Note: Descriptions are shown in the official language in which they were submitted.
2178364
MALEIMIDE COPOLYMERS AND METHOD FOR INHIBITING HYDRATE
FORMATION
FIELD OF THE INVENTION
The present invention relates to novel compounds and a method for inhibiting theformation of clathrate hydrates in a fluid. More specifically, the invention relates to
maleimide copolymers and a method for inhibiting the formation of gas hydrates in a pipe
used to convey oil or gas.
BACKGROUND OF THE INVENTION
Carbon dioxide, hydrogen sulfide, and various hydrocarbons, such as methane, ethane,
propane, normal butane and isobutane, are present in natural gas and other petroleum fluids.
However, water is typically found mixed in varying amounts with such petroleum fluid
constituents. Under conditions of elevated pressure and reduced temperature clathrate
hydrates can form when such petroleum fluid constituents or other hydrate formers are mixed
with water. Clathrate hydrates are water crystals which form a cage-like structure around
guest molecules such as hydrate-forming hydrocarbons or gases. Some hydrate-forming
hydrocarbons include, but are not limited to, methane, ethane, propane, isobutane, butane,
neopentane, ethylene, propylene, isobutylene, cyclopropane, cyclobutane, cyclopentane,
cyclohexane, and benzene. Some hydrate-forming gases include, but are not limited to,
oxygen, nitrogen, hydrogen sulfide, carbon dioxide, sulfur dioxide, and chlorine.
Gas hydrate crystals or gas hydrates are a class of clathrate hydrates of particular
interest to the petroleum industry because of the pipeline blockages that they can produce
during the production and/or transport of the natural gas and other petroleum fluids. For
example, at a pressure of about lMPa ethane can form gas hydrates at temperatures below
4C, and at a pressure of 3MPa ethane can form gas hydrates at temperatures below 14C.
Such temperatures and pressures are not uncommon for many operating environments where
natural gas and other petroleum fluids are produced and transported.
217~364
As gas hydrates agglomerate they can produce hydrate blockages in the pipe or conduit
used to produce and/or transport natural gas or other petroleum fluid. The formation of such
hydrate blockages can lead to a shutdown in production and thus substantial financial losses.
Furthermore, restarting a shutdown facility, particularly an offshore production or transport
facility, can be difficult because significant amounts of time, energy, and materials, as well as
various engineering adjustments, are often required to safely remove the hydrate blockage.
A variety of measures have been used by the oil and gas industry to prevent the
formation of hydrate blockages in oil or gas streams. Such measures include maintaining the
temperature and/or pressure outside hydrate formation conditions and introducing an antifreeze
such as methanol, ethanol, propanol, or ethylene glycol. From an engineering standpoint,
m~int~ining temperature and/or pressure outside hydrate formation conditions requires design
and equipment modifications, such as insulated or jacketed piping. Such modifications are
costly to implement and m~int~in. The amount of antifreeze required to prevent hydrate
blockages is typically between 10% and 30% by weight of the water present in the oil or gas
stream. Consequently, several thousand gallons per day of such solvents can be required.
Such quantities present h~n(lling, storage, recovery, and potential toxicity issues to deal with.
Moreover, these solvents are difficult to completely recover from the production or
transportation stream.
Consequently, there is a need for a gas hydrate inhibitor that can be conveniently
mixed at low concentrations in the produced or transported petroleum fluids. Such an
inhibitor should reduce the rate of nucleation, growth, and/or agglomeration of gas hydrate
crystals in a petroleum fluid stream and thereby inhibit the formation of a hydrate blockage in
the pipe conveying the petroleum fluid stream.
One method of practicing the present invention uses gas hydrate inhibitors which can
be used in the concentration range of about 0.01% to about 5% by weight of the water present
2178364
in the oil or gas stream. As discussed more fully below, the inhibitors of this invention can
effectively treat a petroleum fluid having a water phase.
SUMMARY OF THE INVENTION
According to one aspect of the present invention, there is provided a novel copolymer
having at least two monomeric units, wherein one of the monomeric units is a maleimide unit
and another of the monomeric units is a vinyl unit having a pendant group, the pendant group
having two to twenty-one carbon atoms, at least one nitrogen atom and at least one oxygen
atom; the copolymer having an average molecular weight between about 1,000 and about
6,000,000.
The term "copolymer" as used herein will be understood to include polymers having
two or more different monomeric units.
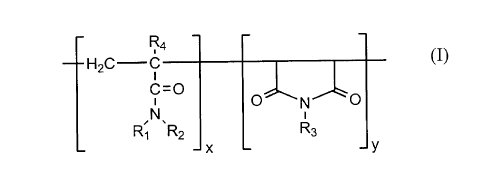
Preferably, the copolymer is selected from the group consisting of:
a) acrylamide/maleimide copolymers having the following general formula:
,R4
--H2C C
,CN=O O~N~b
R, ~R2 R3
-- X _ y
where,
R, is hydrogen or a branched, normal or cyclic hydrocarbon group having one to ten
carbon atoms,
R2 is a branched, normal or cyclic hydrocarbon group having one to ten carbon atoms,
R3 is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms,
217836~
R4 is hydrogen or a methyl group, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000;
b) N-vinyl amide/maleimide copolymers having the following general formula:
H2C Cl I (II)
c-o1 O~N~O
R2 R3
-- x _ y
where,
R, is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms,
R2 is a branched, normal or cyclic hydrocarbon group having one to six carbon atoms,
wherein R] and R2 have a sum total of carbon atoms greater than or equal to one but
less than or equal to eight,
R3 is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000;
c) vinyl lactam/maleimide copolymers having the following general formula:
--H2C Cl I (III)
o OJ~ N~lO
\~/~ n -x ~ R3
where,
'' ~ 217836g
n ranges from one to three,
R3 is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000;
d) alkenyl cyclic imino ether/maleimide copolymers having the following general formula:
--H2C C ~ ,l (IV)
N~\O N O
\~n _x - -Y
where,
n ranges from one to four,
Rl is hydrogen or a methyl group,
R2 is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000; and
e) acryloylamide/maleimide copolymers having the following general formula:
R
--H2C C I I (V)
CN=O O~N~~O
R1 R2 R3
-- ~J --x -- _y
2178364
where Rl and R2 are linked to form a nitrogen-cont~ining cyclic structure having from three to
ten carbon atoms,
R3 is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms,
R4 is hydrogen or a methyl group, and
x + y is an average number of monomeric units for producing an average molecular
weight for the copolymer between about 1,000 and about 6,000,000.
One or both of the Rl and R2 groups of the acrylamide/maleimide copolymers and
the acryloylamide/maleimide copolymers may have one to four heteroatoms selected from the
group consisting of oxygen, nitrogen, sulfur, and combinations thereof.
According to another aspect of the present invention, there is provided a novel
polymer composition comprising a copolymer having at least two monomeric units, wherein
one of the monomeric units is a maleimide unit and another of the monomeric units is a vinyl
unit having a pendant group, the pendant group having two to twenty-one carbon atoms, at
least one nitrogen atom and at least one oxygen atom; the copolymer having an average
molecular weight between about 1,000 and about 6,000,000. Preferably, the polymer
composition contains one or more maleimide copolymers selected from the group consisting
of acrylamide/maleimide copolymers, N-vinyl amide/maleimide copolymers, vinyl
lactam/maleimide copolymers, alkenyl cyclic imino ether/maleimide copolymers, and
acryloylamide/maleimide copolymers, as described above, and a carrier solvent.
According to a further aspect of the present invention, there is provided a method
for inhibiting the formation of clathrate hydrates in a fluid having hydrate-forming
constituents. The method comprises treating the fluid with an inhibitor comprising a
substantially water soluble maleimide copolymer having at least two monomeric units,
wherein one of the monomeric units is a maleimide unit and another of the monomeric units
is a vinyl unit having a pendant group, the pendant group having two to twenty-one carbon
2178364
atoms, at least one nitrogen atom and at least one oxygen atom; the copolymer having an
average molecular weight between about 1,000 and about 6,000,000. Preferably, the
copolymer is selected from the group consisting of acrylamide/maleimide copolymers, N-vinyl
amide/maleimide copolymers, vinyl lactam/maleimide copolymers, alkenyl cyclic imino
ether/maleimide copolymers, and acryloylamide/maleimide copolymers, as described above.
DETAILED DESCRIPTION OF THE INVENTION
INVENTIVE METHOD
The inventive method inhibits the formation of clathrate hydrates in a fluid having
hydrate-forming constituents. Formation of clathrate hydrates means the nucleation, growth,
and/or agglomeration of clathrate hydrates. Such clathrate hydrates may be formed in a fluid
whether it is flowing or substantially stationary, but are often most problematic in flowing
fluid streams conveyed in a pipe. For example, flow restrictions arising from partial or
complete blockages in a fluid stream can arise as clathrate hydrates adhere to and accumulate
along the inside wall of the pipe used to convey the fluid. Nonetheless, the invention can be
used for inhibiting formation of clathrate hydrates in substantially stationary fluids.
In one embodiment of the invention, a concentrated solution or mixture of one or more
of the inhibitors of the type described below is introduced into a petroleum fluid stream
having an aqueous phase. As the inhibitor solution or mixture of this invention is
substantially dissolved in the aqueous phase or dispersed in the fluid stream it reduces the rate
that clathrate hydrates are formed, and thereby reduces the tendency for a flow restriction to
occur.
In a preferred embodiment, the solid polymer is first dissolved in an appropliate
carrier solvent or liquid to make a concentrated solution or mixture. It should be understood
that many liquids may effectively facilitate treatment of the fluid stream without dissolving
the inhibitor. For convenience, such liquids are referred to hereafter as solvents whether they
2178364
produce an inhibitor solution, emulsion, or other type of mixture. The principal purpose of
the solvent is to act as a carrier for the inhibitor and to facilitate the absorption of the
inhibitor into the aqueous phase of the petroleum fluid. Any solvent suitable for delivering
the inhibitor to aqueous phase of the fluid may be used. Such carrier solvents include, but are
not limited to, water, brine, sea water, produced water, methanol, ethanol, propanol,
isopropanol, glycol, and mixtures of such solvents. Other solvents familiar to those skilled in
the art may also be used.
It should be understood that the use of a carrier solvent is not required to practice the
invention, but it is a convenient method of introducing the inhibitor into the fluid. In many
applications the use of a carrier solvent will facilitate treatment of the fluid stream.
Any convenient concentration of inhibitor in the carrier solvent can be used, so long as
it results in the desired final concentration in the aqueous phase of the petroleum fluid.
Higher concentrations are preferred, since they result in a reduced volume of concentrated
solution to handle and introduce into the petroleum fluid. The actual concentration used in a
specific application will vary depending upon the selection of carrier solvent, the chemical
composition of the inhibitor, the system temperature, and the solubility of the inhibitor in the
carrier solvent at application conditions.
The inhibitor mixture is introduced into the aqueous phase of the petroleum fluid using
mechanical equipment, such as, chemical injection pumps, piping tees, injection fittings, and
other devices which will be apparent to those skilled in the art. However, such equipment is
not essential to practicing the invention. To ensure an efficient and effective treatment of the
petroleum fluid with the inhibitor mixture two points should be considered.
First, an aqueous phase is preferably present at the location the inhibitor solution is
introduced into the fluid. In some petroleum fluid systems (particularly natural gas systems),
an aqueous phase does not appear until the gas has cooled sufficiently for water to condense.
2178~64
If this is the case, the inhibitor solution is preferably introduced after the water has
condensed. Alternatively, in the event that an aqueous phase is not available at the point the
inhibitor solution is introduced, the inhibitor solution concentration should be selected to
ensure that the viscosity of the inhibitor solution is sufficiently low to facilitate its dispersion
through the fluid and permit it to reach the aqueous phase.
Second, because the inhibitor primarily serves to inhibit the formation of clathrate
hydrates, rather than reverse such formation, it is important to treat the fluid prior to
substantial formation of clathrate hydrates. As a wet petroleum fluid cools, it will eventually
reach a temperature, known as the hydrate equilibrium dissociation temperature or Teq~ below
which hydrate formation is thermodynamically favored. The Teq of a petroleum fluid shifts as
the pressure applied to the fluid and the composition of the fluid change. Various methods of
determining a fluid's Teq at various fluid compositions and pressures are well known to those
skilled in the art. Preferably, the fluid should be treated with the inhibitor when the fluid is
at a temperature greater than Teq. It is possible, but not preferable, to introduce the inhibitor
while the temperature is at or slightly below the Teq of the fluid, preferably before clathrate
hydrates have begun to form.
The quantity of inhibitor introduced into a petroleum fluid with an aqueous phase
solvent is typically in the range of *om about 0.01 wt% to about 5 wt% of the water present
in the fluid. Preferably, the inhibitor concentration is about 0.5 wt%. For example, a
laboratory study has shown that adding 0.5 wt% of a copolymer of dimethylacrylamide and
propylmaleimide (DMAM/PME) to a petroleum fluid allowed the fluid to cool to a
temperature which was about 9.7C below its Teq without rapid formation of a hydrate
blockage. A higher inhibitor concentration can be used to lower the temperature at which a
hydrate blockage is obtained. A suitable concentration for a particular application, however,
can be determined by those skilled in the art by taking into account the performance of the
inhibitor under such application, the degree of inhibition required for the petroleum fluid, and
the cost of the inhibitor.
2178364
INHIBITOR DESCRIPTION
Compounds belonging to the group of polymers and copolymers of maleimides, and
mixtures thereof, are very effective inhibitors of hydrate nucleation, growth, and/or
agglomeration (collectively referred to as hydrate formation). In accordance with the present
invention, the copolymer has at least two monomeric units, wherein one of the monomeric
units is a maleimide unit and another of the monomeric units is a vinyl unit having a pendant
group, the pendant group having two to twenty-one carbon atoms, at least one nitrogen atom
and at least one oxygen atom; the copolymer having an average molecular weight between
about 1,000 and about 6,000,000. Generic structures of preferred maleimide copolymers are
depicted below:
Acrylamide/Maleimide Copolymers
~4
--H2C C
C = O o~ ~o
R1 ~R2 R3
-- X
where,
Rl is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to ten
carbon atoms,
R2 is a branched, normal, or cyclic hydrocarbon group having one to ten carbon atoms,
R3 is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms,
R4 is hydrogen or a methyl group, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000.
One or both of the Rl and R2 groups may have one to four heteroatoms selected from
the group consisting of oxygen, nitrogen, sulfur, and combinations thereof.
- 10 -
2178364
N-Vinyl Amide/Maleimide Copolymers
H2C Cl I (II)
N - R1 J~ ~lo
R2 R3
--x _ y
where,
R, is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms,
R2 is a branched, normal, or cyclic hydrocarbon group having one to six carbon atoms,
wherein R, and R2 have a sum total of carbon atoms greater than or equal to one but
less than or equal to eight,
R3 is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000.
Vinyl Lactam/Maleimide Copolymers
--H2C Cl I l l (m)
~0 o~~ N~O
\ /~R3
n -x - -Y
where,
n ranges from one to three,
2 1 7 8 3 6 4
R3 is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000.
Alkenyl Cyclic Imino Ether/Maleimide Copolymers
--H2C C ~ 1~ (IV)
N~\O N O
\~ n _ x - - Y
where,
n ranges from one to four,
Rl is hydrogen or a methyl group,
R2 is hydrogen or a branched, normal or cyclic hydrocarbon group having one to six
carbon atoms, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer ranging from about 1,000 and about 6,000,000.
Acryloylamide/Maleimide Copolymers
R
--H2C C I I (V)
~CN~- o 0~ N~O
R1 R2 R3
-- ~J --x -- _ y
2178364
where Rl and R2 are linked to form a nitrogen-cont~ining cyclic structure having from three to
ten carbon atoms,
R3 is hydrogen or a branched, normal, or cyclic hydrocarbon group having one to six
carbon atoms,
R4 is hydrogen or a methyl group, and
x + y is an average number of monomeric units for producing an average molecular weight
for the copolymer between about 1,000 and about 6,000,000.
One or both of the R, and R2 groups may have one to four heteroatoms selected from
the group consisting of oxygen, nitrogen, sulfur, and combinations thereof.
Maleimide copolymers of interest as gas hydrate inhibitors comprise a maleimide
monomer copolymerized with one or more monomers chosen from a class of monomers
including, but not limited to, other maleimides, acrylamides, N-vinyl amides, alkenyl cyclic
imino ethers and vinyl lactams, such that the resultant copolymer is substantially water
soluble. For example, various maleimides can be copolymerized with various acrylamides,
such as dimethylacrylamide, to produce an effective inhibitor in the class of copolymers
described above.
These maleimide copolymers may be used in mixture with other substantially watersoluble polymers including, but not limited to, poly(vinylpyrrolidone) (PVP),
poly(vinylcaprolactam) (PVCap), copolymers of vinylpyrrolidone and vinylcaprolactam,
poly(N-methyl-N-vinylacetamide), copolymers of N-methyl-N-vinylacetamide and
isopropylmethacrylamide, copolymers of N-methyl-N-vinylacetamide and acryloylpiperidine,
copolymers of N-methyl-N-vinylacetamide and methacryloylpyrrolidine, and copolymers of N-
methyl-N-vinylacetamide and acryloylpyrrolidine.
Without limiting the scope of the invention, and for the purpose of illustrating the
invention, various maleimide copolymers were synthesized and tested, including
~178364
acrylamide/maleimide copolymers where dimethylacrylamide (DMAM) was copolymerized
with maleimide (ME), ethylmaleimide (EME), propylmaleimide (PME), and butylmaleimide
(BME).
Other maleimide copolymers of interest and synthesized include other
acrylamide/maleimide copolymers, such as DMAM/methylmaleimide (DMAM/MME), and
DMAM/cyclohexylmaleimide (DMAM/CHME), N-vinyl amide/maleimide copolymers, such
as N-methyl-N-vinylacetamide/ethylmaleimide (VIMA/EME), and lactam/maleimide
copolymers, such as vinylcaprolactam/ethylmaleimide (VCap/EME).
INHIBITOR SYNTHESIS
General Procedure
Dimethylacrylamide (DMAM) used in the synthesis procedures described below was
obtained from Polysciences. Standard laboratory procedures f~mili~r to those skilled in the art
were used to synthesize the evaluated polymers identified above. Benzene, tetrahydrofuran
(THF), or other low molecular weight solvents were used as either reaction or purification
solvents.
Example 1
Copolymerization of N~N-Dimethylacrylamide (DMAM) and Maleimide (ME) (70/30 mol
char~e)
Raw materials: DMAM was purified by passing it through an Aldrich MEHQ InhibitorRemover Column (Catalogue # 30,631-2). ME was purchased from Aldrich and used without
any further purification. Water was deionized by passing it through a Millipore~ ion filtration
unit (Model #ZWDJ02511); it was degassed by rapid boiling while purging with N2, then
cooled to room temperature while m~int~inin~ the N2 purge. 2,2'-Azobis[2-
- 14 -
217836~
methylpropionamide] dihydrochloride (V-50, Monomer-Polymer Laboratories) was used as
recelved.
Copolymerization procedure: ll.Og (0.111 mol) purified DMAM and 4.62g (0.0476 mol) ME
were combined with 141ml deionized, degassed water in a three-necked reaction kettle
equipped with a thermometer, a stirrer, and a N2 inlet/outlet. (The kettle had been previously
purged with N2.) The solution was heated to 60C. Then, 0.1682g (0.62mmol) V-50
dissolved in 3ml water was added to the monomer solution. This reaction mixture was stirred
overnight at 60C under an N2 blanket. The next day, the reaction mixture was slightly
viscous. The polymer was precipitated in acetone, collected by filtration, and dried in a
vacuum oven at 60C overnight. IH NMR and 13C NMR spectra were consistent with the
formation of a 70/30 DMAM/ME copolymer.
Example 2
Copolymerization of DMAM and Methylmaleimide (MME) (50/50 mol char~e!
Raw materials: DMAM (purchased from Polysciences) was purified by passing it through an
Aldrich MEHQ Inhibitor Remover Column (Catalogue # 30,631-2). MME, also from
Aldrich, was used as received. Anhydrous benzene (Aldrich) was transferred using a cannula
or a syringe under a positive pressure of nitrogen. AIBN (2,2'-azobis[2-isobutylnitrile],
Johnson-Matthey) was recryst~llize~l from methanol.
Copolymerization procedure: 8.65g (0.872 mol) of the de-inhibited DMAM described above
was combined with 9.69g (0.872 mol) methylmaleimide and 200 ml benzene in a three-
necked reaction kettle equipped with a thermometer, a stirrer, and a N2 inlet/outlet. (The
kettle had been previously purged with N2.) The solution was heated to 60C. Then, an
AIBN solution (0.1444g (0.88 mmol) in 4ml benzene) was injected into the monomersolution. The reaction mixture was m~int~ined at 60C for 24 hr. The polymer was isolated
217836~
by pouring the reaction mixture into diethylether and collecting the polymer by filtration. The
product was dried in a vacuum oven overnight at 60C. The polymer was re-purified by
dissolution into THF, followed by precipitation into hexane, collection by filtration, and
drying under vacuum as described above. The final purified product yield was 16.4g (89%).
The IH and 13C NMR spectra were consistent with the formation of a 49/51 DMAM/MME
copolymer. This copolymer had a cloud point of 39.5C at a concentration of 0.5% in brine.
Example 3
Copolymerization of DMAM and Ethylmaleimide (EME) (50/50 mol char~e)
Raw materials: DMAM was purchased from Polysciences and purified as described below.
EME (Aldrich) was used without any further purification. Anhydrous benzene (Aldrich) was
transferred using a cannula or a syringe under a positive pressure of nitrogen. AIBN
(Johnson-Matthey) was recry~t~lli7e~1 from methanol.
DMAM purification: 32.9g DMAM and 21.5g benzene were combined and passed throughan Aldrich MEHQ Inhibitor Remover Column (Catalogue # 30,631-2). A 39.2 g solution was
collected.
Copolymerization procedure. 14.9g of the de-inhibited DMAM/benzene solution (9g active
DMAM (0.091 mol)) described above was combined with 11.4g (0.91 mol) ethylmaleimide
and 209 ml additional benzene in a three-necked reaction kettle equipped with a thermometer,
a stirrer, and a N2 inlet/outlet. (The kettle had been previously purged with N2.) The solution
was heated to 60C. Then, an AIBN solution (0.1509g (0.92 mmol) in 4ml benzene) was
injected into the monomer solution. The reaction mixture was m~int~ined at 60C for 24 hr.
The polymer was isolated by pouring the reaction mixture into hexane and collecting the
polymer by filtration. The product was dried in a vacuum oven overnight at 60C. The
polymer was re-purified by dissolution into THF, followed by precipitation into diethyl ether,
- 16 -
2178364
collection by filtration, and drying under vacuum as described above. The final purified
product yield was 15.3g (75%). The IH and 13C NMR spectra were consistent with the
formation of a 51.6/48.4 DMAM/EME copolymer.
Example 4
Copolymerization of DMAM and EME (79/21 mol char~e)
The same procedure was followed as in Example 3, except that the monomer ratio
charged was adjusted to 79/21 DMAM/EME and the AIBN used as the initiator was changed
to 0.1451 g (0.88 mmol). 17g of purified polymer was collected, representing a 87% yield.
The 'H and 13C NMR spectra were consistent with the formation of a 82.6/17.4 DMAM/EME
copolymer.
Example 5
Copolymerization of DMAM and Propylmaleimide (PME) (70/30 mol char~e)
Raw maferials: DMAM was purified as described below. PME was purchased from Fluka
and used as received. Anhydrous benzene (Aldrich) was transferred using a cannula or a
syringe under a positive pressure of nitrogen. AIBN (Johnson-Matthey) was recrystallized
from methanol.
DMAMpurification: 32.9g DMAM and 21.5g benzene were combined and passed through
an Aldrich MEHQ Inhibitor Remover Column (Catalogue # 30,631-2). A 39.2 g solution was
collected.
Copolymerization procedure: 18.7 g of the de-inhibited DMAM/benzene solution (11.4 g
active DMAM (0.1151 mol)) described above was combined with 6.86g (0.049 mol)
~178364
"
ethylmaleimide and 188 ml additional benzene in a three-necked reaction kettle equipped with
a thermometer, a stirrer, and a N2 inlet/outlet. (The kettle had been previously purged with
N2.) The solution was heated to 60C. Then, an AIBN solution (0.1357g (0.83 mmol) in 4ml
benzene) was injected into the monomer solution. The reaction mixture was m~int~ined at
60C for 24 hr. The polymer was isolated by pouring the reaction mixture into hexane and
collecting the polymer by filtration. The product was dried in a vacuum oven overnight at
60C. The polymer was re-purified by dissolution into THF, followed by precipitation into
diethyl ether, collection by filtration, and drying under vacuum as described above. The final
purified product yield was 15.8g (87%). The 'H and 13C NMR spectra were consistent with
the formation of a 74/26 DMAM/PME copolymer.
Example 6
Copolymerization of DMAM and Butylmaleimide (BME) (70/30 mol char~e)
Raw materials: DMAM was purified as described below. BME (Fluka) was used as
received. Anhydrous benzene (Aldrich) was transferred using a cannula or a syringe under a
positive pressure of nitrogen. AIBN (Johnson-Matthey) was recrystallized from methanol.
DMAM purification: 32.9g DMAM and 21.5g benzene were combined and passed throughan Aldrich MEHQ Inhibitor Remover Column (Catalogue # 30,631-2). A 39.2 g solution was
collected.
Copolymerization procedure: 18.7 g of the de-inhibited DMAM/benzene solution (11.4 g
active DMAM (0.1151 mol)) described above was combined with 7.55g (0.049 mol)
butylmaleimide and 195 ml additional benzene in a three-necked reaction kettle equipped with
a thermometer, a stirrer, and a N2 inlet/outlet. (The kettle had been previously purged with
N2.) The solution was heated to 60C. Then, an AIBN solution (0.1408g (0.86 mmol) in 4ml
benzene) was injected into the monomer solution. The reaction mixture was m~int~ined at
~178364
60C for 24 hr. The polymer was isolated by pouring the reaction mixture into hexane and
collecting the polymer by filtration. The product was dried in a vacuum oven overnight at
60C. The polymer was re-purified by dissolution into THF, followed by precipitation into
diethyl ether, collection by filtration, and drying under vacuum as described above. The final
purified product yield was 14.6g (77%). The 'H and 13C NMR spectra were consistent with
the formation of a 72.4/27.6 DMAM/BME copolymer.
Example 7
Copolymerization of DMAM and BME (90/10 mol charge)
The same procedure was followed as in Example 6, except that the monomer ratio
charged was adjusted to 90/10 DMAM/BME and the AIBN used as the initiator was changed
to 0.1133 g (0.69 mmol). 14g of purified polymer was collected, representing a 92% yield.
The 'H and 13C NMR spectra were consistent with the formation of a 91.9/8.1 DMAM/BME
copolymer.
Example 8
Copolymerization of DMAM and Cyclohexylmaleimide (CHME) (90/10 mol charge)
Raw materials: DMAM (purchased from Polysciences) was purified by passing it through an
Aldrich MEHQ Inhibitor Remover Column (Catalogue # 30,631-2). CHME (purchased from
Aldrich) was used as received. Anhydrous benzene (Aldrich) was transferred using a cannula
or a syringe under a positive pressure of nitrogen. AIBN (Johnson-Matthey) was
recrystallized from methanol.
Copolymerization procedure: 12.45g (0.131 mol) of the de-inhibited DMAM described above
was combined with 2.5g (0.014 mol) CHME and 154 ml benzene in a three-necked reaction
- 19 -
2178364
kettle equipped with a thermometer, a stirrer, and a N2 inlet/outlet. (The kettle had been
previously purged with N2.) The solution was heated to 60C. Then, an AIBN solution
(0.1112g (0.68 mmol) in 4ml benzene) was injected into the monomer solution. The reaction
mixture was m~int~ined at 60C for 24 hr. The polymer was isolated by pouring the reaction
mixture into diethylether and collecting the polymer by filtration. The product was dried in a
vacuum oven overnight at 60C. The final purified product yield was 14g (93%). The lH
and 13C NMR spectra were consistent with the formation of a 89/11 DMAM/MME copolymer.
Example 9
Copolymerization of N-methyl-N-vinylacetamide (VIMA) and EME
Raw materials: VIMA monomer (Aldrich) was purified by distillation. EME monomer was
used as received from Aldrich. Anhydrous benzene (Aldrich) was transferred under an inert
atmosphere.
Copolymerization procedure. 11.4 g (0.115 mol) VIMA monomer and 3.6 g (0.029 moles)
EME monomer were dissolved in 154 ml benzene, loaded into a three-necked reaction kettle
equipped with a condensor, a thermometer, and a N2 inlet/outlet, and purged with N2 for one
hour. The solution was then heated to 60C. The reaction mixture was maintained at 60C
overnight, which resulted in a slightly viscous solution. The next day, the polymer was
precipitated into hexane, dissolved into acetone, then reprecipitated into hexane. The reaction
product was then dried at 40C and 10-3 torr overnight. The reaction product wascharacterized by IH and 13c and gel permeation chromatography. The VIMA/EME ratio
produced in the copolymer was about 62/38.
- 20 -
217836~
Example 10
Copolymerization of VIMA, DMAM and EME
Raw materials: VIMA (Aldrich) was purified by passing it through an Aldrich MEHQInhibitor Remover Column (Catalogue #30, 631-2). DMAM (Polysciences) was purified in a
similar manner. EME (Aldrich) was used without further purification. HPLC grade t-butanol
(packaged under nitrogen) was purchased from Aldrich and used without further purification.
2,2'-Azobis[2-methybutylnitrile] (V-67) (Dupont) was used without further purification.
Copolymerization procedure: 0.7g (7.67 x 10-3 mol) VIMA, 0.76g (7.67 x 10-3 mol) DMAM
and 0.48g (3.84 x 10-3 mol) EME were loaded into a Schlenk tube equipped with a magnetic
stirring bar. 8ml t-Butanol was added to the monomer solution and the mixture was purged
with N2 for 1 hour. The solution was then heated to 80C with stirring. Once thetemperature was reached, an initiator solution of an N2-purged solution of 20mg V-67 in lml
t-butanol was injected into the monomer solution. The reaction proceeded under N2 at 80C
overnight. The next day, the reaction mixture was precipitated into diethylether. The reaction
product was collected by vacuum filtration and dried at 50C and 10-3 torr overnight. The 'H
and l3C NMR spectra were consistent with the formation of a 30/54/16 (mol%)
VIMA/DMAM/EME terpolymer.
Copolymerization of Various Maleimides with Vinyl Lactams and Other Acrylamides and N-
Vinyl Amides
It will be apparent to those skilled in the art of polymer synthesis that various
maleimides can produce copolymers with vinyl lactams, such as N-vinylpyrrolidone (VP) and
N-vinylcaprolactam, various other acrylamides, such as acryloylpyrrolidine (APYD), N,N-
diethylacrylamide (DEAM), N-ethylacrylamide (EAM), and N-isopropylacrylamide (iPAM),
other N-vinyl amides, such as N-vinyl-N-n-propylpropionamide (VPP), and alkenyl cyclic
~17836~
imino ethers, such as 2-iso-propenyl-2-oxalizone, using procedures substantially similar to
those described above.
INHIBITOR EVALUATION
Mini-Loop Testin~ Procedure
One method for evaluating the effectiveness of an inhibitor uses a bench-scale high
pressure apparatus referred to as a mini-loop apparatus. A mini-loop apparatus consists of a
loop of stainless steel tubing with about a one-half inch inside diameter and about ten feet in
length. The loop also has a transparent section for observing the fluid flow in the loop and
the onset of hydrate formation in the loop. Fluid comprising about 40% by volume SSW
(Synthetic Sea Water) solution having about 3.5% total ionized salts, 40% by volume
hydrocarbon condensate (i.e., C6+), and 20% by volume hydrocarbon gas mixture is circulated
around the loop at constant pressure. The hydrocarbon gas mixture is comprised of about 76
mole% methane, 9 mole% ethane, 7 mole% propane, 5 mole% n-butane, 2 mole% iso-butane,
and 1 mole% of C5+. The inhibitor is typically injected into the loop as an aqueous solution
to produce the desired weight percent concentration of inhibitor in the aqueous sea salt/gas
solution. Generally, many hydrate inhibitors are evaluated at about 0.5 wt.% of the aqueous
sea salt/gas solution.
The fluid is circulated at a constant velocity of about 2.5 feet/second. The loop and its
pump lay in a controlled temperature water bath for controlling the temperature of the fluid
circulating in the loop. Water is circulated to ensure uniform temperature throughout the bath
and rapid heat transfer between the bath water and the loop. As the loop temperature changes
or as hydrates form, the gas volume in the loop will change accordingly. Therefore, to
mzlint~in constant pressure in the loop, a pressure compensating device is required. Such a
device can be comprised of a gas cell and a hydraulic oil cell separated by a floating piston.
So as the gas volume in the loop changes, oil may be added or removed from the oil cell to
produce a commensurate addition or removal of gas to the loop. Mini-loop tests are typically
2~78364
,
run at a pressure of about 1,000 pounds per square inch gauge (p.s.i.g.). However, any
pressure between 0 and 3,000 p.s.i.g. could be selected for evaluating the performance of an
inhibitor.
The temperature of the water bath is reduced at a constant rate, preferably about 6F
or 3.3C per hour, from an initial temperature of about 70F or 21C. At some temperature,
clathrate hydrates begin to rapidly form. As the dissolved gas is used to form clathrate
hydrates there is an abrupt and corresponding decrease in the volume of dissolved gas in the
aqueous sea salt/gas solution. The temperature at which this abrupt decrease in the volume of
dissolved gas is observed is known as the temperature of onset for hydrate formation (Tos).
Rec~lling from the discussion above, the hydrate equilibrium dissociation temperature or Teq is
the temperature below which hydrate formation is thermodynamically favored in an aqueous
sea salt/gas solution without an inhibitor present. Therefore, another measure of an inhibitor's
effectiveness is the difference between Teq and Tos which is known as the inhibitor's
subcooling temperature, Tsub Therefore, for a given pressure, the greater the subcooling
temperature the more effective the inhibitor. Typically, an aqueous sea salt/gas solution with
no inhibitor present produces a Tsub of about 6-7F or 3.3-3.9C.
Mini-Loop Test Results
Without limiting the scope of the invention, and for the purpose of illustrating the
invention, various maleimide copolymers were evaluated using the mini-loop testing procedure
described above. The results of these evaluations, where available, are presented below:
- 23 -
2178364
TABLE I
MINI-LOOP TEST RESULTS WITH POLYMERIC INHIBITORS
INHIBITOR Ratio CONC. MINI-LOOP MINI-LOOP
within Wt% SUBCOOLING SUBCOOLING
Polymer ~T (F) I~T (C)
None NA NA 7.0 3.9
PDMAM* NA 0.5 11.4 6.3
PVIMA* NA 0.5 12.5 6.9
DMAM/ME 70:30 0.5 16.2 9.0
DMAM/EME 52:48 0.5 17.2 9.6
DMAM/PME 74:26 0.5 17.5 9.7
DMAM/BME 74:28 0.5 16.8 9.3
VIMA/EME 62:38 0.5 20.0 11.1
VIMA/DMAM/EME30:54:16 0.5 18.0 10.0
* Values for maleimide homopolymers where R3 is not hydrogen are not given due to
insolubility in water.
Generally, copolymerizing DMAM with various maleimides, such as ME, EME, PME,
and BME, enhanced inhibitor activity relative to the inhibitor activity for PDMAM and
PVIMA. Other maleimides, such as BME and CHME, may similarly enhance inhibitor
activity when copolymerized with DMAM or VIMA. Also, other maleimide copolymers
formed with vinyl lactams, such as N-vinylpyrrolidone (VP), N-vinylcaprolactam (VCap), and
other N-vinyl amides, such as N-vinyl-N-n-propylpropionamide (VPP), acrylamides, such as
N,N-diethylacrylamide (DEAM), acryloylpyrrolidine (APYD), and alkenyl cyclic imino ethers,
such as 2-iso-propenyl-2-oxazoline (iPpenOx) may enhance inhibitor activity.
The means and method of the invention and the best mode contemplated for practicing
the invention have been described. It is to be understood that the foregoing is illustrative only
and that other means and techniques can be employed without departing from the true scope
of the invention as claimed herein.
- 24 -