Note: Descriptions are shown in the official language in which they were submitted.
~ 21 78258
P-303 TRI-NARK -- 1 -
ME~r~OD AND APPARA~ru8 FOR R . ~
VOLATI~}: ORGANIC COMPO~IND~ ~Y COLD OXIDATION
FIFr n OF TTTF INVFNTION
This invention relates generally to
controlling environmental pollution and, more
10 particularly, to a method and apparatus for reducing and
destroying volatile organic compounds (V. O. C . s) .
BACKGROTJND OF TT~r' INVE~TION
V.O.C.s have long been a major source of air
pollution as an inevitable contaminant exhausted from
many industrial processes including, for example, large
industrial paint shops used in the automotivc industry.
Legislative efforts have established emission standards
20 to control emission of V.O.C.s into the environment.
Current and future, 1 i~nre with such standards places
a continuing demand on industry and creates an on-going
need to reduce, degrade, and eventually destroy V.o.C.
emissions in a manner that is not cost prohibitive.
25 This is particularly critical for manufacturers in
industrialized countries who compete against sources
oper2ting in countries that do not have strict air
pollution control laws.
3 o In automotive paint shops, large volumes of
solvent (V. O. C. ) laden air must be removed from the
paint spray booths and, to a lesser extent, from other
paint shop operations, such as holding and quiet zones
and paint bake ovens. For automotive paint shops, large
35 quantities of solvent-laden air mUst be processed.
2178258
P- 3 0 ~3 TRI ~ 2
Various techniques and combinations thereof have
heretofore been used for V.o.C. abatement in paint
shops .
Typically, scrubbers are used to capture
inorganic chemicals and particulate paint from process
exhaust air using liquids pumped through the scrubber.
Any rF-~; n i n~ paint particles are then removed from the
exhaust air by filter banks with progressively
increasing efficiencies. Less expensive filters are
used in the initial stages to trap most of the larger
paint particles. After filtering, the exhaust air
stream is heated to reduce the humidity for a subsequent
adsorbtion process. In the adsorption processes,
solvent-laden air is concentrated into smaller
quantities, typically 10% of the main exhaust airstream,
and then processed. Typically, the concentration is
accomplished by adsorbing the V. 0 . C. s into a carbon bed
and then desorbing the carbon bed with hot air, hot
inert gas or steam. The concentrated desorption product
can then be finally processed through rl~Pm;c~l
treatment, solvent recovery or incineration.
Various incineration apparatus can be used to
oxidize the solvents in a concentrated solvent-air
mixture taken from the carbon beds. ~owever, typically
the mixture is heated to temperatures in excess of
1,400F. When held at these high temperatures, the
solvents react with oxygen, with the final reaction
3 0 production products theoretically being harmless water
vapor and carbon dioxide. Various types of thermal
regeneration heat exchangers and the like are used to
recoup heat from the incinerator exhaust to improve
thermal efficiency. Direct incineration could be used
35 but it generally has a low thermal efficiency,
2 1 782-58
p--308 TRI-MARX -- 3 --
particularly for processing large volumes of
V. 0 . C. -laden air. However, such prior art systems
require large capacity carbon beds and have high energy
costs f or incineration .
Except in the most advanced systems, some
off-site treatment and/or disposal is frequently
required. For smaller installations, as contrasted to
large automotive assembly plants, off-site carbon bed
lO desorption may be most cost effective. In general,
carbon beds, when used alone, are not effective or cost
efficient for processing large volumes of V.O.C.-laden
air. Special systems are required to desorb the carbon
beds and, for many applications, this is accomplished
15 off-site. Additionally, the desorption concentrate must
still be treated for solvent removal and/or
incineration. Incineration generally generates NOx or
carbon - r~;dP and, without thermal recovery systems,
has a direct thermal impact on the environment as well
20 as requiring off-site disposal. host importantly, prior
art systems relying on high temperatures to complete
oxidation are expensive to operate and may still require
of f -site 1~ qpO~ 1 . These disadvantages, particularly
when coupled with current and anticipated air and
25 environmental pollution control, create an on-going need
f or improved V . 0 . C . abat~ment .
Another technique that has been utilized for
abatement of V. 0 . C. s in industrial process air involves
30 the use of ultraviolet ~uv) light to break down the
V.o.C.s directly and to form activated air containing
oxygen in the form of ozone and other oxidants that also
work to break down the V.O.C.s. As used herein,
"activated air" should be understood to refer to air
3 5 that has been treated, whether by exposure to
2 ~ 78258
P--308 TRI-~ 4 --
ultraviolet light or some other method, to increase the
concentration of oxidants in the air. Commercial
systems are available that utilize this technique for
abatement of solvents contained in process air exhausted
5 from industrial paint booths, ovens, conformal coating
areas, etc. A typical system includes a two-stage pre-
filter, a photolytic reactor, an aqueous reactor, a
coalescer, and a pair of granular carbon beds.
Particulates of one micron and greater in size are
10 collected and removed from the process air by the pre-
filters. The air flow then passes through the
photolytic reactor, where it is exposed to tuned
ultraviolet light. Exposure of the process air to the
ultraviolet light results in photochemical reactions
15 that form ozone from the oxygen contained in the air, as
well as peroxides from the moisture content within the
air .
Oxidative degradation begins in the photolytic
20 reactor due to both the newly formed oxidants and the
direct exposure of the V.O.C.s to the ultraviolet light.
The air stream is then scrubbed with ozonated water in
the aqueous reactor. The ozonated water is generated by
subjecting air to the ultraviolet lights and then
25 injecting and mixing the activated air into the water.
At this stage, water soluble hydrocarbons will collect
in the water and will thereby be removed from the air
stream. After passing through the aqueous reactor, the
water vapor contained in the air stream is removed by
3 o the coalsecer . The f inal stage in this process is to
pass the air stream through a carbon bed f or adsorption
of any remaining V. O. C . s . A second carbon bed i3
utilized so that while one carbon bed in on-line to
adsorb the V. O . C . s, the other is in the process of being
35 regenerated using activated air containing ozone,
2 1 78258
P-308 TRI-MAR~C - 5 -
hydrogen peroxide, and other oxidants produced
photochemically by exposure of clean air to ultraviolet
l ight .
The use of ultraviolet light to generate
activated air containing ozone has also been implemented
in various systems for treating water. For example, the
following U. S . Patents are each directed to the use of
ozone and other oxidants in the wash water of a laundry
washing system: 3,065,620, issued November 27, 1962 to
P.H. Houser; 3,130,570, issued April 28, 1964 to P.M.
Rentzepis; 3,194,628, issued July 13, 1965 to P. Cannon;
5,097,556, issued March 24, 1992 to R.B. Engel et al.;
and 5,241,720, issued September 7, 1993 to R.B. Engel et
15 al. In these systems, ozone is produced by exposing air
to ultraviolet radiation that is produced by either a
corona discharge or ultraviolet lamps. The activated
air containing ozone and, in some cases, hydrogen
peroxide is mixed with the wash water to improve the
2 o cleaning of laundry and reduce or even eliminate the
need for detergents.
The literature also suggests that substantial
laboratory efforts have been directed to using
25 ultraviolet radiation for other types of water
treatment. See Legrini, Oliveros and Braun,
"Photochemical Processes For Water Treatment, " Chem.
Rev. 1993 at pages 671 through 698, American Chemical
Society Document No. 0009-2665/93/0793-0871.
30 Ultraviolet radiation for water treatment is potentially
useful not only for treating drinking water, but also
for treating contaminated surface water, ground water
and waste water. However, based upon the 221
biographical references cited and reviewed, the authors
35 suggest that most such laboratory experimentation, with
~ 2 1 78258
P-3 08 TRI-M~ - 6 --
a f ew noted exceptions, have not been evaluated on a
prototype basis, much less commercially.
Althoug~ the Chemical Review article is
5 directed to water treatment as contrasted to V. O . C.
abatement in industrial process air, some of the
mechanics of oxidative degradation considered therein
may be useful as background for the present invention.
For example, Table I at page 674 ~reproduced as "TABLE
10 1" below) c~nf i rTnc the oxidation potential Or various
oxidants believed to be available from the activated air
and undoubtedly generated elsewhere in the system and
process of the present invention as will be described.
TABLE 1
oxidation Potent; A l ~ of Some ~ nts
- S~ecies Oxidation Potçntial (V~
fluorine 3 . 03
hydroxyl radical 2 . 8 0
atomic oxygen 2 . 42
ozone 2 . 07
2 5 hydrogen peroxide 1. 7 8
P~LI1YdLU~Y1 radical 1.70
permanganate 1. 68
hypobromous acid 1. 59
chlorine dioxide 1. 57
30 hypochlorous acid 1. 49
hypoidous acid l . 45
chlorine 1. 3 6
bromine 1. os
iodine o . 54
Each of the foregoing references, as well as
40 the literature describing the commercially available air
treatment systems described above, all espouse the
virtues Or ozone and the use of ultraviolet radiation to
p-308 TRI-MARX - 7 _ 2 1 78258
generate that ozone. However, as shown in Table 1
above, ozone has a lower oxidation potential than
hydroxyl radicals. Thus, ozone has less tendency to
cause oxidation of the V. O . C . s than the hydroxyl
5 radical; that is, it is less active than the hydroxyl
radical. U.S. Patent No. 4,214,962, issued July 29,
1980 to A.J. Pincon, sets forth other disadvantages of
creating ozone in addition to other oxidants formed by
ultraviolet radiation; namely, the increase in surface
10 tension of water with which it is mixed and the possible
formation of carcinogenic substances. In that patent,
an apparatus is disclosed for using ultraviolet light
under 200 nanometers to generate an undisclosed
activated oxygen product without the production of
15 o~one. When used for treating water for human
consumption or swimming pools, the apparatus can include
a polyvinyl chloride enclosure to permit liberation of
free chloride to provide chlorination of the water.
Not unexpectedly, however, since the Pincon
patent is directed to the use of ultraviolet radiation
for water treatment, it does not address the problems
associated with processing large guantities of
industrial process air laden with V. o . C. s, much less
offer any direct solution to the problems and
disadvantages of the various commercial processes that
rely at least in part on the presence Or ozone for
V.O.C. abatement in the exhaust ~rom paint spray booths.
SUMMARY OF THE TNVENTIO~
The invention provides a method and apparatus
for reducing, abating, and destroying volatile organic
compounds on a continuous ~asis, wherein a V.O.C.-laden
2178258
P-308 TRI-~ - 8 --
air stream is mixed with and maintained in a fog or mist
of activated air in aqueous solution while the mixture
is repeatedly exposed to ultraviolet radiation in a
series of chambers along a tunnel. The activated air
5 contains oxidants such as hydroxyl radicals, hydroperoxy
radicals, and hydrogen peroxide. Preferably, some
activated air is introduced into the tunnel in gaseous
form. The activated air is generated by exposing humid
air to ultraviolet radiation and the activated air is
lO then dispersed in an agueous solution in sparger tanks.
The sparger tanks also include ultraviolet lamps to
generate and maintain a high oxidant level in the
aqueous solution and promote other reactions just before
the aqueous solution is introduced into the tunnel as a
15 f og or mist .
Preferably, upon discharge from the tunnel,
the air stream is still in a highly active state and is
delivered through an expansion chamber to large carbon
20 beds where rr--;n;n~ V.O.C.5 are l:d~Lu~:d and further
oxidatively destroyed. Clean air and water vapor and
quite possibly harmless carbon dioxide are exhausted
from the carbon beds to the atmosphere. Preferably two
carbon beds are used so that one carbon bed is on-line
25 to capture any re~~;n;n~ V.O.C.s while the second carbon
bed is being regenerated. Activated air is used to
regenerate the carbon beds 50 that thc regeneration not
only desorbs the carbon bed but also deposits and
replenishes the carbon bed with oxidants from the
30 activated air. This regeneration as~ures that oxidants
are available for surface reactions with any rr~-;nin~
V. 0 . C . s when the regenerated carbon bed is brought
on-line. Preferably exhaust gases from the regeneration
process are recirculated back through the tunnel, along
35 with activated air vented from the sparger tanks so that
2 1 7`8258
P--308 TRI-MARK - 9 -
any reaction products generated in the carbon beds are
continuously treated while being recirculated through
the system.
The final end products from the cold oxidation
process of the present invention are believed to be
water (H20~ and carbon dioxide (C02~ as in an ideal
incineration process. However, although some harmless
water vapor and gaseous carbon dioxide is undoubtedly
released to the environment, most of the water is
retained in the system. As compared to incineration,
less carbon dioxide than expected is exhausted from the
carbon bed which is on-line. In any event, exhaust from
the system to the environment is not a thermal
pollutant.
While not ascribing to any one theory, it is
strongly believed that the present invention destroys
V. 0 . C . s by a combination of two phenomena, undoubtedly
producing a synergistic effect. First, the activated
air generated by ultraviolet radiation and further
ultraviolet radiation of the active fog produces highly
reactive oxidants which then subsequently oxidatively
degrade and destroy the V.O.C.s. Secondly, direct
ultraviolet photolysis of the V. 0 . C . s causes
photochemical decomposition of the V.O.C.s with the
resulting products interacting with oxygen, the oxidants
and other intermediate radicals in the activated air and
the activated air fog in the tunnel and preferably in
3 o the sparger tanks and the carbon beds .
These two phenomena occur alone and in
combination in various degrees, not yet fully
determined, in the various stages described hereinabove,
principally:
~ 2 ~ 78258
P--308 TRI-N~RK - 10 -
1. Reaction of the V . 0 . C . s, intermediates,
and oxidants in the vapor stage occurring in the tunnel
chambers;
2. Reactions with the V.O.C.s, intermediates,
5 and oxidants in the aqueous sparger oxidation tanks;
3. Sur~ace reaction and final destruction of
V. 0 . C . s at the carbon beds; and
4. To a lesser extent, reactions o~
intermediates and oxidants in supply tanks and PVC
lO piping used throughout the system.
Enhancement of the activity level of the
activated aLr is believed to occur by one or more
effects that serve to decompose ozone generated by
15 irradiation from the ultraviolet lamps and thereby
permit the liberated atomic oxygen to combine with
available atomic hydrogen to form hydroxyl radicals.
These effects include photolysis of the ozone using
ultraviolet light at a wavelength of 254nm, degradation
20 of the ozone as a result of creating and maintaining the
activated air in a high humidity environment, and
degradation of the ozone by radicals such as Cl- that are
made available by ultroaviolet radiation of a PVC or
other catalyst.
Regardless of precisely where and how total
V. 0 . C . destruction occurs and how it progresses, no
waste residue is generated, due in part to continuous
recirculation of the aqueous solution through the
3 0 tunnel, the storage tank, the sparger tanks, and the
circulation of the carbon bed regeneration exhaust air
into the tunnel.
Components of the cold oxidation system, and
3 5 particularly the tunnel, are of modular construction to
- 2 1 7~258
P-3 08 TRI-MARK - 11 -
achieve economical original installation, future system
expansion and retrofitting existing systems with a
minimum capital expense and down time. Although
particularly suited f or V . O . C . abatement of exhaust air
5 from paint spray booths for which they were designed,
the method and apparatus of the present invention are
potentially useful for V.O.C. destruction in exhaust air
from many other laboratory and industrial processes, for
example plating, phosphating and other bath-type
10 treatment processes and the manufacture of powdered
resins and other resin processing operations. Moreover,
the present invention is potentially useful for
destroying a wide variety of V. O . C. s other than those
used commercially in paint solvents, for example various
15 undesirable hydrocarbons and harmful carcinogens.
Objects, features and advantages of the
present invention are to provide a method and an
apparatus rOr V. O . C. air pollution abatement which
20 overcome the disadvantages of prior art techniques for
V. O. C. pollution abatement in exhaust gases; which
effectively and efficiently, both in cost and result,
abate, reduce and/or destroy volatile organic compounds
bef ore being exhausted into the environment; which
25 effectively destroy volatile organic compounds in
exhaust gases to levels well below current accepted
6tandards; which facilitate installation, operation and
maintenance at relatively low cost; which provide fast
and cost-efficient installation, part replacement,
30 system expansion and retrofitting with low capital
expenditures and short down times; which do not produce
any detectable NOx, carbon monoxide, and/or ozone or
other noxious gases exhausted to the atmosphere or
undesirable final reaction products such as nitrogen
35 oxides and nitrous acid which would either remain in the
~ 2 1 78258
p-308 TRI-~RR - 12 --
system and/or which would require off-site ~ pos~l
treatment; which reduce carbon bed absorber size,
capacity and cost, as contrasted to prior art carbon
beds used either with or without subsequent
5 incineration; which operate on a continuous basis with
high volumes of contaminated air and eliminate batch
processing; which do not require high oxidation
temperatures and are energy ef f icient; and which are
particularly cost-effective and efficient for V.O.C.
10 pollution abatement in industrial processes involving
large quantities of V.O.C.-laden exhaust air,
particularly automotive and other large industrial paint
shops and the like.
BRIEF DESCRIPTIQN OF THE DRAWINGS
A preferred exemplary ~rho~;r~nt of the
present invention will hereinafter be described in
20 conjunction with the a~pended drawings, wherein like
designations denote like elements, and:
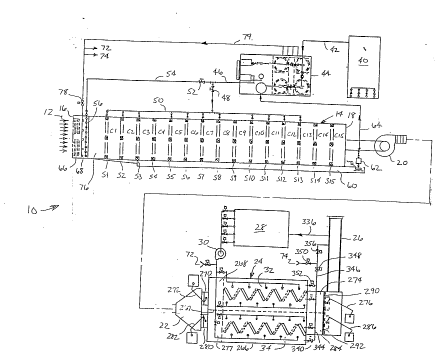
Figure 1 is a schematic diagram of a V. O . C .
abatement system of the present invention that is useful
25 in carrying out the method of the invention;
Figure 2 is a diagrammatic view of a primary
treatment tunnel of the abatement system of Fig. 1
showing the location and relative positioning of the
3 0 various components used in the tunnel;
Figure 2A is a perspective view of an
ultraviolet lamp and deflector used in the tunnel of
Fig. 2;
2 1 78258
P-3 0 8 TRI-MARK - 13
Figure 3 i5 a schematic diagram of a liquid
subsystem used in the abatment system of Fig. 1 to
generate activated fog and continuously recirculate
liquid through the system;
Figure 4 is a sectional view through a sparger
tank of the liquid subsystem of Fig. 3;
Figure 5 is a view, partly broken away and in
10 section, of a cell for generating activated air in the
systcms of Figs. 1 and 3;
Figure 6 is a perspective view of a baffle
plate subassembly of the generator cell of Fig. 5;
Figure 7 is a schematic view of an air f low
system f or regenerating carbon beds and exhaust
recirculation in the abatment system of Fig. 1; and
2 0 Figure 8 is a schematic view of regeneration
piping for the carbon beds of Fig. 7.
DFTATTT~n DEs~`-RTpTIoN
Overview of the V~. Q . ~ . Ah~teme~t Svstem
Fig . 1 schematically shows a pref erred
Pmhnrl;r-rlt of a V.O.C. abatement system 10 of the
present invention as it would be used for h~n~ll;ng large
volumes of V.O.C.-laden process air exhausted from large
automotive paint spray booths. As shown in Fig. 1, a
V. O. C . -laden air stream 12 is drawn through a primary
treatment tunnel 14 between an inlet 16 and an outlet 18
35 using a large centrifugal blower 20. Tunnel 14
- 2 ~ 78258
P--3 0 8 TRI-~ARK -- 14
continuously treats the air stream to destroy the
V. 0. C. s within the air stream by oxidative deqradation
and photolysis. In general, this is accomplished within
tunnel 14 by mixing the air stream with activated air
5 and by direct exposure of the air stream to ultraviolet
light. Blower 20 pushes the air exiting tunnel 14,
along with any r --i nin~ V.O.C.s, into an expansion
chamber 22 and then through a carbon bed
absorber-reactor system 24 which exhausts to the
10 environment through exhaust stack 26.
As will be described below in greater detail,
an activated air generator 28 produces activated air
that is supplied via a second blower 30 to carbon bed
15 system 24 and the upstream end of tunnel 14. Carbon bed
system 24 comprises a pair of separate and isolated
carbon beds 32, 34 so that when one of the carbon beds
is on-line in the process and treating exhaust air from
tunnel 14, the other carbon bed is being regenerated
20 using the activated air from generator 28. The
activated air from generator 28 contains a high level of
oxidants which desorb and regenerate the off-line carbon
bed .
PrimarY Treatment Tunnel
Referring back to the primary treatment tunnel
14, activated air containing a high level of oxidants is
fed from a second activated air generator 40 via pipe 42
30 to a sparger tank farm 44 which, using ultraviolet
radiation, generates an aqueous solution laden with
newly-generated and reactivated oxidants. The oxidant
laden solution is then fed via pipe 46, solenoid
operated valve 48 and header 50 to various selected
35 modular sections Sl through S15 where it is introduced
-
2 1 78258
p--308 TRI-~$~RK - 15 -
as an activated f og into various selected tunnel
chambers C1 through C15 to maintain an oxidant-enriched,
high-humidity environment within tunnel 14. This
oxidant-enriched, high-humidity environment performs
5 various important functions, as will later be described
in greater detail. Oxidant laden solution from sparger
tank farm 44 is also fed via pipe 46, solenoid valve 52
and pipe 54 to an array of nozzles 56 which also provide
an oxidant-enriched, high-humidity environment
10 downstream of a set of primary particulate filters 66 at
the inlet 16 of tunnel 14. Also located downstream of
inlet 16 is an array of activated air nozzles 68 that
are connected to a tap 74 to receive partially spent
activated air exhausted from the regeneration of carbon
15 ~ed system 24. In this way, any V.O~C.s contained in
this regeneration exhaust gas will be recirculated
through abatement system 10. These nozzles also receive
activated air vented from sparger tank farm 44, as will
be described below. Further, these nozzles are also
20 connected to a second tap 72 that can be used to provide
newly generated activated air from generator 28 in the
event greater oxidative activity is re~uired in tunnel
14 .
Activated aqueous solution introduced into
tunnel 14 via nozzles 56 and nozzle header 50, which
precipitates, condenses and/or is filtered out of the
air stream 12, is collected in a supply tank 60 from
which it can be recirculated via a pump 62 and a pipe 64
back to tank farm 44 where it is remixed with activated
air from generator 40 and exposed to ultraviolet
radiation to provide a continuous recirculating supply
of oxidant enriched fog to tunnel 14.
2 1 78258
.
P--308 TRI-NARK - 16 -
Advantageously, each of the modular sections,
S1-S15 can be fully or partially constructed off site.
The modular sections Lnclude framing, structural
members, plumbing, fittings and some electrical wiring
5 for various functional components, i . e., ultraviolet
lamps, mist nozzles, Viledon filters, carbon filters and
catalyst plates, some of which can also be preassembled
in the modules. Sparger tank farm 44 and carbon bed
system 24 can also be partially assembled off site as
10 modules. External housing panels for tunnel 14 are
fabricated and erected on site, preferably of stainless
steel sheet metal to form chambers C1-C15. After
completing all of the sheet metal fabrication and
ductwork, the necessary piping, valving and electrical
15 wiring connections are made and the system cleaned and
flushed before final installation of nozzles and
ultraviolet lamps.
Although an understanding of the present
20 invention will be more fully apparent from the detailed
descriptions to follow, in general, the V.O.C. air
stream 12 entering tunnel 14 is filtered at filters 66
to remove particulate paint and other particles. It is
then blended with the oxidant-enriched fog from nozzles
25 56 and with gaseous activated air from nozzles 68. The
~ixture then enters an expansion chamber 76 which allows
the air stream to egualize and load the unit to a
uniform flow. As the air stream continues moving from
left to right as viewed in Fig. 1, it passes through the
30 series of treating chambers, C1 through C15, defined
between corr~cpnn5in~ modular sections S1 through S15,
where the V. 0 . C . s are at least partially destroyed by
photodecomposition and oxidative degradation.
2 1 78258
P--308 TRI-MARX - 17 -
As the V.O.C.-laden stream 12 passes through
chambers S1 through S15, it is repeatedly exposed to
ultraviolet radiation in the oxidant-laden,
high-humidity environment. Activated air fog is
5 repeatedly replenished via header 50 in selected modular
sections S1 through S15. As the V.O.C. laden air stream
moves through various f ilters, precipitators and
coalescers, particulate water, together with
water-soluble compounds, are collected in supply tank
10 60. Preferably, selected coalescers are perforated
plates coated with a catalyst, such as titanium dioxide,
which provides hydrogen f or production of hydroxyl and
other oxidants to thereby enhance the oxidative
degradation of V.O.C.s. The perforated plates, and to
15 a lesser extent the filters, also act as scrubbers to
mix and bring the activated air fog, oxidants and
V . 0 . C. s in intimate contact with one another .
Pref erably, various other components in the system are
made of PVC to serve as catalysts.
Water from supply tank 60 is recirculated into
the sparger tank farm 44, mixed with the activated air
from generator 40, exposed to further ultraviolet
radiation, and reintroduced into tunnel 14 . Except f or
25 high humidity vapor discharged from the tunnel, together
with r.or-in;n~ V.O.C.-laden air, water and any
water-soluble in~prr~ ries from V.O.C. reactions are
recirculated in a substantially closed system until all
remaining intermediate reaction products are reduced to
30 water and/or carbon dioxide.
Activated Air
-
2 1 78258
P-308 TRI-~ARK - 18 -
As will be described in greater detail below,
the activated air supplied by generzltor 40 is produced
by exposing humid air to ultraviolet radiation to
produce oxidant radicals, pref erably under turbulent
5 flow conditions and in the presence of a catalyst to
enhance oxidant generation and produce additional
oxidants. Normally, plant air is used as the fresh air
supply for generator 40 with a humidity level of 85%
being typical. If necessary, humidity can be introduced
10 into the air at generator 40 by, for example, wicking
water into the plant air. The ultraviolet light
preferably includes 184.9nm and 254nm wavelengths, which
can be achieved by enclosing an ultraviolet lamp in a
suitable quartz lens, as will be described below.
Table 2 lists the relative percentages of the
various oxidants and other constituents believed to be
in the activated air. The percentages given indicate
the relative amounts in the approximately 20% of the
2 0 activated air that includes oxygen and oxygen bearing
compounds .
TAB~E: 2
Percent by Volume of Air
Constituent ~xcludinq Nitroqen
Nitrogen Dioxide (NO~,) 20 . 1%
Atomic Oxygen (I) 24 . 4%
3 o Hydrogen Peroxide (H22) 212 . 6%
Hydroperoxy Radical (HO2) 229 . 4%
Hydroxyl Radical (OH) 26. o%
Oxygen (2) 45 5%
Other Oxidants
such as NO2, N2O <1. 0%
Other gases such as
NH2, NH3, C2, N2, HCN, CI <1.0%
As Table 2 above indicates, even though the
40 ultFaviolet light includes the 184.9nm wavelength that
~ 2 1 782~8
P-308 TRI~ RK - l9 -
is known to cause formation of ozone (03) in air, no
ozone is present in the resulting activated air produced
by generator 40. This is believed to result from the
presence of humidity in the supply air which provides a
5 hydrogen rich environment that allows any ozone to
almost immediately split and form hydroxyl radicals.
This is also believed to result from photolysis of the
ozone caused by the 254nm ultraviolet light.
When the activated air is generated by
exposure to ultraviolet radiation in the presence o~ a
catalyst, such as polyvinylchloride, it is believed that
the following additional radicals are produced:
TABLE 3
RA~i;cals
C2Cl-
cl-
Cl2
ClOH-
H203Cl-
H2Cl-
HOCl-
These radicals further help split the ozone, freeing up
oxygen that can then combine with available hydrogen to
form the highly active hydroxyl radicals.
3 0 Without wishing to be limited to any
theoretical explanation, it is believed that, by
splitting up the ozone almost as soon as it is
generated, the oxygen from the ozone is available to
f orm hydroxyl radicals that have a higher oxidation
potential and are therefore more effective than ozone at
destroying the V. o . C. s . In this way, the activity level
of the activated air can be PnhAnf Pd. Furthermore, the
oxidants produced are negatively charged, and this is
~ 21 78258
P--3 o 8 TRI-MARK - 2 0
believed to result in l~nhP~nr~rl degradation of the
V.o.C.s. Preferably, the relative humidity of the plant
or other fresh air used to generate activated air is at
least 25% and, more preferably is within the range of
75-100% and, even more preferably is in the range of 90-
100% .
The activated air is then dissolved as best as
possible into an aqueous solution at sparger tank farm
44. The resulting activated aqueous solution is then
introduced into tunnel 14 as an activated fog that mixes
with the V.O.C.-laden air stream 12 passing through
tunnel 14. The air stream is kept at a high level of
activity via direct ultraviolet radiation in the high
humidity environment. Photodecomposition initiates
cleaving of the V.O.C.s and oxidative degradation begins
in at least the f irst chamber, Cl . As the air stream
moves through the tunnel, cleaving of the compounds and
oxidative destruction continue at an accelerated rate as
2 0 reaction products in solution are f iltered and
precipitated out of the air stream and returned to
supply tank 60.
As indicated above, V. 0 . C. destruction
according to the present invention is believed to occur
by a combination of two phF~nn-~n~ ~ namely oxidative
degradation of the V. o . C . s by oxidants contained in the
activated air in gaseous and fog states, and by
photolysis occurring simultaneously and undoubtedly
synergistically, in tunnel 14 due to repeated exposure
of the V. o. C . -laden air stream to ultraviolet radiation
in a high-humidity environment. Consequently, tunnel 14
would be useful in and of itself to provide acceptable
V. 0 . C. abatement without further treatment at carbon
beds 32, 34.
~ 2 1 78258
p--308 TRI-MARK - 21 -
Tunnel Chambers
In general, tunnel 14 incorporates particulate
filtration, misting or fogging, ultraviolet light
5 activation, coalescers, carbon filters for surface
reactions, and preferably one or more catalysts. One or
more of these functions is incorporated in each of the
modular sections S1 through S15. Further, these
functions are carried out in a predet~rm;n~d sequence so
10 that the processes and reactions occurring in chambers
C1 through C15, individually and in se~[uence, optimize
V. O . C . destruction occurring in tunnel 14 .
As shown in Fig. 2, the modular sections S1
15 through S15 in tunnel 14 are as follows:
Section S1: Mist nozzles, mixing cones, and
ultraviolet lamps.
Section S2: Ultraviolet lamps and mist nozzles.
20 Section S3: Ultraviolet lamps and mist nozzles.
Section S4: Viledon filters and ultraviolet lamps.
Section S5: Carbon filters, ultraviolet lamps and
mist noz z les .
Section S6: Ultraviolet lamps and mist nozzles.
25 Section S7: Perforated catalyst plates, ultraviolet
lamps, and mist nozzles.
Section S8: Perf orated catalyst plates, ultraviolet
lamps, and mist nozzles
Section S9: Viledon filters and ultraviolet lamps.
30 Section S10: Carbon filters, ultraviolet lamps and
mist nozzles.
Section S11: IJltraviolet lamps and mist nozzles.
Section S12: Ultraviolet lamps and mist nozzles.
Section S13: Perforated catalyst plates, ultraviolet
lamps, and mist nozzles.
2 1 78258
P--3 0 8 TRI -MARK -- 2 2
Section S14: Double Viledon filters and ultraviolet
lamps .
Section S15: Carbon f ilters and ultraviolet lamps .
The illustrated Pmho~l;r^nt is based on an air
stream flow rate of 22,500 ACFM to 36,000 ACFM at an
average l,vt:la~y of 22 lbs. per hour to reduce V.O.C.
concentrations from upwards of lOOppm to no more than
12ppm per volume, which is less than half the current
EPA standards of 25ppm. This system is manufactured in
modular sections so that it can be easily sized for any
flow rate or volume of V.O.C.s. For example, for
smaller flows (22,500 to 36,000 ACFM), a single
generator 40, tank farm 44, and tunnel 14 can be used.
For larger flows (50, 000 ACFM), a pair of tunnels 14
could be connected in parallel and run from a single
generator 40 and tank farm 44. For very large flows
(72 , 000 ACFM), a pair of tunnels could be used with a
pair of generators 40 supplying activated air to tank
20 farm 44 at the rate needed to provide a suitable level
of activated air within the water provided to the two
tunnels. For a capacity of 36,000 ACFM, tunnel 14 is 12
ft. wide, 12 ft. high, and 56 ft. long, preferably made
into two modular sections, each 28 ft. long. The walls
25 and roof are st:l;n~ steel, welded air tight.
Entrance sections are separ~tely fabricated to
house filters 66, activated air nozzles 68, fog nozzles
56, and expansion chamber 76. In the ~rh~;r-nt
30 described, inlet 16 is designed so that the
cross-sectional velocity does not exceed 450 ft. per
minute and three separate f ilter sections are used to
progressively filter out particulate paint, e.g., the
average ef f iciency of the f irst f ilter is seventy-two
35 percent (72%) on paint particles of 4 micron size and
78258
P-308 TRI-MARK - 23 -
greater, the second filter has an average efficiency of
eighty-five percent (85%) on paint particles of 2 micron
size and greater, and the third filter had an average
efficiency of no more than ninety-six percent ~96%) on
paint particles of 1 micron size and greater. All of
the f ilters were made of synthetic organic f ibers to
operate at one-hundred percent ~1009G) relative humidity
withcut deterioration or chPr~r~;nq of the fibers. The
filters can be built up of sections, about two feet by
two feet, two to four inches deep, to fill the cross
section of the tunnel, e.g., six filters across for a
width of 12 ft. and six filters high for a 12 foot
height. Suitable filters are available from Eaton Air
Filter of 2338 Cole Street, Birmingham, Michigan 48009.
Similar filter sections are typically used in prior art
carbon bed systems with incineration.
The array of activated air nozzles 68 consists
of forty-eight nozzles capable of delivering up to about
150 cubic feet per minute of air from tank farm 44, tap
74, and/or generator 28 via tap 72. A normally open,
electronically controlled valve 78 is used to control
the flow of air to nozzles 68. The array of fogging
nozzles 56 at inlet 16 is designed to instantly achieve
high humidity in expansion chamber 76, for example by
using 192 fog nozzles such as stainless steel nozzles
with ruby orifice to produce a fog, ASI #006 at 0.033
gallons per minute at 1, 000 psi. Expansion chamber 76
may be about two feet long to allow the air stream 12 to
equallze and load the system to a uniform air flow.
In each of the Ilwdular sections Sl through
S15, the lamp portion of the modular section includes an
array of 16 ultraviolet lamps 80, one of which is shown
35 in Fig. 2A. Each of the lamps include a lamp tube 80a
-
2 1 78258
.
P-308 TRI-MAR~C - 24 -
made from a high-output ozone ~ quartz that permits
transmission of radiation in the wavelength range of
from 184 . 9 nanometers to 254 nanometers. Lamps of this
type are available from Voltrac Technologies, 186
Linwood Avenue, Fairfield, Connecticut 06420-0688. The
lamps are ~our feet long and are mounted in a
two-section frame, each frame section holding eight
lamps extending in a vertical direction. Alternatively,
lamps 8 0 can be mounted such that they extend in the
horizontal direction, with access openings in the sides
of tunnel 14 to allow the lamps to be changed from the
outside of tunnel 14 wLthout having to shut down
operation of abatement system 10. Each lamp 80 also has
an upstream shield or deflector 82, cut from 3-inch PVC
pipe, leaving a 240 radiant section intact and a 120
window through which radiation is emitted into the
downstream chamber. The PVC deflector operates as a
catalyst, providing the various radicals listed
previously in Table 3 that aid in the destruction of the
V.o.C.s within air stream 12. Preferably, the PVC used
is Schedule 80 which will provide the desired radicals,
yet is dense and will not break down too quickly.
Section S1 includes an array of mixing cones
70 comprising 180 relatively small double-cone venturies
to optimize mixing of the V.O.C.-laden air stream 12
with the aqueous fog from nozzles 56 and gaseous
activated air from nozzles 68. These cones homogenize
the V. 0 . C. -laden air stream with activated fog and
activated air from nozzles 56 and 68, respectively, to
form a homogenous mixture.
The nozzle portion of sections S1-S3, S5-S8,
and S10-S13 each include thirty-five nozzles directed
3 5 downstream and mounted on seven vertical headers with
2 1 78258
P--308 TRI-~RK - 25 --
five nozzles per header. The seven vertical headers are
staggered between the eight vertical lamps, in the space
between the lamp shields. As the air stream moves
through tunnel 14, it is def lected around the lamps and
5 into the fog exiting from the nozzles. The nozzle
section is made of stainless steel mounted on stainless
steel struts. Again, the mist nozzles are of stainless
steel with ruby orifice to produce a fog, ASI #006 at
0 . 033 gallons per minute at 1, 000 psi.
In the f ilter and lamp sections S4, S9, and
514, the filter can be made up of 25 sections,
approximately 24" x 24" x 1" thick, mounted in stainless
steel frames and backed on both sides by stainless steel
15 wire mesh. A suitable filter material is Viledon, sold
commercially by Eaton Air Filter of Birmingham,
~ichigan. Preferably, the Viledon filters remove
particulate water and other particles down to at least
five microns in size and, even more preferably, down to
20 about one micron in size. The ultraviolet lamp portions
of these sections can be identical to the lamp array
previously described, and can be located immediately
downstream of the f ilters .
In each modular section 55, 510, and 515,
twenty-five coconut carbon filters approximately 24" x
24" x 1-7/8" thick are installed in a st~inlPcs steel
framework so that all of the air moving through the
tunnel 14 must pass through the carbon f ilters .
3 0 Pref erably the f ilters have interior cross-bracing and
are backed at the downstream side by a perforated
stainless plate that supports the coconut carbon filters
and ensures that all of the air moving in tunnel 14
passes through the carbon f ilters . In modular sections
s5 and 510, the ultraviolet lamp portion and the mist
-` 2 ~ 782~8
P--308 TRI-MARK - 26 --
nozzle portions are immediately downstream of the carbon
filters and have the construction previously described.
The perforated catalyst plate portions of
sections S7, 58, and S13 are each fabricated from 6iX
standard ferrous punch plates, each approximately 4 ' x
6', 16 gauge, perforated with %" holes, %" off center
and mounted in a frame to fill the tunnel. The
perforated plates are heavily coated with a high
hydrogen shift catalyst, for example, titanium dioxide
applied with an ultraviolet-resistant adhesive. Other
catalysts can be used, including a titanium-ferrous
catalytic mix, a copper zinc catalytic mix, a copper
silver catalytic mix, and copper zinc-silver catalytic
mix. The lamp and nozzle portions of sections S7, S8,
and S13 are of the same construction as set forth
hereinabove and are located just downstream of the
perforated catalyst plates. Preferably, only the
upstream surface of the plates are coated with the
catalyst, leaving the ferrous material of the inner
surface of the perforations and the backside of the
plates exposed . By providing an exposed f errous
surface, the ultraviolet light and peroxide radicals can
react with the iron in the plates to liberate hydrogens
needed to produce the highly active hydroxyl radicals.
With the arrangement of tunnel 14 described
hereinabove, as soon as the V.O.C.-laden air stream 12
enters tunnel 14, wetting of the V. O . C. s and the
3 0 oxidants and some oxidative degradation begins
immediately upon air stream 12 hPrn~;ng mixed with the
activated air from nozzles 68 and the activated fog from
nozzles 56. This step may be viewed as a pre-treatment
for further oxidative degradation and photolysis in
tunnel 14 and may not be required for some applications.
2 1 782~8
P--308 TRI-MARK - 27 -
In the illustrated embodiment, this initial step
provides a convenient and preferred location for
recirculating some of the active solution in supply tank
60 in a closed loop through sparger tank farm 44 and
tunnel 14. Similarly, inlet 16 of tunnel 14 provides a
preferred location for introducing vented activated air
from tank farm 44 and also for continuously
recirculating partially spent activated air exhausted
from carbon bed system 24.
As the air stream progresses through chambers
C1 through C15, it is progressively and continuously
treated by direct exposure to ultraviolet light and
oxidant laden fog. The ultraviolet radiation in chamber
Cl not only continues to activate the fog by
regenerating oxidants, but it also begins photolysis of
the V.o.C.s, at least initiating wetting, cleaving, and
photod~~ osition of the V.o.C.s. Simultaneous
oxidative decomposition of the V.O.C.s occurs due to the
high level of oxidants in the activated fog. Further
oxidative degradation and photolysis occurs as air
stream 12 moves through chambers C2 and C3.
As the stream passes out of chamber C3 and
through the Viledon filters in section s4, the filters
remove particulate water, together with water-soluble
int~ te radicals, compounds and the like, and the
filtrate is collècted in supply tank 60. The Viledon
filters allow only humidity, gaseous V.o.C.s, and
activated air in gaseous form to pass into chamber C5
where the air stream is again irradiated by ultraviolet
radiation still in a high-humidity environment but
without dense activated fog. This is believed to
enhance more direct irradiation of the V. 0 . C. s due to
reduced scattering and absorbtion of the radiation as
-` - 2 1 78258
.
P--308 TRI~ - 28 --
compared to a dense fog environment. However, the
humidity in chamber C5 is sufficiently high (95-10096
relative humidity) to cause formation of new hydroxyl
radicals from any ozone generated therein by the
5 ultraviolet lamps. ~loreover, it ls believed that
oxidants are continuously deposited on the carbon filter
surfaces in Section 55 at the downstream end of chamber
C4 for surface reaction with photod~ osed V.o.C.
products. To some extent, these surface reactions may
10 be influenced by ultraviolet radiation reaching the
carbon filters.
In chambers C5 and C6, the air stream again
moves through a high-humidity, dense fog environment
15 with prolonged activation by ultraviolet radiation.
This causes further oxidant generation and photochemical
and oxidative ~ - 2 ition of the V . o . C . s . As the air
stream reaches section s7 at the downstream end of
chamber C6, the catalytic plates serve as scrubbers to
20 force activated fog, oxidants, and V.O.C.5 into intimate
contact with one another. Activated fog also condenses
on the catalytic plates to remove water-soluble
compounds, intermediate radicals and the like. The
reactions occurring have been found to be ~nh~n~ed by
25 the catalysts present. It is belicved that the
catalysts cause a high hydrogen shift which promotes
breakdown of the V.o.C.s, oxidative degradation and/or
oxidant generation.
3 o Chambers C7 and C8 are also highly active due
to ultraviolet radiation combined with additional
infusions of activated fog. Cleaving, wetting and
photochemical and oxidative decomposition continue,
probably at an accelerated rate, before being f iltered
35 at section S9. In chamber C9, as in chamber C4, the air
2 1 78258
P-308 TRI-MARK - 29 --
stre2m enter6 in a high-humidity condition but without
dense active fog. As in chamber C4, ultraviolet
irradiation of the V.o.C.s is more direct and efficient.
Fresh oxidants are produced, photochemical and oxidative
5 decomposition continues, but ozone production is still
inhibited. Carbon filters at the upstream end of
section S10 perform as in section S5, followed by a high
level of activity in chambers C10-C12, as in chambers C5
and C6, due to replenished activated dense fog and
10 repeated ultraviolet radiation . As the V. 0 . C.
concentration levels continue to drop, photoche_ical and
oxidative decomposition undoubtedly are more effective
and efficient as the V.O.C.s migrate through the
activated f og and are exposed to ultraviolet radiation
15 and oxidants in chambers C10-C12.
At section S13, as at section 57, the air
stream, oxidants and fog are scrubbed and coalesced in
the presence of a catalyst by perforated catalyst plates
20 with any crn~pnq~tion flowing into tank 60. Extended
exposure to ultraviolet radiation and dense active fog
is repeated in chamber C13, as in chamber C8,
progressively achieving still further photorh~mir~l and
oxidative V.O.C. destruction before filtering at section
25 S14.
At section S14, particulate water is removed
by Viledon filters, along with water-soluble reaction
radicals, compounds and the like. In chamber C14, as in
3 0 chambers C4 and C9, the air stream is exposed to
ultraviolet radiation in a high humidity environment,
but without dense fog before passing through the carbon
filter in section S15. Chamber 14 therefore provides a
direct and efficient irradiation of the r~;nin~
35 V. o . C . s, not only in the air stream but also on the
2 1 782~8
P--308 TRI-MAR~ - 30 --
surface of the carbon filters in section 515.
Reactivation of the air stream in chambers Cl4 and C15
by ultraviolet radiation continues V. 0 . C . destruction
and provides additional oxidants that aid in any further
5 V. 0. C. oxidative destruction required at the carbon bed
system 24.
As will be apparent from the above process
description, repeated ultraviolet radiation of the
10 V. 0 . C . laden air stream 12 occurs in a high-humidity
environment, whether as a dense fog, as in chambers C1-
C3, C5-C8, and C10-C13, or as a gaseous humidity, as in
chambers C4, C9, C14, and C15. This insures a
continuously replenished supply of oxidants for reaction
15 with photochemically decomposed V. 0. C. products .
Additionally, the sustainable formation of ozone, as
would be expected with dry air, does not occur in the
high humidity environment maintained throughout tunnel
14. Experimental analysis has not revealed any
20 detectable ozone exiting from tunnel 14. Analysis
conf irmed that the exiting gas from chamber Cl5 does not
contain ozone. It is believed that any ozone produced
is quickly decomposed by one or more of a variety of
rhGn' -n~, including the presence of high humidity,
25 photolysis by the 254nm ultraviolet radiation, and
cleavage due to chlorine and other radicals that were
liberated from the catalysts as a result of being
exposed to the ultraviolet light.
It has been de~tnn;ne~l empirically that the
present invention effectively and efficiently destroys
V. o . C. s to levels well below the current accepted
standards as indicated in Table 4.
~ 2 1 7825
P--308 TRI-~IARK -- 31 _ 8
T}~3LE 4
SYSTEIS INLET' BEFORE CMBON BED2 OUTLET
F<EDUCTION CARBON
BED
5 SWlthout
tunnel 14 56 ppm 56 ppm 50,000 ppm3 14 ppm
('3:1) 25,000 ppm3
With tunnel 14
10 Xylene s6 ppm 30 ppm 0 130 ppm3 0.688 ppm
~ Mea~3ured by Photorae loni3ation Lnstrument averaged over A 33 hour
run.
l~easured by an ~ r9~ 1 lal oratory, Swanson Environmental Inc.
samplen were removed after 8 hours of V.O.C. spray prLor to
regeneratLon. The amounts ~3hown therefore reprenent the re~ldual
~Imount left on carbon before the regeneration process.
Although the present invention preferably utilizes
further oxidative degradation in carbon bed sy5tem 24,
it will be apparent from Table 4 that the treatment in
tunnel 14 alone resulted in a reduction from 56 parts
per million to 30 parts per million which, even without
subsequent treatment in the carbon bed system 24, may be
more than adequate to meet applicable standards.
Liauid Svstem
Referring now to Fig. 3, fresh water,
preferably filtered tap water, is supplied to tank 60
through a solenoid valve 102 and inlet pipe 104. Valve
102 is opened and closed by a suitable liquid level
control, including a float-operated liquid level sensor
106 to initially fill tank 60 and maintain a liquid
level between an upper limit 108 and a lower limit 110.
The upper limit 108 is established by an overflow outlet
pipe 107 which is also connected to a normally-closed
solenoid valve 109 so that tank 60 can be drained.
Fresh water can also be supplied to tank 60 via a manual
2 1 78258
P--308 TRI-~ C - 32 -
valve 112. Liquid from tank 60 is supplied to four
low-pressure sparger tanks 114, 116, 118, and 120 in
tank farm 44 by pipe a 122, pump 62, normally-open
solenoid valves 126, 128, a low-pressure filter 130,
5 normally-open solenoid valve 132 and pipes 64 which
discharge liquid into the top portion of tank 114.
Filter 130 can be bypassed by a normally-closed solenoid
valve 138 and drained into supply tank 60 via a
normally-closed solenoid valve 140. Tank 114 can also
10 be supplied directly with fresh tap water via a
normally-closed valve 141 to f lush out the system .
Liquid pumped into tank 114 is transf erred by
gravity to tank 116 by a pipe 142, from tank 116 to tank
118 by an overflow pipe 144, and from tank 118 to tank
120 by a pipe 146. Pump 62 is a low ~LeS2~UL~:, high
capacity pump. Overflow from tank 120 is returned to
tank 60 via overflow pipe 152 and a normally-open
solenoid valve 154. Each sparger tank 114-120 has a
sight tube 156 so that an operator can visually monitor
the liquid level in each of the tanks. Sparger tanks
114-120 can be drained into tank 60 via drainpipe 189
and normally-closed valves 190, 192 when it is desired
to purge or f lush the system .
Liquid from tank 120 is delivered to nozzle
array 56 and nozzle header 50 through piping 172, filter
176, pump 182, and normally open valves 170, 174, 178,
180, 184, 48 and 52. Filter 176 can be bypassed via a
normally-closed valve 186 and drained into tank 60 via
normally closed valve 188. Preferably, filter 176 traps
particulates larger than l micron to ensure that
particulates do not enter tunnel 14 and prevent clogging
of the nozzles. Header 50 feeds nozzles Nl-N3, N5-N8,
35 and N10-N13 in corresponding modular sections, Sl-S3,
2 1 78258
P--3 o 8 TRI -MARK -- 3 3
55-S8, and S10-S13, as previously described ln
connection with Figs. 1 and 2, to maintain a high
humidity environment throughout tunnel 14.
S As generally described in connection with the
overall system of Fig. 1, the liguid supplied to nozzle
header 50 and nozzle array 56 is an aqueous solution
containing dispersed bubbles of oxidant-laden activated
air from generator 40. As shown in greater detail in
Fig. 3, compressed air is delivered to generator 40 from
a source of fresh plant air 194 through a filter and
coalescer 196, a compressor 198, a variable Venturi flow
regulator 200 and normally-open solenoid valve 202.
Filter 196 is used to remove particulate moisture and
oils down to 0. 01 microns in size. Air entering
generator 40 passes serially through a series of
individual activated air cells 210 (Figs. 3, 5 and 6)
where it is exposed to ultraviolet radiation to generate
the various oxidants. Activated air from generator 40
2 0 is then delivered via header pipe 42 to each of the
sparger tanks 114-120 via an associated variable flow
regulator 212. Regulators 212 control the activated air
entering each tank and balance the distribution to all
of the tanks.
Since tanks 114-120 are of similar
construction, only tank 116 will be described in detail.
Compressed activated air, at relatively low volume and
low pressure, enters the lower portion of tank 116
through an inlet tube 214 and a spider of sparger pipes
216. Sparger pipes 216 cause the activated air to be
dispersed in minute bubbles and agitate the liquid in
tank 116. As shown in broken lines in Fig. 3 and in
cross section in Fig. 4, four ultraviolet lamps 218 are
mounted on the top of tank 116 and extend downwardly
2 1 78258
P--308 TRI-MARK - 34 -
through a major portion of the tank, terminating just
above the spider sparger tubes 216. Lamps 218 also
consist of an ultraviolet lamp tube, protected from the
liquid in tank 116 by a separate quartz lens tube 217
5 and can be identical to lamps 80 used in tunnel 14.
Each of the quartz lens tubes 217 are preferably made
from the same high-output ozone LEI material used to make
lamps 218. For purposes of simplification, an
electrical power source 219 is illustrated with
10 connections to only two of the lamps 218 in each of the
tanks 114, 116, it being understood that source 219 is
connected to all of the lamps in all of the tanks.
As the activated air from spider sparger tubes
15 216 bubbles somewhat violently and migrates upwardly
through tank 116, the activated air bubbles are exposed
to further ultraviolet radiation to generate and
reactivate oxidants. Oxidant generation and
reactivation occurs not only as fresh activated air is
20 introduced into each tank, but also as activated liquid
moves progressively through the tanks. Additionally,
ultraviolet radiation of the aqueous solution in tanks
114-120 is also believed to contribute to total V. O. C .
destruction by promoting further int~rr~ ry and
25 radical reactions which occur after photochemical
degradation of the V . o . C. s in tunnel 14 .
As mentioned above, relatively large
quantities of water are required to maintain high
30 humidity in tunnel 14. In the illustrated (~mho~;r-nt~
tank 60 holds 1,028 gallons, with pump 62 providing 60
gallons per minute at low pressure and pump 182
delivering 12.7 gallons per minute at 1,000 p.s.i. For
supplying about 12 . 7 gallons per minute to pump 182,
35 tanks 114-120 may have capacities of about 113-200
2 1 78258
P - 3 0 8 TRI -~ARK - 3 5
gallons per tank, with compressed activated air from
generator 4 0 being introduced into each tank at a rate
of 1.5 cubic feet per minute at ten to fifteen psi per
minute. Progresslve circulation through tanks 114-120
as described herein insures an adequate supply of
freshly activated, oxidant-laden liquid required to
maintain the dense activated air fog and the high
humidity in tunnel 14. Most of the piping in the low
pressure portions of the system of Fig. 3 is made of PVC
which is believed to provide a catalytic action for
reactions occurring in the liquid as the liquid
recirculates from tank 60 through tank farm 44, resides
in tunnel 14 as a fog, and then is precipitated back
into tank 60.
As shown in Figs. 1 and 3, activated air that
collects at the top of each of the sparger tanks 114-120
is vented to a pipe 79 that supplies the activated air
to air nozzles 68 along with regenerated exhaust air
that is supplied from carbon bed system via tap 74.
Preferably about 100-120 cubic feet per minute is
delivered to nozzles 68, with tanks 114-120 supplying
about 12-16 cubic feet per minute at lOpsi and about 90-
100 ACFM through tap 74.
Activated Air Generator Cell ~
Referring to Figs. 5 and 6, there is
illustrated in greater detail one of the generator cells
210 in generator 40. Each cell includes an ultraviolet
lamp 220, which can be the same as lamps 80 in tunnel 14
and lamps 218 in tank farm 44. Each lamp 220 is
generally concentrically retained in a tubular outer
casing 221 of its associated cell 210. Compressed air
35 from generator 40 enters cell 210 through an inlet
2 1 78258
P--308 TRI-MARK -- 36 -
fitting 226 on an end cap 222 and is exhausted from cell
210 through an outlet fitting 228 on an end cap 224.
Lamp 220 is carried on a subassembly 230, slidably
received in casing 221 and comprising a plurality of
5 baffle plates 232 which provide a serpentine flow path
that introduces turbulence into the air stream f lowing
through cell 210. Baffles 232 are mounted on three rods
234 that are spaced apart and extend axially within
casing 221 about lamp 220. Each baffle 232 is generally
10 a circular disk having a cutaway flat edge 236 and the
baffles are mounted on rods 234 with alternating flats
23 6 facing in opposite directions to provide the
serpentine f low path . All of the baf f les 2 3 2, except
one end baffle 232 ', have a central clearance hole 238
15 through which lamp 220 is received, with one end of lamp
220 being received in and supported by a socket 240 on
end baffle 232 ' . Lamp 220 is energized at its other end
via t~rm;n~l~ 242, 244, wires 246, 248 and socket 250.
Casing 221 has an inside diameter-to-axial
length ratio of between 1 to 8 and 1 to 16, and
preferably about 1 to 12. To insure turbulent flow,
preferably the baffles 232 are equidistant and at a
distance of between one-half and one-times the inside
diameter of the casing 221. Preferably the area of the
cutaway flaps 236 is not greater than about 1096 of the
baf f le plate area to enhance turbulent f low and to
provide a scrubbing action. Preferably casing 221, end
caps 222, 224, baffles 232 and retainer rods 234 are
3 0 made of PVC f or the reasons set f orth above to resist
oxidation and also, it is believed, serve as a catalyst.
With this construction, the baffles 232 are cemented to
rods 234 and the subassembly 230 tacked to casing 221
with PVC cement.
2 1 78258
P--308 TRI~ 37 --
In one practical construction of the generator
cell 210, casing 221 is made from commercially available
PVC tubing having a nominal outside diameter of six
inches and, with end caps 222, 224 an overall length of
5 about 5-1/2 feet. The baffle s~h~c~mhly, also made of
PVC, had 14 baffles having a diameter of about six
inches and a thickness of 3/16 of an inch. About 3/8 of
an inch was removed to form flaps 236. A suitable
ultraviolet lamp tube having an overall length of about
10 61 inches is commercially available from Voltarc
Technologies of Fairfield, Connecticut.
A generator 40 of twelve of these cells
connected in series had an output of about 42 standard
15 cubic f eet per minute when operated with a compressed
air inlet pressure of about 20 psi. The activated air
produced by this generator is believed to typically have
same the oxidants as other radicals in the proportions
set forth in Tables 2 and 3. No bacterial maintenance
20 is required because of the biological treatment by
ultraviolet radiation by lamps 220 in cells 221, lamps
218 in tank farm 44, and the numerous ultraviolet lamps
in chamber 14.
Carbon Bed Reqeneration
In prior applications using carbon beds for
V.O.C. abatement, when low concentration, high volume
air is passed through the carbon bed, the V.O.C.s are
3 o absorbed into the bed. The bed is later desorbed by
steam, etc., yielding a higher concentration, smaller
volume effluent which must then be further processed by
incineration or other solvent removal techniques. For
some prior applications, carbon bed desorption must be
35 performed offsite, and other prior applications require
" 2178258
P--308 TRI-MARK -- 38 -
offsite disposal of waste products. Moreover, when
using the method and apparatus of the present invention,
carbon beds may not be required for all applications
where treatment in tunnel 14 has reduced V . 0 . C . s
5 sufficiently meet applicable standards. When carbon
beds 32, 34 are utilized according to the present
invention, they may be constructed in a manner similar
to that of prior art carbon beds. However, the
utilization and function of carbon beds according to the
10 preferred omhoA;r^nt of the present invention differs
significantly from prior art carbon beds used only as an
absorber .
Referring back to Fig. 1, it will be
15 r ^-hP~ed that in the preferred Pmho~i -nt, the air
stream exiting tunnel 14 has just been reactivated with
ultraviolet radiation in chambers C14 and C15 and
filtered by carbon filters in modular section 15. The
air stream entering expansion chamber 22 is still highly
20 active with oxidants, together with some V.O.C.s and,
most likely, int~ te compounds resulting from
photochemical and oxidative degradation in tunnel 14.
Continued oxidant activity at a high level can be
maintained by using additional ultraviolet lamps in
25 expansion chamber 22.
Preferably two c2rbon beds 32, 34 of modular
construction and assembled off-site are used. During
final on-site as6embly, beds 32, 34 are enclosed by a
30 suitable external housing, generally designated at 266
and isolated from each other by an internal partition
267. An inlet chamber 268 for bed 32 is connected to
expansion chamber 22 via an inlet duct 270 and an inlet
damper 272. Exhaust from bed 32 to an exhaust stack 26
35 is through an outlet duct 274 and outlet damper 276.
2 ~ ~8258
P--308 TRI-MARK - 39 -
Similarly, an inlet chamber 277 for bed 34 is connected
to expansion chamber 22 via a duct 280 and damper 282
and bed 34 is exhausted to stack 26 through an outlet
duct 284 and outlet damper 286. Bed 32 is brought on-
line by opening dampers 272, 276 and closing dampers
282, 286 and bed 34 is brought on-line by opening
dampers 282, 286 and closing dampers 272, 276. Each of
the carbon beds 32 and 34 include a respective template
290 and 292 located just upstream of dampers 276 and
286, respectively. Each of the templates 290 and 292
provides a restriction in the flow path that results in
a backpressure within its associated carbon bed. This
backpressure equalizes the flow through the carbon
filters to help maximize the effectiveness of the carbon
beds. Each of these templates can be implemented using
a pair of perforated plates with the degree of alignment
of the perf orations being varied as necessary to obtain
the desired backpressure.
Referring also to the schematic of Fig. 7,
blower 30 draws fresh plant air 300 through a filter 302
and into generator 28 through a five-port manifold 304.
Each port of manifold 304 feeds a respective row of
seven serially-connected activated air cells 306 whose
exhaust streams flow through respective solenoid
operated valves 308 to a five-port exhaust manifold 310
and then through piping 312 to the inlet of blower 30.
Blower 30 delivers compressed air at about 300 ACFM to
activated air tap 72 via delivery piping 314 and
solenoid valve 316; to perforated tube arrays 320, 321
in carbon bed 34 via piping 314 and respective solenoid
valves 3I8, 319; and to perforated tube arrays 326, 328
in carbon bed 32 through piping 314 and respective
solenoid valves 322, 324. Preferably, each of the
35 perforated tube arrays 320, 321, 326, and 328 has
2 ~ 78258
P-308 TRI-MARK - 40 -
multiple rows of perforated outlet tubes, for example
arranged in a vertical array of three tubes 330, 332,
334, as shown in Fig. 8 for the array 328. This
arrangement uniformly disperses activated air into the
carbon beds during regeneration. In the exemplary
embodiment being described, each carbon bed 32, 34 can
be fabricated in two modular sections, each about 30 ft.
long x 10 ft. wide x 10 ft. high and finally assembled
on site end to end into a 60 ft. unit. The carbon bed
base material can be 500 cu. ft. of natural grain
coconut activated carbon shell.
As ~;~rll~s~cl above in connection with
generator 40, the generation of activated air is
preferably carried out using humid air. Thus, humidity
can be added to the fresh plant air 300 used by
generator 26. Optionally, the cleaned process air
exiting carbon bed system 24 can be used in lieu of
plant air 300 and can therefore be taken directly from
stack 26 via a supply line 336 shown in Fig. 1. This
cleaned process air is well filtered and rises to a
humidity level of approximately 95% after only three
minutes of operation of abatement system 10. Thus, the
cleaned process air neither requires added humidity nor
filtering and filter 302 can therefore be eliminated.
As shown in Figs. l and 7, regeneration
eYhaust gases from carbon bed 34 are delivered to supply
tap 74 through a pair of solenoid valves 340, 342,
exhaust header 344, piping 346 and solenoid valves 348,
350. Regeneration exhaust gases from bed 32 are
similarly delivered to tap 74 through a pair of solenoid
valves 352, 354, manifold 344, piping 346 and solenoid
valves 348, 350. Exhaust manifold 344 may also be
35 directly connected to stack 26 through piping 346, valve
~ 2 1 78258
P--308 TRI-NARK - 41 -
348 and a solenoid valve 356 for applications where the
regeneration exhaust is sufficiently clean for
exhausting it directly into the environment.
Advantageously, activated air supplied to the
carbon beds 32, 34 during regeneration not only desorbs
the carbon beds but also deposits oxidants on carbon bed
surfaces for further V.O.C. destruction by surface
reactions when the regenerated carbon bed is brought on-
line. Consequently, it is also desirable to recirculate
the regeneration exhaust gas from carbon beds 32, 34
through tap 74 and back into tunnel 14 to promote
continuing int~ ry reactions and destroy any
re--; n; n~ V. O . C . S and V. o . C. decomposition products .
Cells 306 in generator 28 can be constructed
substantially as described in connection with cells 210
in generator 40. Cells 306 are serially connected in
six respective rows so that the number of cells on-line
can be selected via valves 308 according to the system
demands for regenerating either cell 32 or 34 and also
supplying activated air via port 28 to activated air
nozzles 68 in tunnel 14. Blower 30 has a variable speed
control (vfd~ 370 so that the output from blower 30 can
be varied in accordance with 6ystem demands.
Photochemical and Oxidation Reactions and Results
Based on testing of abatement system 10, it
3 o has been demonstrated that the present invention
ef f ectively destroys V . o . C . s of the type encountered in
an automotive paint spray booth exhaust system. Much of
this testing was done to simulate a percentage ratio
based on a commercial unit which could handle 72,000 cfm
35 at 45 lbs. of V.O.C.s per hour. In order to get
2 ~ 78258
P - 3 0 8 TRI ~ K - 4 2
representative results for paint spray solvents, the
results shown in Table 4 are for a ratio of two parts
methylamylketone (l~AK) to one part Xylene. Although the
pathways of oxidative degradation and V.O.C. destruction
5 have not been completely elucidated, the various
reactions occuring for these solvents are known and the
results of Table 4 show that the present invention can
successfully reduce or destroy V. O . C. s .
As one example of V.O.C. degradation, the
following reactions show methane (CH4) reduction by
hydroxyl (Ho ) . As shown, this is accomplished by
replacement of each hydrogen atom. ~he first step i5
formation of methyl alcohol (CH30H or H-CH20H), followed
15 by formation of formaldehyde (HCl(OH)2) which, by 10s5 of
water gives CHlO or H CHO, and, finally, formation of
formic acid (CH(OH)3) which, by loss of water gives
CHO (OH) 2, HO-COOH, or H2CO3.
CH4 + HO -- C^H3 + H20
C^H3 + HO' - CH,OH
CH,OH + HO' -- C^H20~0H) + HzO
C^ H2 ( OH) + HO - C^ H2 ( OH) 2 + H20
C^H2(0H)2 + HO - CH~2 + H20
CH,02 + HO ~' CHZO + H20
CH20 + HO - C HO + H20
C^HO + HO' - HzCO2
H2CO2 + HO - HC^ 2 + H20
-
~ 2 l 782~8
P-3 0 8 TRI -~ARK - 4 3
HC^ Oz + NO' - N2 CO3
N2CO3 + HO- ~ HzO + COz
It should be noted that the a~ove reactions are
representative of hydroxyl intrusion only and that they
do not consider any reactions or ~nhAnr -nts of
reactions that may occur due to intrusion of the
ultraviolet light, other radicals and oxidants in the
activated air, as well as electron/photon transfer. A
similar process of chemical reduction to water and
carbon dioxide is believed and expected to occur with
similar compounds, such as: N-butyl alcohol; aromatic
hydrocarbons; mineral spirits; 1,1, 1 trichloroethylene;
BTX's in light ppm; methanol; MEK; V, I~, and P, Naptha;
N-butanol; perchloroethylene; and butyl ether.
As indicated earlier, it is strongly believed
that V. 0. C. destruction into harmless water and carbon
dioxide is achieved by the combination of two ~ht~nt -n
undoubtedly producing a synergistic effect. First,
ultraviolet radiation produces highly active oxidants
which then subsequently oxidatively degrade the V.O.C.s.
The oxidants are generated not only in the activated air
from generators 28, 40 but they are reactivated,
regenerated and freshly generated by ultraviolet
radiation in the sparger tank farm 44 and within the
numerous chambers in tunnel 14 having ultraviolet lamps.
Generation of the activated air is carried out in a high
humidity environment and using ultraviolet wavelengths
of 184 . 9nm to produce ozone and 254nm to help quickly
break down the ozone so that the liberated oxygen can
form highly active hydroxyl radicals. The generation of
activated air is also carried out in the presence of a
catalyst to help liberate hydrogen for hydroxyl radical
2 ~ 78258
P--3 0 8 TRI -MARX - 4 4
production. oxidants in the activated air from
generator 28 are deposited on surfaces in carbon beds
32, 34 during carbon bed regeneration and for surface
reaction with V. 0 . C. s when the carbon beds are brought
5 on-line in the process. These surface reactions can be
enhanced by additional ultraviolet radiation in
expansion chamber 22, ~ust before any ro~-;n;n~ V.O.C.s
in the air stream are passed through carbon beds 32, 34.
Secondly, direct ultraviolet photolysis of the V.O.C.8
10 causes a photochemical decomposition of the V.O.C.s,
with the resulting products interacting with the
oxidants and chemical intor~o~ tes constantly being
generated, reactivated and reacting throughout the
system.
It should also be apparent that ultraviolet
radiation for phot~do(-~~rosition and for oxidant
generation takes place under a variety of conditions.
In tunnel 14, ultraviolet radiation occurs in the
20 various heavily fogged chambers and also in chambers
where, after particulate water is filtered, only high
gaseous humidity is present in the chambers just before
the carbon filters. Residence time varies in different
chambers. Ultraviolet radiation impinges directly on
25 catalyst plates and other PVC components and on carbon
filters where surface reaction can occur. ~eavy
homogeneous mixing occurs at cone array 7 0 and heavy
scrubbing occurs at the perf orated catalyst plates .
Varying degrees of turbulence also exist in tunnel 14
30 due to the different filter arrangements and
particularly the perforated catalyst plates, as well as
the effect of the nozzle sprays and diversion around the
lamp shields. Experimentation has shown that oxidant
production, as well as V.O.C. destruction, is onh~nce~
35 by catalytic action provided, in the example described
2 ~ 782~8
P-308 TRI-MARK - 45 -
hereinabove, by the PVC. In addition to the lamp
shields and catalytic plates, various other internal
supports that are exposed to the ultraviolet lamps
within tunnel 14 can be made of catalytic materials.
5 For example, PVC pipe can be used in the frame structure
that supports the ultraviolet lamps and can be used for
piping of the activated air and the oxidant-laden
aqueous solution created in tank farm 44. Due to the
high level of oxidants present in the system, slight
10 oxidation of the PVC may also be occurring, with some
reaction products entering the system. Indeed, analysis
has yielded some reaction products confirming this and,
after extended use, a slight surface erosion can be
detected at some of the PVC components.
When either carbon bed 32 or 34 is on-line,
oxidants are continuously deposited onto the surface of
the carbon bed for continued destruction of any V.O.C.s
r~-;n;n~ in the air stream passing through the carbon
20 bea. During regeneration, oxidants produced by
activated air generator 2~3 are not only deposited on the
carbon bed for V.O.C. destruction when the carbon bed is
brought on-line but, additionally, regeneration exhaust
is recirculated back into tunnel 14 for further
25 reactivation. At sparger tank farm 44, an aqueous
solution is irradiated as the air bubbles through the
sparger tank and the solution moves from tank to tank
and then into tunnel 14 so that the f og or mist
delivered into tunnel 14 is highly active with oxidants.
3 0 Particulate water f iltered out of tunnel 14 and
otherwise precipitated into tank 60 is believed to still
be suf f iciently active to contribute to reactions
occurring in tank 60. Dissolved oxidants and
intermediary compounds are recirculated through sparger
- 2 i 78258
P-3 08 TRI--MARK -- 4 6
tank farm 44 to be exposed to ultraviolet radiation and
recirculated into tunnel 14.
some of the oxidants produced by ultraviolet
radiation at generators 28, 40 and in sparger tanks 44
and in tunnel 14, are potent oxidizing agents but their
life as a radical, for example the hydroxyl radical, may
be relatively short, liPr~n~l; ng on the competition for
reaction and recombination throughout the system.
lo Hence, for high-volume industrial processes, oxidant
production is continuous throughout the system by
repeated exposure to ultraviolet radiation and by the
introduction of fresh oxidants from generators 28 and
40. Also, the various oxidants available in abatement
system 10 are available not only for direct reaction
with photod~r~roce~l V. 0 . C. s, but they serve as
intP -';Ate radicals in radical to radical reactions as
various chain reactions occur in the system. In any
event, the desired end result of total destruction of
V.O.C.s is achieved, yielding final end products of
carbon dioxide and water.
Since water is a neco~sAry part of the system
operation and processes, generation of water has a
beneficial result, leaving only the relatively harmless
carbon dioxide to be dealt with, at least from a
theoretical standpoint. Analysis to date has not
det~rm;ni rl the fate of carbon dioxide, if any, generated
by abatement system 10. Although some experimentation
has shown expected increases of C02, anticipated
increased presence of C02 at exhaust stack 26 cannot
effectively be detected. This would be desirable for
direct comparison of the V.o.C. destruction efficiency
by the present invention relative to V. o . C. destruction
efficiency by incineration, whose end products are also
2 1 78258
P--308 TRI-~IARK - 47 -
carbon dioxide and water. However, with the system of
the present invention, the absence of a detectable
expected increase in Co2 at exhaust stack 2 6 has sever~l
possible explanations. First, it is possible, but
5 unlikely, that no carbon dioxide is produced by the
system. Secondly, in an oxidant-enriched environment,
carbon dioxide might well react ;~ccording to the
following reaction scheme as suggested in the
aforementioned t`h~mi cal Review article:
HO- + C2 - HCO3
HO' + HCO3 - H20 + CO3
HO- + HCO32 - HO- + CO3
15 In a hydroxyl radical enriched environment, any
bicarbonate or carbonate which is formed by the
interaction of water or hydroxide with C02 can react with
the hydroxyl radical to give a carbonate radical anion.
This radical anion is itself an oxidant, although of
20 less oxidation potential than the hydroxyl radical.
Thirdly, carbon dioxide is readily absorbed into the
carbon beds 32, 34 and this absorbed carbon dioxide is
not easily displaced but it will slowly release over
time. Finally, because of the large amount of water
25 present in and passed through sparger tank farm 44,
carbon dioxide may be made "solubleized" into the
aqueous phase and never appear at exhaust stack 2 6 .
As discussed above, no detectable levels of
30 ozone are present in the activated air produced by
generators 28 and 40. Moreover, no ozone is gene+ated
in sparger tank 4 4 because the air is dissolved in an
aqueous solution and no ozone is generated in tunnel 14
--` 2 1 782~8
P-308 TRI-M~RK - 48 -
due to the f og and high humidity present throughout the
tunnel. This is believed to result from one or more of
the following rh~nl -n~: decomposition of ozone due to
the presence of high humidity; selection of ultraviolet
light wavelengths, and ~he presence of radicals from the
PVC catalysts. It is generally believed that, in a dry
environment, ultraviolet radiation at wavelengths
shorter than about 220 nanometers will produce ozone and
decompose l into O~. It is also believed that
ultraviolet radiation at a wavelength of 184 . 9
nanometers is particularly effective in producing ozone.
It is further believed that ultraviolet radiation at
wavelenght of 254 nanometers decomposes ozone. The
ultraviolet lamps used in practicinq the present
invention have a spectral wavelength distributed between
184 and 254 nanometers, with the 184.9nm light helping
to generate ozone and the 254nm light helping to break
down that ozone. The lamps are relatively low wattage,
for example 0 . 425 amps at 120 volts for each lamp in
each of the cells 306 in generator 28 and cells 210 in
generator 240. Similarly, each of the lamps used in
tunnel 14 and sparger tank farm 44 is relatively low
wattage .
It is expected that the process of the present
invention can be optimized for a wide range of V.O.C.s
and V. O . C. s other than those expected in paint spray
booth applications by varying the amount (higher
wattage) and radiation wavelengths, either distributed
over different wavelength ranges or possibly even
monochromatic radiation at selected wavelengths. By
using modular sections such as sections S1 through S15,
when new technology is developed, it can easily be
retrof itted to an existing system . Exposure time to
ultraviolet radiation in tunnel 14 can be varied by
2 1 78258
P--3 o 8 TRI-MARK -- 4 9
varying the number and length of chambers included in
the tunnel and by varying the turbulence in various
chambers .
The present invention effectively and
efficiently achieves the desired end products, namely
water and undoubtedly some CO2. No nitrous oxides, ozone
and other objectionable pollutants, including thermal
pollution, are exhausted into the environment. Original
installation costs are competitive with competitive
systems for a variety of reasons. System components and
system design do not have to meet the demands of high
temperature systems such as incinerators. Smaller
carbon beds can be used because substantial V . O . C .
reduction and destruction occur in the tunnel and V.O.C.
destruction continues in the carbon bed by surface
reactions with oxidants from the generator. The
apparatus of the present invention is constructed with
relatively ;nP~ ncive materials including extensive use
of low-cost, commercially available PVC pipe.
Operating and maintenance costs are also
reduced as compared to competitive systems. No special
~h~ are required as might be used for chemical
V.o.C. destruction. Indeed, the only "starting~
materials are tap water and plant air. The oxidants
utilized, for example as shown in Tables 1 and 2,
represent 20g~ of the atmosphere. Carbon bed
regeneration time is reduced by using activated air,
3 o regeneration is done on site and no large quantities of
waste by-products need to be hauled away for offsite
treatment and/or ~;qposAl. In this regard, with the
present invention it has been noted that some small
granules about the size of a grain of f ine sand
eventually collect in tank 60, but the quantity produced
2 ~ 78258
P--3C8 TRI-~$~RK - 50 -
is insignificant and possibly due to some incidental
mineralization occurring in the process. Energy
efficiencies are expected to compare favorably with
incineration, even though that comparison has not been
5 quantif ied to date .
It will thus be apparent that there has been
provided in accordance with the present invention a
method and apparatus f or abatement of volatile organic
10 compounds which achieve the aims and advantages
specified herein. It will of course be understood that
the foregoing description is of a preferred exemplary
embodiment of the invention and that the invention is
not limited to the specif ic Plnhorl; 1- ~t shown . Various
15 changes and modifications will become apparent to those
skilled in the art and all such variations and
modif ications are intended to come within the scope of
the ~rpPnr~Prl claims.