Note: Descriptions are shown in the official language in which they were submitted.
- 21~8~fi8
DIFFERENTIAL CONDUCTIVITY HEMODYNAMIC MONITOR
Field of the Invention
This invention relates to measurement of multiple
hemodynamic variables. More particularly, this invention
relates to measurement of the hemodynamic variables
during a medical procedure or for diagnostic purposes
using a differential conductivity monitor to measure or
detect at least one of recirculation efficiency, flow
rate or the presence of air bubbles.
Background of the Invention
In many medical situations it is desirable to
quantitatively determine, or measure, various hemodynamic
parameters, such as the recirculation rate or the
recirculation efficiency of a biological or medical fluid
to increase the benefits of, or decrease the time
required for, a therapeutic treatment, or for diagnostic
purposes. For example, hemodialysis (herein "dialysis")
is an inconvenient, expensive, and uncomfortable medical
procedure. It is, therefore, widely recognized as
desirable to minimize the amount of time required to
complete the procedure and to achieve a desired level of
treatment.
In dialysis, a joint is typically surgically created
between a vein and an artery of a patient undergoing
dialysis. The joint provides a blood access site where
an inlet line to a dialysis apparatus and an outlet line
from the dialysis apparatus are connected. The inlet
line draws blood to be treated from the patient, while
the outlet line returns treated blood to the pat.i.ent.
This joint may be an arteriovenous fistula, which is
a direct connection of one of the patient's veins to one
of the patient's arteries. Alternatively the joint may
2~7~46~
be a synthetic or animal organ graft connecting the vein
to the artery. As used herein, the term "fistula" refers
to any surgically created or implanted joint between one
of the patient's veins and one of the pati.ent's arteries,
however created.
In the fistula a portion of the treated blood
returned to the patient by the outlet line may
recirculate. Recirculating treated blood will co-mingle
with untreated blood being withdrawn from the patient by
the inlet line. This recirculation, and the resulting
co-mingling of treated and untreated blood, is dependent,
in part, on the rate at which blood is withdrawn from and
returned to the patient. The relationship is typically a
direct, but non-linear relationship. It can be readily
appreciated that the dialysis apparatus will operate most
effectively, and the desired level of treatment achieved
in the shortest period of time, when the inlet line is
drawing only untreated blood at the maximum flow rate
capability of the dialysis apparatus consistent with
patient safety. As a practical matter, however, as flow
rate through the dialysis apparatus is increased, the
proportion of recirculated treated blood in the blood
being drawn through the inlet line is increased. In
order to select the flow rate through the dialysis
apparatus, it is desirable to know the proportion of
recirculated treated blood in the blood being withdrawn
from the patient by the inlet line. This proportion is
referred to herein as the "recirculation ratio". The
recirculation ratio can also be defined as the ratio
between the flow of recirculated blood being withdrawn
from the fistula to the flow of blood being returned to
the fistula. Recirculation efficiency may then be
defined by the relationship:
2
- 2178468
E = 1 - R (Equation 1)
where
E = Recirculation efficiency
R = Recirculation ratio
Alternatively, recirculation efficiency may be
equivalently expressed as the ratio of blood flow being
returned to the fistula, but not being recirculated, to
the total blood flow being returned to the fistula.
Knowing the recirculation efficiency, the dialysis
apparatus operator can adjust the flow rate through the
dialysis apparatus to minimize the time required to
achieve the desired level of treatment.
In the prior art, quantitative determination of
recirculation ratio or recirculation efficiency has
typically required laboratory testing, such as blood urea
nitrogen tests, which take considerable amounts of_time
and which require withdrawing blood from the patient,
which is recognized as undesirable.
A method and apparatus for qualitatively detecting
the presence or absence of recirculation in a fistula is
described in "FAM 10 Fistula Flow Studies and their
Interpretation" published by Gambro, Ltd. based on
research performed in 1982. The Gambro method and
apparatus injects a quantity of a fluid having an optical
density less than the optical density of treated blood
into the dialysis apparatus outlet line. A resulting
change in the optical density of the blood being drawn
through the dialysis apparatus inlet line is
qualitatively detected as indicative of the presence of
recirculation. The Gambro method and apparatus does not
quantitatively determine or measure a recirculation ratio
or recirculation efficiency.
3
CA 02178468 1999-10-OS
Devices which qualitatively determine recirculation
by thermal techniques are also known.
A quantitative measurement of the recirculation
efficiency of a bodily or medical fluid is useful in
other therapeutic and diagnostic procedures as well. For
example, recirculation ratios and efficiencies are useful
for determining cardiac output, intervascular
recirculation, recirculation in non-surgically created
access sites, and f.ialyzer performance from either the
blood side or the dialysate side of the dialyzer, or
both.
It is known that the electrical conductivity of a
fluid in a closed non-metallic conduit can be measured
without contact with the fluid by inducing an alternating
electrical current in a conduit loop comprising a closed
electrical path of known cross sectional area and length.
The magnitude of t:he current thus induced is
proportional to the' conductivity of the fluid. The
induced current magnitude may then be detected by
inductive sensing t:o give a quantitative indication of
fluid conductivity. A conductivity cell for measuring
the conductivity of: a fluid in a closed conduit without
contact with the f7_uid is described in U.S. Patent No.
4,740,755 entitled "Remote Conductivity Sensor Having
Transformer Coupling In A Fluid Flow Path," issued April
26, 1988 to C>gawa and assigned to the assignee of the
present invention.
It is further desirable to have a way of detecting
the presence of air in a dialysis apparatus outlet line
to minimize t:he probability of air being returned to a
patient in tree outlet line. It is further advantageous
to have a means of determining a volume flow rate of
4
CA 02178468 1999-10-OS
fluid flowing in the inlet and outlet tube of the
dialysis apparatus.
Air bubble detectors which detect the presence of an
air bubble sonically, ultrasonically or optically are
known, but a :more sensitive device that is not subject to
sonic or optical shadows or distortion is desirable.
It is further desirable to measure a flow rate of a
fluid in a tube, either as a part of a recirculation
monitoring procedure, or as a separately measured
hemodynamic parameter.
It is still further desirable to provide a
hemodymamic monitoring device which is capable of
monitoring more than one hemodynamic parameter, in order
to reduce system cost and increase system flexibility.
It is against this background that the differential
conductivity hemodynamic monitor of the present invention
developed.
Summary of the Invention
A significant aspect of the present invention is a
method and apparatus for detecting the presence of air
bubbles by monitoring the conductivity of a fluid in
which the air bubbles may be entrained. In accordance
with this aspect the present invention comprises a method
for detecting the presence of air bubbles entrained in a
liquid flowing through a tube by flowing the liquid
through a first conduit, said first conduit comprising a
first conductivity cell with a continuous path
configuration, inducing a first electrical current in the
liquid in thE~ first. conductivity cell, sensing the first
electrical current in the liquid in the first
conductivity cell, and interpreting a decrease in the
first electrical current as indicative of the presence of
an air bubblE~ in the liquid.
5
CA 02178468 1999-10-OS
Preferably, the inducing step further comprises
positioning an Exciting electromagnetic coil in proximity
with the conducv~ivity cell at an inducing location and
inducing the first electrical current along the continuous
path of the first conductivity cell. In accordance with this
aspect of the invention the sensing step may comprise
positioning a sensing electromagnetic coil in proximity with
the first conductivity cell at a sensing location.
The method may :Further comprise flowing the liquid
through a second conduit, said second conduit comprising a
second conductivity cell with a continuous path
configuration, inducing a second electrical current in the
liquid in the ~,econd conductivity cell, and sensing the
second electrical current in the liquid in the second
conductivity cel~_, the interpreting step further comprising
subtracting the second electrical current from the first
electrical current to produce a signal representative of the
difference in tree conductivity between the liquid in the
first and second conductivity cells, a decrease in the
conductivity of the liquid in the first conductivity cell
with respect to t:he conductivity of the liquid in the second
conductivity cell. being indicative of the presence of an air
bubble in the liquid in the first cell.
The inducing step may further comprise positioning the
exciting electromagnetic coil in proximity with the first and
second conductiv_Lty cells at an inducing location, inducing
the first electrical current in an electrical direction along
the continuous ~~ath of the first conductivity cell, and
simultaneously inducing the second electrical current to flow
in the same electrical direction along the continuous path of
the second conductivity cell as the direction of the first
electrical current.
6
CA 02178468 1999-10-OS
The sensing and subtracting steps may further comprise
positioning a sensing electromagnetic coil in proximity with
the first and second conductivity cells at a sensing location
with the first conductivity cell oriented at the sensing
location with th.e first electrical current in an opposite
electrical dirE:ction with respect to the sensing
electromagnetic ~~oil i_rom the electrical direction of the
second electrical current with respect to the sensing
electromagnetic coil.
Another significant aspect of the present invention is
an apparatus for detecting the presence of an entrained air
bubble in a liquid having a conductivity flowing in a tube,
comprising a first conduit through which the liquid flows,
said first conduit comprising a first conductivity cell with
a first conductivity cell upstream connection, a first
conductivity cell downstream connection, and two branches
connecting the upstream connection to the downstream
connection with a continuous path configuration from the
upstream connect_!_on to the downstream connection through one
of the two branches and returning to the upstream connection
through the other one of the two branches, means for inducing
a first electrical current in the liquid in the first
conductivity cell, means for sensing the first electrical
current in the liquid in the first conductivity cell, and
means for interpreting the sensed electrical current, a
decrease in electrical current being indicative of the
presence of entrained air.
The inducing means may comprise an exciting
electromagnetic coil in proximity with the first conductivity
cell at an exiting location and the
7
CA 02178468 1999-10-OS
sensing means may further comprise a sensing electromagnetic
coil in proximity with the first conductivity cell at a
sensing location.
Further in accordance with this aspect of the
invention the apparatus may comprise a second conduit
through which the liquid flows, said second conduit
comprising a second conductivity cell with a second
conductivity cell upstream connection, a second
conductivity cell downstream connection, and two branches
connecting the upstream connection to the downstream
connection with a continuous path configuration from the
upstream connection to the downstream connection through
one of the two branches and returning to the upstream
connection through the other one of the two branches,
means for inducing a second electrical current in the
liquid in the second conductivity cell, means for sensing
the second electrical current in the liquid flowing in
the second conductivity cell, and means for subtracting
the second electrical current from the first electrical
current to produce a signal representative of the
difference in the conductivity between liquid in the
first and second conductivity cells, a low conductivity
of the liquid in the first conductivity cell with respect
the conductivity of the liquid in the second cell being
indicative of the presence of entrained air.
Still further in accordance with this aspect of the
invention the inducing means may comprise an exciting
electromagnetic coil in proximity with the first and
second conductivity cells at an exciting location, the
first conductivity cell being oriented at the exciting
location with respect to the second conductivity cell
with the fir~;t electrical current in an electrical
direction with respect to the exciting electromagnetic
8
CA 02178468 1999-10-OS
coil and the ~>econd electrical current in the same
electrical direction with respect to the exciting
electromagnet7_C CO1:L. The sensing means and the
subtracting means may further comprise a sensing
electromagnetic coi:L in proximity with the first and
second conductivity cells at a sensing location, the
first conductivity cell being oriented at the sensing
location with respect to the second conductivity cell
with the elect:rical direction of the first electrical
current with respect to the sensing electromagnetic coil
opposite the electrical direction of the second
electrical current with respect to the sensing
electromagnet_Lc coil.
A further significant aspect of the present
invention is <~n apparatus capable of performing a
plurality of hemody:namic parameter determinations. In
accordance wii=h this aspect of the invention the
apparatus detf~cts the presence of entrained air in the
tubing and further is suitable for use as a recirculation
monitor for determining a degree of recirculation of a
fluid in a zone of a vessel.
Brief Description of the Drawings
Fig. 1 is a schematic diagram of a dialysis system
incorporating a differential conductivity recirculation
monitor in accordance with the present invention.
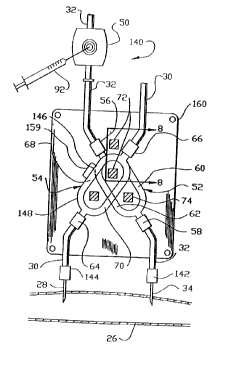
Fig. 2 is a partial perspective view illustrating
the functional elements of the differential conductivity
recirculation monitor shown in Fig . 1.
Fig. 3 is an electrical schematic diagram of the
differential conductivity recirculation monitor shown in
Fig. 2.
9
21~846~
Fig. 4 is an electrical block diagram of sensing
logic usable with the differential conductivity
recirculation monitor illustrated in Figs. 2 and 3.
Fig. 5 is a graph illustrating differential
conductivity versus time during a recirculation test
employing the differential conductivity recirculation
monitor shown in Fig. 2.
Fig. 6 is a graph illustrating the integral of
differential conductivity versus time during a
recirculation test employing the differential
conductivity recirculation monitor shown in Fig. 2,
having substantially the same time scale as Fig. S.
Fig. 7 is a partial el.evational view of a tubing set
and sectional view of an excitation and sensing unit for
use with the dialysis system shown in Fig. 1,
incorporating the differential conductivity recirculation
monitor in accordance with the present invention.
Fig. 8 is a partially diagrammatic sectional view
taken substantially at line 8-8 in Fig. 7.
Fig. 9 is a partially diagrammatic perspective view
of the excitation and sensing unit of the differential
conductivity recirculation monitor of the present
invention.
Fig. 10 is a diagrammatic representation of the
passage of an ideal bolus of saline and an actual bolus
of saline through a conductivity cell of the present
invention.
Fig 11 is an illustration of the output signals from
the conductivity cell of Fig 10.
Detailed Description of the Preferred Embodiment
Fig. 1 illustrates a dialysis system 20
incorporating a differential conductivity recirculation
21~84s8
monitor 22 for determining and displaying recirculation
efficiency in accordance with the present invention. The
dialysis system 20, which is one example of a medical
system with which the present invention may be
advantageously used, comprises a dialysis apparatus 24
connected to a fistula 26 surgically formed in a dialysis
patient (not shown). Untreated blood is drawn from the
fistula 26 through a dialyzer inlet needle 28 and a
dialyzer inlet line 30. Treated blood is returned to the
fistula through a dialyzer outlet line 32 and a dialyzer
outlet needle 34. The recirculation monitor 22 is
located in the dialyzer inlet and outlet lines 30 and 32
at a point intermediate between the fistula 26 and the
dialysis apparatus 29.
The dialysis apparatus 24 comprises a blood pump 36
typically a peristaltic pump, a dialyzer 38 having a
blood compartment 40 and a dialysate compartment 42
separated by a semi-permeable membrane 44, a bubble trap
46 and a dialysate generator 48. Blood is drawn from the
fistula 26 by the action of the blood pump 36 and passed
through the blood compartment 40 of the dialyzer 38. The
membrane 44 allows transfer of impurities in the blood,
such as urea and creatinine, from the blood compartment
40 to the dialysate compartment 42 of the dialyzer 38.
The dialysate compartment 42 is connected to a di.alysate
generator 98 which generates the dialysate, a liquid
isotonic to blood, and circulates it through the
dialysate compartment 44.
The principles of operation of the differential
conductivity recirculation detector 22 of the present
invention are explained in conjunction with Figs. 2 and
3. The recirculation detector 22 comprises a needle
access site 50 in the dialyzer outlet line 32. A first
11
217848
or outlet conductivity cell 52 is located in the dialyzer
outlet line 32 downstream of the needle access site 50.
A second or inlet conductivity cell 54 is located in the
dialyzer inlet line 28. The first conductivity cell 52
comprises an upstream connection 56, a downstream
connection 58 and first and second tubing branches 60 and
62, respectively, each of which interconnect the upstream
connection 56 with the downstream connection 58. Treated
blood from the dialyzer flows in the dialyzer outlet 7_ine
32 through the needle access site 50 to the upstream
connection 56. At the upstream connection 56 the flow
splits approximately equally with a portion of the
treated blood flowing in each of the two tubing branches
60 and 62 of the outlet conductivity cell 52. The flow
rejoins at the downstream connection 58 and flows through
the dialyzer outlet line 32 to the fistula 26 (Fig. 1).
Similarly, the inlet conductivity cell 54 comprises an
upstream connection 64, a downstream connection 66 and
third and fourth tubing branches 68 and 70, respectively,
which each connect the upstream connection 64 to the
downstream connection 66. Untreated blood from the
fistula 26 flowing in the dialyzer inlet line 30, enters
the inlet conductivity cell 54 at the upstream connection
64 divides approximately equally between the third and
fourth tubing branches 68 and 70 and rejoins at the
downstream connection 66 to the inlet conductivity cell
59. Each one of the tubing branches 60, 62, 68 and 70
has the same cross sectional area and length as each
other one of the tubing branches.
The blood, or other biological or medical fluid,
flowing in each conductivity cell 52 and 59 comprises an
electrical circuit. The electrical circuit is a path for
circulation of an electrical current from the upstream
12
. - 217~4~8
connection, through one of the tubing branches, to the
downstream connection and from the downstreafi connection
through the other one of the tubing branches to the
upstream connection.
The outlet conductivity cell 52 and the inlet
conductivity cell 54 are positioned adjacent to each
other in an angular relationship resembling a pretzel so
that the first tubing branch 60 of the outlet
conductivity cell 52 is positioned parallel to the third
tubing branch 68 of the inlet conductivity cell at an
excitation location. The conductivity cells are further
positioned so that the second tubing branch 62 of the
outlet conductivity cell 52 crosses the fourth tubing
branch 70 of the inlet conductivity cell 54 at an angle,
approximately sixty degrees in the preferred embodiment,
at a sensing location. An excitation coil 72 enc.i.rcles
the first tubing branch 60 of the outlet conductivity
cell 52 and the third tubing branch 68 of the inlet
conductivity cell 54 at the excitation location. A
sensing coil 74 encircles the second tubing branch 62 of
the outlet conductivity cell 52 and the fourth tubing
branch 70 of the inlet conductivity cell 54 at the
sensing location.
An electrical circuit, as is illustrated
schematically in Fig. 3, is thus formed. The excitation
coil 72 is inductively coupled to the outlet conductivity
cell 52 and the inlet conductivity cell 54. When a
source of excitation energy 76 causes an alternating
excitation current, illustrated by direction arrow 78, to
flow in the excitation coil 72 a changing magnetic field
is generated which causes an electrical current,
illustrated by the direction arrow 80, to flow in the
blood in the outlet conductivity cell 52 and causes
13
2178468
another electrical current, illustrated by direction
arrow 82, to flow in the same electrical direction in the
blood in the inlet conductivity cell 54. Since the
conductivity cells 52 and 54 are formed to create
electrical paths of equal cross sectional area and equal
path length the electrical conductance of the paths, as
illustrated by the schematic resistors 84 and 86, and
thus the magnitude of the induced currents 80 and 82,
will be related to the conductivity of the blood in the
respective conductivity cells 52 and 54.
The induced currents 80 and 82 flowing in the outlet
and inlet conductivity cells 52 and 54 generate a
changing magnetic field at the sensing location that
induces a sensed current, illustrated by direction arrow
88, in the sensing coil 74. The induced currents 80 and
82 are in opposite electrical directions so that the
magnetic field at the sensing location has a magnitude
proportional to the difference between the induced
currents. The sensed current 88 is proportional to the
magnetic field at the sensing location where the sensing
coil 74 encircles the second and fourth tubing branches
62 and 70, respectively. The sensed current 88 induced
in the sensing transformer 74 is therefore proportional
to a difference between the induced currents 80 and 82 in
the outlet and inlet conductivity cells 52 and 54,
respectively. The induced currents 80 and 82 in the
outlet and inlet conductivity cells 52 and 54,
respectively, are related to the conductivity of the
fluids in those chambers. Therefore, the magnitude of the
sensed current 88 induced in the sensing coil 74 will be
related to the difference between the conductivities of
the fluids in the outlet and inlet conductivity cells 52
and 54. The sensed current 88 is delivered to, and
19
. - 217468
interpreted by a sensing logic and display circuit 90,
which displays the recirculation efficiency..
It should be appreciated that the present invention
will function in substantially the same way if the
locations of the exciting coil 72 and sensing coa.J_ 74 ar_e
reversed.
Referring now to Figs. 1 and 2, to use the
recirculation monitor 22 to perform a recirculation test
the dialysis system operator injects a bolus of a marker
fluid into the treated blood in the dialyzer outlet line
32 at the needle access site 50 using a typical
hypodermic needle 92. The marker fluid may have an
electrical conductivity that is higher or lower than the
fluid flowing in the outlet line 32. In the preferred
embodiment a high conductivity marker fluid is used to
avoid damaging blood cells. In the preferred embodiment
the bolus is 1 milliliter of 29 percent hypertonic saline
solution. The conductivity of the treated blood being
returned to the patient through the dialyzer outJ.et line
32 and the outlet conductivity cell 52 of the
recirculation monitor 22 is altered. This altered
conductivity blood enters the fistula through the outJ_et
needle 34.
If the flow balance in the fistula 26 is such that
no flow is recirculating the altered conductivity blood
will exit the fistula, as illustrated by the flow
circulation arrow 94, without altering the conductivity
of the blood within the fistula. If, however, the flow
balance within the fistula 26 is such that blood is
recirculating, as illustrated by flow circulation arrow
96, a portion of the blood withdrawn from the fistula 26
by the pump 36 will be the altered conductivity blood.
The recirculation monitor 22 measures the conductivity of
2178468
the blood flowing in the outlet line 32 and the
conductivity of the blood flowing in the inlet line 30
and quantitatively determines the difference between
those conductivities continuously throughout the
recirculation test. The sensing logic and display
circuit 90 interprets the quantitative conductivity
differences measured by the recirculation monitor 22 to
determine recirculation efficiency.
The determination of recirculation efficiency will
be explained by reference to Figs. 4, 5 and 6. The
outlet conductivity cell 52 and the inlet conductivity
cell 59 may be thought of as signal generators generating
the induced currents 80 and 82 in the outlet and inlet
conductivity cells. The induced current 82 of the inlet
conductivity cell 54 is inverted 98 and added 100 to the
induced current 80 in the outlet conductivity cell 52, by
virtue of the physical relationships between the
conductivity cells, excitation coil 72 and sensing coil
74, to produce the sensed current 88.
The sensing logic and display circuit 90 performs a
zeroing operation 102, a dialyzer outlet flow determining
operation 109, and unrecirculated flow determining
operation 106, and a dividing operation 108, and includes
a visual display device 110, preferably a liquid crystal
display. Alternatively the functions of the sensing
logic and display circuit 90 may be performed by a
digital computer (not shown).
Fig. 5 is a graph illustrating differential
conductivity (reference 112) as a function of time
(reference 119) during a typical recirculation test.
Fig. 6 is a graph illustrating the integral of
differential conductivity (reference 116) as a function
of time 114 during the typical recirculation test. Prior
16
r _ 2178468
to the beginning of the recirculation test there may be
some normal difference (reference 118) between the
conductivity of the treated blood in the dialyzer outlet
line 32 (Fig. 2) and the untreated blood i.n the dialyzer
inlet line 30 (Fig. 2). This normal conductivity
difference 118 is subtracted from the sensed current 88
by the zeroing operation 102 of the sensing logic and
display circuit 90 to remove the effect of the normal
difference in conductivity 118 from determination of
recirculation efficiency. The recirculation test begins
(reference time Tl) when the bolus of high conductivity
fluid is injected into the dialyzer outlet line 32 (Fig.
2) at the needle access site 50 (Fig. 2). The
conductivity of the treated blood in the dialyzer outlet
line 32 (Fig. 2) is increased. As the bolus passes
through the outlet conductivity cell 52 (Fig. 2) the
differential conductivity 112 increases (reference 120)
and then decreases (reference 122) until the normal
conductivity difference 118 is reached (reference time
T2). The outlet flow determining operation 104
calculates the integral of conductivity from the start of
the test (reference time T1) until the differential
conductivity returns to the normal value 118 (reference
time T2). The integral 116 of the conductivity increases
(reference 124) until a first steady state value
(reference 126) of the integral 116 is reached when the
differential conductivity 112 returns to the normal value
118 (reference time T2). The first steady state value
126 is stored by the outlet flow determining operation
104 and is representative of the flow of treated blood in
the dialyzer outlet line 32 (Fig. 2). After the treated
blood with the altered conductivity enters the fistula 26
(Fig. 1) a portion of it may recirculate and be withdrawn
17
. - ~1~8468
from the fistula 26 (Fig. 1) through the dialyzer inlet
line 30 (Fig. 2). The conductivity of the untreated
blood in the inlet conductivity cell 54 is increased
(reference time T3), causing the differential
conductivity to decrease 128 and then increase 130,
returning to the normal value of conductivity difference
118 (reference time T4). The integral of differential
conductivity from the beginning of the recirculation test
(reference time T1) until the normal value of
conductivity difference 118 is reached again (reference
time T9) is calculated by the unrecirculated flow
determining operation 106 of the sensing logic and
display circuit 90. The integral of differential
conductivity 116 decreases (reference) to a second steady
state value 134 (reference time T9. The second
steady state value 134 of the integral of differential
conductivity is stored by the unrecirculated flow
determining operation 106 of the sensing logic and
display circuit 90 and is representative of the portion
of the bolus of high conductivity liquid that was not
recirculated. The second steady state value 134 is thus
representative of the unrecirculated portion of the
treated blood flow. The dividing operation divides the
second steady state value 134 by the first steady state
value 126 to calculate a recirculation efficiency 136.
The recirculation efficiency 136 is provided to the
operator as a visual output by the display device 110.
It will be apparent to those skilled in the art that
the sensing logic and display circuit 90 may be
implemented using analog or digital circuit devices and
that other calculation algorithms may be used to
calculate recirculation efficiency 138. Further, the
recirculation efficiency 138 may be calculated in real
18
2178468
time or, alternatively, the necessary data stored and the
calculations performed on the stored data.
Further details of the preferred embodiment of the
differential conductivity recirculation monitor will be
explained by reference to Figs. 7-11.
Fig. 7 illustrates a portion of a typical disposable
tubing set 140 incorporating conductivity cells 52 and 54
in accordance with the present invention. As is well
known in the art, it is highly desirable for all portions
of the tubing set 140 to be used with a dialysis system
to be disposable, in order to prevent cross contamination
and infection between patients. This is true of most
blood and other biological or medical fluid processing
systems.
Disposable tubing sets may be formed from a
plurality of plastic tubes, connectors, needles and
medical devices using techniques that are well known i.n
the art. The discussion of the tubing set 140 will
therefore be limited to a discussion of the differential
conductivity recirculation monitor 22 (Fig. 1) portion of
the tubing set.
The dialyzer outlet line 32 is a plastic tutee which
extends through the needle access site 50, into the
outlet conductivity cell 52. The outlet conductivity
cell 52 comprises a plastic conduit loop and includes the
upstream connection 56, elongated divided first and
second tubing branches 60 and 62, and the downstream
connector 58. The downstream connector 58 has mounted in
it an extension of the dialyzer outlet line 32, which is
mounted through a connector 192 to the outlet needle 34.
The dialyzer inlet needle 28 is connected through a
connector 144, to the dialyzer inlet line 30. The
19
2178468
dialyzer inlet line 30 is connected to the inlet
conductivity cell 54, which includes the upstream
connection 64, elongated divided third and fourth tubing
branches 68 and 70 respectively, and downstream connector
66. The dialyzer inlet line 30 extends from the
downstream connector 66 to the dialyzer apparatus 24
(Fig. 1) .
In the preferred embodiment the portion of the
dialyzer outlet line 32 between the dialyzer outlet
needle 34 and the downstream connector 58 of the outlet
conductivity cell 52 and the portion of the dialyzer
inlet line 30 between the dialyzer inlet needle 28 and
the upstream connector 64 of the inlet conductivity cell
54 must be sufficiently long so that the bolus of marker
fluid passes completely through the outlet conductivity
cell before any altered conductivity fluid from the
fistula 26 enters the inlet conductivity cell.
The conductivity cells 52 and 54 have the overall
shape of links in an ordinary chain, straight side
portions 146 being joined at their ends by semicircular
portions 148. In cross-section at the excitation
location, as shown in Fig. 8, the wall of each
conductivity cell 42 and 54 defines a D, the insides of.
the Ds providing conduit portions 150 and 152. ~1 flat
portion 154 of the D of the outlet conductivity cell 52
is abutted and adhered to a flat portion 156 of the D of
the inlet conductivity cell 54 along one pair of
semicircular portions 198 of the conductivity cells. The
other pair of circular portions 148 are separated so that
axes of the conductivity cells 52 and 54 define
therebetween an angle of approximately sixty degrees.
The flat portions 154 and 156 of the conductivity cells
52 and 54 are further joined along two of the straight
21784fi8
portions 146 at a location along the second and fourth
tubing branches 62 and 70, respectively at the sensing
location. An orientation tab 159 is formed on the inlet
conductivity cell 54.
Mating with tube set 140 is a tubing set acceptor
160. As shown in Fig. 9, the tubing set acceptor 160
comprises a portion of an excitation and sensing unit 1.62
which also includes a logic circuit module 164. The
tubing set acceptor 160 comprises a portion of a first,
or rear, acceptor plate 166 and a second, or front,
acceptor plate 168 joined by a hinge 169 for motion
between open and closed positions and provided with a
latch or spring (not shown) to hold the acceptor plates
in the closed position. The first acceptor 166 plate is
relieved to accept into appropriately-shaped indentations
170 thereof the outlet conductivity cell 52 (Fig. 2) and
portions the tubing set 140 (Fig. 7). The second
acceptor plate 168 is relieved to accept into
appropriately-shaped indentations 172 thereof the inlet
conductivity cell 54 and portions of the tubing set 140
(Fig. 7). An orientation tab recess 173 is defined by at
least one of the appropriately shaped indentations 170
and 172. The orientation tab recess 173 cooperates with
the orientation tab 159 (Fig. 7) of the tubing set 140
(Fig. 7) to assure that the tubing set is correctly
oriented when installed in the tubing set acceptor 160.
The tubing set acceptor 160 is sufficiently large to
support the conductivity cells 52 and 54 and enough of
the dialyzer outlet line 32 and dialyzer inlet line 30 so
that fluid flow patterns through the conductivity cells
are substantially repeatable, being relatively unaffected
by bends, curves, tubing movement, and other disturbances
or variations in the positions of the outlet and inlet
21
2178468
lines with respect to the conductivity cells during
measurement. ,
The excitation coil 72 and sensing coil 74 are
mounted to the tubing set acceptor 160. The excitation
coil 72 and sensing coil, 74 are positioned at right
angles to each other to minimize magnetic interference
between the coils. The excitation coil 72 comprises a
first, or rear, and a second, or front, half core 174 and
176, respectively. Similarly the sensing coil comprises
a third, or rear, and a fourth, or front, half-core 178
and 180 respectively. The first and third half-cores 174
and 178, respectively are mounted to the first acceptor
plate 166 and the second and third half cores 176 and 180
respectively are mounted to the second acceptor plate
186.
As illustrated in Fig. 8, each half core has a U-
shaped configuration, with short legs 182 having ends 184
and connecting legs 186. The tubing set acceptor 160
holds a portion of the tubing set 140 which includes the
conductivity cells 52 and 54 in a fixed relationship with
the excitation coil 72 and sensing coil 74.
The first and second half cores 174 and 176 are
oriented so that their ends 184 abut when the first and
second acceptor plates 166 and 168 are brought to the
closed position. The excitation coil 72 thus formed is
in the shape of a rectangle defining a rectangular
window. The third and fourth half cores 178 and 180 ar.e
similarly oriented so that their ends abut when the first
and second acceptor plates 166 and 168 are brought to the
closed position. The sensing coil 74 thus formed is a.l.so
in the shape of a rectangular ring defining a rectangular
window (not shown). When a tubing set 140 is placed in
the tubing set acceptor 160 the first and third tubing
22
2~7s4ss
branches 60 and 68 are engaged in the window of the
excitation coil 72 and the second and fourth tubing
branches 62 and 70 are engaged in the window of the
sensing coil 74 so that the coils encircle the
corresponding tubing branches. Biasing springs 188 may
be provided to hold corresponding half-cores in firm
contact when the acceptor plates 166 and 168 are closed.
The legs 182 and 186 of the coil 72 and 74 are
square in cross-section. At least one connecting leg 186
of each coil 72 and 74 is transformer wire wrapped 190.
The logic circuit module 164 of the excitation and
sensing unit 162 may be mounted to one of the acceptor
plates 168 or may be separate from the tubing set.
acceptor 160 with wiring interconnections (not shown) to
the tubing set acceptor 160. Further, either or both of
the logic circuit module 164 or the tubing set a.rceptor
160 may be incorporated into the dialysis apparatus 24.
The logic circuit module houses the sensing logic and
display circuit 90, with the display device 110 and one
or more manual input switches 192 to enable the operator
to perform such functions as turning the recirculation
monitor on and off, testing the operation of the monitor
and initiating recirculation test, and may also include
switches and displays associated with other hemodynamic
monitoring functions.
Although the display device 110 and manual input
switches 192 are shown in Fig. 9 as being on a side 194
of the logic circuit module 164 adjacent to the second
acceptor plate 168, in the preferred embodiment the
display device and manual input switches may be on a side
,196 opposite the second acceptor plate 168, or any other
side of the logic circuit module.
23
CA 02178468 1999-10-OS
The circuitry for conductivity measurement and
calibration may suitably be as set forth in the Ogawa
patent .
The apparatus and methods described above may
optionally be adapted to measure and detect other
hemodynamic parameters such as the presence of entrained
air in the treated blood returned to the patient from the
dialysis apparatus 24 through the dialyzer outlet line
32. For this use it is not necessary to inject saline at
the needle access site 50. Entrained air in the blood in
the form of a larger bubble will cause an electrical
discontinuity in the outlet conductivity cell 52 as it
passes through either of the tubing branches 60, 62 of
the outlet conductivity cell 52. This will cause the
magnitude of induced current 80 flowing in the outlet
conductivity cell _'~2 to be greatly reduced or turned off
completely, d.ependi.ng on the size of the bubble.
Further, a plurality of small bubbles will effectively
reduce the conducting volume of the blood in the tubing
branches 60, 62 of the conductivity cell, decreasing the
conductance, and therefore the induced current 80, in the
outlet conductivity cell 52.
By sensing th~_s reduction in the outlet conductivity
cell 52 induced current 80 the passage of a bubble or a
plurality of bubbles can be detected, and corrective
action taken, if nE~cessary, to minimize their
introduction into i~he patient through the outlet line 32
and outlet nE~edle :?8. Corrective action may include
turning off t:he dialysis apparatus 24, closing a venous
clamp (not shown) and/or activating indicator or alarm
devices to a::ert a human operator of the presence of the
air bubble of bubb:Les.
24
. - 2~784~8
In the preferred embodiment, a difference in the
conductivity of the blood in the outlet conductivity cell
52 of the outlet line 32 and the blood in the inlet
conductivity cell of the inlet line 30 is substantially
constantly monitored. When one or more air bubbles enter
the outlet conductivity cell 52, causing the conductance,
and thus the induced current 80 and resulting sensed
conductivity of the fluid in the cell 52, to decrease
relative to the conductivity of the blood in the inlet
conductivity cell 59, this decrease is sensed by logic in
the sensing logic and display circuit 90 of the logic
circuit module 169 of the excitation and sensing unit
162. If the conductivity of the blood in the outlet
conductivity cell 52 is sufficiently lower than the
conductivity of the blood in the inlet conductivity cell
54, this conductivity difference is interpreted as the
presence of entrained air in the outlet line 32.
The apparatus and methods described above may
optionally be adapted to measure the hemodynamic
parameter of blood volumetric flow in the outlet line 32.
Blood volumetric flow rate may be measured and displayed
as and incident to the measurement of a degree of
recirculation, as described above, or may be measured in
a separate blood volumetric flow monitoring procedure.
The measurement of blood volumetric flow using the
differential conductivity sensor of the present apparatus
will be explained by reference to Figs. 10 and 11. The
conductivity of a fluid is directly proportional to the
concentration of conductivity producing ions in the
fluid. Consider an ideal bolus 202 of hypertonir saline
solution having a known volume vol and a known mass of.
conductivity altering ions M. The ion concentration of
this ideal bolus 202 would be:
2178468
C= o (Equation 2)
If this bolus were injected at the needle access site 50
into the outlet line 32, which is a tube of known cross-
sectional area a, into fluid flowing at a flow rate Q,
corresponding to a velocity V, the ideal bolus would pass
through the outlet line 32 in the form of a cylinder
having a length L, L being defined as:
vol
L = (Equation 3)
a
As this ideal bolus 202 passes through the outlet
conductivity cell 52 it would cause the conductivity cell
to sense a square pulse 204 of altered differential
conductivity having a magnitude proportional to the ion
concentration C of the bolus and a duration t,
proportional to the length L of the bolus 202 and the
flow rate Q of the fluid. The flow rate of the fluid can
then be calculated as:
Q=Va= ~~ _ ~ (Equation 4)
Note that Ct, is the area under the sensed square pulse
204.
In reality the bolus 202' of known volume vol and
known mass of conductivity altering ions M will not take
the form of a perfect cylinder, but will exhibit gradual
leading edge curve 206 and trailing edge curve 208, and
will further diffuse into the fluid in the outlet line
32. The differential conductivity pulse 204' caused by
the passage of the bolus 202' through the outlet
conductivity cell 52 will deviate substantially from a
square pulse and will have gradually increasing and
decreasing leading and trailing edges 210, 212
corresponding to the leading and trailing edges 206, 208
26
2178468
of the bolus. Furthermore, the time t2 that the bolus
202' takes to pass through the outlet conductivity cell
will be longer than the time tlfor an ideal bolus 202.
In order to determine the flow rate, Q it is necessary to
determine the area under the differential conductivity
curve by integrating the output over time as follows:
M
(Equation 5)
f C( t )dt
0
Thus, if a bolus of saline of a known volume vol and
a known concentration of conductivity altering ions Ck is
injected into the needle access site 50, the flow rate of
the fluid can be determined to be:
Q- Ck*vol (Equation 6)
~z
f C(t)dt
0
When the fluid flowing in the conductivity cell 32
has a background conductivity, representing a background
concentration Cbof conductivity, measured by the outlet
conductivity cell 52 immediately prior to the passage of
the bolus 202' through the cell 52, representing a
background level of conductivity producing ions, the
effect of the background level must be subtracted to
obtain the correct value of flow:
~- (Ck-Cb)*vol
(Equation 7)
f (C(t)-C6)dt
0
In the differential conductivity cell 22 of the preferred
embodiment Cb is representative of a difference in
background concentration, and hence conductivity, between
the fluid in the outlet conductivity cell 52 and the
fluid in the inlet conductivity cell. If, under steady
state conditions, the conductivity of fluid in the outlet
27
~1~~4~8
cell 52 is the same as the conductivity in the inlet
cell, then the background concentratin C~ is,zero. The
preferred embodiment of the present invention may
optionally be provided with selectably engageabl_e logic
to analyze a differential conductivity pulse from the
bolus 202.' of saline passing through the outlet
conductivity cell 52 and generate a value indicative of
the flow rate through the conductivity cell. This value
may be selectively displayable on the same display device
110 as is used to display a degree of recirculation. The
bolus of saline 202' may optionally be the same bolus
used to determine a degree of recirculation, in which
case the flow rate will be determined substantially
simultaneously with the degree of recirculation and
displayed simultaneously of sequentially therewith.
The apparatus and methods described above may
optionally be further adapted to incorporate the
capability of measuring or detecting more than one
hemodynamic parameter into a single differential
conductivity measuring apparatus.
The preferred embodiments of the present invention
has been described by reference to determination of
recirculation efficiency in a surgically created blood
access site during, or in conjunction with, a
hemodialysis procedure. It should be understood that the
present invention is not so limited. The present
invention may be used in a variety of medical and non-
medical circumstances where it is desirable to determine
recirculation efficiency. Further, it should be
understood that the present invention may be used in a
variety of medical and non-medical circumstances where it
is desirable to compare the electrical conductivities of
two fluids. Presently preferred embodiments of the
28
~1'~~4G8
present invention and many of its aspects, features and
advantages have been described with a degree of
particularity. It should be understood that this
description has been made by way of preferred example,
and that the invention is defined by the scope of the
following claims.
29