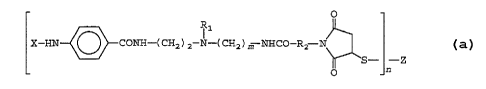Note: Descriptions are shown in the official language in which they were submitted.
VVO 95116894 PCTlUS94114484
-1-
4
The present invention relates to novel~derivatives
of procainamide and N-acetylprocainamide (NAPA). The
derivatives include immunogens used to stimulate antibody
production and polypeptide conjugates useful in
immunoassays for detecting procainamide and NAPA. Also
provided are hapten intermediates in the synthesis of the
immunogena and polypeptide conjugates.
The cardiac depressant drugs procainamide and N-
acetylprocainamide are used clinically to treat or
prevent cardiac arrhythmia. N-acetylprocainamide
(abbreviated as NAPA and also known as acecainide) is the
major.metabolite of procainamide in man. The
concentration of this metabolite in the plasma of
patients receiving procainamide often exceeds the
concentration of the parent drug itself. Metabolism is
by in vivo acetylation, and much genetic-based variation
has been observed in the rate at which individual
patients transform the drug to its-metabolite. This
phenomenon is of importance in the clinical use of the
" drugs because of the lower incidence of aide effects
associated with NAPA. Both the therapeutic usefulness
and the toxicity of the drugs are better correlated with
their blood levels than with their-dosages. The
relationship between the amount of drug administered and
WO 95116894 PCTIUS94114484
-2-
the blood levels is quite variable. It is influenced by
completeness of absorption, distribution characteristics
and rates of metabolism and excretion. ,
Because of these considerations, numerous analytical
methods have been developed to determine blood levels of
these drugs, including high pressure liquid
chromatography (HPLC), quantitative thin layer
chromatography (TLC), and immunoassay, including enzyme
immunoassay and immunoassay using fluorescence
techniques. Competitive binding immunoassays have proved
to be particularly advantageous. In such assays, an
analyte in a biological sample competes with a labeled
reagent, or analyte analog, or tracer, for a limited
number of receptor binding sites on antibodies specific
for the analyte and analyte analog. Enzymes, fluorescent
molecules, and radioactive compounds are common labeling
substances used as tracers. The concentration of analyte
in the sample determines the amount of analyte analog
which binds to the antibody. The amount of analyte
analog that will bind is inversely proportional to the
concentration of analyte in the sample, because the
analyte and, the analyte analog each bind to the antibody
in proportion to their respective concentrations. The
amount of free or bound analyte analog can then be
determined by methods appropriate to the particular label
being used.
CA 02178915 2000-11-28
- 3 -
One type of competitive binding immunoassay is based
upon the reassociation of polypeptide fragments to form
active enzymes as a :step of generating a detectable
signal utilized. to determine the amount of analyte
present in a sample. This type of assay, known as cloned
enzyme donor. immunoassay (CEDIA), is described in U.S.
Pat. No. 4,708,929. In particular, a (3-galactosidase
enzyme donor polypept:ide combines with a (3-galactosidase
enzyme acceptor polypeptide to form active ((3-
galactosidase enzyme. Conjugating a hapten, or a small
analyte or an analyte analogue, to the enzyme donor
polypeptide at certain sites does not affect the ability
to form (3-galactosidase by a complementation reaction and
hence does not affec:t~ the rate of ~3-galactosidase
activity when in the. presence of a substrate for (3-
galactosidase. However, when the enzyme donor-hapten
conjugate is bound by anti-analyte antibody, the
complementation rate is impeded, and thereby the enzyme-
catalyzed reaction rate during the initial phase of the
reaction is reduced. This reduction in enzyme-catalyzed
reaction rate can be monitored and has been used to
quantitate the detex-urination of a plurality of analytes
on the principle of competitive inhibition where enzyme
donor-analyte conjugate present in a reaction mixture and
analyte present in t:he sample compete for anti-analyte
antibody prior to the addition of enzyme acceptor. The
WO 95/16894 PCTIUS94I14484
zo~~T~
-4-
complementation-rate of.(3-galactosidase formation, and
hence enzyme catalyzed reaction rate, is increased as the
r
amount of analyte present in the sample is increased.
In accepted clinical practice, procainamide and NAPA
are analyzed separately. Therefore immunoassays for NAPA
and procainamide require antibodies with a high degree of
specificity for either the metabolite or the drug. Since
the metabolite and-drug differ only by the presence or
absence of an acetyl function, the generation of specific
antibodies is a particularly challenging problem.
Surprisingly, however, the immunogens of the present
invention have been found.-to be especially useful for
this purpose.
The preparation-of antibodies to procainamide and
NAPA for use in immunoassays to determine the drugs has
been accomplished in the prior art by essentially three
different approaches. One-approach has been to couple
procainamide through the benzene ring amino group by
diazotization and subsequent condensation to an albumin
carrier [A. S. Russel et al., Clin. Exp. Immunol. 3:901
(1968) and Mojaverian et al., J. Pharm. Sci. 69.721
(1980)]. The resulting antibodies show a high degree of
cross-reactivity with NAPA, however, and-are therefore '
unsuitable foruse in immunoassays specific for one or
the other drug.
The second approach involves coupling of the drugs
at the opposite end oftheir structures, at the N-
CA 02178915 2000-11-28
_ 5 -
diethylamino group, by modification of one of the ethyl
substituents for subsequent coupling to an antigenic
carrier. As a result., antibodies are raised against an
immunogen in which a major functional group of the drugs
has been modified in order to couple them to the carrier.
An example of this approach is described in U.S. Pat. No.
4,235,969 issued to Singh. et al., in which one of the N-
alkyl groups is rep7_aced with a nonoxocarbonyl-alkyl
substituent. The nonoxocarbonyl functionality, a linking
l0 group containing a carbonyl or imino function, is
employed for conjugation to antigens and enzymes.
Similarly, European Latent Specification No. 199042,
published October 29,, 1986 (Heiman et al.) discloses
antigenic conjugate~~ and enzyme conjugates of
procainamide analog: modified at the terminal
diethylamino group. A specific linking group is attached
to a poly(amino acid;i or a fluorescein tracer.
In a third approach, Buckler et al., U.S. Pat. No.
4,673,763, describe derivatives of procainamide or NAPA
coupled at the a-position of the amide side chain to an
immunogenic carrier material or to a label.
The conjugates described in the Singh, Heiman and
Buckler publications all utilize amide bond condensation
chemistry with amino- or carboxyl-functionalized haptens.
The present invention, however, differs from the linker
chemistry of the pr_Lor art. In the conjugates of the
present invention, rnaleimide modified haptens are reacted
with sulfhydryl groups on carrier proteins, enzymes or
WO 95116894 PCTIUS94/14484
217'915
-6-
enzyme donor polypeptides to give thioether linked hapten
conjugates. Maleimide/sulfhydryl chemistry [M. Brinkley,
Bioconjugate Chem. 3:5 -(1992)] is more easily controlled
than amide bond condensation, thus allowing the
preparation of immunogens and enzyme or enzyme donor
conjugates with defined, targeted degrees of
substitution, a feature which is very important to the
functional efficacy of the conjugates.
Two other metabolites of procainamide and NAPA that
have recently been identified are desethyl procainamide
(PARE) and desethyl N-acetylprocainamide (NAPADE) [Ruo et
aI., Ther. Drug Monitoring, vol. 3:231 (1981) and Ruo et
al., J. Pharm. Exp. Ther., vol. 216:357 (1981)]. Since
the immunogens of the present invention are hapten-
derivatives of the desethyl compounds, it would be
expected that antibodies derived from such immunogens
would show a high degree of- cross-reactivity with PARE
and NAPADE and would therefore be unsu~,table for use in
the assay of-procainamide and NAPA. Quite surprisingly,
however, the antibodies of-the present invention show a
low cross-reactivity, less than about l0 percent, with
PARE and NAPADE. Such low cross-reactivity with desethyl
metabolites is highly desirable for accurate clinical
analysis, and the prior art- has failed to address the
problem of cross-reactivity with these metabolites.
WO 95/16894 ~ ~ ~ PCflUS94114484
The present invention provides novel activated
hapten derivatives of the following formula:
0
~ i
X-HN~CONH-(CHz)2 ~-(CHa)~ NHCO-Rz N
O
wherein:
X = hydrogen or acetyl;
R1 = an alkyl group having 1 to 3 carbon atoms,
preferably 2 carbon atoms;
m = an integer from 2 to 10, preferably 2; and
R2 = an alkyl, cycloalkyl or aryl group having 2 to
10 carbon atoms, preferably (CH2)2~
The present invention further provides novel hapten
conjugates of the formula:
O
R1
X-HN~CONH-( CHZ ) 2 N-( CH2 ) ~ NHCO-R2 N
'S- -Z
O n
wherein:
X = hydrogen or acetyl;
R1 = an alkyl group having 1 to 3 carbon atoms,
preferably 2 carbon atoms;
m = an integer from 2 to 10, preferably 2;
R2 = ~n alkyl, cycloalkyl or aryl group having 2 to
10 carbon atoms, preferably (CH~)2;
WO 95/16894 PCTYUS94J14484
~17~~915'
_8_
Z = a poly(amino acid) or polysaccharide; and
n = 1 to p where p = MW of Z/1000.
The present invention uniquely provides reagents for
use in procainamide and NAPA immunoassays involving the
coupling to or derivatization of the maleimide modified
activated hapten precursor compound via sulfhydryl groups
on a poly(amino acid). The immunogens of the present
invention, which comprise the haptenic drug covalently
linked via its maleimide moiety and a sulfhydryl bridge
to an immunogenic carrier material, are used to stimulate
the production of antibodies to the respective drugs.
Antibodies prepared using the novel immunogens of .the
invention have been found to show surprisingly low croas
reactivity with the desethyl metabolites of procainamide
and NAPA.
In another aspect, the present invention provides
immunoassay methods and reagents for the determination of
NAPA and procainamide using the novel antibodies. The
present invention also provides novel hapten-enzyme or
hapten-enzyme donor conjugates for particularly preferred
embodiments of the assay methods.
$ D.R RT TTON O TH D AWTNCQ
The present invention-will be better understood by
reference to the following -detailed description of the
invention when considered in combination with the .
drawings that form part of thespecification, wherein:
W095/16894 21 ~ 8 91 ~ P~~S94J14484
_g_
Figs: 1 and 2 illustrate particular synthetic
schemes for preparing maleimide derivatives of NAPA and
procainamide and for preparing particularly useful
precursor compounds for use in such derivatizations of
the drugs .
Fig. 3 is a graph showing a dose response curve at
varying levels of procainamide using enzyme donor
conjugates and antibodies of the present invention in a
CEDIA assay.
Fig. 4 is a graph showing a dose response curve at
varying levels of NAPA using enzyme donor conjugates and
antibodies of the present invention in a CEDIA assay.
The present invention, in all of its interrelated
embodiments, is focused on the preparation of maleimide
derivatives of NAPA and procainamide which can then be
used to form immunogens by coupling the derivatives to
conventional antigenic poly(amino acid) or other carrier
materials and subsequently used to obtain antibodies, or
the derivatives can be used to form enzyme, enzyme donor
or labeled conjugates which are useful as detection
reagents in immunoassays for the drugs.
The chemical structures of NAPA and procainamide are
represented by the formula:
CA 02178915 2000-11-28
- 10 -
X-HI~1--~~CONH-( CH2 ) 2-N-( C2H5 ) 2
wherein X is hydrogen for procainamide and acetyl for
NAPA.
In a preferred embodiment of the present invention,
maleimide haptens of. the drugs and immunogen conjugates
are synthesized according to the scheme shown in Fig. 1.
Compound I is synthesized according to the method of,
e.g., Ruo et al., Ther. Drug Monitoring 3:231 (1981).
Compound III is generated. from I by alkylation with an N-
carbobenzoxy bromoa7_kylamine. This is reduced to V by
catalytic hydrogenation. The reactive intermediate IX is
obtained by acylation of V with maleimido-alkanoic acid
N-hydroxysuccinimide ester, which is then coupled through
the free sulfhydryl groups of a poly(amino acid) to
generate compound XI.
In another pref=erred embodiment of the invention,
maleimide derivatives of the drugs and immunogen
conjugates are synthesized according to the scheme shown
in Fig. 2. Catalytic: hydrogenation of compound I yields
II. Selective acety7_ation of compound II with acetic
anhydride gives compound IV. This compound is then
alkylated with an N--carbobenzoxy bromoalkylamine to
produce VI. Compound VI is hydrogenated to remove the
carbobenzoxy (Cbz) croup and generate free amine VII.
Compound VII is acy7_ated with maleimido-alkanoic acid N-
WO 95/16594 ~ ~ ~ PCT/US94114484
i
-11-
hydroxysuccinimide ester to give VIII. Finally, VIII is
coupled to the free sulfhydryl groups of a poly(amino
acid) to give product X.
Examples of suitable R2 linking groups include
ethyl, propyl, butyl, cyclopentyl, cyclohexyl, methyl-
cyclohexyl, phenyl, benzyl, and substituted derivatives
thereof. . __
In preparing the immunogens of the invention, a
thiol-containing carrier protein or other substance
having immunogenic properties is coupled to the maleimide
hapten. Although thiolated keyhole limpet hemocyanin
(KLH) is an especially preferred antigenic poly(amino
acid), or carrier protein, it should be understood that
various protein carriers may be employed, including
albumins, serum proteins, e.g., globulins, ocular lens
proteins, lipoproteins and the like. Illustrative
protein carriers include bovine serum albumin, egg
ovalbumin, bovine gammaglobulin, thyroxine binding
globulin, etc.- -Alternatively, synthetic poly(amino
acids) having a sufficient number of available sulfhydryl
groups such as cysteine may be employed, as may other
synthetic or-natural polymeric materials bearing reactive
functional groups. In particular, carbohydrates, yeasts,
or polysaccharides may be conjugated to the hapten to
produce an immunogen.
Enzyme donor conjugates are prepared by coupling the
donor polypeptide-to the maleimide hapten. The enzyme
WO 95/16894 PCT/US94/14484
-12-
donor peptides suitable for maleimide-hapten conjugation
are,those which contain cysteine groups. The hapten to
enzyme donor conjugation ratio should be at least a 10-
fold excess of hapten.
Conjugates ofthe activated hapten and a labelling
group such as an enzyme, a substance having fluorescent
properties, or a radioactive label may also be prepared
and used as reagents in immunoassays. As with the
immunogen and enzyme donor-conjugates, the label employed
must have available thiol-containing groups to be
suitable for use in the present invention. The thiol
groups may be naturally occurring or they may be
artificially introduced using a thiolating agent such ae
N-succinimidyl-3-(acetylthio) propionate (SATP).
In order to generate antibodies, the immunogen is
conveniently prepared for injection into a host animal by
rehydrating lyophilized immunogen to form a solution or-
suspenaion of the immunogen. The immunogen solution is
then combined with an adjuvant such as Freund's. The
immunogen may be administered in a variety of sites, at
several doses, oneor more times, over many weeks.
Preparation of polyclonal antibodies using the
immunogen may follow any of the conventional techniques
known to those skilled in the art. Commonly, a host
animal such as a rabbit, goat, mouse, guinea pig, or
horse is injected with the immunogen mixture. Further
injections are made, with serum being assessed for
WO 95/16894 ~ ~ PCTlUS94114484
-13-
antibody titer until it is determined that optimal titer
has been reached. The host animal is then bled to yield
a suitable volume of specific antiserum. Where
desirable, purification steps may be taken to remove
undesired material such as nonspecific antibodies before
the antiserum is considered suitable for use in
performing assays.
Monoclonal antibodies may be obtained by hybridizing
mouse lymphocytes, immunized as described above, and
myeloma cells using a polyethylene glycol method such as
the technique described in Methods ~n Enzymology, vol. 73
(Part B), pages 3-46 (1981).
Synthesis of n-amino-N-f(2-ethylaminnlAr__h_~r11-N~-
Referring to Fig. l, in which R1 = CH2CH3, R2 =
(~2)2, and R3 = (CH2)2, p-amino-N-[(2-ethylamino)ethyl]-
N~-[2-(3-maleimido-propionamido)ethyl]-benzamide (Ig) was
synthesized according to the following scheme:
p-Nitro-N-[2-(ethylamino)ethyl]benzamide (I) was
first synthesized from N-ethyl-ethylenediamine and p-
' nitrobenzoyl chloride using the method of Ruo et al.,
ibid. Of this intermediate, 233 mg was dissolved in 2.5
ml of N,N-dimethylformamide (DMF) and treated with 266
mg of N-carbobenzoxy-2-bromoethylamine [E. Katchalski and
D.B. Ishai, J. Org. Chew. 15:1067 (1950)] in the presence
CA 02178915 2000-11-28
- 14 -
of 282 mg of anhydrous potassium carbonate. After
stirring 20 hr. at room temperature, the suspension was
rotary evaporated to a volume of 1 ml and reacted.
another 20 hr. The reaction mixture was then filtered and
rotary evaporated to an oil. The crude product was
dissolved in chloroform, 25m1, and washed sequentially in
a separatory funnel with 10 ml portions of water, 1 M
sodium bicarbonate .and saturated sodium chloride
solution. The chloroform solution was dried over sodium
sulfate; filtered a:nd evaporated to an oil. The product
was purified from u:nreact:ed starting materials by silica
gel chromatography 'with chloroform/methanol, 95:5 v/v, as
eluent to give 167 mg of p-nitro-N-[(2-ethyl-amino)-
ethyl]N'-[2-carbobe:nzoxy-aminoethyl]-benzamide (III). The
latter intermediate was dissolved in a mixture of 10 ml
ethanol and 0.8 ml of 1 N hydrochloric acid. The mixture
was hydrogenated in a Parr reactor at 50 psi hydrogen in
the presence of 121 mg oi= 10% palladium/charcoal
catalyst. After shaking i_or 2 hr. at room temperature,
the hydrogenation was stopped and the suspension was
filtered through a :bed of Celite (trade-mark) to remove
catalyst. The ethanol filtrate was rotary evaporated to a
film. The product was precipitated from ethanol/diethyl
ether to give 75 mg of p-amino-N-L(2-ethylamino)ethyl]-
N'-[2-aminoethyl]-benzamide dihydrochloride (V). This
amine intermediate, 39 mg, was dissolved in 1.4 ml of
DMF. Triethylamine, 0.034 ml, was added followed by 32 mg
of 3-maleimido-propionic acid N-hydroxysuccinimide ester.
CA 02178915 2000-11-28
- 15 -
The resultant mixture wa:~ stirred at room temperature for
18 hr. The DMF solution of maleimide adduct was loaded on
a preparative HPLC ~~olumn (Vydac, 2.2 x 25 cm C18)
equilibrated with 0.1 % aqueous trifluoroacetic acid
(TFA). The column was eluted at 4m1/min with a 20 min
linear gradient of 0-15 ~ acetonitrile containing 0.1
TFA, while monitoring at 260 and 280 nm. The major peak
was collected and lyophilized to yield 29.7 mg of
maleimide adduct based upon UV extinction at the maximum
of 279 nm. 1H-NMR of the product confirmed the target
structure IX [i.e., maleimide resonance at 6.8 ppm
(singlet), p-aminob~~nzamide resonances at 7.2 and 7.8 ppm
(doublets), alkyl r~~sonances at 1.4 ppm (CH3 triplet), 2.5
ppm (CHZ-CO triplet), and 3.3-4.0 ppm (CH2-N overlapping
multiplets)]. Vydac is a trade-mark.
EXAMPLE 2
Synthesis of p-acetamido-N-f(2-ethylamino)ethyll-N'
f2- (maleimid.o-propionamido) ethyl] -benzamide
Referring to Fig. 2, in which X = CH3C0, R1 - CH2CH3,
RZ = (CHZ) 2 and R3 = (CH2) z, p-acetamido-N- [ (2-ethyl-
amino)ethyl]-N'-[2-(maleimido-propionamido)ethyl]-
benzamide (VIII) was synthesized according to the
following scheme:
p-Acetamido-N-[(2-ethylamino)ethyl]-benzamide or
desethyl-NAPA (IV) was first synthesized from p-nitro-
R'O 95116894 PCT/US94/14484
.~ _16- ,
N[(ethylamino)ethyl] benzamide (I) by reduction to
desethyl procainamide followed by acetylation using the
methods of Ruo et al (ibid.). The desethyl NAPA, 152 mg,
was dissolved in 2 ml of DMF and treated with 160 mg of
N-carbobenzoxy-2-bromoethylamine and 166 mg anhydrous
potassium carbonate by stirring at room temperature for
53 hr. An additional 169 mg of N-carbobenzoxy-2-
bromoethylamine in 2 ml DMF was then added and the
mixture was stirred for 21 hr. Finally, a third portion
of N-carbobenzoxy-2-bromoethylamine was added in 2 ml DMF
and the reaction was continued for 3 more days, at which
time TLC indicated nearly complete reaction. The
reaction mixture was then filtered and rotary evaporated
to an oil. The oil was redissolved in chloroform, 20 ml,
and washed sequentially in a separatory funnel with 10
ml portions of 1 M sodium bicarbonate and saturated
sodium chloride solution. The chloroform solution was
dried over anhydrous sodium sulfate, filtered and rotary
evaporated to give crude product. Purification was
accomplished by silica gelchromatography with
chloroform/methanol elusnts (95:5 and 90:10 v/v) to give
100 mg of p-acetamido-N-[(2-ethylamino)ethyl]-N'-[2-
carbobenzoxy-aminoethyl]-benzamide (VI) ae an oil_ The
latter intermediate was dissolved in 20 ml of methanol
and hydrogenated in a Parr reactor at 38 psi hydrogen for
1.5 hr. in the presence of-100 mg of 10 ~
palladium/charcoal catalyst. The catalyst was filtered
WO 95/16894 ~ PCTIIJS94114484
-17-
off and the filtrate was diluted with 0.8 ml of 1 N
hydrochloric acid. Rotary evaporation gave a film which
was rediasolved in ethanol and precipitated with diethyl
ether to give 54 mg of solid p-acetamido-N-[(2-
ethylamino)ethyl]-N~-(2-aminoethyl)-benzamide
dihydrochloride (VII). This amine intermediate, 44 mg,
was dissolved in 1.4 ml of DMF. Triethylamine, 0.034 ml
was added followed by 34 mg of maleimido-propionic acid
N-hydroxysuccinimide ester.- The reaction mixture was
stirred at room temperature for 6 hr. Purification was
accomplished by preparative HPLC as-in Example 1 above
with the exception that a 5-20% gradient over 15 min. was
employed. The major peak from the HPLC was lyophilized
to give 29 mg based upon W extinction at the maximum of
268 nm. 1H-NMR confirmed the target structure (VIII)
[i.e., maleimide resonance at 6.8 ppm (singlet), p-
acetamidobenzamide resonances at 7.55 and 7.7 ppm
(doublets), acetyl methyl resonance at 2.2 ppm, and alkyl
resonancea at 1.35 ppm (triplet), 2.4 ppm (triplet) and
3.3-4.0 ppm (overlapping multiplets)].
Procainamide and NAPA immunogens were prepared by
coupling the activated maleimide haptens, Compounds VIII
and IX, respectively, with thiolated keyhole limpet
hemocyanin (KLH). For the maleimide modified haptens to
WO 95116894 PCTIUS94I14484
2i789i5 1
-18-
be coupled to the KLH, sufficient sulfhydryl groups must
be present on-the KLH. This was accomplished through the
use of the thiolating agent Id-succinimidyl-3-(acetylthio)
propionate (SATP).
Thiolation of KLH
KLH was solubilized by dissolving 100 mg in 20 ml of
phosphate buffered saline and then mildly sonicating and-
stirring at room temperature. A 300 molar excess of SATP
was used to thiolate the xr,u for coupling to Compounds
10- VIII and IX. In 1 ml dimethylformamide (DMF), 73.5 mg of
SATP was dissolved. The KLH was slowly added to the SATP
while stirring at room temperature. The reaction mixture
was incubated for 1 hour at room temperature and then a
1/10 volume of 1 M lysine was added (final concentration
100 mM) and incubation was continued for an additional 15
min. The KLH-SATP was dialyzed against 50 mM phosphate,
pH 7.4 in 50,000 molecular weight cutoff SpectroporeT"~
(Spectrum Medical) dialysis tubing overnight (4L x 4
changes). Thiol incorporation was determined
apectrophotometrically by reaction with 5,5~-dithio-bis-
2-nitrobenzoic acid (DTNB) and was found to be 33 moles
per mole of KLH.-
One quarter of the-KLH-SATP was-used for compound
VIII coupling and 1/4 for compound IX. The remainder was
saved for later use. To 1/2 of the dialyzed xr,u-SATP, -a
1/10 volume of 1 M hydroxylamine-(final concentration 100
CA 02178915 2000-11-28
- 19 -
mM) was added to dep rotect the sulfhydryl, and the
reaction was incubal~ed for 1 hour at room temperature.
The deprotected matE=_rial was divided in half and placed
into two test tubes. Twenty mg of compound VIII and 20 mg
of compound IX were dissolved in 1.67 ml DMF each, and
one was added to each of the two deprotected KLH-SATP
samples in test tubf=_s, i.e., one tube contained, compound
VIII arid KLH-SATP a:nd the other tube contained compound
IX and KLH-SATP. Ths=_ reaction mixtures were incubated for
4 hours at room temperature while stirring slowly. The
mixtures were trans:Eerred to 50,000 molecular weight
cutoff Spectropore l~~ubinc~ and dialyzed against distilled
water, 4 L x 3 time;. UV analysis indicated the thiols on
the KLH were saturated with hapten. Samples were
aliquoted into vial; (1 mg/vial), frozen at -70°C, and
lyophilized. The lyophilized samples were then stored at
-20°C for later use as immunogens.
EXAMPLE 4
Conjugation of compound VIII (or compound IX) to a
peptide fragment of ~3-galactosidase
The peptide fragment. of ~-galactosidase that was
used in this example=_ (ED28) consists of (3-galactosidase
amino acids 1-46 wii~.h cysteines at positions 1 and 46, as
described in Manning et al., European Patent Specifi-
cation No. 413561, published February 20, 1991. To remove
reducing reagent th<~t is used in the storage buffer for
this compound, 3.3 mg of ED28 was
VI'O 95116894 PCT/US94114484
-20-
desalted on a NAPST"~ (Pharmacia) desalting column into 1
ml 50 mM sodium phosphate, pH 7Ø One mg, or l ~tmol, of
this material was used forthe coupling reaction. One
wmol of compound VIII was dissolved in 100 ul of
dimethylformamide (DMF). To 1 ~tmole of compound VIII, 1
mg of desalted ED28 was added drop.dise while stirring
slowly. This was incubated for 1 hr at room temperature.
To prepare the sample for HPLC-purification, the
conjugate mixture was desalted-on a NAPS column
equilibrated with water; 0.1 % TFA to remove excess
hapten.- The conjugate was purified on a C4 semi-
preparative HPLC column (Vydac) at 4 ml/min using a 25-
40% gradient over 15 minutes with solvent A being
water/0.1% TFA and B being acetonitrile/0.1% TFA, and the
major peak was collected and stored at -20°C. In this
procedure, two moles of-compound VIII were coupled to
each mole of ED28 through the two thiols on the peptide.
The same procedure was followed for compound IX-ED28
coupling.
Tmmunization of Host
Preparation of the immunogen-and immunization of the
host are accomplished using techniques which will be
known to those skilled in the art. The immunogen can be
prepared for injection into the host animal by
WO 95/16894 ~ ~ ~ PCTlUS9dl14d84
-21-
,..
rehydrating lyophilized immunogen in phosphate buffered
saline (PBS). The antigen solution is then combined with
equal- amounts by volume of- Freund's adjuvant to form an
emulsion. The first immunization can be completed with
Complete Freund~s Adjuvant and all subsequent
immunizations with Incomplete Freund~e Adjuvant. The
immunogen may be administered in a variety of sites, at
several doses, one or more times, over many weeks.
In this example, supernatants were selected from 96-
well culture plates using a CEDIA homogeneous assay. As
previously described, the CEDIA assay utilizes two
genetically engineered, enzymatically inactive fragments
of (3-galactosidase. The smaller polypeptide, designated
the enzyme donor, can recombine spontaneously with the
larger fragment, the enzyme acceptor, to form active (3-
galactosidase, in a process called complementation. When
a specific antibody to the ligand attaches to the enzyme
donor conjugate, complementation is inhibited. The
addition of free ligand to this system will modulate the
inhibition of complementation. This assay principle was
used to screen fusion products in a 96-well format.
A primary screening of the fusion products was first
performed to evaluate the ability of the antibodies to
bind to enzyme donor conjugate prepared in Example 4 and
inhibit complementation. The number of inhibition-
positive clones were then narrowed further by performing
WO 95116894 PCTIUS94I14484
21~78~~1'5': _z2_
a secondary screening assay to determine whether-the free
drug would modulate orcompete with the enzyme donor
conjugate for the antibody. The modulation assay also
identified specific clones when screened against cross
reacting analytes. The clones which modulated with the
specific analytes of choice were then grown for further-
study. The culture supernatant containing the monoclonal
antibody was col-lected and-evaluated on the HITACHI 717
autoanalyzer (BOehringer Mannheim Corp., Indianapolis,
IN) as described inExample 6 below.
CEDIA assays for procainamide and NAPA were
performed using the enzyme-donor conjugates prepared in
Example 4 and the antibodies produced according to
Example 5_ The following reagents were prepared:
CA 02178915 2000-11-28
- 23 -
Donor reagent
Enzyme donor conjugate 0.5 nM
Antibody 1:10-1:100
CPRG (chlorophenylred-(3-n- 1 mg/mL
galactopyranosude)
NaCl 500 mM
K2HP04 3 0 mM
EGTA 10 mM
EDTA, Disodium 0.6 mM
Na Azide 20 mM
TWEEN-20 0.02%
pH 6.80
0
Registered TM of ICI Americas, Inc. for
polyoxyethylene sorbitan
Acceptor ream°nt:
Enzyme acceptor 220 U/ml
Magnesium acetate 5 mM
NaCl 500 mM
K2HP04 3 0 mM
EGTA 10 mM
L-methionine 10 mM
Na Azide 20 mM
TWEEN-20 0.02%
pH 6.80
Assays were pe=rformed using an HITACHI 717 (trade-
mark) autoanalyzer. 'The instrument dispensed 3~.1 of
sample containing NAPA on procainamide into a cuvette,
and 2001 of donor :reagent was added. The mixture was
allowed to incubate at 37°C for 5 minutes, after which
150,1 of acceptor rc=_agent: was added. The absorbance rate
was measured over the time period of 243.4 sec to 302.75
sec following the addition of the acceptor reagent. The
WO 95/16894 PCTIUS94114484
~~ '~~915
-24-
primary wavelength used was 570nm, with 660nm used as the
secondary wavelength. The absorbancerate at 570 nm was
plotted against procainamide or NAPA concentration to
construct a dose response curve. The curves obtained are
shown in Fig. 3 and Fig. 4.
The cross-reactivity for each of the clones was
assayed as described in Example 6 using an HITACHI 717
autoanalyzer. Each clone was tested for cross-reactivity
with the following analytes and concentrations: NAPA, 5C
~tg/ml; procainamide, 50 ~g/ml; PADE, 50 Pg/ml; NAPADE,--50
wg/ml; p-aminobenzoic acid (PABA), 50 wg/ml; and
acetaminophen, 500 Pg/ml. The percent cross-reactivity
was calculated from the rate produced by each of the
above analytes, and the apparent dose was found using
Figures 3 and 4 as standard curves. The apparent dose of
the analyte divided by the actual dose multiplied by 100
gives percent cross-reactivity.
Clone 13<PA>6 was checked-for-cross-reactivity with
the above substances using the procedure in Example 6-and
the standard curve in Fig. 3. The results found were as
follows:
sec following the additi
WO 95/16894 PCTIUS94114484
i
-25-
Rate Apparent Actual Dose % Cross-
Analyte (mAU/min) Doae (~g/ml) (ug/ml) reactivity
NAPADE 242 0 50 0
NAPA 256 0.56 50 1.1
PADE - 275 , 1.379 50 2.8
. ,
PABA 239 0 50 0
Aceta- 235 0 500 0
minophen
In another example, clone NAPA>11.1 also
24< was
checked for cross-reactivity with these substances
using
the proc edure in Example 6 and standard cur ve in Fig.
the
4. The following results were
found:
Rate Apparent Actual Dose % Croas-
Analyte (mAU/min) Dose (gg/ml) (wg/ml) reactivity
NAPADE 30.1 2.58 50 5.2
Procain- 29.4 0.56 50 0
amide
PADE 30.3 2.74 50 5.5
PABA 29.2 0 50 0
Aceta- 29.2 0 500 0
minophen
It will be understood that the specification and
examples are illustrative but not limitative of the
present inventian, and that other embodiments within the
spirit and scope of the invention will suggest themselves
to those.skilled in the art.