capacity for a relatively strong flow of flo1diz1ng gas will be able to
fluidize
larger alumina particles than an apparatus having a weaker flow of flo1diz1ng
gas. In this invention, the diameter of the alumina particles in microns
should range from 10 to 500, preferably 10 to 400 and most preferably 10 to
200. Most fluidized bed systems having adjustable flo1diz1ng gas flow rates
3o will be capable of flo1diz1ng alumina support particles of this size.
The alumina particles are introduced to a fluidized bed apparatus.
The initial introduction of alumina may occur before fluidization gas begins
to flow or, alternatively, the alumina particles may be introduced while the
fluidization gas is flowing. Alumina particles may be introduced to the
fluidized bed by means known to those skilled in fluidized bed technology,
including without limitation com~eyor belts, screw feeders, and gravity feed
from a storage hopper. The process may be operated in batch, semi-
CA 02176343 2002-04-09
- ,
continuous or continuous modes. In continuous operation, alumina will be
introduced to the fluicGzed bed at a predeterHnined usually constant rate
selected to provide appropriate residence time for the alumina in he
fluidized bed as further described herein. At the same time, alumiina
particles coated with alkali metal will be withdrawn from the fluidization
zone.
Parameters to be determined for successful operation of a fluidized
bed are described in the literature, e.g: Chemical Engineer's Handbook, Fifth
Edition, Perry & Chilton, McGraw-Hill, 1973,
1o Pspecially pp. 5-54 and 20-65 to 20-75, M. Leva, Fluidizanon,
McGraw-Hill, New York, 1959, and , G. F. Froment and K. B. Bischofi~
Chemical Reaction Analysis and Desr'gn, p. 667, John-Wiley & Sons, New
York, 1979. A fluidized bed may be established in a vessel having a gas inlet
located below the fluidization zone and a gas outlet located above the
is fluidization zone. During operation of the fluidized bed apparatus,
fluidization gas flows into the fluidization zone from the gas inlet upward
through the fluidized bed and then out of the fluidization zone through the
gas outlet. As the downward force of the earth's gravity on the alumina
particles is counterbalanced by the opposing flow of fluidizing gas, the
2o alumina particles are suspended in the fluidization zone. Preferably the
fluidization zone is vertical, having a fluidizing gas inlet at the bottom and
a
fluidizing gas outlet at the top of the fluidization vessel. ~.~
The flow of fluidizing gas should be maintained at a rate sufficient to
suspend the alumina particles in the fluidization zone and below a rate where
25 gas slugging occurs. Gas slugging is an undesirable condition in a
fluidized
bed where large pockets of fluidization gas travel upward through the bed:
Proper suspension of alumina particles in the fluidization zone will not occur
unless the flow rate of the fluidization gas is maintained within certain
ranges. Several variables will affect the flow rate that is optimum, as
3o descn'bed in the Chemical Engineers Handbook, including without limitation
the density, shape and amount of alumina particles in the fluidization zone;
and the density of the fluidizing gas. The flow rate should be just above the
minimum fluidization velocity and below the terminal fluidization velocity.
Terminal fluidization velocity can be calculated by Stokes law or other
35 similar methods. The object of fluidization in this invention is to achieve
agitation and mixing without causing attrition of support particles.
w0 95113136 , , . ' '_ 217 6 3 4 3 PCT~S94/13045
Ideally the conditions are such that no more than 15 wt.%, preferably
no more than 10 wt.%, and most preferably no more than 5 wt.%, of the
support particles after preparation of the catalyst are smaller than the
minimum particle size of those support particles before the preparation
method is begun. Alternatively stated, the wt.% of particles below a desired
minimum particle size of support particles prior to performing the
preparation method, preferably does not rise by more than 10 percentage
points. For example, if the starting material contains 7 wt.% particles being
less than 10 microns in size, then the finished catalyst will contain
preferably
to no more than 17 wt.% of particles being less than 10 microns in size.
Generally, the optimum flow rate for the fluidization gas can be easily
determined by testing at various fluidization gas flow rates to determine
which rate provides the best fluidization under the particular circumstances.
Usually the flow rate (superficial velocity) of the fluidizing gas will be
maintained from 0.15 to 6.1 meters per minute (0.5 to 201inear feet per
minute), preferably 0.61 to 6.1 m/minute (2 to 20 ft/min), more preferably
1.2 to 3.7 meters per minute (4 to 12 linear feet per minute), calculated
based
on the apparent flow rate of ffuidization gas through the fluidization zone
while empty, i.e., without support particles present. This flow rate is
2o customarily referred to as the superficial velocity of the fluidization
gas. At
these flow rates the fluidized bed is referred to as a dense fluidized bed.
The fluidization gas that should be used during the dispersion phase of
this invention is one that is inert to reaction with an alkali metal. Inert
gasses
such as nitrogen, helium, neon, argon, natural gas (methane) ethane, krypton,
xenon, radon and mixtures of any two or more of these are suitable. Nitrogen
is preferred. During the time alkali metal is introduced to the fluidized bed
and dispersed (evenly distributed) on the support particles, the entire
fluidized bed should be maintained in an inert atmosphere. Utilizing a
fluidizing gas consisting essentially of the inert gasses Listed above will
3o maintain an acceptably inert atmosphere.
The fluidization gas should be relatively dry since water vapor is
reactive with alkali metals. Generally the fluidization gas will be
sufficiently
dry if tile water content of the fluidization gas is below 10 ppm. If
necessary,
the fluidizing gas may be dried prior to contact with alkali metal. Means for
drying a gas are known in the art, including passing the fluidization gas
through a bed of molecular sieves, anhydrous alumina or anhydrous calcium
sulfate.
w0 95!13136 217 6 3 4 3 P~~S94113045
Optionally, the fluidized bed may be utilized to pretreat the stamina.
The pretreatment of stamina will prepare the stamina to receive the alkali
metal, and adjust the water content of the stamina to appropriate levels.
~ This pretreatment step is a type of preparation generally described in the
art
as calcination. The stamina pretreatment is accomplished by introducing
~ stamina to the fluidized bed apparatus and then beginning ffuidization
without adding alkali metal to the stamina. Preferably the fluidizing gas is
heated to promote removal of water from the stamina during pretreatment.
Due to the good heat transfer between fluidizing gas and stamina that is
to inherent with a fluidized bed, the stamina particles will be maintained at
the
same temperature as the fluidizing gas. The temperature of the fluidizing gas
during the pretreatment step should be maintained from 100° to 40(l~'C,
preferably 200' to 400' C, most preferably 300' to 400° C. The
residence time
of stamina particles in the fluidization zone during the pretreatment step
should range from 0.5 to 5, preferably 1 to 4 and most preferably 1 to 2
hours.
If the water content of stamina is to be reduced during the pretreatment step,
the residence time may need to be adjusted to accommodate removal of the
appropriate amount of water. If more water is to be removed, increasing the
residence time and/or temperature of the ffuidizing gas will facilitate the
2o appropriate water removal. If relatively large amounts of water are to be
removed, pretreatment may be extended beyond 4 hours. After the
pretreatment step and before alkali metal is contacted with the stamina
particles, the temperature of the fluidizing gas can be reduced to the
appropriate temperature for contact between stamina and the alkali metal.
Continued fluidization at the lower temperature will cool the stamina to a
temperature that is appropriate for contact between alkali metal and
stamina.
Any alkali metal or combination of alkali metals may be contacted
with the stamina particles to produce the isomerization catalyst of this
3o invention. In this application, the term alkali metal means such individual
alkali metals and/or combinations of alkali metals unless the context in
which the term is used expressly indicates a contrary intention. Specific
alkali
metals include lithium, sodium, potassium, rubidium and cesium. Sodium
and potassium are preferred, and of the two, sodium is preferred. The alkali
metal should be contacted with stamina particles at a temperature that is
above the melting point of tile particular alkali metal. For sodium the
preferred contact temperature is 100' to 200' C.
w0 95!13136 , ' 21 l 6 3 4 3 pCT~S94/13045
Pure alkali metal may be introduced to the fluidization apparatus in
either a solid or molten liquid form. Once the alkali metal is introduced, the
temperature in the fluidization zone will melt any solid alkali metal
introduced and maintain any molten alkali metal in the molten liquid state.
Preferably, the alkali metal is introduced to the fluidization zone in a
molten
(liquid) state. The alkali metal is preferably introduced to the fluidization
zone at a constant rate, continuously over a period of 1 to 3 hours.
Preferably
the temperature at which sodium is added ranges from 110' to 200' C.
The amount of alkali metal that should be added to make the
1o isomerization catalyst of the invention is generally in the range of 5 to
20
parts based upon 100 parts of alumina by weight (a ratio in the range of 1:20
to 1:5), preferably 5 to 15 and most preferably 10 to 15 parts of alkali metal
to 100 parts of alumina. In a preferred embodiment of the invention, the
alkali metal is added to the fluidized bed at a certain optimum rate ranging
from 0.01 to 0.45 kg/hr per kg (0.01 to 0.45 lb/hr per !b) of allunina support
particles, preferably 0.05 to 0.20, while maintaining the temperature in the
fluidization zone within the range of 110' to 200'C. Optionally, during the
addition of alkali metal, mechanical agitation of the fluidized bed at low
speeds with a turbine agitator at a tip speed of 0.003 m/s to 0.61 m/s (0.01
to
2 ft/s tip speed) may help break up larger agglomerates of alkali metal and
support particles that may otherwise form in the bed.
Suspension of the alumina particles in the ffuidization zone should
continue during the addition of alkali metal and usually for a time
thereafter.
The period of time after substantially all the alkali metal has been added
z5 during which fluidization continues at a temperature above the melting
point
of the alkali metal is referred to herein as the mixing period, and is
preferably at least 30 minutes in duration. The continued fluidization of the
alumina particles during the mixing period breaks larger alkali metal/support
particle agglomerates into smaller individual particles of support material
3o coated with alkali metal and provides for uniform dispersion of the alkali
metal onto the alumina particles, and may optionally be continued for 1 to 4
hours. Generally, continued fluidization of the alumina for a mixing period
of 1 to 2 hours after all of the alkali metal has been introduced to the
fluidization zone will provide sufficient time for the alkali metal to be
evenly
35 dispersed on the alumina particles. The degree of uniformity of the
dispersion of alkali metal can be determined by measuring the conversion of
alkali metal when contacted with oxygen.
WO 95113136 217 6 3 4 3 P~~594I13045
_g_
Another optional feature provided by this invention is the ability to
produce an oxidized alkali metal isomerization catalyst. In accordance with
this feature of the invention, oxygen or an oxygen containing gas is mixed
- with the fluidizing gas in certain proportions. As the fluidizing gas
supports
the alkali metal/alumina particles, the oxygen in the fluidizing gas reacts
with
the alkali metal in an exothermic reaction that liberates 50 kcal of heat per
g
atom of alkali metal reacted. Oxygen or oxygen containing gas is introduced
to the ffuidized bed at a relatively constant rate that is determined by heat
transfer characteristics of the fluidized bed system. The temperature of the
to particles in the fluidized bed during oxidation is maintained below
25(1° C,
and preferably below 20(YC. The total amount of oxygen (02) added should
range from 0.001 to 0.2 moles based upon each mole of alkali metal
dispersed on the support particles. The oxygen is preferably added to the
fluidizing gas prior to the gas distributor in the fluidizing apparatus. The
temperature of the fluidized bed is preferably maintained from 150° to
200'C. The optimum molar oxidation level is from 0.15 to 0.20:1 (02:Alkali
metal). The rate at which oxygen is introduced to the fluidization zone is
preferably in the range from 0.01 to 2 moles 02 per hour per mole of alkali
metal, more preferably 0.01 to 02 and most preferably 0.01 to 0.1 moles/hr
2o per mole of alkali metal on the support particles.
Optionally, a helical, paddle type or turbine agitator may be used to
provide supplemental agitation to the alumina particles. The ffuidized bed
apparatus is constructed so the agitator is deployed in the fluidized bed
portion of the support particles. The agitation velocity should be low to
avoid
a attrition.
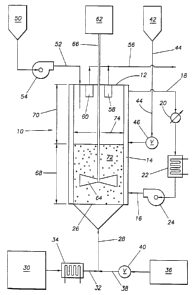
The invention may be further understood by reference to Figure 1
which shows a fluidizing apparatus 10. The ffuidizing apparatus 10 comprises
a vessel 12, an alkali metal reservoir 50, a support particle reservoir 42 and
a
gas supply 30. Support particles may be charged to vessel 12 from support
3o particle reservoir 42 through feed conduit 44 which connects support
particle
reservoir 42 with vessel 12. The flow of support particles into vessel 12 may
be controlled or restricted by control valve 46. Control valve 46 may be a
slide valve, star valve, table feeder, screw feeder, cone valve or any other
sflmilar device used to control flow of solid particles.
35 Support particles may be charged to vessel 12 either continuously
during fluidization or support particles may be charged as a batch all at once
to vessel 12 prior to or during fluidization. After support particles have
been
WO 95113136 ' ~.. 217 6 3 4 3 PCT~S94I13045
-10-
charged to vessel 12, they may be ffuidized with a fluidizing gas. In Figure
1,
gas supply 30 provides the fluidizing gas to gas inlet 28 which is in
communication with the bottom of vessel 12. Gas inlet 28 is connected with
gas supply conduit 32 through which fluidizing gas flows from gas supply 30.
If necessary, the fluidizing gas may be heated by passing it through gas pre-
heater 34.
Fluidizing gas enters vessel 12 through gas inlet 28 and then passes
through gas distributor plate 26 which is positioned near the bottom of vessel
12. Support particles are maintained in fluidized bed 72 in the space above
w gas distributor plate 26. Fluidizing gas travels up through gas distributor
plate 26 through fluidized bed 72 and out of vessel 12 through gas outlet 56.
Gas outlet 56 is connected to the upper portion of vessel 12 to avoid
removing support particles that are entrained in the flow of fluidizing gas
through vessel 12. Optionally, filters 58 and 60 allow for passage of the
ffuidizing gas therethrough but filter out entrained support particles so that
they stay within vessel 12. The fluidizing gas may be discharged from the
system or recycled back to gas supply 30.
The ffuidized bed depth is the distance between gas distribution
plate 26 and the top of ffuidized bed 72 and is represented in Figure 1 by
line
68. Preferably, vessel 12 provides for disengaging space above the fluidized
bed 72. The disengaging space is the volume above the top of fluidized
bed 72 and below the top of vessel 12, and the disengaging space depth is
represented by reference character 70. The disengaging space provides for
separation of support particles (which fall back down into ffuidized bed 72)
from the flowing fluidized gas which is withdrawn through gas outlet 56.
Alkali metal is added to fluidized bed 72 from alkali metal reservoir
SO through feed conduit 52. Feed pump 54 may be used to meter the proper
amounts of alkali metal into vessel 12.
Optionally, vessel 12 may be equipped with a turbine agitator 64
3o connected to motor 62 by shaft 66. In operation, turbin agitator 64 rotates
to
provide an agitation in the fluidized bed in addition to that provided by
ffuidization. '
After all of the alkali metal that is to be added to the support particles
has been added, and the alkali metal has been uniformly distributed upon the
support particles,' oxygen may be added to vessel 12 to react with the alkali
metal. Oxygen from oxygen supply 36 is introduced to vessel 12 through
supply conduit 38 which is connected to gas inlet 28. The flow of oxygen
w0 95113136 _ . _ _,. ,_.
~ ~ PCTlU594l13045
-11-
through supply conduit 38 may be controlled by control valve 40. Addition of
oxygen should be controlled at a rate so that the temperature of fluidized bed
72 does not exceed temperature limits as described in this speciflcadon.
~ In order to provide for better temperature control of vessel 12, it may
be necessary to install a heat exchanger 14 around vessel 12. In Figure 1,
heat exchanger 14 is an oil jacket having an oil inlet 16 and an oil outlet
18.
gn operation, oil circulates through heat exchanger 14 to add or remove heat
from fluidized bed 72 as needed. Circulation of the oil may be provided by
means of oil pump 24. The temperature of the oil may be adjusted using
w alternatively oil cooler 20 or oil heater 22 as needed. During the
pretreatment step and also during the mixing period, oil heater 22 may be
used to maintain fluidized bed 72 at the desired temperature. Alternatively
or in conjunction therewith, gas preheater 34 may be used. During the
addition of alkali metal, it may be necessary to use oil heater 22 to keep the
system at temperatures to maintain the alkali metal in a molten form.
During oxygen addition, because of the exothermic oxidation reaction, it may
be necessary to use oil cooler 20 to maintain the temperature of fluidized bed
72 within appropriate limits.
As discussed in the specification, the superficial velocity of the
2o fluidizing gas is calculated with reference to vessel 12 in an empty
condition.
If vessel 12 is circular in cross section, the fluidizing gas flow superficial
velocity may be calculated by determining the cross sectional area from
diameter 74, measuring the ffuidization gas flow rate and pressure and
calculating the gas flux across the cross section of vessel 12.
The invention may be more fully understood by reference to the
following examples.
EXAMPLES
3o Example 1. Compau~ative
This example describes a catalyst preparation where sodium metal
and alumina are mixed with a stir paddle at 250 rpm in a 300 cc reactor. The
tip speed of the paddle was 0.79m/s (2.6 ft/s). First, gamma-alumina (30 g),
with a surface area of 150 m2/g is dried in a nitrogen flow at 400'C for 1
hour and then cooled to room temperature under nitrogen. Then, small
pieces of metallic sodium (4.5 g) and the alumina are placed in the nitrogen
w0 95!13136 217 6 3 4 3 PCTIUS94/13045
-12-
blanketed reactor and heated under nitrogen to 150PC. At the point when
metallic sodium starts to melt, stirring is started and continued throughout
the preparation. The mixture is stirred for 30 minutes at 15(J~'C and then the
temperature is raised to 300'C where stirring is continued for 60 minutes.
Then the mixture is cooled to room temperature and a mixture of 5% 02 in
N2 is added at 126 mL/minutes for 123 minutes. Total stirring time was 213
minutes. Measurement of particle size showed 25 wt%a of the finished
catalyst had a diameter less than 10 microns. The starting alumina bad 7
wt% of particles with less than 10 micron diameter.
i0 Particles with diameters less than 10 microns in diameter cause
problems when the catalyst is used in a liquid slurry isomerization processes
because the rate at which these particles will settle is too slow for
efficient
catalyst/liquid-product separation.
Example 2 - Comparative
This example shows catalyst preparation in a commercial double cone
rotating mixer. Gamma-alumina powder (11.3 kg) (25 !b) having a surface
area of 170 m2/g was dried at 400'C in a flow of nitrogen. The powder is
2o then heated to 150'C under a N2 blanket and molten sodium (1.7 kg) (3.75
!b) is added over a period of 30-60 minutes while rotating the equipment to
provide agitation. Mixing and heating at 15(P C are continued for 120
minutes. Next, a mixture of 5% 02 in N2 is added over a period of 120-240
minutes while maintaining mixing action and temperature between 150-
200'C. The total agitation time is between 270 and 420 minutes. Catalyst
prepared in this manner was found to have 2 wt% of particles with diameters
greater than 600 microns compared to the starting alumina which had none.
There was no change in the amount of particles with diameters less than 10
microns. Particles with diameters greater than 600 microns cause processing
3o problems because they tend to plug transfer lines that handle catalyst or
liquid slurries in commercial isomerization plants.
Example 3 - The Invention
This example shows catalyst preparation in a fluidized bed reactor
with auxiliary mechanical mixing. Gamma-alumina powder (36.3 kg) (80 !b)
with a surface area of 170 m2/g was added to the reactor and then ffuidized
w0 95/13136
~ 17 6 3 4 3 P~.~S94~I3045
_ . . -.
_~_
with N2 at a linear superficial velocity of 9 ft/min and stirred with a
helical
turbine at a tip speed of (0.23 m/s) (0.75 ft/s). The fluidization and
stirring
was maintained throughout the remainder of the preparation. The alumina
was heated to 350' C for 60 minutes, and then cooled to 150° C over a
period
of 120 minutes. Then, molten sodium (5.4 kg) (12 1b) was added at a
- constant rate over 120 minutes at 150'C. Neat, 0.954 m3 (33.7 ft3) of oxygen
was added over a 370 minute period while maintaining the temperature
between 150' and 200'C. Total mixing time was 670 minutes. Compared to
the starting alumina, there was no increase in amounts of particles with
1o diameters greater than 600 microns or less than 10 microns.
Example 4 - Comparative
This example describes a catalyst preparation similar to example 1
I5 except the alumina and reaction temperatures were chosen to match those in
example 3. Sodium metal and alumina are mixed with a stir paddle at 250
rpm in a 300 cc reactor. The tip speed of the paddle was 0.79 m/s (2.6 ft/s).
Fn'st, gamma-alumina (21.8 g), with a surface area of 170 m2/g is dried in a
nitrogen flow at 400' C for 1 hour and then cooled to room temperature
2o under nitrogen. Then, small pieces of metallic sodium (3.3 g) and the
alumina are placed in the nitrogen blanketed reactor and heated under
nitrogen to 150'C. At the point when metallic sodium starts to melt, stirring
is started and continued throughout the preparation. The mixture is stirred
for 150 minutes at 150'C. Then, a mixture of 5% 02 is added at 126 mL/min
25 for 82 minutes at 150'C.
Example 5 - Compares Example 3 (Invention) with Example 4
(Comparative) With Respect to the Degree of Sodium Dispersion
3o One skilled in the art knows that the degree to which the amount of
metal can be exhaustively oxidized on a catalyst indicates the degree to which
the metal is dispersed on the catalyst. The greater the degree of sodium
dispersion on the catalyst, the lower the amount of sodium which remains
after oxidization. The catalysts in examples 3 and 4 were subjected to
35 exhaustive oxidation by adding 5% 02 in N2 so that 'the theoretical
stoichiometric ratio for Na20 of 0.25/1 (moles 02: moles Na) was achieved.
The amounts of metallic sodium remaining on the catalysts after exhaustive
WO 95!13136 . L 7 7 6 3 4 3 PCT~S94/13045
-14-
oxidation were determined by H2 evolution after hydrolysis. Catalyst from
example 3 after exhaustive oxidiztion had 0.9 wt% metallic sodium remaining
and catalyst from example 4 had 4.6 wt% metallic sodium remaining. This
example demonstrates that the use of gas fluidization as a mixing technique
of the present invention results in a higher degree of sodium dispersability.
Example 6 - Compares Example 3 (Invention) with Example 4
(Comparative) With Respect to Conversion of VNB Feed
l0 The catalysts in examples 3 and 4 were measured for activity to
isomerize S-vinyl-2-norbornene (VNB) to 5-ethylidene-2-norbornene (ENB)
using VNB that contained various impurities that might be expected in
commercial practice. The impurities (and their level in the VNB) were
vinylacetylene-cyclopentadiene adducts (720 ppm), tetrahydroindene
(570 ppm), cyclooctadiene (300 ppm), and alkyl-vinylcyclohexenes (600 ppm).
The vinylacetylene-cyclopentadiene adducts are known to be catalyst poisons.
The isomerizations were run by stirring 25 g of VNB with 035 g catalyst for 2
hours at ambient temperature. Products were analyzed by gas
chromatography: The VNB conversions were 98.5% for the example 3
a0 catalyst and 24% for the example 4 catalyst. The gas fluidization technique
for mixing results in a catalyst with greater activity.
Example 7 - Compares Example 3 (Invention) with Example 2
(Comparative) With Respect to Conversion of Impure VNB Feed
The catalysts in examples 2 and 3 were measured for activity to
isomerize VNB to F.NB using VNB that contained 1250 ppm indene as a
poisoning agent. The isomerizations were run by stirring 25 g of VNB with
0.35 g catalyst for 2 hours at ambient temperature. Products were analyzed
3o by gas chromatography. The VNB com~ersions were 98% for the example 3
catalyst and 30% for the example 2 catalyst. This again shows the gas
ffuidization technique for mixing results in a catalyst with greater activity.