Note: Descriptions are shown in the official language in which they were submitted.
~ 2176452
METHOD FOR REDUCING THE SURFACE AREA
OF CARBONACEOUS POWDERS
FIELD OF THE INVENTION
This invention pertains to methods for reducing the
surface area of carbonaceous powders. Specifically, it
pertains to methods for reducing the surface area of
carbonaceous anode powders for use in non-aqueous
rechargeable lithium ion batteries and thereby improving
the safety of the batteries.
BACKGROUND OF TFiE INVENTION
Carbonaceous powders exist in many different forms,
including amorphous carbons (eg. soot), cokes, graphites,
and speciality carbons. These powders are used widely
throughout many varied industries. In many applications,
high surface area carbonaceous powders are desired to
provide a maximum reactive surface (eg. activated char-
coals). Alternatively, it can be desirable to employ
powders with minimal surface area. Since smaller particles
present larger surfaces per unit volume or mass, the use of
the largest particles possible is generally preferred in
minimizing surface area. Use of powder with a uniform
particle size (diameter) can be most preferred. Spherical-
ly shaped particles theoretically can provide the lowest
possible surface area for a given particle size if the
particle surface is smooth. Spherically shaped carbon- _
aceous powders that are relatively smooth are commercially
available as speciality products (eg. mesocarbon
microbeads, hereinafter referred to as MCMB). However, in
practice, it can be difficult to achieve surface area
values close to the theoretical. There is usually some
significant surface roughness on the particles and often
there can be significant variation amongst different
production batches.
Lithium ion rechargeable batteries have been available
commercially since about 1991 and represent a preferred
2176452
2 -
rechargeable power source for many consumer electronics
applications. These batteries are characterized by a large
energy density (Wh/L) and high operating voltage (typically
above 3M volts). Such batteries use two different inser-
tion compounds with ample capacity for reversible lithium
insertion but with differing lithium insertion potential
for the active cathode and anode materials. At this time,
a lithium transition metal oxide (eg. LiCOOZ, LiNiO2,
LiMn2O4) is usually employed as the cathode material and a
carbonaceous compound (eg. coke, graphite, hard disordered
carbon) is usually used as the anode material.
The relatively low ionic conductivity of the typical
non-aqueous electrolytes employed in lithium ion batteries
makes it necessary to use thin electrodes (of order of 100
micrometer thick) in order to allow battery operation at
reasonable rates. Additionally, the diffusion coefficients
for lithium in the typical electrode material is not so
great. As a consequence, electrodes of the active inser-
tion compounds are typically made of small particles (eg.
20 micrometers in size).
The use of small particles not only desirably
increases the battery rate capability but also unfortunate-
ly increases the amount of and/or rate of certain undesir-
able chemical-reactions that occur on the particle sur-
faces. For instance; an irreversible loss of lithium
occurs on the carbonaceous anode surface in order to form
a protective passivation film against further decomposi-
tion. Thus, the more surface, the more the irreversible
loss of lithium capacity. Additionally, the safety level
of lithium ion batteries can be higher when low surface
area electrode materials are used. For instance, U.S.
patent number 5,246,201 discloses the effect of the surface
area of a LiNiO2 electrode on battery behaviour during
thermal abuse.
The spherically shaped mesocarbon microbeads carbon-
aceous powder, known as MCMB (a product of Osaka Gas) , has
been advantageously employed by many in the art as the
2176452
3 -
active anode material in lithium ion batberies (see, for
example, European patent application number EP 474,183).
As a result of its uniform size and low surface area for a
given particle size, such MCMB powder can allow high
electrode packing densities and good battery rate capabil-
ity while still presenting a minimal surface area for
undesirable chemical reactions.
Generally, it is known in the art that pyrolyzing
organic precursors, such as benzene, in the presence of
carbonaceous compounds can modify the surface conditions of
the carbonaceous compound, including the surface area
thereof. However, it is not possible to predict whether
such pyrolysis will result in a high surface area or
'fluffy' deposit or, alternatively, result in a smooth low
surface area deposit.
In published Japanese patent application number JP05-
307959, Mitsubishi discloses the use of a substantial
amount of pyrolyzed pitch (preferably 30 to 4016 of the
total weight) to agglomerate and coat the particles of a
core material (an active carbonaceous anode powder),
thereby producing a coated agglomerate anode powder with
certain advantageous properties. The coated agglomerates
are substantially larger in size than the original core
particles and have, as expected, a smaller surface area.
In an example therein, core particles that were 6 microme-
ters in diameter were treated in this manner and were then
ground up into agglomerates that were about 20 micrometers
in diameter. A reduction in geometric surface area is thus
inherently expected.
SUMMARY OF THE INVENTION
A method has been discovered for reducing the surface
area of carbonaceous powders in general and specifically
for reducing the surface area of electrode materials used
in rechargeable lithium batteries. The method involves
pyrolyzing an amount of petroleum or coal tar pitch in the
217(p) 45 2
4 -
vicinity of the carbonaceous powder. The carbonaceous
powder becomes coated with decomposition products of the
pitch and the coating can have substantially lower surface
area than the original carbonaceous powder.
Specifically, an amount of petroleum or coal tar pitch
is mixed with the carbonaceous powder. The mixture is then
pyrolyzed in an inert atmosphere at a temperature above the
decomposition temperature of the pitch. Some mild agglom-
eration may occur as a result of the pyrolysis.
Consequently, any agglomerates are broken up such that the
particle size distribution of the coated carbonaceous
powder is essentially unchanged by the pyrolysis or the
coating.
The method can be successfully used on carbonaceous
powders such as spherical graphite, flaky graphite, or hard
disordered carbon. The average particle size of the
carbonaceous powders can be in a range from about 20 to 75
micrometers and the surface area of the carbonaceous
powders before pyrolysis can typically be in a range from
about 1 to 210 m2/g. The method of the invention can reduce
the surface area substantially such that the surface area
of the coated carbonaceous powder after pyrolysis may be in
a range from about 0.3 to 12 m2/g.
The amount of pitch employed can range from about 7
to 3396 wt. of the weight of the carbonaceous powder.
Depending on the pyrolysis yield, the pitch decomposition
product coating can then be in a weight range from about 2
to 9% wt. of the weight of the carbonaceous powder. Since
these relatively small amounts can result in a substantial
surface area reduction, the bulk properties of the coated
carbonaceous powder remain largely unaffected by the
coating.
The pyrolysis can be performed at a temperature from
about 1000 to 2650 C, and for times of about 30 minutes.
Ramping rates of about 10 C per minute can also be
employed.
~ 2176452
-
It can be particularly advantageous to apply the
method of the invention in the construction of certain
batteries. For instance, as mentioned above, the spherical
carbonaceous powder MCMB is commonly employed as the active
5 anode material in lithium ion batteries. A particular
particle size of MCMB may be selected for purposes of
obtaining high electrode packing densities and good battery
rate capability. However, the surface area of commercial
MCMB is generally somewhat higher than that expected for
smooth spherical particles of similar size. Additionally,
the MCMB surface area may occasionally be substantially
higher than that expected for equivalent smooth particles.
(The reason for this is unknown, but it may relate to
scaleup and/or control difficulties during manufacture of
the MCMB.) Thus, there is generally some theoretical
possibility for the reduction of the surface area of the
MCMB and, often, there is substantial possibility for such
reduction.
We have discovered that by using the method of
the invention, the surface area of such anode powders can
indeed be reduced thereby still providing the desired
advantages for that particular particle size selection of
powder while simultaneously minimizing any disadvantages
arising from chemical reactions at the powder surface.
The battery can therefore be a non-aqueous
rechargeable lithium ion battery and can have a carbon-
aceous powder electrode comprising spherical graphite. A
preferred average particle size for such battery electrode
powders is about 20 micrometers and the surface area of
such powders before pyrolysis treatment can be about 2 m2/g.
The use of a minimal amount of pitch may be desirable
in such applications in order to obtain a substantial
surface area reduction without significantly altering the
bulk properties of the anode powder. Thus, the amount of
pitch can be about 7%- wt. of the weight of the carbonaceous
powder. Depending on the pyrolysis yield, the pitch
w 2176452
6 -
decomposition product coating can then be from about 2 to
4%~ wt. of the weight of the carbonaceous powder. Such
pyrolysis treatment can reduce the surface area such that
the coated carbonaceous powder after pyrolysis is less than
about 0.5 m2/g.
BRIEF DESCRIPTION OF THE DRAWINGS
In drawings which illustrate specific embodiments of
the invention, but which should not be construed as
restricting the spirit or scope of the invention in any
way:
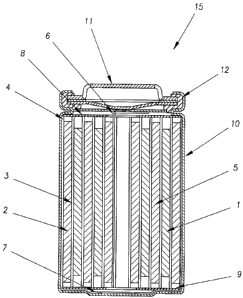
Figure 1_depicts a cross-sectional view of a conven-
tional cylindrical spiral-wound lithium ion battery.
Figures 2a and b show the temperature versus time data
during hot box testing of the batteries having different
anode surface area in the Battery Examples.
DETAILED DESCRIPTION OF SPECIFIC
EMBODIMENTS OF THE INVENTION
We have discovered that the surface area of carbon-
aceous powders can be reduced by pyrolyzing an amount of
petroleum or coal tar pitch in the vicinity of the carbon-
aceous powder such that the powder becomes coated with
decomposition products of the pitch. Volatile components
of the pitch apparently deposit on the powder and fill
'cracks' or other surface irregularities during the
pyrolysis such that the final decomposed pitch components
unexpectedly form a smooth, thin coating on the powder.
This coating can have a substantially lower surface area
than the original carbonaceous powder.
Generally, according to the invention, a carbonaceous
powder is first identified as having a surface area that is
significantly higher than that desired for a particular
CA 02176452 2007-01-24
- 7 -
application. Additionally, of course, the surface area
must also be significantly higher than the theoretical
lower limit for the particle shape and size distribution
for that powder. The method then comprises mix-
ing/dispersing a suitable amount of petroleum or coal tar
pitch with the carbonaceous powder and pyrolyzing the
mixture. The preferred amount of pitch is selected on the
basis of overall results from a few non-inventive empirical
trials.
The pyrolysis is performed in an inert atmosphere at
a temperature above the decomposition temperature of the
pitch. Petroleum and coal tar pitch contain mainly high
molecular weight aromatic compounds which do not vaporize
below about 200 C (for example, see "Carbon and Graphite
Handbook", edited by C.L. Mandell, published by John Wiley
and Sons, 1968). Typical pyrolysis temperatures are
substantially higher, about 900 C and even up to about
3000 C. However, the structure of the bulk carbonaceous
powder itself can be significantly affected by treatment
temperatures as high as the latter 3000 C. Thus, unless it
is desirable to additionally anneal or graphitize the
carbonaceous powder as well as reduce its surface area, a
pyrolysis temperature close to the former 900 C is gener-
ally employed. A typical suitable pyrolysis time is about
30 minutes and a typical suitable temperature ramping rate,
which can be achieved using common furnaces, is about 10 C
per minute. After pyrolysis, the mixture can be cooled at
a rate of about 10 C per minute.
Following the pyrolysis, some mild agglomeration may
be encouraged. The extent of any agglomeration will depend
in part on the amount of pitch employed. Any such agglom-
erates are easily broken up by a mild grinding operation
such that the original particle size distribution of the
carbonaceous powder is essentially maintained.
The method can be successfully used on a variety of
carbonaceous powders including spherical graphite (eg.
MCMB), flaky graphite, and hard disordered carbon. The
CA 02176452 2007-01-24
- 8 -
average particle size of such powders can be in a range
from about 20 to 75 micrometers with surface areas that are
typically in a range from about 1 to 210 m2/g. For use in
the method, preferred amounts of pitch can be from about 7
to 33% of the weight of the carbonaceous powder with a
resulting pyrolyzed coating weight from about 2 to 9% of
the weight of the powder, depending on the yield. Reduc-
tions in surface area below 12 mZ/g and down to 0.3 m2/g can
be expected to be achieved.
In battery applications, a similar method is employed
in the preparation of carbonaceous powders for use as an
electrode material. A preferred application is in the
manufacture of commercial lithium ion batteries. These
batteries come in a variety of sizes and formats, including
coin cell and prismatic constructions. Figure 1 shows a
typical construction of a conventional cylindrical spiral-
wound battery lithium ion battery. Therein, a jelly roll
4 is created by spirally winding a cathode foil 1, an anode
foil 2, and two microporous polyolefin sheets 3 that act as
separators.
Cathode foils for the jelly-roll are prepared by
applying a mixture of a suitable powdered cathode material,
such as LiCoO2, a binder, and a conductive dilutant onto a
thin aluminum foil. Typically, the application method
first involves dissolving the binder in a suitable liquid
carrier. Then, a slurry is prepared using this solution
plus the other powdered solid components. The slurry is
then coated uniformly onto the substrate foil. Afterwards,
the carrier solvent is evaporated away. Generally, both
sides of the aluminum foil substrate are coated in this
manner and subsequently the cathode foil is calendered.
Anode foils are prepared in a like manner except that
a reduced surface area powdered carbonaceous insertion
compound prepared by the method of the invention (typically
about 20 micron size) is used instead of the cathode
material and thin copper foil is usually used instead of
aluminum. Anode foils are often slightly wider than the
CA 02176452 2007-01-24
- 9 -
cathode foils in order to ensure that anode foil is always
opposite cathode foil.
The jelly roll 4 is inserted into a conventional
battery can 10. A header 11 and gasket 12 are used to seal
the battery 15. The header may include safety devices if
desired (eg. overpressure vent, positive temperature
coefficient device, and/or a pressure activated electrical
disconnect device). The external surface of the header 11
is used as the positive terminal, while the external
surface of the can 10 serves as the negative terminal.
Appropriate cathode tab 6 and anode tab 7 connections
are made to connect the internal electrodes to the external
terminals. Appropriate insulating pieces 8 and 9 may be
inserted to prevent the possibility of internal shorting.
Prior to crimping the header 11 to the can 10 in order to
seal the battery, a suitable non-aqueous electrolyte 5 is
added to fill the porous spaces in the jelly roll 4.
Suitable electrolytes include those having such lithium
salts as LiPF6 or LiBF4 dissolved in mixtures of linear
and/or cyclic carbonate solvents such as ethylene carbonate
(EC), ethyl methyl carbonate (EMC), and diethyl carbonate
(DEC) solvents.
The header 11 is then crimped to the can 10. Lastly,
an electrical conditioning step involving at least a single
charging of the battery is usually performed next as part
of the assembly process.
As mentioned earlier, the spherical carbonaceous
powder MCMB is commonly employed as the active anode
material in lithium ion batteries. MCMB with a nominally
uniform particle size is often chosen for purposes of
obtaining high anode packing densities and good battery
rate capability. Also, the lowest surface area possible
for the anode material is desirable, since it can provide
the greatest level of safety in certain circumstances. For
instance, during high temperature abusive conditions, the
non-aqueous electrolyte in these batteries can react
exothermically with the lithium in the anode at a reaction
CA 02176452 2007-01-24
- 10 -
rate determined in part by the anode surface area. The
threshold value at which a thermal runaway occurs can be
significantly raised with a reduction in the reactive
surface area of the anode.
However, the surface area of commercial MCMB is
generally, and not unexpectedly, somewhat higher than the
theoretical value for any given nominal particle size.
Also, we have found that the surface area of an occasional
batch of commercial MCMB may be substantially higher than
usual for reasons unknown. Consequently, the method of the
invention can be useful in reducing, to some extent, the
surface area of an MCMB batch generally and can be particu-
larly useful in 'correcting' the surface area of any such
occasional high surface area batch.
MCMB having an average particle size of about 20
micrometers may be desirable for lithium ion battery anode
applications. The surface area of such powder can typi-
cally be about 2 m2/g. The use of a minimal amount of pitch
while still obtaining a substantial surface area reduction
is generally desirable. Thus, the amount of pitch can be
about 7% of the weight of the carbonaceous powder which can
result in a decomposition product coating from about 2 to
40 of the weight of the powder, depending on pyrolysis
yield. Such pyrolysis treatment can reduce the surface
area markedly to less than about 0.5 mZ/g, without signifi-
cantly affecting the particle size distribution of the
powder.
The following Examples are provided to illustrate
certain aspects of the invention but should not be con-
strued as limiting in any way.
Inventive Examples
We have been successful in reducing the surface areas
of several carbonaceous powders using the method of the
invention as summarized below in Table 1. The powders used
were: MCMB (a product of Osaka Gas), a flaky graphite
CA 02176452 2007-01-24
- 11 -
denoted KS75TM (a product of Lonza), a flaky graphite
denoted SFG75TM (a product of Lonza), and a hard disordered
carbon prepared by pyrolyzing Plenco 11760TM resin (a
product of Plastics Engineering Co.) at 800 C in argon. An
amount of either petroleum pitch or coal tar pitch (indi-
cated as a o by weight of the powder) was added to the
powder and mixed therewith. The mixture was then pyrolyzed
at a temperature and for a time as indicated.
In order to determine the amount of decomposition
product remaining on each powder after pyrolysis, samples
of pure petroleum and coal tar pitch were pyrolyzed under
similar conditions in a thermal gravimetric analyzer. The
yield in each case after pyrolysis was about 28% and 50o by
weight respectively. A similar yield for each pitch type
is expected in these inventive examples.
Samples 1 to 5 agglomerated slightly and the agglomer-
ates were broken up in the following manner. First, the
pyrolyzed product was passed through a mesh screen (140
size mesh for samples 1 to 3, and 80 size mesh for samples
4 and 5). Greater than 80% by weight went through easily
on this initial screening. The remainder was lightly
ground using a manual mortar and pestle. The lightly
ground agglomerates then went through a second screening
using the same mesh. Greater than 95% of the pyrolyzed
product in total passed through the screen. The screened
powder was then used to determine the characteristics in
Table 1.
Sample 6 agglomerated somewhat more than the other
samples. Significantly more grinding was performed using
a manual mortar and pestle until >95% by weight went
through a 140 size mesh screen. The significant grinding
of sample 6 was evidently excessive for purposes of break-
ing up agglomerates only, based on the increase in the
average particle size after treatment.
Surface areas were determined by BET, a standard
nitrogen adsorption technique, using a commercial analyti-
CA 02176452 2007-01-24
- 12 -
cal machine made by Quantachrome. Before measuring, all
samples were outgassed by preheating at 150 C under pure
nitrogen for 30 minutes.
Particle size distributions were determined via laser
diffraction methods using a commercial analytical machine
made by Horiba. In Table 1, only the average particle size
of each sample is reported for comparison. In samples 1 to
5, there was no substantial change in particle size distri-
bution as evidenced by little change in the average parti-
cle size. In sample 6, the powder after treatment and
excessive grinding actually showed a marked reduction in
average particle size. Nonetheless, data for this sample
has been included since it still shows a marked reduction
in surface area as well.
In all instances, the surface area was reduced by a
factor of over 2 without a significant increase in average
particle size. The surface area of the spherically shaped
MCMB was reduced a factor of over 4 in sample 1. Sample 6
showed an order of magnitude reduction in surface area.
2176452
13 -
U ~~"
M O\ Cf GD eh
=a~i
y~ M M
~
tV
t7 s O O O "' "" ~-+
rA
~
O oo O oo a
.~ r,+ N N N N M t,
4y
cn N
%O ~O N N N
Lr
N
E,,, == t~ .. '. .. ..
=~ .~' " ~n v~
[- r~ n M
tn y cn
~ a w a a.
cn
~
in ~o
~
F
~ 14 - 2176452
-
Comparative Example
A sample of MCMB similar to samples 1 and 2 above
(Table 1) was obtained and was determined to have a BET
surface area before pyrolysis treatment of 1.1 m2/g. Then,
7%- by weight of table sugar was mixed with this sample and
the mixture was pyrolyzed at 1000 C for 0.5 hours. As in
the Inventive Examples above, slight agglomeration of the
powder occurred. The agglomerates were broken up in the
same manner as samples 1 to 3. The surface area after
pyrolysis treatment was found to be 20.2 m2/g, that is,
substantially higher than that of the starting powder.
This example demonstrates that not every pyrolyzed
organic precursor suitably reduces the surface area of
certain carbonaceous powders, particularly that of MCMB, a
powder having a relatively low surface area to begin with.
Battery Examples
18650 size cylindrical batteries (18 mm diameter, 650
mm height) were fabricated as described in the preceding
and shown generally in Figure 1. Cathodes comprised a
mixture of LiCoOZ powder, graphite conductive dilutant, and
polyvinylidene fluoride (PVDF) binder that was uniformly
coated on both sides of a thin aluminum foil. Anodes were
made using either Inventive Sample No. 1 after treatment
or, for comparison, Sample No. 3 before treatment (ie. a
material similar to Inventive Sample No. 1 but without a
pyrolyzed pitch coating). The active anode material was
mixed with Super S (trademark of Ensagri) carbon black and
PVDF binder and uniformly coated on thin copper foil to
prepare an anode. Celgard' 2400 microporous polypropylene
film was used for the separators 3. The electrolyte 5 used
was a solution of 1M LiPF6 salt dissolved in a solvent
mixture of ethylene carbonate (EC), ethyl methyl carbonate
(EMC),and diethyl carbonate (DEC) in a volume ratio of
21764S2
- 15 -
30/50/20 respectively. All batteries were made in the same
manner except that some anodes were pitch treated according
to the method of the invention and thus had lower surface
area.
After assembly, the batteries were electrically
conditioned (ie. a controlled charging) and cycled once
under similar conditions. Both types of battery showed
similar capacities and rate capabilities.
A representative fully charged battery made with each
anode was then subjected to a thermal abuse test. Bat-
teries were placed in a convection oven set at 150 C and
the battery skin temperature versus time was monitored.
Figures 2a and b show the temperature versus time data for
the Comparative battery (made with Sample No. 3 before
treatment, namely an untreated higher surface area MCMB)
and the Inventive battery (made with Sample No. 1 after
treatment, namely a pitch treated lower surface area MCMB)
respectively. As can be seen in Figure 2a, the Comparative
battery skin temperature continues to climb above the 150 C
set point of the oven as a result of exothermic reactions
taking place in the battery. Thermal runaway occurs just
before 0.7 hours into the test whereupon the battery vents
violently with flame. The temperature is seen to spike
upwards and then falls as the battery cools after burning.
In comparison, the Inventive Battery as seen in Figure 2b,
shows much less heating from internal exothermic reactions
and does not undergo thermal runaway. (The drop in the
temperature plot at about 1.2 hours is due to the opening
of the pressure relief vent of the battery.)
This comparative example demonstrates the safety
advantage associated with use of lower surface area anode
material.
As will be apparent to those skilled in the art in the
light of the foregoing disclosure, many alterations and
modifications are possible in the practice of this inven-
tion without departing from the spirit or scope thereof.
Accordingly, the scope of the invention is to be construed
21.76452
- 16 -
in accordance with the substance defined by the following
claims.