Note: Descriptions are shown in the official language in which they were submitted.
~17 ~ 3 61 CT-2311A/BMMC7A
This application discloses novel fluorene compounds having
amidoethyl substituents at the Cg position. It also concerns the
preparation of these compounds, as well as methods and compositions
which use them. The compounds have melatonergic properties that are
10 believed to make them useful in treating sleep disorders, e.g., jet-lag and
the like.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone
synthesized and secreted primarily by the pineal gland. Melatonin levels
15 show a cyclical, circadian pattern with highest levels occurring during the
dark period of a circadian light-dark cycle. Melatonin is involved in the
transduction of photoperiodic information and appears to modulate a
variety of neural and endocrine functions in vertebrates, including the
regulation of reproduction, body weight and metabolism in photoperiodic
20 m~mm~ls, the control of circadian rhythms and the modulation of
retinal physiology.
Recent evidence demonstrates that melatonin exerts its biological
effects through specific receptors. Use of the biologically active,
25 radiolabelled agonist [l25I]-2-iodomelatonin has led to the identification of high affinity melatonin receptors in the central nervous systems of a
variety of species. The sequence of one such high affinity melatonin
receptor, cloned from frog dermal melanophores, has been reported
(Ebisawa, et al., Proc. Natl. Acad Sci. 91: 6133-6137, 1994). In mammalian
30 brain, autoradiographic studies have localized the distribution of
melatonin receptors to a few specific structures. Although there are
significant differences in melatonin receptor distribution even between
closely related species, in general, the highest binding site density occurs
in discrete nuclei of the hypothalamus. In humans, specific [l25I]-2-
35 iodomelatonin binding within the hypothalamus is completely localizedto the suprachiasmatic nucleus, strongly suggesting the melatonin
receptors are located within the human biological clock.
~ 1 7 8 3 61 CT-2311A/BMMC7A
Exogenous melatonin administration has been found to
synchronize circadian rhythms in rats (Cassone, et al., J. Biol. Rhythms, 1:
219-229, 1986). In humans, administration of melatonin has been used to
5 treat jet-lag related sleep disturbances, considered to be caused by
desynchronization of circadian rhythms (Arendt, et al., ~r. Med. J. 292:
1170, 1986). Further, the use of a single dose of melatonin to induce sleep
in humans has been claimed by Wurtman in International Patent
Application WO 94/07487.
Melatonin binding sites have been found in several diverse tissues
of the body--i.e., in the retina, superchiasmatic nucleus, spleen, etc. Thus,
melatonin exerts multiple physiological effects, is not highly selective, and
has a significant potential for producing side effects. Melatonin agonists
15 should be more selective than melatonin and give fewer side effects.
In addition, melatonin's metabolic profile can be problematic in
that the compound degrades rapidly in vivo and its oral bioavailability is
often low and variable. Suitable melatonin agonists could overcome
20 these drawbacks, resulting in products having more predictable activity.
Thus, melatonin agonists should be particularly useful for the
treatment of sleep disorders and other chronobiological disorders.
Melatonin agonists would also be useful for the further study of
25 melatonin receptor interactions as well as in the treatment of conditions
affected by melatonin activity, such as depression, jet-lag, work-shift
syndrome, sleep disorders, glaucoma, reproduction, cancer, immune
disorders, and neuroendocrine disorders.
30U.S. Patent 5,206,377 to McAfee discloses compounds having
melatonin antagonist activity which conform to formula 1:
R ~ R,.
R6 R2 R1-
~ 1 7 8 ~ 6 ~ CT-2311A/BMMC7A
wherein R1 is C1~ alkanoyl; R1 is hydrogen, C1 6 alkyl or optimally
substituted phenyl; R2 is hydrogen or phenyl substituted C1~ alkylene;
and R3, R4, Rs and R6 are Cl 6 alkyl, Cl~ alkoxy or optionally substituted
phenoxy. The McAfee compounds do not contain N-amidoethyl
5 substituents.
Stamm, et al., at Chem. Ber. 111: pp. 2665-6 (1978), show the
amidoethylation of fluorene with N-acylaziridines to yield compounds of
formula 2:
~3
HNC(O)Z
wherein Z is ethoxy, diphenylamine, diethylamine or phenyl.
Assithianakis et al., disclose compounds of formula _ in Arch.
Pharm. Vol. 320, (1987), pp. 604-8:
,~
H3C>~ z
~ NHCO~R
Z=HorOH;andR=HorBr.
Hansen et al. disclose, in EPO Patent publication 0215297A2,
compounds of formula 4:
Ar
11
HN--C--C--N--C--C--N--R9
I 1 11 1 /\ I
R4 R5 O R6 R7 Rs ~alkylene)-9H-fluoren-9-yl
wherein Ar is an optionally substituted phenyl group, and R4 through R9
are H or lower alkyl. These dipeptides are used as analgesics.
~ 17 8 3 61 CT-2311A/BMMC7A
Severin et al., show in Chem. Ber. Vol. 110, (1977), p. 491-8, the
preparation of the fluorenyl ethyl amine of formula 5:
CH2CH2NH2
None of these publications discloses the compounds of this
inventlon.
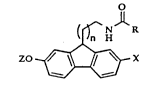
The invention is concerned with substituted fluorenyl compounds
of formula I and compositions and methods which employ them.
lS
Formula I is:
(¦~ H
zo ~ x (I)
wherein:
X= H, halogen, OH or OZ;
Z= Cl~ alkyl; -(CH2)m-CF3 (m=0-2); CD3; or
O-alkyl
--(CH2)m ~ (m'=1-3);
n=1 or 2; and
R= C1 6 alkyl, C3~ cycloalkyl, C2~ alkenyl, halogen substituted C1 6 alkyl,
or C1 6 alkoxy substituted Cl 6 alkyl.
The melatonergic agents of the invention have several advantages
over similar agents. They perform well in tests which demonstrate
affinity for the melatonin binding site found in human suprachiasmatic
~2178361
CT-2311A/BMMC7A
nucleus (SCN). Many of the compounds have ICs~ values for melatonin
binding of 250 nM or less.
The instant compounds are agonists as determined by their
5 melatonin-like ability to block the forskolin-stimulated accumulation of
cyclic AMP in certain cells. Also, many of these compounds are able to
affect activity rhythms in rodents, indicating the ability to moderate
circadian rhythms in mammals.
These ànd other advantages will become more apparent after
consideration of the specification and claims.
The new melatonergic agents described herein conform to
formula I:
( ~ N R
zo ~ x (I)
wherein:
20 X= H, halogen, OH or OZ;
Z= C1~ alkyl; -(CH2)m-CF3 (m=0-2); CD3; or
~ O-alkyl
~ (CH2)m ~ (m'=1-3);
n=lor2;and
R= C1 6 alkyl, C3-6 cycloalkyl, C2 4 alkenyl, halogen substituted C1 6 alkyl,
2~ or Cl 6 alkoxy substituted C1 6 alkyl.
By "alkoxy", applicants mean alkoxy groups, -O-alkyl, having
branched or stralght chains. Methoxy groups are among the preferred
alkoxy groups.
By "alkyl" is meant branched or straight chain Cn H(2n +l) group
moieties and cyclic Cn H(2n~-l) moieties. n' is the number of C atoms in
~1 7 ~ 3 6 ~ CT-2311A/BMMC7A
these moieties. All alkyl groups in R or Z, except for cyclic ones, contain
from 1 to 6 carbon atoms. Preferred alkyl groups include methyl, ethyl,
isopropyl, n-propyl, cyclopropyl, and cyclobutyl.
"Alkenyl" denotes monovalent straight or branched chain
moieties containing one site of unsaturation and at least 2 carbon atoms.
These moieties conform to the formula Cn H(2n~-1), with n" being the
number of carbon atoms present. Preferred alkenyl moieties include
vlnyl.
Alkoxy substituted phenyl groups in compounds of the invention
will contain from 7 to 10 carbon atoms. They may be linked to a ring of
the fluorene moiety via 1 to 3 methylene (-CH2-) groups. (Methoxy
phenyl)propyl substituents are preferred.
Alkoxy substituted alkyl groups found in compounds of the
invention contain a total of 2 to 8 carbon atoms. Any of the alkyl groups
may be straight, branched or cyclic, as set out above. Preferred groups of
this type include methoxymethyl.
The term "halogen" refers to Cl, Br, F or I atoms. Generally, there
will be from 1 to 3 halogen substituents present in each halogen
substituted alkyl moiety. Preferred halogen substituents include Cl and F.
Halomethyl groups, e.g., chloromethyl and trifluoromethyl groups are
preferred.
"D" refers to deuterium
m can be 0, 1 or 2 and designates the number of CH2 groups. m is
preferably 1.
m' refers to 1, 2 or 3 and is preferably 3.
n is 1 or 2, preferably 1.
One preferred group of formula I compounds includes those
wherein Z is a methyl group and X is hydrogen or a methoxy group.
~ :17 8 3 ~ ~ CT-2311A/BMMC7A
Among these, those in which R is methyl, n-propyl, methoxymethyl,
i-propyl and vinyl are preferred. Some compounds in this group are:
N-[2-(2-methoxyfluoren-9-yl)ethyl]butanamide;
N-[2-(2-methoxyfluoren-9-yl~ethyl] acetamide;
N-[2-(2-methoxyfluoren-9-yl)ethyl]-2-propenamide;
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]methoxyacetamide; and
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]-2-methyl propanamide.
A second preferred group of compounds of formula I are
compounds in which Z is a methyl group and X is a methoxy group.
Included in this group are:
N-[3-(2,7-dimethoxyfluoren-9-yl)-prop-1-yl]propanamide;
N-[3-(2,7-dimethoxyfluoren-9-yl)-prop-1-yl]butanamide;
N-[3-(2,7-dimethoxyfluoren-9-yl)-prop-1-yl]acetamide;
N-[3-(2,7-dimethoxyfluoren-9-yl)-prop-1-yl]cyclobutane carboxamide;
N-[3-(2,7-dimethoxyfluoren-9-yl)-prop-1-yl]cyclopropane carboxamide;
N-[2-(2,7-dimethoxyfluoren-9-yl)-ethyl]but-2-enamide; and
N-[2-(2,7-dimethoxyfluoren-9-yl)-ethyl]cyclopentane carboxamide.
Among the compounds in the second group, the following are
more preferred:
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]butanamide;
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]propanamide;
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]cyclopropane carboxamide;
N-~2-(2,7-dimethoxyfluoren-9-yl)ethyl]acetamide;
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]cyclobutane carboxamide; and
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]choroacetamide.
A third preferred group are compounds wherein Z is a methyl
group, X is alkoxy or alkoxyphenylalkyl and R is alkyl.
Yet another preferred group are compounds wherein
X=OZ=OCH2CF3.
Compounds of formula I also encompass all solvates, particularly
hydrates, thereof.
217836~
CT-2311A/BMMC7A
The invention also encompasses geometric and optical isomers
which arise as a consequence of structural asymmetry. Separation of
individual isomers is accomplished by the application of various
methods known to practitioners in the art.
The compounds of the invention are made using the following
general scheme:
General Synthetic Scheme
CN
zo~, (EtO)20PCH2CN _~,X
NaH, THF
PtO2 / ¦ NaH, DMSO
/ CHCI3 Me3S(O)I
--NH2~HCI EtOH CN
zo~,X zo~,X
3 5
Et3N
CH3CN 1) Raney-Ni. H2
, RCOCI EtOH, EtOAc, NH40H
O 2)HCI
~ N~ Et3N 1~ NH2-HCI
ZO~3~3,X RCOCI Z~3,
4 6
.
n = 1 or 2
X = H, halogen or alkoxy
Z= C1~ aL~cyl; -(CH2)mCF3; CD3; or--(CH2)m' ~ O-alkyl
15 THP = tetrahydrofuran
The appropriate fluorenone is converted to the cc"~-unsaturated
nitrile which is then catalytically reduced to give the ethylamine
hydrochloride. Alternatively, the a, ~-unsaturated nitrile can be
- ~1 7 8 3 6~ i CT-2311A/BMMC7A
cyclopropanated and then reduced to provide the propylamine
hydrochloride. Either type of amine is then converted to the desired
amide using any one of a variety of acylating conditions. The use of this
scheme is described in greater detail below.
The fluorenones are puchased commercially or prepared by the
following general scheme:
General Synthesis of Fluorenones
Pd(dba)3
YO~ CO2Me ~¢~ ~ yo~ C2Me
Br (HO)2B Na2CO
Y=HorMe
1) when Y= H, then alk~late with R'X, base,
2)1N NaOH, EtOH CO2H SOCI2 ll
R O~_~ 1) SOCI2 ~X
2) AICI3
R'= alkyl or alkylaryl
An appropriately substituted o-bromoester and phenylboronic acid
are coupled using palladium catalysis to provide a biphenyl intermediate
15 which can be alkylated on oxygen in the cases where a free phenol exists
on one of the aromatic rings. The ester is then hydrolyzed to the acid
which is cyclized to the desired fluorenone. The use of this scheme is
described in greater detail below.
20 ADMINISTRATION
The compounds of the invention may be administered to patients
in need of melatonergic treatment i.e., patients suffering from sleep
disorders and the like, in a variety of ways. Thus, oral, transdermal,
25 subcutaneous, intravenous, intramuscular, rectal, buccal, intranasal, and
ocular routes can be used.
~1 7 8 3 ~i I CT-231 1A/ BMMC7A
One or more of the compounds of the invention is mixed with
pharmaceutically acceptable amounts of one or more conventional
pharmaceutical excipients to produce a formulation to be administered by
the desired route. Generally, such formulations will contain one or
5 several carriers or diluents. Useful carriers include solids, semi-solids
and liquids which have miscibility, or other compatibility, with the active
agent(s) so that they can deliver same to a patient or host.
Suitable carriers include lactose, dextrose, sucrose, sorbitol,
10 mannitol, starches, gum acacia, calcium phosphate, alginates, tragacanth,
gelatin, calcium silicate, microcrystalline cellulose, polyvinylpyrrolidone,
cellulose, water, syrup, methyl cellulose, methyl- and propyl-
hydroxybenzoates, talc, magnesium stearate, mineral oil and the like.
Mixtures are operable.
Other useful excipients include lubricants, wetting agents, gellants,
emulsifiers, preservatives, colorants, perfumes, flavor enhancers, drying
agents and the like. Mixtures can be employed.
Generally, compositions which include the compounds of the
invention will contain from about 0.10 to about 10% of active
compound(s) and 99.9 to 90%, or other suitable amounts, of excipient(s).
Dosage levels will be dictated by the patient's needs and by the
medical judgment of the treating physician. Generally, however, dosages
of about 0.1 mg to about 100 mg per day are useful to treat sleep or
circadian rhythm disorders.
While human patients are most preferred, the compounds of the
invention may be used to treat other subjects, i.e., animals preferably
mammals.
~17 ~ 3 ~1 CT-2311A/BMMC7A
SPECIFIC EMBODIMENTS
The compounds which constitute this invention, their methods of
preparation and their biologic actions will appear more fully from
5 consideration of the following examples, which are given for the purpose
of illustration only and are not to be construed as limiting the invention
in sphere or scope. In the following examples, used to illustrate the
foregoing synthetic processes, temperatures are expressed in degrees
Celsius and melting points are uncorrected. The nuclear magnetic
10 resonances (NMR) are spectral characteristics refer to Chemical shifts (~)
expressed as parts per million (ppm) versus tetramethylsilane (TMS) as
rererence standard. The relative area reported for the various shifts in the
lH NMR spectral data corresponds to the number of hydrogen atoms of a
particular functional type in the molecule. The nature of the shifts as to
15 multiplicity is reported as a broad singlet (bs), singlet (s), multiplet (m), doublet (d), or triplet (t). Abbreviations employed are DMSO-d6
(deuterodimethylsulfoxide), CDCl3 (deuterochloroform) and are
otherwise conventional. The infrared (IR) spectral descriptions include
only absorption wave numbers (cm~l) having functional group
20 identification value. The IR determinations were employed using the
compound neat as a film or by employing potassium bromide (KBr) as
diluent. The elemental analyses are reported as percent by weight.
Unless otherwise noted, all percentages recited herein are weight
25 percents, based on total composition weight.
The following examples describe in detail the preparation of
compounds of Formula I. It will be apparent to those skilled in the art
that modifications, both of materials and methods, will allow preparation
30 of other compounds disclosed herein. From the foregoing description
and the following examples it is believed that one skilled in the art is able
to use the invention to the fullest extent.
The appropriate starting materials such as 2-hydroxy-9-fluorenone
35 were purchased from commercial sources; 2,7-dihyroxy-9-fluorenone was
prepared according to the methods described by Andrews et al (Journal of
h) 17 8 3 g ~ CT-2311A/BMMC7A
Medicinal Chemistry, 1974, 17, 882) and Agarwal (Journal of MedicinaZ
Chemistry, 1967, 10, 99) or purchased from commercial sources.
5 EXAMPLES
These examples discuss-methods of making various compounds of
formula I and their biological activity.
10 Example 1: 2-[9-(2-Methoxyfluorenyl)]ethylamine hydrochloride: A
solution of 2-hydroxy-9-fluorenone (0.042 mol), potassium carbonate (0.20
mol), and methyl iodide (0.042 mol) in acetonitrile was heated to reflux
overnight. The reaction mixture was cooled and the solvent removed in
vacuo to yield a solid. This was dissolved in methylene chloride, washed
15 with saturated sodium carbonate solution, dried over MgSO4 and the
solvent removed to yield a solid which was identified as 2-methoxy-9-
fluorenone. To a suspension of NaH (1.61 g, 0.067 mol) in THF (200 mL)
at RT was added via syringe, diethyl cyanomethylphosphonate (7.43 g,
0.042 mol); the reaction was allowed to stir for 15 min after which a pale
20 yellow solution was observed. To this was added dropwise a solution of
2-methoxy-9-fluorenone (8.89 g, 0.042 mol) in THF (100 mL). The reaction
was allowed to stir overnight at RT. The solvent was removed in vacuo
and the residue was dissolved in methylene chloride, washed with water,
dried over MgSO4, and concentrated to yield an orange solid. This was
25 dissolved in acetonitrile and washed with hexane; the acetonitrile
solution was concentrated to yield an orange solid shown to be the
a,~-unsaturated nitrile by 1H NMR. (79%) A solution/suspension of the
a,~-unsaturated cyano compound (7.72 g, 0.033 mol), PtO2 (0.77g), CHCl3
(23 mL), in EtOH (150 mL) was charged with H2 (50 psi) and allowed to
30 shake on a Parr Hydrogenation Apparatus for 18 h. The reaction mixture
was then filtered and concentrated in vacuo to yield a white solid. This
was washed with Et2O and dried in vacuo to obtain a white solid (69%).
;~'1 7 g 3 ~ ~ CT-2311A/BMMC7A
Example 2: 2-[9-(2,7-Dimethoxyfluorenyl)]ethylamine hydrochloride:
Prepared analogously to 2-[9-(2-Methoxyfluorenyl)]ethylamine
hydrochloride in Example 1 beginning with 2,7-dihydroxy-9-fluorenone.
lH NMR (300 MHz, CDC13) ~ 7.60 (d, J=8.0 Hz, 2H), 7.00 (d, J=2.0 Hz, 2H),
5 7.85 (dd, J=8.0, 2.0 Hz, 2H), 3.90-4.10 (m, lH), 3.85 (s, 6H), 2.50-2.60 (m, 2H),
2.10-2.30 (m, 2H). Alternatively, the necessary intermediate 2,7-
dimethoxyfluorenone was prepared as follows: Methyl 2-bromo-5-
methoxybenzoate (1.60 g, 6.53 mmol), 4-methoxyphenylboronic acid
(1.30 g, 8.55 mmol), and tris(dibenzylideneacetone) dipalladium (0) (0.20 g,
0.22 mmol) were added to dimethoxyethane t25 mL) and 2 M sodium
carbonate (25 mL). The reaction was stirred at reflux for 16 h then
decanted and the residue extracted with ethyl acetate. The combined
organic layers were dried (MgSO4) and the solvent removed by rotary
evaporation to afford 1.50 g of the coupled product (5.51mmol, 84% yield).
The ester (10.80 g, 39.70 mmol) was hydrolyzed with 1 N sodium
hydroxide (80 mL) in refluxing ethanol (500 mL). The cooled reaction
mixture was extracted with methylene chloride and then acidified with
1 N hydrochloric acid. The acidic solution was then extracted with
methylene chloride. The organic layers were combined and the solvent
was dried and concentrated by rotary evaporation to give 9.00 g
(34.88 mmol, 88% yield) of the acid. 9.00 g (34.88 mmol) of the acid was
dissolved in thionyl chloride (250 mL) and stirred at reflux for 6 h. The
reaction was cooled to ambient temperature and the solvent was
removed by rotary evaporation to give 8.20 g of 2,7-dimethoxyfluorenone
(34.17 mmol, 98% yield) as a red solid.
Example 3: 2-methoxy-7-pentoxyfluorenone. Methyl 2-bromo-5-
hydroxybenzoate (9.30 g, 40.26 mmol), 4-methoxyphenylboronic acid
(6.54 g, 43.00 mmol), and tris(dibenzylideneacetone) dipalladium (0)
(0.30 g, 0.33 mmol) were added to dimethoxyethane (75 mL) and 2 M
sodium carbonate (75 mL). The reaction mixture was stirred at reflux for
16 h then cooled and decanted and the residue washed with ethyl acetate.
The combined organic layers were dried (MgSO4) and the solvent
removed by rotary evaporation to afford 9.45 g of the coupled product
(36.64 mmol, 91% yield).
~17 8 3 61 CT-2311A/BMMC7A
The coupled product (1.06 g, 4.10 mmol) was alkylated by treating it
with pentyl iodide (1.16 g, 5.85 mmol) and potassium carbonate (1.38 g,
10.00 mmol) in DMF at 75 C for 16 h. The cooled reaction mixture was
partitioned between ethyl acetate and water. The solvent was removed
5 from the combined organic layers by rotary evaporation to give the
alkylated product. The alkylated product was hydrolyzed with 1 N sodium
hydroxide (10 mL) in refluxing ethanol (50 mL) until the TLC indicated
that the saponification was complete. The cooled reaction mixture was
acidified with 1 N hydrochloric acid and extracted with methylene
10 chloride. The organic layers were dried (MgSO4) and concentrated by
rotary evaporation to give the acid. The acid was dissolved and warmed
in thionyl chloride (50 mL) at 65 C for 30 min. The solution was cooled to
ambient temperature and the thionyl chloride was removed by rotary
evaporation. The residue was dissolved in methylene chloride (50 mL)
and aluminum chloride (0.67 g, 5.00 mmol) added. The reaction was
stirred at ambient temperature for 2 h then quenched by adding the
reaction mixture to a beaker containing 100 mL lN hydrochloric acid and
ice. The methylene chloride layer was separated and the acidic layer was
washed with methylene chloride. The combined organic layers were dried
20 (MgSO4) and the solvent removed by rotary evaporation to give 1.05 g of
2-methoxy-7-pentoxyfluorenone (3.55 mmol, 87% yield) as a red solid.
IH NMR (300 Mhz, CDCl3) ~ 7.28 (d, J = 8.3 Hz, 2H), 7.14 (s, 2H), 6.93 (d, J =
8.3 Hz, 2H), 3.96 (q, J = 7.5 Hz, 2H), 3.82 (s, 3H), 1.80 (p, J = 7.6 Hz, 2H), 1.42
(m, 4H), 0.93 (t, J = 7.5 Hz, 3H).
Example 4. 3-[9-(2,7-Dimethoxyfluorenyl)]propylamine hydrochloride.
Sodium hydride (0.6 g, 22 mmol) was washed with hexane and suspended
in 20 mL anhydrous THF under a nitrogen atmosphere. Diethyl
30 cyanomethylphosphonate (2.8 g, 16 mmol) was added slowly in portions
over 15 min. and the heterogeneous reaction mixture became a clear
solution after the addition was complete. The reaction stirred for 30 min
at room temperature. The ketone (4.2 g, 16 mmol) dissolved in 30 mL
THF was added in portions and the reaction was refluxed overnight. The
35 crude reaction was cooled to room temperature and then poured into 150
mL water and extracted with several portions of 40 mL CH2Cl2. The
combined organic layers were dried over MgSO4 and concentrated in
~t783~i
CT-2311A/BMMC7A
vacuo to give a wine red solid. This a,~-unsaturated nitrile (3.9 g) was
used in the subsequent reaction.
Sodium hydride (1.1 g, 44 mmol) was washed with hexane and
suspended in 100 mL anhydrous DMSO under a nitrogen atmosphere.
The heterogeneous reaction mixture was stirred well while portions of
trimethylsulfoxonium iodide (9.9 g, 45 mmol) were added. The solution
was stirred until the foaming subsided. The reaction was cooled to -60C
and a solution of the c~ ~-unsaturated nitrile in 40 mL anhydrous DMSO
was added in portions over 20 min. The reaction was warmed to room
temperature and stirred overnight. The crude reaction was slowly poured
into saturated NH4Cl followed by the addition of EtOAc. The aqueous
layer was extracted several times with EtOAc and the combined organic
fractions were dried over MgSO4 and concentrated in vacuo. The
resulting light brown oil was purified by flash chromatography (EtOAc/
Hexane gradient) to give the desired nitrile in 43% after 2 steps (2.1 g;
mp= 131-133C; light yellow solid): lH-NMR (300 MHz, CDCl3) ~ 7.58 (m,
2H), 6.91 (m, 3H), 6.42 (m, lH), 3.85 (s, 3H), 3.80 (s, 3H), 2.33 (m, lH), 2.11
(m, 2H); 13C-NMR (75 MHz, CDCl3) ~ lS8.8, 158.7, 144.9, 142.2, 120.2, 117.8,
114.1, 112.9, 106.8, 104.9, 55.5, 34.8, 21.7, 14.8; ~-llR (KBr) 2833, 2237, 1623,
1582, 1470 cm-l; Anal. Calc. for Cl8H15NO2: C, 77.96; H, 5.45; N, 5.05.
Found: C, 77.80; H, 5.49; N, 5.00.
The nitrile (4.3 g) was dissolved in 200 mL of a 1:1 mixture of
EtOH/ EtOAc and 20 mL of NH40H. The mixture was hydrogenated in
the presence of Raney-Ni for 4hrs (TLC 1:1 EtOAc/ Hex). The crude
mixture was filtered, concentrated in vacuo, redissolved in CH2Cl2 and
acetonitrile, dried over MgSO4, filtered, and concentrated to dryness in
vacuo. The resulting solid was dissolved in MeOH and acetonitrile and
concentrated HCl was added (0.52 mL, 17 mmol). After concentrating to
dryness, the white solid was triturated with hexane, filtered, and dried.
The product was obtained in 46/O (2.3 g; mp= 218-221C): lH-NMR
(300 MHz, CDCl3) ~ 7.64 (d, 2H, J= 8.3 Hz), 7.14 (d, 2H, J= 2Hz), 6.90 (dd, 2H,
J= 8.3, 2.0 Hz), 3.95 (t, lH, J= 5.2Hz), 3.81 (s, 6H), 2.66 (t, 2H, J= 8.8Hz), 2.12-
2.05 (9m, 2H), 1.30-1.20 (m, 2H); 13C-NMR (75 MHz, CDCl3) ~ 158.3, 147.9,
133.5, 119.8, 112.8, 110.3, 55.3, 46.3, 28.9, 22.9; FTIR (KBr) 3425, 2946, 1243
cm-l; Anal. Calc. for Cl8H2lNO2-HCl-0.15 H2O: C, 67.03; H, 6.97; N, 4.34;
Found: C, 66.85; H, 7.03; N, 4.21.
~ 1 7 3 3 ~ ~ CT-2311A/BMMC7A
Example 5: General Procedure, N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]
propanamide. 2-[9-(2,7-Dimethoxyfluorenyl)]ethylamine hydrochloride
(0.0083 mol) was suspended in CH3CN (150 ml) and to this was added
excess Et3N (0.024 mol). This mixture was allowed to stir until starting
5 material was completely dissolved. Propionyl chloride (0.0083 mol) was
then added and the reaction mixture was allowed to stir overnight. The
acetonitrile was removed in vacuo. The residue was then washed with
excess water and extracted into CH2Cl2. The organic layer was separated,
dried (MgSO4), filtered and concentrated in vacuo to obtain a solid which
was purified by recrystallization from EtOAc/hexane. m.p. 139-140 C;
Related compounds were purified by silica gel column chromatography
or reverse phase HPLC. lH NMR (300 MHz, CDCl3) ~ 7.52 (d, J=8.3 Hz,
2H), 7.06 (d, J=2.4 Hz, 2H), 6.87 (dd, J=8.3, 2.3 Hz, 2H), 4.87 (bs, lH), 3.98 (t,
J=5.1 Hz, lH), 3.83 (s, 6H), 3.04 (m, 2H), 2.29 (q, J=6.6 Hz, 2H), 1.96-1.82 (m,2H), 0.92 (t, J=7.6 Hz, 3H); 13C NMR (75 MHz, CDCl3) ~ 173.4, 158.8, 147.7,
133.9, 119.9, 113.0, 110.1, 55.6, 45.7, 36.1, 31.9, 29.5, 9.49; IR (KBr) 3260, 1640,
1240 cm~l; MS (DCI) m/e MH+= 326; Analysis calc'd for C2oH23NO3: C,
73.82; H, 7.12; N, 4.30; found: C, 73.63; H, 7.17; N, 4.22.
16
~ 17 8 3 ~1 CT-2311A/BMMC7A
Examples 6-31
The following compounds were also prepared by the general
procedure outlined in Example 5 using the appropriate amine
5 hydrochloride and acid chloride.
Example Compound MeltingPoint (C)
6 N-[2-(2-methoxyfluoren-9-yl)ethyl] 97-99
butanamide
7 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 152-153
cyclopropane carboxamide
8 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 147
acetamide
9 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 94-95
methoxyacetamide
N-~2-(2,7-dimethoxyfluoren-9-yl)ethyl] 126
butanamide
11 N-~2-(2-methoxyfluoren-9-yl)ethyl]-2- 143-145
propenamide
12 N-[2-(2-methoxyfluoren-2-yl)ethyl] 133-135
acetamide
13 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 145-146
cyclobutane carboxamide
14 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]- 88-89
2,2-dimethyl propanamide
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]-2- 145-146
methyl propanamide
16 N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 122-123
choroacetamide
17 N-[2-(2,7-dimethoxyfluoren-9- 155-156
yl)ethyl]cyclopentane carboxamide
18 N-[2-(2,7-dimethoxyfluoren-9- 130-132
yl)ethyl]but-2-enamide
19 N-[3-(2,7-dimethoxyfluoren-9-yl)prop-1- 110-113
yl]cyclobutane carboxamide
N-[3-(2,7-dimethoxyfluoren-9-yl)prop-1- 139-141
yl]acetamide
~17 ~ i CT-2311A/BMMC7A
Example Compound Melting Point (C)
21 N-[3-(2,7-dimethoxyfluoren-9-yl)prop-1- 88-90
yl]butanamide
22 N-[3-(2,7-dimethoxyfluoren-9-yl)prop-1- 120-122
yl] propanamide
23 N [3-(2,7-dimethoxyfluoren-9-yl)prop-1- 141-142
yl]cyclopropane carboxamide
24 N-[2-(2-fluoro-7-methoxyfluoren-9-yl)- 72-73
ethyl]propanamide
N-[2-(2,7-di(methoxy-d3)f~uoren-9-yl)- 141-143
ethyl] acetamide
26 N-[2-(2,7-di(methoxy-d3)fluoren-9-yl)- 138-140
ethyl]propanamide
27 N-[2-(2,7-di(methoxy-d3)fluoren-9-yl)- 133-136
ethyl]cyclopropane carboxamide
28 N-[2-(2,7-di(methoxy-d3)fluoren-9-yl)- 117-120
ethyl]butanamide
29 N-[2-(2-ethoxy-7-methoxyfluoren-9-yl)- 95-98
ethyl]propanamide
N-[2-(2-hydroxy-7-methoxyfluoren-9-yl)- 50-60
ethyl] propanamide
31 N-[2-(2,7-diethoxyfluoren-9-yl)- 225-228
ethyl]propanamide
18
217 ~ 3 6 ~ CT-2311A/BMMC7A
Example 32: N-2-(2-methoxy-7-(1-pentoxy))fluorene-9-yl)ethyl
propanamide. Prepared by the general procedure outlined in example 5
using propionyl chloride. lH NMR (300 MHz, CDCl3~ ~ 7.53 (d, J = 8.3 Hz,
2H), 7.02 (s, 2H), 6.88 (d, J = 8.3 Hz, 2H), 4.90 (bs, lH), 3.95 (m, 3H), 3.84 (s,
3H), 3.04 (m, 2H), 2.30 (q, J = 5.3 Hz, 2H), 1.87 (q, J = 7.6 Hz, 2H), 1.77 (m, 2H)
1.43 (m, 4H), 0.93 (t, J = 7.5 Hz, 6H); 13C NMR (75 MHz, CDCl3) ~ 173.8,
158.6, 158.2, 147.6, 134.1, 133.8, 119.8, 113.5, 113.0, 110.7, 110.1, 68.2, 55.5j 45.6,
36.0, 31.7, 29.3, 29.0, 28.1, 22.4, 13.9, 9.4. Anal. Calcd for C24H31NO3-0.25
H2O: C, 74.68; H, 8.23; N, 3.63. Found: C, 74.57; H, 8.20, N, 3.68.
Example 33: N-2-(2-methoxy-7-(3-(3-methoxyphenyl)propox-1-
y))fluorene-9-yl)ethyl propanamide. Prepared by the general procedure
outlined in example 5 using propionyl chloride. lH NMR (300 MHz,
CDCl3) ~ 7.54 (d, J = 8.3 Hz, 2H), 7.21 (t, J = 7.8 Hz, 2H), 7.03 (s, 2H), 6.91-6.73
(m, 4H), 4.92 (bs, lH), 4.01 (m, 3H), 3.90 (s, 3H), 3.85 (s, 3H), 3.06 (q, J = 6.2
Hz,2H),2.82(t,J=7.9Hz,2H),2.28(q,J=6.6Hz,2H),2.12(p,J=7.8Hz,2H)
1.89 (q, J = 7.6 Hz, 2H), 0.95 (t, J = 7.6 Hz, 3H); 13C NMR (75 MHz, CDCl3)
173.4, 158.8, 158.1, 147.7, 143.2, 133.9, 130.4, 128.4, 120.9, 118.8, 114.7, 114.2,
112.5, 112.0, 111.2, 110.2, 109.9, 109.2, 67.3, 56.5, 54.2, 46.5, 36.1, 32.2, 31.9,
30.8, 29.4, 10.4, 8.7. Anal. Calcd for C29H33NO4-0.67 H2Q: C, 73.85; H, 7.34;
N,2.97.Found:C,73.81;H,7.02,N,2.84.
Example 34: Measurement of Melatonergic Binding
1. Reagents
(a) 50 mM Tris buffer containing 12.5mM MgCl2 and 2mM E~TA (pH
7.4 at 37C).
(b) Wash buffer: 20mM Tris base containing 2mM MgCl2 (pH 7.4 at
room temperature).
(c) Melatonin (10-5 M final concn.).
(d) 2-[l25I]-iodomelatonin (100 pM final concn.).
Source: NEN
19
~ 1 7 ~ 3 ~1 CT-2311A/BMMC7A
2. Membrane preparation. The receptor cDNA (human ML1A) was
subdoned into pcDNA3 and introduced into NIH 3T3 cells using
Lipofectamine. Transformed NIH 3T3 cells resistant to geneticin were
isolated and single colonies expressing high levels of 2-[125I]-
5 iodomelatonin binding were isolated and characterized. Cell pellets werefrozen at -80C for further use. For preparing membrane homogenates,
pellets are thawed on ice, and resuspended in TME buffer, Tris base,
MgCl2, EDTA (pH 7.4 at 37C), supplemented with aprotinin, leupeptin,
and phenylmethlysulfonylfluoride. The cells were then homogenized
10 using a dounce homogenizer, and centrifuged. The resulting pellet was
resuspended with a dounce homogenizer in TME and frozen. On the day
of assay, the small aliquot was thawed on ice and resuspended in TME
buffer.
15 3. Incubation: 37C for 1 hour. Reaction is terminated by filtration.
The procedure was based on that disclosed in: Reppert, S. M.,
Weaver, D. R., and Ebisawa, R. (1994), Neuron ~,1177-1185 (1994).
~17 ,~ ~ 6 1 CT-2311A/BMMC7A
The following table sets forth selected Formula I compounds and
binding data which demonstrates their usefulness.
Binding Data of Selected Compounds of Formula I
Compound ExampleBinding Affinity4
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 5 +++
propanamide
N-[2-(2-methoxyfluoren-9-yl)ethyl] 6 + +
butanamide
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyll , 9 + +
methoxyacetamide
N-[2-(2-methoxyfluoren-9-yl)ethyl]-2- 11 + +
propenamide
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl] 13 +++
cyclobutane carboxamide
N-[2-(2,7-dimethoxyfluoren-9-yl)ethyl]- 14 +
2,2-dimethyl propanamide
N-[3-(2,7-dimethoxyfluoren-9-yl)prop-1- 20 + +
yl]acetamide
N-[2-(2-ethoxy-7-methoxyfluoren-9-yl)- 29 + +
ethyl]propanamide
* +++ = ICso < 10 nM; ++ = ICso < 100 nM; + = ICso < 500 nM
~1783~1
CT-231 lA/ BMMC7A
Example 35: Measurement of Functional Activity
Cyclic AMP Accumulation in Intact Cells: Melatonin
CELLS:
The media was removed from cell flask and washed with Hank's salt
solution or PBS, as appropriate. The cells were detached from flask.
Enough media was added so that the concentration of cells is 4 x 105/ml
when counted with a hemocytometer. Dialyzed or heat inactivated fetal
bovine serum (FBS) was used in the media when plating the cells. 1 ml
of cell suspension was put into each well, then 2 mls of media. Cells were
incubated overnight.
SOLUTIONS:
1. Stock solution: plain media (no serum or additives) + 20 mM HEPES.
2. IBMX solution: media/HEPES + lmM IBMX.
3. Assay solution: 90% stock solution + 10% IBMX solution.
Each well gets 3 mls of assay solution for preincubation and 3 mls for the
assay. Each test condition is done in triplicate.
4. Drug solutions:
a) Basal assay solution + DMSO
b) Forskolin stimulation: 10 ~M final concentration.
c) Forskolin + competitor (melatonin): 10 ~M final concentration
forskolin plus desired concentration of competitor (melatonin~.
REACTION:
All tests were done in triplicate at 37C. Plates with cells were kept in a
shallow 37 C water bath throughout the reaction. Media was taken from
the wells and 3 ml of preincubation media was added. After 10 min, that
solution was removed and 3 mls of drug solution was added. After 10
min, the media was removed and reaction stopped with HCl. Samples set
for at least an hour at room temperature. 1 ml from each dish was taken
and put into a microfuge tube and spun to remove floating cells. After
dilution to 1:100 for RIA, a radioimmuno-assay was done.
- ~ ~17~36~ -
CT-231 lA/ BMMC7A
The following table sets forth selected Formula I compounds and
intrinsic activity data which demonstrates their usefulness.
Functional Data of Selected Compounds of Formula I
Compound Name Ex. I.A.*
N-[2-(2,7-Dimethoxyfluoren-9-yl)ethyl] 5 1.14
propanamide
N-[2-(2,7-Dimethoxyfluoren-9-yl)ethyl] 10 1.15
butanamide
N-[2-(2,7-Dimethoxyfluoren-9-yl)ethyl] 7 1.02
cylopropane carboxamide
*I.A. (Intrinsic Activity) = Emax (experimental compound)/Emax
(melatonin)
10 Emax = maximal effect
Reasonable variations, such as those which would occur to one
having ordinary skill in the art, can be made herein without departing
from the scope of the invention.