Note: Descriptions are shown in the official language in which they were submitted.
WO 95/19227 21 7 6 4 $ 2 PCT/US94/00510
BLACK ELECTl~OPHORETIC PARTICLES
AND METHOI~) OF MANUFACTURE
,- TECHNICAL FIELD OF THE INVENTION
The present invention relates to dielectric black particles for
~ use in electrophoretic image displays, electrostatic toner or the like and the
S corresponding method of m~nllf~rt~lring the black particles. More
particularly, the present invention relates to polymer latexes, prepared by an
emulsion polymerization technique, wherein the polymer latexes are reacted
with a metal oxide forming black dielectric particles that have physical
characteristics, such as size, density, and surface functionality selectively
determined by varying the polymerization reaction.
BACKGROUND ART
The electrophoretic effect is a well known and the prior art is
replete with a number of patents and articles which describe the effect. As
will be recognized by a person skilled in the art, the electrophoretic effect
operates on the principle that certain particles, when suspended in an
medium, can be electrically charged and thereby caused to migrate through
the medium to an electrode of opposite charge. Electrophoretic image
displays (EPID) utilize the electrophoretic effect to produce desired images.
In prior art EPID colored dielectric particles are suspended in a fluid
medium that is either clear or of an optically contrasting color as compared
to the dielectric particles. The colored electrophoretic particles are then
caused to selectively migrate to, and impinge upon, a transparent screen,
thereby displacing the fluid medium against the screen and creating the
desired image.
As will be recognized by a person skilled in the art, the
selection of the electrophoretic particles used in the EPID is very important
in determining the performance of the EPID and the quality of the viewed
image produced. Ideally, electrophoretic particles should all be of a
wo 95/19227 21 7 6 4 8 2 ~ PCT/US94/00510
unilorm size. IO help in assuring that each of the electrophoretic particles
will behave similarl~. Additionall~, it is desirable to utilize electrophoretic
particles that have essentially the same density as the fluid medium in which
they are suspended. By using electrophoretic particles of essentially the
same density as the suspension medium, the migration of the electrophoretic
particles through the medium remains independent of both the orientation
of the EPID and the forces of gravity.
To affect the greatest optical contrast between electrophoretic
particles and the suspension medium, it is desirable to have either white
particles suspended in a black medium or black particles suspended in a
backlighted clear medium. In the prior art, it has proven difficult to
produce black electrophoretic particles that are dielectric, of uniform size,
and which have a density matching that of a common suspension medium.
As a result, EPIDs commonly use readily manufactured light colored
electrophoretic particles suspended in dark mediums. Such EPIDs are
exemplified in U.S. Patent Nos: 4,655,897to DiSanto et al., 4,093,534to
Carter et al.,4,298,448to Muller et al.,and 4,285,801to Chaing. In such
prior art where light particles are suspended in a dark medium, the
suspension often appears grayish until the application of an electric field.
With the electric field applied, the light colored particles migrate through
the grayish suspension producing a light image on a gray background,
thereby resulting an image that is not highly contrasted.
To produce a more contrasted image, it is desirable to
backlight suspended black particles in a clear medium. However, as has
been mentioned, the development of suitable dielectric black particles
remains a long felt need in the art of electrophoretic image displays. In arts
other than EPIDs, black particles are commonly produced from carbon.
However, carbon blacks are not readily adaptable to EPIDs because carbon
blacks are conductive and the density of carbon blacks is not readily
matched to a suitable suspension medium. Research efforts have been
WO 95/19227 21 7 6 ~ 8 2 PCT/US94/00510
made in an attempt to solve the density and conductivity problems of
carbon blacks. however~ none has succeeded without trading off the
blackness (i.e. energy absorbency) of the particles created. Such efforts to
produce dielectric particles from carbon blacks are exemplified in the
following articles: Fowkes et al. "Electrophoretic Display Medium". a
research project report for the Department of Chemistry at Lehigh
University (August 28, 1989) and Hou et al. "Polymer-Encapsulated
Particles With Controlled Morphologies." PH.D Dissertation, (Lehigh
University, 1991) .
The present invention does not use carbon black as the source
of the electrophoretic particle. Rather, composite latexes stained with a
metal oxide are used to form the dielectric black particles suitable for use in
a EPID. More particularly, the preferred embodiment of the present
invention produces black particles from seeded emulsion polymerization
techniques, used to produce core/shell latex structures with the residual
double bonds, that are stained black with a metal oxide. The density,
blackness, particle size and surface characteristics of the present invention
black particle are controlled by the polymer composition, crosslink density,
residual double bond density and reaction conditions.
The development of particles from synthesized core/shell
latexes has been addressed in numerous technical articles, as exemplified by
the following: Wessling et al. "AStudy Of Emulsion Polymerization
Kinetics by the Method of Continuous Monomer Addition." Journal of
Macromolecular Science., A7 (3), pp. 647-676 (1973); Keusch et al." The
Growth of Polystyrene Latex Particles." Journal of Macromolecular
Science., A7 (3) pp. 623-646 (1973); Grancio et al." The Morphology of the
Monomer-Polymer Particle in Styrene Emulsion Polymerization.i' Journal of
Polymer Science., vol. 8, pp 2617-2629 (1970); Grancio et al. "Molecular
Weight Development in Contrast-Rate Styrene Emulsion Polymerization."
Journal of Polymer Science., vol. 8, pp. 2733-2745 (1970); and Wiener, H.
WO 95/19227 ~ PCT/US94/00510
2176482
- 4 -
"Polymerization in the System Vinylidene Chloride-Potassium Laurate-
Potassium Persulfate"~ Journal of Polymer Science vol. 7, pp. 1-20 (1951).
Of more direct relation to the present invention process of producing black
particles are the below referenced articles.
In Daniels et al. "Preparation of
Acrylonitrile/Butadiene/Styrene Latexes Using Hydroperoxide Redox
Initiators." Journal of Applied Polymer Science~ vol. 41, pp. 2463-2477
(1990). an acrylonitrile/butadiene/styrene composite latex is shown where
the polybutadiene core is uniformly surrounded by a poly(styrene-co-
acrylonitrile) shell. In Daniels, batch and semi-continuous seeded emulsion
polymerization techniques are used with varying core and shell ratios and
other reaction parameters.
In Merkel, M.P. "Morphology of Core/Shell Latexes and their
Mechanical Properties" PH.D. Dissertation (Lehigh University 1986), seeded
emulsion polymerization is utilized to synthesis polybutadiene-poly(methyl
methacrylate) core/shell latexes. The core/shell latexes have various levels
of crosslink density of the core and various thickness of the shell.
In Sundberg et al. Journal of Dispersion Science Technology
vol. 5, pp. 433 (1984), synthesized polybutadiene-polystyrene core-shell
latexes are studied in various conditions, such as monomer/polymer ratio,
initiator level, degree of conversion and concentration of chain transfer
agent, to determine grafting efficiencies of styrene onto polybutadiene
latexes.
The use of staining agents on polymers was first used to form
contrasts helpful in viewing polymer structures through electron microscopy.
The prior art pertaining to such polymer staining is exemplified in the
following articles. In Gaylarde et al. Science, vol 161, pg. 1157 (1968)
ruthenium tetraoxide was used as a staining agent for polymeric materials in
electron microscopy. In Vitali et al. Polymer. vol 21, pg. 1220 (1980)
ruthenium tetraoxide was used to improve image contrast for polybutadiene
- 5 - ~ 4 8 ~
,
lattices, a terpolymer of acrylnitride, butadiene and
styrene, and an acrylnitrile-styrene-acrylnitrile
polymer. In Trent et al, Journal of Polymer Science,
volume 19, pg. 315 (1981), ruthenium tetroxide was used
in vapor staining polystyrene/poly(methylmethacrylate)
blends for electron microscopy studies. Finally, in
Trent et al, Macromolecules, vol. 16, pg. 589 (1983)
ruthenium tetroxide was shown having the capability of
staining both saturated and unsaturated polymer systems
that contain ether, arene, alcohol, aromatic, amide, or
olefin moieties.
However, none of the above referenced prior
art addresses a process of producing dielectric
particles having a high degree of blackness with a
controlled density and particle sizes so as to be
adaptable to an electrophoretic image display. It is,
therefore, an object of the present invention to provide
an improved electrophoretic particle that has a high
degree of blackness, a controlled particle size, surface
functionality and density so as to be readily suspended
in the liquid medium of an electrophoretic image
display.
DISCLOSURE OF THE INVENTION
According to the present invention, there is
provided in an electrophoretic display, having an
electrophoretic dispersion that includes dielectric
electrophoretic particles suspended in a liquid medium,
an improved electrophoretic particle for said electro-
phoretic dispersion comprising a particle body comprised
of a polymer structure wherein the particle body has a
density substantially equivalent to the liquid medium,
and wherein the particle body is stained to a desired
shade of black by a reaction with a metal oxide.
Preferably, the polymer structure includes a
latex. The latex particles are produced using an
emulsion polymerization technique. The latex particles
produced contain residual double bonds which are then
r~
~ ~ 7 ~ 4 ~ ~ ;
reacted with the metal oxide to produce a desired degree
of blackness.
In a preferred embodiment the latex particles
are formed having a core polymer surrounded by at least
S one shell of a differing polymer. The core structures
are polymers with residuals double bonds such as poly-
butadiene and polyisoprene and the shell structures are
polymers with different functionalities such as
poly(methacrylic acid) and poly(methyl
,A
wo 95/l!)Z~7 2~17 6 q ~ 2 PCT/U594/00~10
methacrylate) or copolymers such as poly(styrene-co-methacrylic acid) and
poly(styrene-co-methyl methacrylate). The residual double bonds of the
core structures are then reacted with a metal oxide such as osmium
tetroxide to form a complex structure which efficiently absorbs incident light
and provides a high degree of blackness. The blackness of the core/shell
particles is dependent upon the residual double bond density of the core,
the shell thickness, the core/shell ratio, particle size and the degree of
staining. The surface characteristics of the particles are controlled by the
chemical composition of the shell structure. The black particles also have
controlled mechanical properties which are dependent upon the crosslink
density as well as the composition of the core and shell structures. Since
the core structure and shell structure can be made from differing polymers
and formed in any ratio, the density of the black particle can be controlled
by selecting the size of the particle to be made, the latex material to be
used and the ratio of the core structure to the shell structure. As such, the
present invention black particles can be formed with a desired density so as
to match the density of a desired suspension medium in a electrophoretic
image display.
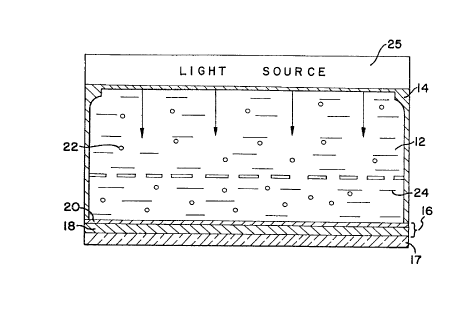
The sole figure is a cross sectional view of an electrophoretic
display conr~ining dielectric black particles produced in accordance with the
one preferred embodiment of the present invention.
BEST MODE FOR CARRYING OUT THE INVENTION
Although the present invention black particles can be used in
many different applications where particles of high blackness and low
density are desired, such as paint, ink, and electrostatic toner, it is especially
suitable for use in connection with electrophoretic image displays (EPID).
Accordingly, the present invention black particles will be described in
connection with a typical EPID.
WO 95/19227 2 ~ 7 6 ~ 8 2 PCT/US94/0051(~
Referring to Fig. 1~ there is shown a cross sectional view of an
electrophoretic image display 10. As will be recognized by a person skilled
in the art, an EPID 10 contains a volume of an electrophoretic dispersion
12 encapsulated between a anode structure 14 and a cathode structure 16.
S The cathode structure 16 is comprised of glass plate 17 on which is
deposited a thin layer 18 of indium-tin-oxide (ITO) or a like compound.
The ITO layer 18 is deposited in such a manner so as to be substantially
transparent when viewed through the glass plate 17. Cathode lines 20 are
etched onto the ITO layer 18 in a pattern of parallel lines. The anode has
a similar set of parallel ITO lines 26, which are perpendicular to those on
the cathode. A conductive mesh 27 sits in the fluid between the cathode
and anode.
In the present invention, the electrophoretic dispersion 12 is
comprised of black dielectric electrophoretic particles 22 suspended in a
clear medium 24. The electrophoretic particles 22 have a density
substantially equivalent to that of the fluid medium 24 so as to remain
randomly disperse in the fluid medium 24, unaffected by the orientation of
the EPID 10 or the effects of gravity. When an electrical bias is applied to
the cathode lines 20, the anode lines 26 and the mesh in proper sequence,
the electrophoretic particles 22 migrate to selected positions on the cathode
lines 20 creating an image viewable through the glass plate 17. The
migration of the black electrophoretic particles 22 to the cathode displaces
the clear medium 24 adjacent to the ITO layer 18, thereby blocking the light
produced by the light source 25. The remainder of the particles 22 sit on
2~ the mesh and do not block light from source 25. Consequently, black
electrophoretic particles 22 can be seen through the glass plate 17, resulting
in a black image contrasted against a light background.
As has been indicated previously in the Background of the
Invention, the production of a black image on a light background is highly
desirable. However, a major problem associated with any EPID is the
WO 95/19227 ~ , PCT/US94/00510
2I764~2
- 8 -
creation of dielectric black parlicles that have a density that can be readily
matched with common suspension fluids. The present invention black
electrophoretic particles 22 are formed from composite latexes using seeded
emulsion polymerization techniques. The composite latexes are then
reacted with a metal oxide which stains the residual double bonds of the
latexes black. Latexes are dielectric materials, as are metal oxides,
consequently the resulting black particles formed from the latexes are
dielectric. Additionally. utili7.ing varying core and shell latexes, black
particles of low density can be readily obtained. By varying the latex
materials used, size of the particles produced and the ratio between the core
and shell polymers and the density of the black particles can be selectively
adjusted. The resulting black particles can thereby be formed to have a
desired density, in the rage of 0.8 to 1.4gm/cm3, allowing the black particles
to have the same density as many suspension media.
To form the present invention dielectric black electrophoretic
particles 22, core/shell latex structures are prepared by batch and semi-
continuous seeded emulsion polymerization techniques. As will be
recognized by a person skilled in the art, the creation of a core/shell
composite latex by seeded emulsion polymerization includes synthesizing a
seed latex by traditional emulsion polymerization, swelling the seed particles
with a second stage monomer and then polymerizing the second stage
monomer during the second stage polymerization to encapsulate the seed
particles within a newly formed shell polymer. In the present invention, the
core structures are manufactured as polymers cont~ining residual double
bonds, such as polybutadiene or polyisoprene. The shell structures are
manufactured as polymers having different functionalities from the core
structure, such as poly(methacrylic acid) and poly(methyl methacrylate) or
copolymers such as poly(styrene-co-methacrylic acid) and poly~styrene-co-
methyl methacrylate). The residual double bonds of the core structures are
then stained with a metal oxide to form a complex structure with a high
WO 95/19227 217 6 4 8 2 PCT/US94/00510
degree of blackness, wherein the degree of blackness is dependent upon the
residual double bond density of the core structure~ the thickness of the shell
structure. the ratio between the core and shell structures, the overall size of
the core/shell structure and the degree of the reaction with the metal oxide.
PROCESS ONE
In an exemplary embodiment of the present invention method for
producing the electrophoretic black particles 22? the core structure,
commonly called a seed latex is prepared by emulsion polymerization in a
closed container. The core structure is a crosslinked poly(butadiene-co-
styrene) latex formed from the polymerization recipe described in Table I
below.
Table I
Materials Wei~ht (~)
styrene 8 . 0
butadiene 35.0
distilled-deionized water 100.0
dioctyl sodium sulfosuccinate0.07
octyl phenoxy polyethoxy ethanol 0.24
potassium persulfate 0.4
divinylbenzene 0.4
Prior to use, the inhibitors of the monomers butadiene and styrene and the
crosslinker divinylbenzene are removed by standard methods of passing each
of the monomers through a column cont~ining the ~propliate inhibitor
remover. In this, and subsequent processes, the butadiene is of the type
commercially available from Air Products and Chemicals, Inc. The styrene
- is of the type commercially available from Fisher Scientific, Inc.. and the
divinylbenzene is of the type commercially produced by Dow Chemicals, Inc.
WO 95/19227 PCT/US94/On510
-~2-17 6 ~;82
- 10 -
The octyl phenoxy polyethoxy ethanol (also known as Triton
X-45 manufac~ured by Rohm and Haas. Inc.) and dioctyl sodium
sulfosuccinate (also known as Triton X-200 manufactured by Rohm and
Haas, Inc.) are used as emulsifiers and are dissolved in the distilled-
deionized water and charged into a closed container. The styrene and
divinylbenzene are combined and mixed with an initiator of potassium
persulfate manufactured by Fisher Scientific, Inc. After the styrene,
divinylbenzene. and potassium persulfate are combined, the combination is
charged to the closed container cont~ining the Triton X-200 and Triton X-
45 emulsifier solution. The closed container is then purged with nitrogen.
The butadiene monomer is then condensed and weighted into the closed
container. The butadiene can be considered with any known method but is
preferably condensed using isopropanol cooled with liquid nitrogen. The
container, cont~ining the composite mixture is the warmed and agitated for
a desired reaction time. In one preferred embodiment the mixture is
tumbled at fifteen revolutions per minute for forty eight hours at seventy
degrees celsius. At the end of the forty eight hour period, the core
structures of poly(butadiene-co-styrene) namely, seed particles, are formed
that are monodisperse in size having a diameter of approximately 286 nm,
as they would appear in a TEM photograph
A shell structure of poly(styrene-co-methacrylic acid) is
formed around the poly(butadiene-co-styrene) core structure by batch
seeded emulsion copolymerization. The shell structure for the poly(styrene-
co-methacrylic acid) polymer is formed from the polymerization recipe
described in Table II below.
Table II
Materials Wei~ht (~)
poly(butadiene-co-stvrene)
seed latex 5.0
WO 95/19227 PCT/US94/00510
176482
- 11
styrene 1. 5
methacrylic acid 0.2
divinylbenzene 0. 1
potassium persulfate 0 . 02
distilled-deionized water 40. 0
To form the poly(styrene-co-methacrylic acid) shell structure around the
poly(butadiene-co-styrene) core structure the poly(butadiene-co-styrene)
seed latex are placed in a container with the styrene~ divinylbenzene and a
methacrylic acid monomer, such as that manufactured by Aldrich Chemical,
Inc. The container is then purged with nitrogen and the poly(butadiene-co-
styrene) core structures are swelled in the presence of the other monomers
at room temperature. The poly(butadiene-co-styrene) core structures are
swelled to a desired degree and are then combined with the potassium
persulfate initiator. In the preferred embodiment, the combination is then
heated to sixty degrees celsius and tumbled at thirty revolutions per minute
for twenty four hours. As a result of the above process, poly(butadiene-co-
styrene)/poly(styrene-co-methacrylic acid) core/shell structures are
produced having a diameter of approximately 480 nm of which
approximately 50 nm is a result of the poly(styrene-co-methacrylic acid)
shell structure thickness.
Two percent, by weight, aqueous solution of osmium tetroxide
is then added to the core/shell latex so as to react with, and stain, the
resulting residual double bonds. The core/shell latex is tumbled with the
osmium tetroxide solution at room temperature for a desired reaction time,
thereby resulting in a core/shell latex, having a desired degree of blackness,
that can be used as the present invention electrophoretic particles 22. In
regard to the advantages set forth hereafter in process two. It should be
- understood that in place and stead of the osmium tetroxide. ruthenium
tetroxide or other metal oxides may also be used.
WO 95/19227 PCT/US91/00510
2 ~ 7 6 ~ ~ 2
The above described method of manul'acture. utilizing the
poly(butadiene-co-styrene) core structure produced by the polymerization
recipe of Table I and the poly(styrene-co-methacrylic acid) shell structure
produced by the polymerization recipe of Table II, produce black particles
S of a given blackness, size, hardness and surface characteristics. By varying
the polymerization recipes of Tables I and II and by varying other reaction
parameters of the method of manufacture, the physical characteristic of the
black particles produced can be selectively altered as needed for a given
application.
By varying the degree of conversion and the amount of the
divinylbenzene crosslinker present in the creation of the poly(butadiene-co-
styrene) core structures, the concentration of the residual double bonds
present in the poly(butadiene-co-styrene) core structure can be altered. The
degree of conversion during polymerization is dependent upon the reaction
time which can be varied from twenty four hours to seventy two hours to
obtain fifty percent to ninety nine percent of conversion. A higher degree
of conversion leads to fewer residual double bonds left in the core structure.
The polymerization recipe set forth in Table I calls 0.4 gms of
divinylbenzene. However, the amount of divinylbenzene can be varied from
O.Ogm to 1.2 gms. Consequently, the concentration of residual double
bonds will vary as a function of the concentration of divinylbenzene within
the given range. Since the residual double bonds present in the core/shell
structure is what reacts with the metal oxide, by varying the concentration of
residual double bonds the blackness of the end product electrophoretic
particles can be selectively varied as desired. Additionally, by varying the
concentration of residual double bonds, the hardness of the end product
electrophoretic particles can also be varied within the available range.
The blackness and hardness of the produced particles may
also be effected by altering the butadiene:styrene monomer ratio used in the
poly(butadiene-co-styrene) core structure polymerization recipe of Table I.
WO 95/19227 PCT/US94/00510
~ 21769~2
- 13 -
In Table 1. the given butadiene:styrene monomer ratio was 35:8. In order to
selectively control the hardness and blackness of the end product
electrophoretic particles. the butadiene:styrene ratio in the poly(butadiene-
co-styrene) core structure can be changed form the Table I value of 35:8 to
39:4, 31:12. 27:16, or 23:20 as desired. In addition to butadiene, other
conjugated diene compounds (e.g. isoprene) or any compound Cont~ining
more than one double bond (e.g. diacrylate, triacrylate, tetraacrylate,
dimethacrylate and trimethacrylate compounds) can also be used as stained
component.
In emulsion polymerization there are many variables such as
swell time, reaction time and polymerization recipe that can be altered to
affect the size of the formed core structure and the ratio between the size of
the core structure and the surrounding shell structure. In the present
invention. the size of the poly(butadiene-co-styrene) core structures are
preferably controlled by varying the concentration and types of emulsifiers
present in the poly(butadiene-co-styrene) core structure polymerization
recipe. In the polymerization recipe of Table I, 0.07 gms of Triton X-200
and 0.24 gms of Triton X-45 emulsifiers are used. It should be recognized
by a person skilled in the art that by varying the concentrations of the
emulsifiers during polymerization, the size of the poly(butadiene-co-styrene)
core structure can be selectively controlled. Preferably in the
polymerization recipe of Table I, the Triton X-200 emulsifier can be varied
from between O.Ogms and 0.14 gms while the Triton X-45 emulsifier can be
varied from 0.16 gms to 0.40 gms. Anionic, cationic and nonionic
emulsifiers or the combination for each type of emulsifier can be used in
the emulsion polymerization. Specific examples of suitable emulsifiers are
sodium lauryl sulfate, sodium dodecyl sulfate, Dowfax surfactants, Igepal
surfactants, Aerosol surfactants, Pluronic surfactants, Cantrez surfactants,
- Arlacel surfactants. Tetronic surfactants~ poly(vinlyalcohol), poly(ethylene
oxide), and the like.
WO 95/19227 , ~ ~ PCT/US94/00510
- 14 -
The physical characteristics of the end product electrophoretic
particles can also be varied by varying the process and polymerization recipe
for the poly(styrene-co-methacrylic acid) shell structure that surrounds the
poly(butadiene-co-styrene) core structure. For example, the surface
S functionality of the final core/shell structure can be varied by varying the
amount of methacrylic acid monomer present during polymerization. By
varying the amount of methacrylic acid monomer from the 0.2 ~ms listed in
Table II to 1.0 gm, the amount of carboxylic acid on the shell structure is
changed thereby which affecting a change in the surface functionality of the
end product electrophoretic particles. Additionally, it should also be
understood that the use of a methacrylic acid monomer is exemplary and
the surface functionality of the end product electrophoretic particles can be
changed by substituting other monomers such as methyl methacrylate,
acrylonitrile, vinyl chloride, acrylic acid, sodium styrene sulfonate vinyl
acetate, chlorostyrene, dimethylamino-propylmethacrylamide,
isocyanatoethyl methacrylate, N-(iso-butoxy-methyl) acrylamide, or other
similar functional monomers in place and stead of the methacrylic acid.
The shell thickness of the poly(styrene-co-methacrylic acid)
shell structure, as well as the blackness and mechanical properties of the
end product electrophoretic particles, can be selectively altered by varying
the monomer:polymer ratio used in the second sta~e polymerization recipe.
In the preferred embodiment of the second stage polymerization recipe,
shown in Table II, the monomer:polymer ratio can selectively adjusted from
80:20 to 20:~0 in order to affect the needed shell structure characteristics.
WO 95/19227 ~ 217 6 9 8 2 PCT/11S94/OOS10
~"_
- 15 -
PROCESS TWO
In an alternative embodiment of the present invention. blacl~
particles are produced by emulsion polymerization which produces
poly(styrene-co-methacrylic acid) latex particles. In this embodiment
S poly(styrene-co-methacrylic acid) latex particles are prepared lltili7.insg the
polymerization recipe shown below in Table III.
TABLE III
Materials Wei~ht (~)
styrene 40
methacrylic acid 2
distilled-deionized water 100
potassium persulfate 0.4
divinylbenzene 0.4
sodium lauryl sulfate 1.2
In preparing the poly(styrene-co-methacrylic acid) latex particles, the
contents of Table III were charged into a container, purged with nitrogen
and ~git~te~l for a desired reaction time to complete polymerization. The
resulting poly(styrene-co-methacrylic acid) latex particles are then either
mixed with a two percent by weight aqueous solution of ruthenium
tetraoxide or exposed to ruthenium tetraoxide vapor to stain the polystyrene
component of the polymer particles. Because the interfacial tension
between poly(methacrylic acid) and water is lower than that between
polystyrene and water the methacrylic acid component will migrate to the
surface of the particles during polymerization, which provides functional
groups for surface charging.
Particles made by this process are spherical and are uniforrn
in size, namely, monodisperse particles, which allow each particle to have
WO 9~/19227 ~ ;, PCT/US94/00510
21-7C~82 -
- 16 -
uniform surface charge and chargeimass ratio to create uniform
electrostatic images in electrophoretic image displays.
Poly(styrene-co-methacrylic acid) latex particles with different
surface functionalities for surface charging can be formed by ch~nging the
functional monomer of methacrylic acid to other functional monomers such
as acrylic acid, methacrylate, vinyl acetate, methyl methacrylate,
acrylonitrile, sodium styrene sulfonate, dimethylaminopropylmethacrylam,
isocyanatoethyl methacrylate, N-(iso-butoxymethyl) acrylamide, or other
similar functional monomers.
Properties such as density, optical property glass transition
temperature and mechanical strength of the poly(styrene-co-methaclryic
acid) latex particles can be selectively altered by substituting the stained
component form styrene to another vinyl monomer such as vinyl methyl
ether, vinyl formal, vinyl alcohol, vinyl methyl ketone, ethylene, propylene or
combination of the above to make homopolymer, or copolymer particles
The size of the poly(styrene-co-methacrylic acid) latex
particles can also be selectively controlled by varying the concentration and
type of emulsifier used during the polymerization reaction. In the present
invention, the sodium lauryl sulfate can be changed with anionic, cationic
and nonionic emulsifiers or the combinations of each. Specific examples of
suitable emulsifiers are sodium dodecyl sulfate, Dowfax surfactants, Igepal
surfactants, Aerosol surfactants, Pluronic surfactants, Cantrez surfactants
and the like.
In view of the above rethinium tetraoxide can be used as a
staining agent in place and stead of the osmium tetroxide. Ruthenium
tetraoxide can stain a wide range of polymeric materials with varying
structures and the density of ruthenium tetroxide is lower than osmium
tetroxide. Consequently, electrophoretic particles having a high degree of
blackness and a relatively low density can be created.
21'76482
~VO 95/19227 ~ ~ PCT/US94/00~10
PROCESS THREE
In a third alterative embodiment of the present invention,
black particles are produced by emulsion polymerization which produces
poly(butadiene-co-styrene-co-methacrylic acid) latex particles. In this
embodiment poly(butadiene-co-styrene-co-methacrylic acid) particles are
prepared lltili7ing the polymerization recipe shown below in Table IV.
Table IV
Materials Wei~eht (~)
styrene 12.0
butadiene 30 . 0
methacrylic acid 1.0
distilled-deionized water100.0
Triton X-200 0.07
Triton X-45 0.24
potassium Persulfate 0.4
divinylbenzene 0.4
The inhibitors of the butadiene, styrene, and divinylbenzene are removed in
the manner previously described. To produce the present invention black
particles the Triton X-200 and Triton X-45 emulsifiers are dissolved in the
distilled-deionized water and charged to a container. In accordance with
Table IV, the styrene, potassium persulfate and divinylbenzene are mixed
and added to the emulsifier solution. The container holding the mixture is
then purged with nitrogen and condensed butadiene is added. The resulting
mixture is then held at 70~cand agitated for a desired reaction time of
preferably forty eight hours. As a result of the process, poly(butadiene-co-
styrene-co-methacrylic acid) latex particles are formed.
The poly(butadiene-co-styrene-co-methacrylic acid) latex
particles are stained black by being reacted with 2% by weight aqueous
solution of osmium tetroxide, ruthenium tetroxide or similar metal oxide.
WO 9S/19227 PCT/US94/00~10
217~i~82 ; - i~8-
The meta~ oxide solution and poly(butadiene-co-slyrene-co-methacrylic acid
latex particles are allowed to react at room temperature for approximately
twenty four hours, thereby staining the poly(butadiene-co-styrene-co-
methacrylic acid) latex particles black. The black particles are then
S collected for use in the electrophoretic displays.
As with previous embodiments, the size of black particles can
be controlled by varying certain process parameters such as reaction time.
emulsifier concentration~ temperature. etc. However. in the present
invention particles size as well as the blackness and hardness of the end
product products can be controlled by varying the
butadiene:styrene:methacrylic acid monomer ratio in the polymerization
recipe. By varying the butadiene:styrene:methacrylic acid monomer ratio
from 30:12:1,as shown in Table IV, to 30:11:2,30:10:3,20:22:1,20:21:2and
20:20:3 the diameter of the resulting poly(butadiene-co-styrene-co-
methacrylic acid) latex particles can be varied between 330 nm to 529 nm as
desired. For example, a poly(butadiene-co-styrene-co-methacrylic acid)
latex particle, produced as described above with a
butadiene:styrene:methacrylic acid monomer ratio of 20:20:3 produces latex
particles having a diameter of approximately 390 nm as measured from a
TEM photograph. Similarly, poly(butadiene-co-styrene-co-methacrylic acid)
latex particles having butadiene:styrene:methacrylic acid ratios of 20:21:2
and 20:21: 1 produce particles having diameters of 480 nm and 510 nm,
respectively, as measured from a TEM photograph.
The size of the poly(butadiene-co-styrene-co-methacrylic acid)
latex particle can also be selectively controlled by varying the concentration
and types of emulsifiers used during the polymerization reaction. In the
present invention, particles can be controlled by varying the amount of
Triton X-200 emulsifier present from between 0.0 gms and 0.14 gms.
Additionally, the amount of Triton X-45 emulsifier can be varied from 0.16
gms to 0.40 gms to vary particle size. Anionic, cationic and nonionic
~0 95/19227 217 6 ~ 8 2 PCT/US94/00~10
,,,,,_ ,
- 19 -
emulsifiers or the combination of each type of emulsifier can be used in the
emulsion polymerization. Specific examples of suitable emulsifiers are
. - sodium lauryl sulfate . sodium dodecyl sulfate, Dowfax surfacants? Igepal
surfactants, Aerosol surfactants, Pluronic surfactants, Cantrez surfactants,
5 Arlacel sufactants, Tetronic surfactants, poly(vinyl alcohol), poly(ethylene
oxide), polyacrylic acid and the like.
The poly(butadiene-co-styrene-co-methacrylic acid) latex
particle. although not technically a core/shell structure, is still dependent
upon the concentration of residual double bonds to determine how black
10 the particle will stain when reacted with a metal oxide. As with previous
embodiments, the concentration of residual double bonds is dependent upon
the degree of conversion, the ratio of monomers and concentration of
divinylbenzene in the polymerization recipe. The amount of divinylbenzene
in the polymerization recipe of Table IV can be varied between 0.0 gms and
15 1.2gms to create a poly(butadiene-co-styrene-co-methacrylic acid) latex
particle with a desired residual double bond concentration and therefore a
desired bl~ckn~oss and hardness. In addition to butadiene monomer, other
conjugated diene compounds (e.g. isoprene) or any compound conr~ining
more than one double bond (e.g. diacrylate, triacrylate, tetraacrylate,
20 dimethacrylate and trimethacrylate) can also be used as a staining
component. The degree of conversion can be varied from 50% to 99%
depending upon the reaction time. A higher degree of conversion leads to
fewer residual double bonds left in the final particles. I)ifferent monomer
ratio give the final particles with varying blackness. A higher percentage of
25 butadiene monomer leads to more residual double bonds left in the final
particles.
The surface functionality of the poly(butadiene-co-styrene-co-
methacrylic acid) latex particle may be effected by varying the amount of
methacrylic acid monomer present in the ori_inal polymerization recipe.
30 Additionally, surface functionality may be controlled as desired by
WO 95119227 PCT/US94/00510
217~:~82-
~o
substituting methyl methacrylate. acrylonitrile. acrylic acid. vinyl acetate
sodium styrene sulfonate. cholorstyrene. Dimelhylamino aminapropyl
propylmethacrylamide, Isocyanatoethyl methacrylate, N-(iso-butoxymethyl)
acrylamide, vinyl chloride or other monomers in place and stead of the
methacrylic acid.
PROCESS FOUR
In a fourth embodiment, black electrophoretic particles are
made having a polystyrene core structure and a poly(butadiene-co-
methacrylic acid) shell structure. The polystyrene core structure, namely,
seed particles, is prepared by emulsion polymerization pursuant to the
polymerization recipe set forth below in Table V.
Table V
Materials Wei~ht (n)
styrene 40
distilled-deionized Water 100
potassium persulfate 0.4
sodium lauryl sulfate 1.2
divinylbenzene 0.4
To produce the polystyrene seed latex, the inhibitors of the styrene and
divinylbenzene are removed and the styrene, water, potassium persulfate,
sodium lauryl sulfate and divinylbenzene are charged into a container,
purged with nitrogen and ~git~ted at 60~cfor a desired reaction time.
Utilizing a seeded emulsion polymerization method the
poly(butadiene-co-methacrylic acid) shell structure is formed around the
polystyrene core structure. The poly(butadiene-co-methacrylic acid) shell
structure is formed utili7ing the polymerization recipe set forth in Table VI
below.
WO 95/19227 217 6'g ~ 2 PCT/US94/00510
Table VI
Materials Wei~Jht ( )
polystyrene seed latex 100
butadiene 20
methacrylic acid 2
distilled-deionized Water 80
potassium persulfate 0.4
divinylbenzene 4
The poly(butadiene-co-methacrylic acid) shell structure is formed by first
placing the polystyrene seed latex in a container with the butadiene,
methacrylic acid and divinylbenzene, whereby the polystyrene seed particles
are allowed to swell to a desired degree. The potassium persulfate initiator
is then added to the container and the polymerization reaction is held at
60~cfor a desired period of preferably forty-eight hours, thereby producing
the desired polystyrene/poly(butadiene-co-methacrylic acid) core/shell
structure.
The resulting core/shell structure is then mixed with a 2% by
weight aqueous solution of osmium tetroxide, ruthenium tetroxide or
another similar metal oxide, to produce a staining reaction with the residual
double bonds of the core/shell structure. The staining thereby producing
particles of a desired blackness for use in the electrophoretic display.
In the present embodiment the core structure is derived from
styrene. It should be understood that core/shell structures having differing
densities reflectivity, glass transition temperature and mechanical strength
can be obtained by substituting either vinyl methyl ether, vinyl formal, vinyl
alcohol, vinyl methyl ketone, acrylate, methacrylate, methyl, methacrylate,
ethylene, propylene, like monomers or combinations thereof in place and
stead of styrene.
WO 95/19227 21 7;6 4 8 2 PCT/US94/00510
- 22 -
Similarly. core/shell structures having different functional
groups for surface charging can be obtained by replacing the methacrylic
acid in the second stage polymerization recipe of Table VI with vinyl methyl
ether, vinyl formal, vinyl alcohol, vinyl methyl ketone. acrylate, methacrylate.methyl methacrylate, dimethylaminopropylmethacrylamide, Isocyanatoethyl
methacrylate, N-(iso-butoxymethyl) acrylamide, like functional monomers or
combinations thereof.
Changes in residual bond density, particle size, blackness,
hardness, and surface functionality can be affected in the manners
previously described in relation to prior process embodiments.
PROCESS FIVE
In a fifth alternate embodiment, a multilayer composite
structure is formed using a three step emulsion polymerization technique.
To create the original core structure a polystyrene core
structure is formed in the manner previously described in connection with
Process Four, following the first stage polymerization recipe set forth in
Table V. The polystyrene core structure is then subjected to a secondary
polymerization process forming a first shell structure around the polystyrene
core. The first shell structure is prepared, as previously described in
Process Four, lltili7ing the second stage polymerization recipe of Table VI,
except the m~th~rylic acid deleted from the polymerization recipe. As a
result of the secondary polymerization process, a polybutadiene shell
structure is formed, thereby producing a polystyrene/polybutadiene
core/shell latex.
The polystyrene/polybutadiene core/shell structure is then
used as the seed for a third stage polymerization, wherein the
polymerization recipe for the third stage polymerization is given in Table
VII below.
WO 95/19227 217 6 4 8 2 PCT/US94/00510
Table VII
Materials Wei~ht ( )
polystyrene/
polybutadiene seed latex 10
- S styrene 10
methacrylic acid 2
distilled-deionized water 40
potassium persulfate 0.4
divinylbenzene
To form the desired particle, the polystyrene/polybutadiene seed latexes are
combined with the styrene, methacrylic acid and divinylbenzene, wherein the
polystyrene/polybutadiene particles are allowed to swell for a given period
to reach a desired degree. The potassium persulfate initiator is then added
to the swelled polystyrene/polybutadiene particles and the combination is
tumbled at 60~cfor a predetermined reaction time. The result of the third
stage polymerization is a multilayered structure having two varied shell
structures, wherein the first shell structure is a polybutadiene polymer and
the second shell structure is a poly(styrene-co-methacrylic acid) copolymer.
The multilayered structures are then combined with 2% by
weight aqueous solution of osmium tetroxide, ruthenium tetroxide, or like
metal oxides to produce the desired black electrophoretic particles.
Particles formed with a multilayer structure, wherein middle
layer is polybutadiene for providing the residual double bond needed for
staining, can be produced to have a higher degree of blackness, a lower
density and better mechanical properties than single shell structures by
adding choices to the latexes that can be used to forrn the electrophoretic
particles. In view of the above description, it should be understood by a
person skilled in the art that multilayered composite latex can be formed
with any multitude of layers, each created by its own unique polymerization
WO 95/19227 217 6 ~ 8 2 PCT/US94/00510
- 24 -
recipe. so long as residual double bonds are formed at some point in the
structure.
The described techniques for ch~nging the particle size,
residual double bond density, blackness, hardness, and surface
characteristics in previous processes can be applied to the current multilayer
structure. Additionally, multilayer structures of varying density, reflectivity,glass transition temperature and mechanical strength may be produced when
the styrene, used in the original polymerization recipe, for the first stage
polymerization process that produces the original core structures, is changed
to vinyl methyl ether, vinyl formal, vinyl alcohol, vinyl methyl ketone,
acrylate, methacrylate, methyl methacrylate, ethylene, propylene, other like
monomers or combinations thereof. The density, reflectivity, glass transition
temperature and mechanical strength of the multilayer structure can also be
controlled by effecting the polymerization recipe for the third stage
polymerization process as shown in Table VII. For example, density,
reflectivity, glass transition temperature, and mechanical strength of the
final particles can be altered as desired by replacing the styrene in Table
VII with vinyl methyl ether, vinyl formal, vinyl alcohol, vinyl methyl ketone,
acrylate, methacrylate, methyl methacrylate, ethylene, propylene, other like
monomers or combinations thereof.
Additionally, the outer shell of the multilayer structure can be
formed to have different functional groups for surface charging by varying
the third stage polymerization recipe. More particularly~ the methacrylic
acid listed in Table VII can be changed to vinyl methyl ether, vinyl formal,
vinyl alcohol, vinyl methyl ketone, acrylate, methacrylate, methyl
methacrylate, ethylene, propylene, dimethylaminopropylmethacrylamide,
isocyaratoethyl methacrylate, N-(iso-butoxymethyl) acrylamide or other
similar functional monomers or combinations of the above.
It should be understood that the processes for forming the
present invention particles, as specifically described in this specification are
WO 95119227 21 7 6 4 8 2 ~ PCTIUS94/00510
~ ':
- 25 -
merely exemplary and a person skilled in the art may make numerous
variations and modifications to the describe processes without departing
from the spirit and scope of the invention. More particularly, it will be
recognized by a person skilled in the art that many of the compounds found
in the various polymerization recipes of Tables I through VII have chemical
equivalents that have not been specifically stated. All such equivalents are
intended to be included in the scope of the invention. Additionally, it will
be recognized by a person skilled in the art that the method set forth in
each described process for varying the size, hardness, blackness, density,
residual double bond density, surface functionality, reflectivity, glass
transition temperature and mechanical strength of the present invention
particles can be applied to each other described process and a person
skilled in the art may vary many other parameters such as the time and
temperature to also vary the characteristics of the final product black
particles. All such variations and modifications are intended to be included
within the scope of the invention.
PROCESS SIX
In a sixth alternative embodiment of the present invention
black electrophoretic particles are produced by creating core/shell
structures wherein there exists a polystyrene core and a poly(methacrylic
acid) shell. In this embodiment the polystyrene core is made pursuant to
the polymerization recipe set forth below in Table VIII.
Table VIII
Materials Wei~ht (~)
styrene 40
distilled-deionized 100
- potassium persulfate 0.4
sodium lauryl sulfate 1.2
WO 95/19227 ~ ; PCTIUS94/00510
~176g82 ~6
divinylbenzene 0 . 4
The contents of Table VIII are charged into a container, purged with
nitrogen and agitated for a desired reaction time and at a desired
temperature. In the preferred embodiment, the contents are tumbled at
60~cfor thirty six hours. As a result of the polymerization reaction,
polystyrene seed latexes are formed.
A second stage polymerization is then conducted utili7ing the
polystyrene particles as the seed latexes. In the second stage polymerization
a poly(styrene-co-methacrylic acid) shell structure is formed around the
polystyrene seed latexes. The poly(styrene-co-methacrylic acid) shell
structure is formed urili7.inP the polymerization recipe listed below in Table
IX.
Table IX
Materials Wei~ht (~)
polystyrene 100
seed latex
styrene 10
methacrylic acid 2
distilled-deionized water 40
potassium persulfate 0.4
divinylbenzene 0.4
The poly styrene seed particles are swelled at room with the above
listed monomers for a predetermined reaction time. The potassium
persulfate initiator is then added to the container and the mixture is
agitated for a desired reaction time. The resulting poly
styrene/poly(styrene-co-methacrylic acid) latex are then stained by adding a
two percent by weight aqueous solution of ruthenium tetroxide to the
WO 95/19227 21 7 6 ~ 8 2 PCT/US94/00510
- 27 -
containers, reacting the same until a desired degree of blackness is obtained
in the latex particles. Alternatively, the poly styrene/poly(styrene-co-
methacrylic acid) latex may be exposed to ruthenium tetraoxide vapor to
stain the poly styrene component.
The stained component in the present embodiment is the poly
styrene. It will be recognized by a person skilled in the art that the stained
component of styrene can be changed to vinyl methyl ether, vinyl formal.
vinyl alcohol, vinyl methyl ketone, ethylene, propylene, other vinyl
monomers or combinations of the above to make polymer particles having
different properties such as density, reflectivity glass transition temperature
and m~ch~nir~l strength.
PROCESS SEVEN
In a seventh embodiment, black, electrophoretic particles are made
having a hollow core structure and a multilayer shell structure. Firstly, a
linear polystyrene core structure, namely, seed particle, is prepared by
emulsion polymerization pursuant to the polymerization recipe set forth in
Table X.
Table X
Materials Wei~ht ( )
styrene 40
distilled-deionized water 100
potassium persulfate 0.4
sodium lauryl sulfate 1.2
The polystyrene seed particles are then subjected to a secondary emulsion
polymerization forming a first shell structure around the polystyrene core.
The first shell structure is prepared, as previous described in Process Four,
lltili7ing the second stage polymerization recipe of Table VI. As a result of
WO 9~!i/19227 21 7 6 4 8 2 ~ PCT/US94/00510
- 28 -
the secondary polymerization process, a polybutadiene shell structure is
formed, thereby producing a polystyrene/polybutadiene core/shell structure.
The polystyrene/polybutadiene core/shell latex used as the seed is
then subjected to a third stage polymerization process forming a second
shell structure around the polystyrene/polybutadiene core. The second shell
structure is prepared, as previously described in Process Five, utili7ing the
third stage polymerization recipe of Table VII. The result of the third stage
polymerization is a multilayered structure having two varied shell structures,
wherein the first shell structure is a polybutadiene polymer and the second
shell structure is a poly(styrene-co-methacrylic acid) copolymer.
Because the polystyrene core is a linear polymer which is soluble in
its good solvents, such as tetrahydrofurane and toluene, the final
multilayered composite particles (after the third stage polymerization) are
mixed with a large amount of good solvent (e.g. toluene) and tumbled at
room temperature for 48 hours to dissolve the linear polystyrene out of the
core of the composite particles. The dissolved polystyrene and the good
solvent are then removed resulting a multilayered composite structure with
a hollow core.
The multilayered hollow composite particles are then combined with
2% by weight aqueous solution of a metal oxide to produce the desired
black electrophoretic particle.
Particles formed with a hollow structure can be produced to have a
density close to or lower than 1 g/cm3 which can be easily dispersed in a
2~ low-density medium (e.g. decane and octane) without adding another high-
density medium (e.g. carbon tetrachloride and tetrachloroethylene) and are
easier to remain a good colloidal stability.
Particles formed with a multilayer structure, wherein middle layer is
polybutadiene for providing the residual double bond needed for staining,
can be produced to have a high degree of blackness, a low density and good
WO 9S/19227 2 17 6 ~8 2 PCT/US94/00510
,._~
- 29 -
mechanical properties by adding choices to the latexes that can be used
form the electrophoretic particles. In view of the above description, it
~ should be understood by a person skilled in the art that multilayered hollow
composite latex can be formed with any multitude of layers, each created by
5 its own unique polymerization recipe, so long as residual double bonds are
formed at some point in the structure.
The described techniques for ch~nging the particle size, residual
double bond density, blackness, and surface characteristics in previous
processes can be applied to the current hollow structure. The density,
10 reflectivity, glass transition temperature and mechanical strength of the
multilayer hollow structure can also be controlled by effecting the
polymerization recipe for the third stage polymerization process as shown in
Table VII. For example, density, reflectivity, glass transition temperature,
and mechanical strength of the final particles can be altered by replacing
15 the~ styrene in Table VII with vinyl methyl ether, vinyl formal, vinyl alcohol,
vinyl acetate, vinyl methyl ketone, acrylate, methacrylate, methyl
methacrylate, ethylene, propylene, other like monomers or combinations
thereof.
Additionally the outer shell of the multilayer structure can be formed
20 to have different functional groups for surface charging by varying the thirdstage polymerization recipe. More particularly, the methacrylic acid listed
in Table VII can be changed to vinyl methyl ether, vinyl formal, vinyl
chloride, vinyl methyl ketone, vinyl acetate, acrylic acid, sodium styrene
sulfonate, methyl methacrylate, dimethylaminopropylmethacrylamide,
25 isocyanatoethyl methacrylate, N-(iso-butoxymethacrylamide), other like
functional monomers or combinations of the above.
Furthermore, in the described processes of the present
invention batch emulsion polymerization was used. In addition to the
latexes or corelshell composite latexes made by the emulsion
30 polymerization or seeded emulsion polymerization, the polymer particles
WO 95/19227 PCT/US94/00~10
2176482
- 30 -
cont~ining ether, arene, alcohol~ aromatic, amide or olefin moieties or any
combination of the above moieties. made by emulsion polymerization,
miniemulsion polymerization, microemulsion polymerization, suspension
polymerization, dispersion polymerization or precipitation polymerization
can also be used for staining with ruthenium tetraoxide to make non-
conductive black polymer particles with controlled properties.
All equivalents, variations and modifications that can be
applied to the described present invention by a person skilled in that art,
are intended to be included within the scope of this invention as defined by
the appended claims.