Note: Descriptions are shown in the official language in which they were submitted.
~ 2l~sa2~ ,
BACK~r~(>llND 5~F TI-IF, INVFNTION
2 1. Tl~c li icl~l of tllc rnYcntisn
3 Thc present invention relates generally to a tobacco substitute for ~mini~t~t rin~ a
4 dose of nicotine. More specifically, the present invention is directed to a nicotine-containing
s dosage-fomm having a holder which may be used as part of an effective tobacco cessation
6 program or in situations where smoking is uuldc~ or not permitted.
8 2. Bs~ ml gf ~ - Inv~>ntt
9 Nicotine is a naturally occurring drug found in tobacco which has both stimulant and
depressant effects in the peripheral and central nervous systems (CNS). Nicotine can thus
be included in a broad category of CNS-acting drugs. Nicotine occurs as a basic, colorless
to pale yellow, very ll~ uaCO,u;C, oily, volatile liquid that has an unpleasant pungent order
13 and a sharp, buming, persistent taste. Nicotine fomls salts with almost any acid and
14 therefore exists in a variety of salt fomms. Nicotine is considered to be very toxic, and toxic
effects develop rapidly following an acute ovadose. When nicotine is obtained from
tobacco, as by chewing, snuffing or smoking, the amount of nicotine absorbed into the body
generally does not build up to a toxic level.
8 Nicotine can be introduced into tlle body through many different routes. One of the
19 most popular versions of nicotine use involves tlle smoking of cigarettes. When the tobacco
in a cigarette is ignited, the c.~,,,l, ~l;,~,, process causes the release of nicotine vapors. The
2I nicotine in cigarette smoke, suspended on minute particles of "tar" is quickly absorbed
22 through the lung. The absorption of nicotine into the body through cigarette smoke is almost
23 as quick as intravenous ~ linn, with the nicotine reaching the brain within eight
2~ seconds after inhalation of the tobacco smoke.
2s Unfortunately, i~ otlu~,;llg nicotine into the body in this manner also introduces
26 many other u~Jlll,Uuullds into the body as well. The combustion process of tobacco is
- Page 2 - r)OC~et NQ 10~04 52
~\pril 19, 199~
~ 2178~321
complex Witll abo~lt 4,000 compounds bcing generated during combuslioll. Among tlle
Z CUIIII~UUIId~ being ~enerated are tllose wllicll produce lligllly undesirable efrects sucll as
3 carbon monoxide, carbon dioxide, nitrogen oxides, ammonia, and many other qllhc~tlnt~t~e
4 In addition, many substances are left in the lungs as "tar." The variety of substances
s generated by burning tobacco include many which are believed to have serious long term
6 health efrects. Because of this, in recent years smoking has been i~ lt à~ ly disfavored,
7 and because of second hand inhalation, restrictions have been placed on where an individual
8 may smoke.
9 Because of these and other tJlld~, t;la~lc side efrects, many attempts have been made
to provide acceptable substitutes to cigarettes. Most of these substitutes contain nicotine
Il which is generally considered to be the ~ producing component in tobacco. Other
12 factors such as social l~h~l.,.,~ ,llvilulull~lltal factors (~, advertising), and learning
13 behavior may also contribute to tobacco ,~
Most heavy smokers seem to behave as if they are aKempting to adjust tlle
,....,1.,.1l1~,, of nicotine witllin relatively narrow limits. For example, when cigareKes Witll
16 a relatively high content of nicotine are given to heavy smokers, they tend to reduce the
17 number of cigarettes smoked and alter their inhalation patterns thereby achieving
18 ~ ornicotine in the blood plasma which are only slightly greater tllan those to
19 wllich they are ~ 1. rl Similarly, when lleavy smokers are given cigarenes with a very
20 IOW nicotine content, tlley change tlleir pattern of inhaling or increase the number of
21 cigarettes smoked in order to avoid declines in plasma nicotine ~ This suggests
22 that smokers may be best served by cigarette substitutes which allow for the regulation of
23 plasma nicotine cont~rntrtltit~nq within specified ranges which mimic cigarettes. Likewise,
2~ users of chewing tobacco or other forms of tobacco need a substitute that is capable of
25 mimicking the plasma levels associated with tlle use of a particular tobacco form.
26
- Page 3 - r)Oc~et No. 10304.52
/~Dnl 19, 1996
~ 2~ 78~2~
Cessation of ~he use ortobacco may be followed by a witlldrawal syndro~ne whicll2 varies rrom person to persoll in intensity and specirc signs and symptoms. Al~lloug~l tllere
3 is wide variability, the most consistent signs and symptoms are a craving ror tobacco,
irritability, anxiety, 1~ , and dirficulty in ~ E Drowsiness, headaches,
s increased appetite, insomnia, and ~a,ll~,;~,,lil,al complaints are also common. The use of
6 nicotine ~ .". "i~ during this withdrawal time ~las been shown, in some cases, to increase
7 the rate of success for those wishing to quit smoking.
8 Substitutes currently available include nicotine gum, sublingual lozenges, tablets,
g nasal sprays, vapor inhalers, and patches. These substitutes rely on the fact that nicotine is
o readily absorbed through the mucosa and skin. Because nicotine is a strong base, its
absorption from the small intestine is limited unless the pH is raised but nicotine is rapidly
12 and c,~t~ ,ly " ,. ~ I during the rlrst pass through the liver.
13 The available substitutes, while eliminating the health risks associated with cigarette
14 smoking, do not fully meet the needs of a smoker.
I5 When nicotine gum is used by smokers, they are often encouraged to chew one piece
16 of gum whenever they have the urge to smoke. The instructions generally suggest that the
17 gum should be chewed very slowly until a slight tingling in the mouth is perceived. Ollce
18 this tingling is relt, it is l~ " ". . ,~ that the user then stop cllewing the gum and wait until
19 this tingling is almost gone (usually within about one minute). This chewing procedure is
then repeated periodically ror about thirty minutes. This chewing technique is designed to
21 provide constant, slow buccal absorption Or nicotine from the gum. By providing slow,
22 constant absorption, nicotine levels in tlle blood stream can be maintained at a constant level.
23 While there is some evidence indicating that low constant blood levels of nicotine relieves
24 some of the symptoms of nicotine withdrawal, a smoker's craving for tobacco is not
mitigated by a relatively low, constant level of nicotine. This is because the nicotine levels
26 derived from smoking are dramatically dirferent in terms of t~le ~ r~ of nicotine in
--Page 4-- Docktl No. 10~04.52
Aplll 19, 1996
2178~21
tlle blood stream over time from t1le nicotine levels ill tlle blood stream achieved wllen
2 nicotille gum is used.
3 When nicotine is received tllrough smoking, the rapid absorption of tlle nicotine
4 tllrough the lungs results in an initial peak of nicotine in the blood stream which then
S ,ui"c~u~ ly trails off. The blood level peak produced by cigareffes is both higher and
6 sharper tilan the steadier levels which are obtained from gum or ~ systems. The
7 initial peak in nicotine t~nnr~rntr~ltif~nc in the blood from smoking is generally between thirty
8 to forty nanograms per milliliter. Furthermore, this peak is attained within about ten
g minutes. Studies have sllown that quick-rise effects are probably necessary for more
o complete relief from craving in the early stages of cigarette withdrawal. See
Russell, M.A.H., In Njrt~tine Rr.~ 1 A ('ritit~ v5tlll~tit~n Pomerleau, O.F. andPomerleau, C.S., Eds., Alan R Liss, Inc., New York, 1988, pp. 63-94. Russell indicates that
13 a rise in the nicotine blood level of at least ten nanograms per milliliter in ten minutes is
14 required to obtain ptJ~; tyllr~ . effects at nicotine receptors in the CNS and at autonomic
s ganglia. These ~otJ~L~ylla~)lic effects may be responsible for the drug-like "high" feelings such
6 as li~hthr r~ri~nt cc or di2~iness . ~ I by cigarette smokers. fhus, when nicotine can
be delivered in a manner which reproduces or mimics the manner in which nicotine is
8 delivered tllrouc~il cigarette smoki~lg, tlle smoker's craving for cigarettes n1ay be red~lced.
19 The slow, constant absorption produced by the intr rmittt nt chewing of nicotine gum, fails
to achieve this result.
21 In an effort to mimic the manner in whieh nieotine is distributed through smoking
z2 a cigarette, a user can more a~ ,1y chew the nicotine gum. Tilis, however, is generally
23 not ~ 11 because chewing tlle gum too rapidly can cause excessive release of
24 nicotine resulting in adverse effects similar to those of excessive smoking such as nausea,
25 hiccups, and irritation of the throat. Chewing nicotine gum agc,l"~ ,ly will result in a large
26 amount of nicotine being swallowed because more nicotine is released than can readily be
- Page 5 - Dcc~t No. 10304.5't
April 19, 1996
2~ 7~n21
absorbed at tlle buccal cavity site. Ir ~oo much nicotil1e is swallowed, tlle resultant nausea
2 Will mos~ likely cause vomiting. Nicotine gum is tllus unable to safely provi.de a nicotine
3 plasma concentration curve similar to tllat achieved through smoking cigarettes.
In addition, the use of nicotine gum does not address the ~ logi~,~l needs of the
s smoker to have something which is placed into the mouth and removed from the moutll in
6 a ritualistic manner. Nicotine gum may also be difficult to tolerate as a long-term treatment.
7 The usefulness of nicotine gum r I "nns are limited because they taste bad, cannot be
8 used effectively by denture wearers, and may Icad to mouth ulcers and heartburn.
9 Fu~ IIIIUIG~ because of the unique chewing regime which must be imposed to adequately
regulate nicotine ~.n ~ ,~, " 1~ ,.1;. ~1.~ in the blood, nicotine gum may be difficult to use in order
to regulate nicotine levels within a relatively narrow plasma ~ l,.l;".. such as that~2 desired by heavy smokers. Tablet-type smoking substitutes suffer from similar drawbacks.
Transdermal patches which contain nicotine have also been developed. These
14 patches are designed to be placed on one's skin. Tbe nicotine in the patch is then absorbed
throughtheskin. Beca~lseofthesimplicityofnicotinepatches,patientcomplianceisusually
16 high. Transdermal patches have been developed that can be changed regularly. For instance,
17 patches which are to be changed once a day or, perhaps once a week, are available. Nicotine
18 patches are able to d~liver nicotille in such a way tllat a steady state nicotine cnnnl~ntr~tinn
19 can be maintained in the blood plasma. This eliminates the n ,~ that can occur when
20 using gum or tablets which must be taken regularly.
2~ As with the nicotine gum, the delivery of nicotine into the body is at a relatively
22 constant rate. These patches are thus unable to duplicate the plasma nicotine co~
23 curve obtained through smoking a cigarette. In addition, severe poisoning can result from
24 improper use of these patches. For example, if an individual has a patch applied and then
25 smokes several cigarettes, tlle plasma nicotine c- ~ ,. . "l l ,,l ;. ,., will be much greater than what
26 the individual is used to. This may result in nicotine overdose.
- Pagc 6 - r~t No. 10304.52
April 19, 1996
2178021
Other (~-nsi~rA~ions must be ~aken into account when nicotine patches are used.
2 Tlle let~lal dose unit for au~ average adult is about sixty milligrams of nicotine. One cigarette
3 delivers about one milligram of nicotine. Therefore, a patch that is to be effective for twelve
4 or twenty-four llours may contain between thirty and sixty milligrams of nicotine. This
s presents a safety concem as tllis represents a potentially lethal dose if nicotine delivery from
6 the patches were S~ rl~ y increased. This might occur if the patient were to lay on a
7 heating pad or warm water bed. In addition, if tlle patch is tampered with or ingested by a
8 child, for example, poisoning may occur. Ful III~IIIIU~ rlicotine patches do not provide oral
9 stimulation to address the ~ llolo~ l aspects of cigarette craving.
o Thus, while current cigarette substitutes are capable of delivering enough nicotine
Il to help alleviate some physical symptoms of nicotine withdrawal, they fail to provide the
12 quick-rise in nicotine blood ~, ,l, ,,l ;, .~ which smoking a cigarette provides. They also
13 fail to address the p~jcllolo~5ic~1 needs of someone who is trying to quit smoking. Many
rituals are developed during years of smoking. One such ritual is the periodic placing of
something into and out of a person's mouth and the associated oral stiml~lAti-n of holding
16 a cigarette in one's mouth.
Additionally, patients are usually under-dosed because the physician does not
mcasure baseline nicotine or cotinine levels and does not instruct the patient ill the proper
19 use of the gums, tablets andlor patches. This may explain the somewhat low level of success
with patches and gum. Some studies indicate that only about twenty percent of tllose using
21 nicotine patches under ul~ ion managed to quit smoking. Otller studies using nicotine
22 gum showed similar results. Nicotine nasal spray also causes problems such as pain or
23 irritation to the nasal mucosa, and while adequate plasma levels of nicotine may be reali~d,
2~ the oral grAtifirAtion and sensory ritualistic behaviors are left l.n~Ati~fi~-~
26
--Page 7-- DrckelNo. 10304 52
Apdl 19, 1996
2178~21
Bl~IF,F SUMMAI~Y AND OBJECTS OF TIIF, INVENTIQN
2 It is one object of ~lle present il1vention to provide a nicotine-containing dosage forn
3 which can be utilized as part of a long-term smoking cessation program.
4 Another object of the present inYention is to provide a nicotine-containing dosage
s form which is suitable for use as a smoking substitute whenever smoking is not allowed or
6 desired.
7 A further object of the present invention is to provide a nicotine-containing dosage
8 form which can maintain nicotine plasma ~". .,ll,.l;.~ll~ within a range which alleviates
9 smoking withdrawal symptoms.
A still further object of the present invention is to provide a nicotine-containing
dosageformwhichcanprovidenicotineplasma~..~"- .l.,,l;,~.,~.similartothoseachievedby
smoking a cigarette.
Yet another object of the present invention is to provide a nicotine-containing
dosage form which addresses some of the ~ 31O~i al needs of an individual who desires
to quit smoking.
A still further object of the present invention is to provide a nicotine-containing
dosage form which is suitable for use by those wearing dentures or other dental appliances.
othcr objecl of tlle prcsent invcntion is to provide a nicotine-containillg dosage
19 form which is easy to use so as to promote patient rornrli~nrP
Yet another object of the present invention is to eliminate the craving for a cigarette
21 by allowing the patient to self-dose the amount of nicotine to overcome the person's
22 individual craving.
23 It is yet anotl3er object of the present invention to provide a nicotine-containing
24 dosage form which can be used in conjunction with a patch so that the individual can control
2s the dosage to treat breakthrough cravings as they occur.
26
--Page ~-- Dock~No. 103~4.
Apnl 19, 1996
~ 2178~21
It is a still further object of tl~e present invention to provide a nicotine-colltaining
2 dosage rorm whicll allows patients experiencing a relapse to control occasional urges Witllout
3 raising the baseline nicotine plasma ~~- ,. f . ~ Ievels.
4 To achieve tlle foregoing objects, and in accordance with the invention as embodied
s and broadly described herein, a nicotine-containing dosage form is provided. The dosage
6 form is configured having a nicotine-containing t~.t~mrt~ on attached to a holder member.
7 Nicotine is released from the dosage form and absorbed tllrough the intra-oral mucosal
g surfaces as the nicotine-containing ~ ., . releases nicotine within a user's mouth. The
9 holder member facilitates insertion and removal of the dosage form into and out of a user's
o mouth The user can selectively insert and remove the dosage form as desired to selectively
I l control tlle release of nicotine to satisfy the user's individual craving. In addition, the user
12 can insert and remove the dosage form in a manner which meets the user's ~ olo~;~l
t3 need or desire for ritualistic oral stimulation similar to cigarette smoking.
14 These and other objects and features of the present invention will become more fully
1S apparent from tlle following description and appended claims, or may be learned by the
16 practice of the invention as set forth hereinafter.
17
18
19
21
22
23
24
26
- Page 9 - Dtlck~t ~o. 10304.52
Apnl 19. 1996
~ 217~2~
BRIF.F l)F,SCRIl'TIOI~ OF Tl:lF Dl~Wll~lGS
2 In order tllat tlle manner in Wllicl1 tlle above-recited and otller advantages and objects
3 of the invention are obtained, a more particular description of the invention briefly described
4 above will be rendered by reference to a specific ~ . . .l ~o~l,, . ,; thereof which is illustrated in
s the appended drawings. U~ that these drawings depict only a typical
6 r mho~limPnt of the invention and are not therefore to be considered to be limiting of its
7 scope, the invention will be described and explained with additional specificity and detail
8 through the use of the dC~,v~ J~ly ;llg drawings in which:
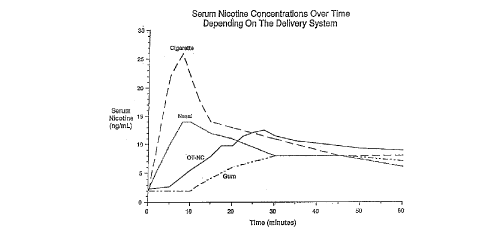
9 Figure I is a serum nicotine ~ the blood
plasma level changes in individuals after ~lminictrAti~n of cigarettes, nasal sprays, gum and
tt the present invention;
t2 Figure2isacomrslricr,ngraph.1.. ,.. ",~1.,.1;.,~bufferedversusunbufferedabsorption
13 rates;
Figure 3 illustrates a nondissolvable dosage forrn;
Figure 4 illustrates a dissolvable dosage form having wafers of varying
rr,nrPn~rr~tionC of nicotine;
17 Figures5-9illustratellvlld;~vlva'vlcdosageformscapableof~.l,,,;,,~l.... ,,,gvariable
18 qllalltities alld concenlralions orlliCotille; and
19 Figures 10-15 illustrate a series of dissolvable dosage forms for adl.. i~ tc.;l,g the
nicotine in varying concentrations, some of which are formed of Cv~ ,d powder.
22
23
24
2s
26
- Page 10 - r~ e~ ~o. 10304.52
Jlpril 19. 1996
~ 2178021
Dl~TAlr,F.l) DF~CRlr'TION OF TIIF. rRlJ.FFRl~F.I) FMBOl)llVlF1~T~
2 The present inventioll rclates to metllods of man~lfacture and compositions ~vl-icl~
3 facilitate tlle l,,.,.~,-"~ delivery of nicotine to a patient for use as either a smoking
4 substitute, an aid to smoking cessation, or, as will be discussed later, in the treatment of
s illnesses such as colitis, Tourette's syndrome, Parkinson's disease; and Al2heimer's disease.
6 Simply stated, the present invention relates to a selectively removable nicotine-containing
7 dosage form permitting ~ delivery of nicotine through the mucosal tissues of the
8 mouth, pharynx, and esophagus. The nicotine-containing dosage form of the present
g invention is capable of delivering nicotine into a patient's blood in a manner which results
in attainment of blood nicotine ~ similar to the blood nicotine ~ nf~ntrAtit~n~tattained through smoking cigarettes to thereby address the physical cravings for nicotine
which a smoker develops. In addition, the nicotine-containing dosage form of the present
invention provides a patient the uyyul LtLtliLy~ if desired~ for physical ~ and oral
stimulation associated with repeated insertion and removal of the dosage form into and out
of the patient's mouth to thereby address some of the y~ y~llOlOgiC~I cravings which a smoker
develops:
17 The present invention overcomes several of the limitations associated with either
18 nicotine-containing ~ delivery systems or nicotine-containing gum, tablet, nasal
19 spray, or lozenge delivery systems. One of tlle primary advantages of the present invention
is the ability to selectively vary the amount of nicotine released from the dosage form over
2I time through, for example, patient-controlled behavior such as varying the rate of
22 consumption of the dosage form or removing the dosage form from the mouth and
23 reinserting it, andlor &ough design adaptations which affect the ~ . ,. ,, . l . ~ l i ~- ,1 of absorbable
24 nicotine released from particular portions of tlle dosage form.
2s With the nicotine-containing dosage form of tlle present invention, it is possible to
26 acllieve a relatively rapid irlitial increase in blood nicotille ~ . . followed by a period
- Page 11 - rOc~e- No. 10304.5~
ADril 19. 199~i
2l7sn2l
of m~ t~n~n.~ of a lower blood nicotine collcentratio~l and tllereb~ sim~llate tlle pattern
2 attained by smoking a cigarctte. Tllus, tlle nicotine-containing dosAge fonn of the present
3 invention may provide a more satisfying alternative to smoking tllan presently available
4 nicotine delivery systems. This ability is ~ -I in Figure I in which the effect of the
s ~ n of several popular dosage fonms is ~irrn~nctr~t~l The initial peak followed
6 by a gradual .1;., .;, .:~1, ,1 of serum nicotine after smoking a cigarette is shown. Although
7 the nasal spray most closely emulates the cigarette line on the graph, nasal sprays do not
8 provide the P~ ~ ~,llolOgicàl benefits of an oral dosage fonm. Of the oral dosage forms tested,
9 the present invention (indicated as OT-NC) has tlle greatest potential for a~ u~dlllaiillg the
o physiological and psychological effects of cigarettes. By moderating the speed and intensity
Il of sucking on the dosage form, the serum level can be altered to satisfy an individuals unique
12 craving. Thus, the peak on the graph for the instant invention may be altered to a~)~lU~
the curve of a cigarette. The l~all~d~ àl patch infnrmr~tinn is not included on the chart but
requires several hours to reach therapeutic blood nicotine levels and provides a 1~ .Iy
state nicotine crmrr ntrr~tion
16 Figure I also reveals that the ~.1,, ,;";~1, ,.1 ion of conventional nicotine gum results
in a slow, gradual increase in serum nicotine tllat levels out over a prolonged period. Since
18 tlle a-~ministration orgum fails to produce an initial peak, tlle g~m dosage forms fails to
19 emulate tlle effect of smoking. Similarly, patches produce a slow, gradual increase in serum
nicotine that plateaus and stays constant over a prolonged period of time. Like gum, the
21 patches do not produce an initial peak of serum nicotine and thus do not emulate the effect
22 of cigarettes.
23 One advantage of the present invention is the ability to address the psychological
24 cravings of a cigarette smoker to handle an obiect which is rit~ i ctir~ y inserted into~ held
25 within, and removed from the mouth. The nicotine-containing dosage forms of the present
26 invention comprise a holder member, sucll as a stick, and a nicotine containing composition
--Page 12-- DorkelNo. 1030~.52
~pril 19, 1996
21'~8(~21
attached to tlle stick. Tl1e holder member faci~itates selective insertion and removal of the
2 dosage form into and oul of a patient's moutll sucl1 that a desired pllysical and p.sychological
3 erfect may be achieved. Ulllike nicotine-containing gum, tablets, or lozenges, tlle dosage
4 form of the present invention can easily be removed to assess the physical errects of the
s absorbed nicotine, temporarily cease the absorption of nicotine, or to inspect the size and
6 condition of the dosage form at any time. In addition, the holder member prevents
7 inadvertent swallowing of the dosage form and facilitates positioning of the dosage form in
8 a . . ~ .1 r~ and adjustable fashion within the oral cavity. Thus, local mucosal in itation
9 from continued contact with nicotine-containing gum, lozenges, or tablets may be avoided
o by using the holder member to re-position the dosage fonm within the oral cavity as desired.
The present invention may be used as a smoking substitute by a person in a situation
12 where smoking is either not permitted or not desirable. In this situation, the nicotine-
containing dosage form of the present invention provides a satisfying altemative to smoking
a cigarette by permitting both physical and ~ ologic~l simulation of the smokingl5 experience. The present invention may also be used by persons who desire to stop smoking
16 but experience difficulties due to the physical ,lnl~1,. ~ on nicotine and the psychological
d.,~,~"dclice on the rituals of smoking which have been developed. Such persons must go
18 tllrough a withdrawal period during wllich the smoking habit is gradually overcome. In this
19 situation, the nicotine-containing dosage form of the present invention provides a means to
satisfy botll tlle pllysical and psychological cravings and, hopefully, permit the person to
21 resist the craving to smoke cigarettes during a withdrawal period sufficient to free the person
22 from the smokin~ habit.
23 Nicotine is available in the present invention in eitller a free base or salt forrn.
2~ Nicotine base is readily absorbed through mucosal ,,,,.1", ,. c but is highly volatile.
25 Nicotine salts, on the other hand, are not readily absorbable through mucosal " .. "l ,. ,.. ,. ~
26 but are much more stable. Nicotine is also available as an ionic complex in the form of a
- Page 13 - rJoc~et~o. 10304.52
April 19, 1996
217~021
polyacrylic cation exchange resin. Pharm~rf l~ir~lly acceptable nicotine salts include, but
2 are not limited to nicotine hydrochloride, nicolille dillydrocl~loride, nicotine sulfate, nicotille
3 monotartrate, nicotine bitartrate, nicotine citrate, nicotine zinc chloride monohydrate and
4 nicotine salicylate. In an alkaline ~ UII~ i.e., pH above about 7, and in the presence
s of an aqueous medium, such as saliva within the oral cavity, nicotine salts react to form
6 nicotine base. Because saliva normally has a somewhat neutral pH, the incorporation of an
7 all~aline salt into the dosage-form of the present invention will buffer the pH and facilitate
8 the reaction to form readily absorbable nicotine base. Preferred alkaline salts include sodium
9 carbonate, sodium b;call), , potassium carbonate, potassium l ~ , trisodium
o phosphate, disodium hydrogen phosp~late, sodium oxylate, sodium succinate, sodium citrate,
~, il"~ ".",.. and sodium salicylate. Buffering may also affect stability. The results of
2 buffering are graphically ,1~ "~r~ r~l in Figure 2.
13 In addition to nicotine in a releasable form which is readily absorbed ~ . ". ,. ~.~,,lly,
14 the nicotine-containing f r,mrf~lei~inne in accord with the present invention may contain otller
ingredients such as flavorings, sweeteners, flavor enhancers, lubricants, binders and fillers.
6 Witll respect to flavorings, it should be noted that it is desirable to discourage nicotine use
by young people. Accordingly, it may be desirable to flavor the nicotine-containing
romrr~ci~irln in a manner wllich is unattractive to young people. It may even be desired to
19 provide an unflavored nicotine-containing C~ r~ wllich would be palatable to smokers
20 ~ f d to the taste of nicotine yet unattractive to others not so :~f, l~l, ,., ,f fl
21 The nicotine-containing dosage form of the present invention consists of any
22 nicotine-containing rr,mrr,ci~irn capable of delivering readily absorbable nicotine to the
23 intra-oral mucosal tissues in rrmhin~ir~n with an attached holder member. The nicotine
24 form that is ill~,ul~u~ ,l into the nicotine-containing f rmrf)ei~if n may be pure nicotine or
2s any compound thereof. The method of Illallura~,~ul~: may be any suitable method known in
26 the art including, but not limited to, the addition of a form of nicotine to a dissolvable or non-
--Page 14-- Doe~el No. 10~0~.52
Arril 19, 1996
-- 2178021 -
dissolvable matrix buccal dosage form intended to be sucked or passively dissolved in tlle
2 mo~ltll. Tlle melllod of a~tachment of tlle llolder l~lember si~llilarly may be.aily sui~able
3 metllod knowll in the art including, but not limited to, positioning of the holde~ member
within a non-solidified nicotine-containing cr~ o which is ~Ubaf UU~ y solidified by
CUIIIIJIC;~:/IUII~ injection molding or other means, and attachment by gluing, as witll
6 ( ~ r ~ f . ''i glue, or bonding by other means, to the pre-manufactured nicotine-containing
7 rnmrn~iti~n
8 One method in accord witll the present invention comprises mixing the desired
9 ingredients to form a powdered compressible matrix material w~lich is l;UllllJIC~ ,d into an
integral solid mass under high pressure. This process is referred to as "powder cf~mr:lf t~
Duringl..".l"..~...,.,themassisattachedtoaholdermembertofommanicotine-containing
12 dosage fomm. Altematively, the mass may be attached to a holder member in another
13 manner, such as by gluing with f.OI . r. ~1 ;l ,, ,. ~ glue.
14 More specifically, each of the C~ O~ including COIIIIJIC~ carbohydrate
fillers and binders, is mixed with the other ~ lf~ ll' in dry fomm to produce the
16 compositions of the present invention. It is presently preferred to use the method of
17 geometric dilution in adding the various .~ .. 1 ll .l l .1~ together prior to the mixing process.
18 Otller ~ llc that are typically ~lsed in powder ct~nnr~tion include ingredients
19 sucll as flavorings, sweeteners, flavor enhancers, releasing agents, and buffers. It is preferred
that these ingredients be provided in a powder form to facilitate mixing even if the
21 ingredients llappen to be insoluble or otherwise chemically incompatible.
22 Once complete mixing is ~ ~ ni . ,~ , tlle mixture is UUIl~ ,cd under relatively
23 high forces to provide a coherent dosage form. Compressive forces in the range of from
24 ~ lux.illl;LtclylooNewtollsto~ lu~ tf-l.~llsoNewtonsarepreselltlypreferred~however~
any force which is sufficient to compress tlle ingredients into a coherent, integrated mass
26
--Page 15-- Doc~elNo. 1~33~4.52
Arril 19, 1996
~ 2178021
could be used. As a result, ~lle ~ ss~d mass is held together by physical, rather ~han
2 c~lemical means.
Tlle extent of the compressive force can be modified to vary the dissolution rate, i.e.,
the rate at which tlle ~ . dissolves within the oral cavity. In particular, tlle greater
s the ~x)llly,~:,lv~ force used, the slower the dissolution rate will be. The dissolution rate may
6 also be affected chemically with other ingredients. For example, the dissolution rate may be
7 decreased by adding lly~ yllOb;~. agents such as ealcium stearate or the dissolution rate ean
8 be inereased with the addition of hydropllilie agents.
9 When employing the present invention, there is no need to heat the mixture to a
molten mass as has been tlle practice in the past in fomming drug-containing (~r nfecti/~n~ .~s
a result, the present method avoids the use of high ~ wllieh volatilizes nieotine
2 and also avoids undesired ehemieal reaetions whieh may oeeur between the various
ingredients in a heated or liquid ~ UI~ . Nevertheless, good mixing and unifomm
produet are provided.
The ronf~r~tionr~ry mass may be attached to a holder such as a stick or other similar
6 type of holder. The holder may be glued to the eonfeetion by , f~ ~ I l, '~ glue or other
food grade glues. Altematively, the holder may be ~,ulllylr ~a~,d into the dosage fomm by the
8 compressive forces described above.
19 One metllod of ereating an emhorlimr~nt of the present invention utili2es a mold
block with an anterior having a cavity formed in any desired shape so that the ingredients
21 deseribed above ean be ~OIIlyl~ ~r;d suffieiently to fomm an ~yyluyli_'~ly shaped dosage.
22 Eaeh half of tlle mold bloek can be removed in order to remove the confection once it is
23 sufficiently ~ d. A ram is used to compress the cavity. Following ~,~,lllyl~ ,ll of
2~ the eonfeetion, the stiek is seeurely bound in plaee.
The stick may take various shapes. For example, it may be desirable for the stick
26 to be oval or triangular in cross section.
--Page 16-- Doc~el No. 11333~.52
Arril 19, 1996
~ 2178~21 -
Another method utilizcd to produce an embodiment of the present inven~ion
2 illvolves a nondissolvable matrix into wllich tlle nicotille is placed.
3 The nicotine may be i-lcul~u,.. tcd intû a variety of pûssible nondissolvable
.:olltailull~lll matrixes. For example, tlle nicotine may be ;Il~ Olal~d into a sponge-like
s matrix; the nicotine may be l~;C~U ~ 1 t~l, the nicotine may be held within a
6 microsponge; the nicotine may be contained within a pemmeable membrane or screen-like
7 barrier; or the nicotine may be held within other nondissolvable ~ "1 vehicles
8 capable of rele~ing the nicotine for ~
9 The scope of the present invention includes I ,ho-l;,,,- ~.t~ having a permeable
o membrane or screen-like barrier which retains the nicotine containing vehicle.
Il The barrier may be screen-like with relatively large pores or membrane-like with
12 relatively small pores. The barrier preferably h~ a pore si~ sufficient to permit the nicotine
13 to pass ~ th~ou~l~. It is important that the nicotine be retained within the barrier under
conditions outside the patient's mouth and that the nicotine be capable of permeating the
s barrier within the patient's moutll.
16 For example~ ill olle preferred rl ~ withill the scope of the presellt illvelltioll~
17 tlle l l l ~ ll medium viscosity is sufficielltly high outside the mouth such that the surface
18 tension at the barrier pores prevents the nicotine from permeating the barrier. But once the
19 dosage-form is placed within the patient's mouth, the viscosity of the m~rliAAml-nt medium
20 is lowered so that the nicotine permeates the barrier. In one .Illbo~ the viscosity of tlle
21 ~ medium is lower within the mouth due to saliva contact with the ,.,~
22 medium In other G., .l~h~ tlle viscosity of the l l lr~ medium is lower witllin the
23 mouth due to an increased ~ aLulG within the mout~l.
2~ In another embodiment within the scope of the present invention, tlle nicotine within
25 the ...~ medium permeates the barrier in response to pressure effects within the
26 mouth. For instance, negative pressure caused by sucking the dosage-form draws the
-Page 17- Doc~e~No.10~04.52
April 19, 1996
~ 2~78~21
medicament tl~rougll tl~e barrier. Alternatively, positive pressure caused by squeezing the
2 dosrlge-rorm rorces the m~ Am~nt through tlle barrier.
3 Olle embodiment utilizes microonrS~r~ to~l drug particles retained within a
permeable barrier. Miulu ~ od drugs are nicotine particles or droplets which have
s been coated with a protective coating material. Typical coating materials include fats,
6 waxes, Ll;~;lyu~id~ fatty acids, fatty alcohols, ~I.u~L.~ fatty acids and alcohols,
7 stearates, sugars, poly(ethylene glycol), certain metals, gums, hydrocolloids, latexes, and
8 various polymer-based form-llAtion~ such as polyethylene, ethyl cellulose, eLll~l~.lc~
9 acetate, ethylene-acrylic acid, polyamides, and some enteric polymers.
The protective coating material of 1ll..,ll . ' ' nicotine prevents nicotine
~le~rA~lAtirln by moisture, retards oxidation of the nicotine, decre~es ~,V~AUUIaLivll and
12 ~,.1.1;"._I;r.", protects the nicotine from reaction with other in~rertiont~ and m~ks urlple~ant
13 t~te of nicotine. Nicotine III;I.~IU' "' ''I''' ,1,.1 i on techniques are known in the art.
4 Another emho~imont utili~s a plurality of drug-containing sponge-like matrixes
s which are retained within the barrier. Sponge-like matrixes, which include Il~ lUi~lJOl~g~
are devices capable of entrapping a ", 1;.. ~ and then releasing the ,.. rl - A~ over
time. Tl ese sponge-like matrixes are biologically inert, non-irritating, non-mutagenic, non-
allerg~nic, non-toxic, and non-biodegradable. They can even improve ",~lli. ,,.,...,1 stability.
Suitable Illi~,lu ,l~ullg~ or sponge-like matrixes are known in the art.
~o Like true sponges, the sponge-like matrixes or Illil.,lU~UUllg~,. contain a myriad of
21 illt~l.,vl.llc~lillg voids within a non-collapsible structure with a large porous surface. The
22 size of tlle sponge-like matrix as well as the number and size of tlle internal pore structure
23 can be varied depending on the .". .li. ,..". .,~ size and viscosity.
2~ The ",. .li~ l iS rele~ed from a sponge-like matrix in response to a suitable
25 "trigger". For example, rubbing or pressing the sponge-like matrix, elevating the tulll,u~lalul~
26 of tlle matrix (as within the patient's mouth vis-a-vis ambient t~ .lalulc), or introducing
-Page 18- r~ie~NO.~03o4s2
Ap~,l 19, 1996
~ . ~
2178t~21
suitable solvent such as saliva can cause a controlled release of tlle ~nPr~ f~mPnt Pressure
2 may also be used to rclcase l11e drug rrom the sponge-like matrixes. Squeezing and s~lcking
3 a dosage-fornl containing the sponge-like matrixes saturated with the ",.~1;, ~..,~.,1 will
4 releasethel"f l;~ll
s In other 1,l.boli,l,.,l,6 within the scope of the present invention, the sponge-like
6 matrix or l-,i~"u . n~ 1 nicotine particles may be held togetller Witll a hiocf~nnr~ltihle
7 bindi~lg material or adhesive (either dissolvable or nondissolvable) such as sodium
8 c~fl~u~y~ ylcellulose~ sodium alginate, and tragacanth.
9 . In yet another l.."l.oll;,.,. .,1 of the present invention, the sponge-like matrix or
0 III;~,IU~ nicotine particles may be retained witllin a col,lu-~ss-,d powder dosage-
11 form or other dissolvable using the previous discussed powder f ~", ll".~ ~ i. ,. . process.
12 In these l.. l~c.. l;.. ,:~, a plurality of ~ . ' ' nicotine particles are
13 ~;ulll,ul-, >a~,l together in a dosage-forrn with compressible sugar and other ingredients as
14 previously described. A handle is also preferably attached to the dosage-form.
The handle or holder may be attached to the nù~ ùl~lc matrix by ill~,Ul~Ul~lLil~g
16 the holder into tlle nondissolvable matrix as the dosage-form is being formed.
17 Alternativcly, ~lle holder may be glued, Culll~ ~l, screwed, snapped, or otherwise
18 atlacllcd ~0 Ille nondissolvable matri:c once tlle matrix is rormed. In yet olller embodiments,
19 dosage-forms may be assembled il,llllcdl;_~uly prior to uses by sliding nondissolvable
20 I ~ hl~Jr dosage elemerlts containing a suitable l~ lt onto an ,u,ulu~ y
21 configured holder. Optionally dissolvable or nondissolvable flavored Crll1ll. ~.1 nl,lf elements
22 may also be slid onto the holder.
23 In one rl 1 ll lOl ~;l l l. . Il illustrated in FIG. 3, a perfneable barrier 40 defines a chamber
24 42 and an opening 44 to the chamber. The chamber is filled witll a nicotine rnlmro~itiorl 46
in the form of Ill;~,lu .,uull~f~S, lll;~ '-'~ ' drug particles, a .. 1;~ n.l.. ll medium, or
26 other similar drug-containing ff~nm~ tif~n A holder 48 includes a cover 50 for opening 44.
--Page 19-- Doc~clNo. 10304.52
ADril 19, 1996
2178~21
Cover 50 is conf gured to securely seal opelling 44 wllile at tlle same time provide means for
2 a~taclling llolder 48 ~o tllc dos tge-for~n. I~l tllis way, tlle quantity and concentration of
3 nicotine may be placed wi~llin thc dosage-form prior to use. Tlle nicotine may even be
4 replenished or replaced during use if necessary.
s It will be appreciated tllat attachment of the nicotine-containing matrix onto a holder
6 canfacilitatethell,."~.,l".r,~,.labsorptionofavarietyoftherapeuticagents. Attachmentto
7 a holder also facilitates verifiable transfer of the medication of the patient. For instance the
8 medication may be bound to a dye such tilat loss of color indicates transfer of the medication
9 to the patient. The holder permits the nicotine-r.~ :",l~ ,l matrix to be positioned at the
o desired location within the patient's mouth and provides a convenient point of reference
enabling the medical professional to Yerify the proper placement of the matrix.
Dosage-form 60, illustrated in FIG. 4, contains a plurality of c~ dosage
13 elements 62. Dosage elements 62 include a solid core 64 defining a male coupling 66 and
a female coupling 68. A dosage cap 70 is configured substantially the same as dosage
elements 62, except that the solid core does not define a male coupling. The dosage elemehts
16 are preferably constructed of a screen-like material such as woven fabric or a perforated sheet
17 of material which is mo~ded or fabricated around the solid core. The solid core may be
,ull~Llu-,Lt,d of a suitable bircrmr~ihle material such as polyethylene. The screen-like
19 material defines a chamber for l~olding the desired ",~ ". .,l and releases the ", l;, ~
20 in sh~f~n~i~.lly tlle same manner as described above in connection with FIGS. IA-IC.
21 Dosage-form 60 is constructed by ;~ a plurality of dosage elements
22 through their respective male and female couplings. A holder 72 which includes a male
23 coupling 74 constructed at one end thereof is preferably coupled to the ~:ol.n~ dosage
2~ elements. The ability to assemble a dosage-form prior to use permits the dosage-form to be
"customi~d" to the individual patient or ~ Various concentrations of a drug,
26 or even multiple drugs may be ~J,il.i ,t~l~d in this manner.
--Page ~0-- D~ck~tN~ 10304.52
Apnl 19, 1996
~ 2i7sn2l .
FIGS. 5 and G illustrate anotl1er possible dosage-form embodiment witl~in tlle scope
2 of ~lle plesent invention. Dosage-form 80 includes a covering material 82 molded around
3 a semisolid core 84. Tlle semisolid core is preferably mounted to a holder 86. Covering
4 material 82 is preferably a thick mesh or perforated sheet having the desired ~ ,t 88
s embedded tllerein which will permit the ~lf .1:. A.ll. .~1 to leach out or otherwise enter the
6 patient's mucosal membrane. The . " .~ . ,. "l may be powdered, liquid, ~
7 Or otherwise trapped in the covering material 82 so that the " .~;. A.~l~ will be released
8 within the oral ~,llV;lOlltll~
g The PmhortimPntc illustrated in FIGS. 7-9 permit the nicotine A~ -I;r~ rate too be controlled by adjusting the pressure applied to a "~.1;. ---,. .,l medium. Dosage-form 90
I l shown in FIGS. 7 and 8 includes a holder 92 and a screw 94 internally threaded within holder
12 92. Secured to holder 92 and to screw cap 96 is a s ~ l, Al lr membrane 98 which
13 provides a ~:.. ,.lh:.".,r.. l barrier for a quantity of III-~;~A-I-- '1l medium 100. Membrane 98 is
14 similar to those described above by having pore size sufficient to permit ,.,rf1i~Ar: to pass
tll~ lluugl- within an oral ~IIV;IU~ .ll. The l,~ f.~l medium may be a liquid
16 III~.I;IA.II. 1l solutionoras--cl~Pn~ion
17 In operation, dosage-form 90 is placed within the patient's mouth and screw 94 is
18 twisted such tllat .,.~.li, All~. .~t medium 100 is placed underpressure thereby increasing tlle
19 rate tlle mP~irAment permeates membrane 98.
The Pmhor~imPnt illustrated in FIG. 9 is similar to that shown in FIGS. 7 and 8
21 except that the ~r.li~ i.. ~l. .1l is embedded within a semisolid ~,.~ .li, A-~ - .~1 medium 102
22 embedded with l ~ ~r ~ which is capable of bein~i compressed~ In operation, the dosafAje-
23 form is placed within the patient's moutll and screw 94 is twisted such that mPr1irAmPnt
24 medium 102 is compressed thereby directly releasing the ~ r-~t for absorption across
the patient's mucosal membrane.
26
- Page 21 - r~ock~tNo. 10304.52
April 19, 1996
2178~,21
FIGS. 10 and 11 sllow yet another possible embodilllent Witl~il1 the scope of the
present invcntioll. Dosage-form ~10 incl~ldes a pl~1ralit! oft~lbe-like members 112 located
3 around tlle peripllery of a semisolid core 114. The semisolid core is preferably mounted to
a holder 116. A layer of ~ lf material 11 g may optionally be located between the
tube-like members and the semi-solid core.
6 Tlle tube-like members are formed from a screen-like material 120, such as nylon
7 or dacron mesh, which is molded in a ~ ylil,1li.,AAl shape. The tube-like members are
8 mounted to expandable material 118 such that the screen-like material provides a barrier for
9 a quantity of Ill.. l;~.A.II. 1l 122. ~frAnf~ lf material 118 is preferably uu.. ~llu~,~ed of
o methylcellulose or similar material encased in a porous mesh which will hydrate and expand
Il when placed irl the patient's mouth. Upon expansion increased pressure is exerted on the
12 porous tube-like members, thereby increasing the rate ' is released form the
13 dosage-form.
The . ~ - " illustrated in r IG. 12 is similar to that shown in FIGS. 10 and 11,except that the ".~ .l:. A.~l. .1~ is embedded directly within an expandable material 124 such as
16 methylcellulose. The ""A,~,~ ,A.II. ..t is released as material 124 expands v~ithin the patient's
17 mouth.
18 Anotller optional ~lllbo,li~ lL wllich is not sho~n in the figures replaces semisolid
19 core 114 with a hollow tube constructed of polyetllylene or similar material which can be
20 injected witll air such that it expands against the tube-like members containing the
21 . ~ The pressure (from a know volume of injected air) and the pore size covering
22 the tube-like members governs the delivery rate of the " ,. .1,. A 11 If . ~1
23 FIGS. 13 and 14 show a dosage-fomm which is a variation of the clllbodi.,.~.... L
2~ illustrated in r'IG. 10. Dosage-fomm 130 of r'rGs. 13 and 14 includes a plurality of tube-like
members 132. Tube-like members 132 are shown in cross-section in FIG. 14. Members 132
26 include a screen-like material 132 which f~nf sr~ a quantity of ml flir~gnnf nf medium
- Page 22 - I)rlc~c~ No. 10304.52
Arril 19, 1996
~ 2178~21
136. A ri~id stem 138 is attachcd to screen-like material 134 and is confi~ured to be slid and
2 locked into correspondinL slo~s rormed in a solid core 138. A l~andle 140 is plefcMbly
3 secured to the solid core to facilitate placement and removal of tlle dosage-form.
Dosage-form 130 may be assembled prior to use by sliding the rigid stems of a
s plurality of tube-like members 132 into cullr ~ mdillg slots formed in tlle solid core. The
6 ability to assemble a dosage-form prior to use permits the dosage-form to be ". ~l":,. 1"
7 tO the indiYidual patient or ~ Various ~ s of nicotine, or even
8 multiple drugs may be adlll;";~ l in this manner.
9 FIG. 15 illustrates another possible dosage-form ~Illbol;~ lL which may be
o individually assembled prior to use. Dosage-form 150 of FIG. 15 is assembled from a
Il plurality of dosage elements 152. Each dosage element includes a ring 154 which is
12 positioned around a semi-solid disk 156. Rings 154 are fabricated from u~ v~.;al1 porous
13 material such as woven nylon or dacron or sheets of perforated nylon, polypropylene, or
14 pOI~r~ ,llC. Rings 154 are filled with ~ either liquid or powder. The semisolid
disks define a hole 158 tllerein such that a plurality of dosage elements may be assembled
16 on a holder. The ability to assemble a dosage-form 150 prior to use permits the dosage-fomm
17 to be "cl-ctr mi7~ d" to tlte individual patient or ~ Various l onr~r ntrAfionc of
18 nicotine, or even mulliple drugs, m~y be a~ .;st. l~d in tllis manner.
19 The foreLoinL dosage-forrns are Liven to illustrate v~rious rl l l~ which may
20 be made in accordance with tl~e present invention. It is to be understood tl~at the foregoing
21 dosage-form cnnfi~l~r~ti~nc are not CUIII~IC:II~,II.~;V~ or exhaustive of the many types of
22 r,li;,"~ofthepresentinvention. Itisimportantthatthenondissolvabledosage-form
23 configuration be hir~r~r~nnr~ lr and capable of releasing the nicotine for absorption through
24 the patient's mucosal tissues. The c~,llr~ ,. should preferably have a structure, shape,
and texture which is palatable to the patient.
26
- Page 23 - Dor~el No. 10~04.52
April 19, 199O
~ 2~78~)21
Placing a nicotine dosage-form onto a llolder also facilitates the temporary removal
2 of mcdicatioll for inspection or t~le reduction of tlle effect wl~en llecessary. Unlil;e
3 ~flminictr~iifln of nicotine orally or even sublingually, the present ~U~ /O~iliVll can easily be
4 removed to assess the effect induced at any particular time. When a pill or lozenge is used,
s removal from the patient's mouth at an ;"I, ""~,~; tr stage to assess effect is generally
6 imrr:u tif~1 if notimrnccihlf~
7 In contrast to a lozenge the nvlldi~ .vl~al,lc dru~-f fl"l ~;, .. ". .,l matrixes attached to
8 a holder can also prevent or malfe much more diff cult the aspiration of the dosage-form.
9 One major problem with existing lozenges and the like is their tendency to crumble. Once
the lozenge .I;~. . ,t. ~,. . .t. ~, controlled l., "~" .". r,~.~l delivery is less ideal and the particles are
swallowed. Many of the dosage forms set forth herein dissolve but do not or cannot
12 di
13 In addition, either or both the drug and the permeation enhar cer may be dispersed
uniformly throughout the dissolvable solid matrix fflmroeitif~n or may be selectively
s dispersed, i.e., stratifled or coated, to thereby vary the absorption of the drug from selected
16 portions of the dissolvable solid matrix. It will be appreciated that a stratified nicotine-
containing f flmrflcitifm could be formulated to effect an initial relatively rapid rise in blood
nicotine concentration followed by l"...l,...,~-,.-. of a relatively lower blood nicotine
19 concentration. T~lis may be ~-~,1.1;~1, ~1, for example, by fflrm~ tinE a two-layer
composite matrix comprising an inner matrix and an outer matrix. Tl~e solubility of tlle
21 matrices could be different such that the outer matrix is more rapidly dissolved within tlle
22 oral cavity than the inner matrix.
23 Alternatively, or in addition, the c-~n-Pntr~tifln of nicotine compound within the
24 matrices could be different such tllat the outer matrix releases a higher quantity of nicotine
tllan the inner matrix. In any of these multilayer fmhorlimf-nlc, t~le blood nicotine
26
--Page 24-- Doc~.~ No. 1030~.5~
April 19, 1996
2178û21
concentration will rise relatively more rapidly while the patient consumes the outer matrix
2 tllan wlli~e the patient consumes tlle inner matrix.
3 Additional selectiYity and control of nicotine release and absorption rates could be
4 obtained witl1 more matrix layers and/or c-~mhinP tinnS of the above-described methods, i.e.,
s varying the matrix dissolvability, varying the pemmeation ~ . ,1 IA 1~1 ~ ' I " ' I ~, or varying the drug
6 ç~nrPntrAtinn throughout the matrices. It will be appreciated that more than two layers could
7 also be i~ Jula~td into the multilayer nicotine-containing f f~ .v~ ., to further vary the
8 effect as desired. The presence of additional layers can be indicated by color or flavor
9 changes.
o In the fi.,.. , ~l~';.. i disclosed, the amount of nicotine in each dosage form is
Il preferably less than 30 mg., and most preferably between 0.5 to 20.0 mg. to avoid accidental
~12 overdosage by swallowing. ~fhe presently preferred Pmho~imPnt utilizes nicotine bitartrate.
13 Alt~lough the dosage fomm of tlle present invention comprises a holder member which
14 prevents swallowing of the dosage fomm, it may nevertheless be preferable to keep the
tS nicotine dose in individual dosage forms low to thereby allow a patient to easily selectively
16 control the amount of nicotine ingested by controlling the number of dosage forms used.
17 As disclosed in the above-referenced patent, the nicotine-containing comro~iti~ns
18 intended to be sucked are prcferably buffcred to increase and n1aintain tlle percentage of
19 unionized drug to facilitate transport and absorption through the oral mucosa and thereby aid
in l l n~ 1 absorption. A preferred formulation is at a pH of 6.8-11. .As described
21 above, buffered f,.,. ~ will include sodium carbonate, sodium phosphate, calcium
22 carbonate, ~ .,." hydroxide, trimPth~rninP and other substances known in the art.
23 Tl~e addition of a buffer can result in a decrease in the stability. Altematively, the
24 buffer can be ~ in a water soluble material to improve stability but still provide
the optimal pH in the mouth as the unit dissolves.
26
--Page 25-- Docke~lo. 1030~.52
Ap~l 19, 1996
2l7~a~l
Tlle buffered l~ico~ine-containing compositions formulated by direct compression
2 may comprise a mixf~lre of directly -,-JI"~ 5ibl~ excipienls, a ~ y aCceptable
3 salt of nicotine and a base sufrlcient to maintain a specif ed pH level within the patient's
4 mouth. The preparation is mixed with suitable diluents and compressed or ~olll,UI. ~;f with
s a granulated "core" comprising a portion of the buffering base ihgredient~
6 Anotller method involves cold CO~ lc~;OI~, extrusion, or drying of a mixture
7 containing an inert filler material, an inert binder material, and a solution of nicotine or
8 nicotine-containing substance dissolved in alcohol to formulate a nicotine-containing dosage
9 form. This form may include a coating which can, for example, comprise a substance to
enhance dissolvability of the c,..,.~ , or a substance to act as local anesthetic and
Il thereby decrease the perceived local irritation from a nieotine absorption site. It will be
12 dlu~ ,;a~etf that a method similar to the "coating" method coufd also be used to provide a
13 multi-layer nicotine-containing co. ~ .- ., . wherein the layers permit nicotine to be released
in different amounts or absorbed at different rates similar to the approach discussed above
s with respect to "stratified" dissolvable solid matrices.
16 Another embodiment of the present invention utilizes a Culll~ d GalbOIl,~ lla~e
7 powder matrix, but llas mixed substantially uniformly t~lerein a coated buffering agent. The
18 buffering agent is coated with a water soluble material so that the buffering agent is released
19 in tlle moutll of the patient. Upon release, tlle buffering agent will modify the pH of the
saliva in the mouth of the patient to enhance absorption of the nicotine. By coating the
21 buffering agent prior to mixing with the matrix, stability of the combined matrix is
22 ms~intllin-~rl
23 In addition, methods for fi-rrn~ tin~ dissolvable nicotine-containing matrices for
2~ buccal dosage forms, which melt in the user's oral cavity but are stable in higher shipment
and storage ~ J~.a~Ul.,. are known and can be ;llcolpula~etl.
26
--Page 26-- Doc~et No. IO~oJ.52
Ap~il 19, 1996
2l7sn2l . ,,
As discussed previously, tob~cco subs~itutes llave several uses. A smoker who does
2 noL wisll to expose otllers to tile cffects orsecond-lland smoke ma~ use a tobacco substit-lte
3 for short periods, such as when traveiing on an airplane, or during working hours.
Long-terrn tobacco users may have incurred damage from chewing tobacco or from
s cigaretles so that they may no longer use those products, yet stili feel the need for nicotine.
6 ~or those who wish to cease smoking or using snuff, tobacco substitutes may be
7 used to wean the individual from the nicotine craving. By slowly reducing the amount of
8 nicotine consumed, the individual may reduce the craving without ~ C;Il~ significant
9 withdrawal side effects.
o A similar group of tobacco substitute users utili~e the substitute oniy to deal with
peak periods of craving. These individuals do not regularly use the substitute, but instead
12 use it oniy occasionally when needed to deal with occasionai cravings beyond their resolve.
13 It will be appreciated that although nicotine is often consumed in cigarette form ~Viti
14 the a~,cull~ g deleterious effects of tar and smoke, nicotine has been found to have
IS beneficial effects such as treatment of Alzheimer's disease, ulcerative colitis, Tourette's
16 syndrome, and Parkinson's disease. Altilough presented as a tobacco substitute, the present
invention is also directed to tile benerlcial ~ illlill;aLlaLiO~I of nicotine for treatment of these
18 and other ailments. In these ~rrlir~tion~, the handle of tlle present invention allows tile
19 patient to titrate tlle amount of nicotine to prevent side effects such as nausea, etc. The
present dosage form not only aids in the comfort of the patient by preventing side effects, but
21 also provides an added measure of safety as the dosage forrn may be removed not only by
22 the patient, but also by a care provider. Unlike other dosage forms with slower or delayed
23 absorption rates, tile user may remove the present dosage form at the first signs of nausea and
24 need not consume the entire dosage form. This OIJIJUII~ ;L~ is not available Witil ingested
therapies or with slower acting dosage forms such as patches.
26
--Pa~e 27-- Doc~et No. 10iO4.52
Avril 19, 1996
2l7sn2l
Tlle present invention may be embodied in other specific forms Witllout departing
2 froln i~S spirit or essential characteristics. Tlle described embodiments are to be corlsidered
3 in all respects only as illustrative and not restrictive. Tlle scope of the invention is, therefore,
indicated by the appended claims rather than by the foregoing description. All changes
s which come within the meaning and range of equivalency of the claims are to be embraced
6 within their scope.
Il
12
13
1~
16
17
18
19
21
22
23
24
26
--Page 28-- Dock~t N~. 10304.52
Apnl 19, 1996