Note: Descriptions are shown in the official language in which they were submitted.
21731~1
PREPARATION AND STORAGE OF PANCREATIC ISLETS
This invention was made with government support under
grant DK208?7, awarded by The National Institutes of Health.
The United States government has certain rights in this
invention.
Field of the Invention
This invention is directed at the preparation and storage
of pancreatic islets, for transplantation into diabetic
patients.
Backqround of the Invention
Pancreatic islet transplantation has the potential to be
the most physiologically advantageous and minimally invasive
procedure for treatment of type I diabetes mellitus. However,
despite progressively increasing numbers of islet transplants
in these patients, the endocrine function established by the
transplant is far from optimum. In order for this approach to
be a clinically acceptable diabetes therapy, several technical
and immunological problems need to be solved.
Donor islet preparation is the first critical step to
provide a sufficient number of high quality islets for
transplantation. Large-scale islet preparation from the
pancreas of large animal species, including dogs, pigs and
humans, has become possible through the development of highly
automated procedures. Islet isolation, involving the
digestion of pancreatic tissue and the purification of islets,
is, in particular, the most important process that influences
the outcome of transplants.
Pancreatic islets are usually transplanted into diabetic
patients who also need a kidney or other solid organ
transplant who are already being, or will be treated with
immunosuppressants in order to prevent graft rejection.
Despite many attempts, to date only a small fraction of islet
allografts have functioned for a prolonged period. One of the
2179101
-
major reasons for this failure appears to be an insufficient
number of islets used for transplantation. The current
recommendation by the International Islet Transplant Registry
is to transplant more than 6,000 islets, equivalent to 150 ~m
in size, per kg of the recipient's body weight in order to
achieve long-term maintenance of euglycemia. To fulfill this
requirement, islets from multiple donors have often been used.
However, a more desirable approach would be to derive both
kidney (or other organs) and islets from the same donor in
order to avoid an additional antigenic load and therefore,
decrease the possibility of rejection. This would require the
isolation of a large number of high quality islets from a
single human pancreas.
Large-scale islet isolation from the human pancreas has
become possible with advances in technology and the
availability of high quality collagenase used in their
preparation. However, even with these improvements, the islet
yield from a single pancreas is often insufficient for
transplantation. It is desirable to develop a method for
isolation of pancreatic islets and storage of the islets so
that transplants can be prepared from a single donor.
Summary of the Invention
The present invention is directed at a method, a solution
and a chamber for the preparation and storing of pancreatic
islets.
The method of the present invention comprises contacting
a pancreas with a warm collagenase solution, digesting the
pancreas in the warm collagenase solution to form warm digest,
adding cold preservative solution to the warm digest,
agitating the warm digest/cold preservative solution at a
temperature between about -0~ and 15~C, to thereby further
digest the partially digested pancreas included in the warm
digest, to form cold digest and collecting liquid from the
cold digest to form isolated islets.
--2--
2179101
The solution of the present invention comprises a sugar
derivative such as D-mannitol, K-lactobionate and a buffer.
The chamber of the present invention comprises a lower
chamber for the digestion of pancreatic pieces and a lid
inserted into the lower chamber. The lid comprises a cap, a
filter attached to the cap, wherein the filter is placed
within the lower chamber when the lid is inserted into the
lower chamber, a port attached to the cap wherein digested
pancreatic pieces are decanted from the lower chamber,
filtered through the ~filter and removed from the chamber
through the port and a cover attached to the port to prevent
contamination of the contents of the chamber.
--3--
2179101
Brief Description of the Drawinqs
Features, aspects and advantages of the invention will be
more fully understood when considered with respect to the
following detailed description, appended claims and
accompanying drawing where:
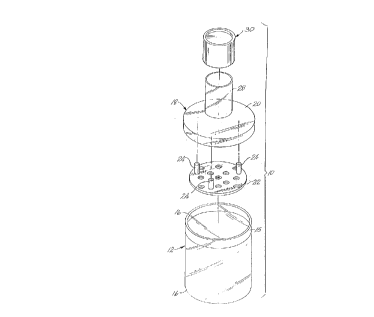
FIG. 1 is an exploded perspective view of a digestion
chamber of the present invention.
--4--
2179101
.
Detailed DescriPtion
The present invention is directed at a new isolation
technique which uses an intermittent two-step digestion
procedure for pancreatic tissue and includes warm and cold
digestion steps. The invention is also directed at a chamber
for use in the digestion procedure and a cold storage solution
for storage of isolated islets and digested pancreatic
tissues. The present invention has lead to the consistent
isolation of a significantly higher number of islets than were
achieved by previously,used methods.
For the isolation of pancreatic islets in accordance with
the present invention tissue is digested by a two-step
procedure, first by warm digestion, then by cold digestion, as
described below.
The pancreas is transported, under refrigeration or on
ice, in a solution such as 30 mM raffinose, 100 mM K-
lactobionate, 15 mM KH2PO4, 5 mM MgSO4, 5 mM adenosine, 3 mM
glutathione, 1 mM allopurinol, 8 mM dexamthazone and 5~ (w/v)
hydroxyethyl starch (HES), pH 7.4 or other suitable
transportation medium. The organ is preferably trimmed of
surrounding membranes, vessels, fat and lymph nodes to reduce
the amount of "contaminating" tissue and material from the
pancreas, cannulated with an angiocatheter and weighed. In a
preferred embodiment of the present invention, the organ is
expanded by injecting 150-250 ml of warm collagenase solution
using a 30 ml syringe and sectioned into approximately 8-10
pieces. In another embodiment of the present invention the
pancreas is soaked in or otherwise contacted with the
collagenase solution. It will also be realized by those
skilled in the art that the pancreas could be processed whole
rather than being cut into pieces. In a preferred embodiment
the collagenase solution includes a collagenase such as
Collagenase P (Boehringer Mannheim CO., Indianapolis, IN),
about 2% (v/v) heat-inactivated newborn bovine serum (NBS,
Sigma Chemical Co., St Louis, MO), about 1 mg/ml calcium
--5--
2179101
.
chloride and about 40 mg/dl DNase in a solution such as 5.6 mM
glucose, 15 mM KH2PO4, 0.33 mM Na2HPO4, 0.82 mM MgSO4, 5.4 mM
KCl, 137 mM NaCl and 1.3 mM CaCl2, pH 7.2, although one
skilled in the art will appreciate that other collagenases and
'5 components of the collagenase solution could be substituted or
used and still successfully digest the pancreas. The
concentration of collagenase is optimized with respect to the
time required to achieve digestion of the pancreas for each
lot collagenase and usually ranges from 1.5 to 2.5 mg/ml.
In a preferred embodiment, pieces of pancreas are placed
in a container, such as that described in ~IG. 1, containing
an agitator such as glass marbles. The chamber is gently
agitated in an incubator or a water bath at about 37~C using
a shaker such as a Wrist Action Shaker Model 175 provided by
Burrell Co. of Pittsburgh, PA, for about 15 minutes, until the
pancreas is partially digested (Step 1: warm digestion phase).
While it is preferred that the digestion be performed at 37~C
other temperatures, at which the collagenase retains its
activity, but do not result in significant damage to the
pancreatic islets, could be used, generally temperatures
within the range of 35~ to 38~C are suitable for pancreatic
digestion.
Preferably the partially digested tissues/collagenase
solution, is filtered through a sheet of large-mesh (about-5
to about 10 mm) screen into a second container and placed on
ice. While filtration is preferred it will be understood that
other methods of separation such as gravity sedimentation or
decanting would also be applicable or, alternatively, the
partially digested tissues/collagenase solution could be
further processed without filtration or separation of the
partially digested tissues/collagenase solution. Cold (about
4~C) preservation solution is added to the second container at
a volume approximately 1/3 of the decanted volume. The
preservation solution of the present invention comprises K-
~5 lactobionate, a sugar derivative such as D-mannitol and a
--6--
2179101
buffer such as KH2PO4. The preservation solution may also
include a membrane stabilizer such as MgSO4, a radical
scavenger such as superoxide dismutase, catalase, and vitamins
such as vitamin C, vitamin E and nicotinamide and combinations
thereof. The concentrations of the components of the
preservation solution are preferably in the range of 25 to 50
mM for D-mannitol, 80 to 120 mM for K-lactobionate, 15 mM for
KH2PO4 and when used, 1 to 5 mM MgSO4, superoxide dismutase at
a concentration where free radicals are eliminated from the
solution but not at a concentration which is so high as to be
wasteful of the material and 1 to 10 mM for nicotinamide (LAP-
1). In a preferred embodiment of the present invention the
preservative solution comprises 30 mM D-mannitol, 100 mM
K-lactobionate, 15 mM KH2PO4 and when used, 5 mM MgSO4, 30,000
units/l superoxide dismutase and 5 mM nicotinamide (LAP-l).
When the method employed uses a separation step as
described above, warm collagenase solution is again added to
cover the remaining undigested tissue and warm digestion is
repeated. After about 5 minutes, all collagenase/digested
tissues in the first container are transferred into the second
container. The second container is gently shaken, for example
by hand, at about 4~C or on ice. About every 5 minutes, 1/3
of the supernatant containing digested tissues is decanted,
from the second container, into a collection bottle containing
a cold preservative solution as described above and preferably
LAP-l solution supplemented with about 20~ (v/v) serum such as
newborn bovine serum. A preservative solution is added to the
second container at about a volume equal to that decanted and
the container is shaken again. This "cold digestion" process
is repeated until all islets are freed (Step 2: cold digestion
phase). If necessary, warm digestion is repeated a third
time, so that only undigested, larger ductal structures
remained.
To avoid harmful effects on digested tissues, collagenase
and other enzymes released from pancreatic acinar cells are
--7--
2179101
inactivated as soon as possible after the cold digestion step.
For this purpose, digested pancreatic tissues containing free
islets are collected from the second container at 5-10 minute
intervals and stored on ice in a large amount of cold
preservation solution which includes a high concentration of
serum, although other methods of inactivating the enzymes are
known to those skilled in the art. Pancreatic digests are
stored in a preservation solution as described above and
preferably cold LAP-l solution supplemented with about 20
(v/v) NBS or other suitable serum.
In a preferred embodiment of the present invention,
pancreatic digests are suspended in a solution such as Euro-
Ficoll solution such as that supplied by Pharmacia with a
density of 1.100 g/cm3 and islets are separated from acinar
cells by discontinuous gradient centrifugation on three layers
of Euro-Ficoll solutions (densities of: 1.100, 1.087 and
1.056) using a COBE2991 cell processor such as that supplied
by Cobe Laboratories, Inc. of Lakewood, CO. After about 10
minutes centrifugation, islet fractions are identified and
collected. Islets are washed twice with a cold preservative
solution such as LAP-l and once with a medium such as RPMI1640
medium, supplied by GIBCO, containing 10~ (v/v) fetal bovine
serum (FBS) at room temperature. Other suitable methods of
cell purification are known to those skilled in the art and
would also be suitable for use in the present invention. The
practice of the present invention typically provides islets
with a purity of at least 70~, islets retain at least 90~ of
their viability and retain their ability to respond to glucose
stimulus.
In a second aspect of the present invention a solution
for the storage and preservation of pancreatic islets has been
developed. The solution has been designated LAP-l and
comprises K-lactobionate, a sugar derivative such as D-
mannitol and a buffer such as KH2PO4. The preservation
solution may also include a membrane stabilizer such as MgSO4,
--8--
2179101
-
a radical scavenger such as superoxide dismutase, catalase,
vitamin C or vitamin E and nicotinamide. The concentrations
of the components of the preservation solution are preferably
in the range of 25 to 50 mM for D-mannitol, 80 to 120 mM for
K-lactobionate, 15 mM for KH2PO4 and when used, 1 to 5 mM
MgSO4, superoxide dismutase at a concentration where free
radicals are eliminated from the solution but not at a
concentration which is so high as to be wasteful of the
material and 1 to 10 mM for nicotinamide. In a preferred
embodiment of the presçnt invention the preservative solution
comprises 30 mM D-mannitol, 100 mM K-lactobionate, 15 mM KH2PO4
and when used, 5 mM MgSO4, 30,000 units/l superoxide dismutase
and 5 mM nicotinamide. Additionally, the solution may include
trypsin inhibitors and other components as desired. The pH of
the solution is adjusted to about 7.3 at room temperature.
Newborn calf serum, or other suitable serum, is added to a
concentration of about 5 to 20~ (v/v) and preferably 20~ (v/v)
to store digested tissues. In a preferred embodiment LAP-l
comprises 30 mM D-mannitol, 100 mM K-lactobionate, 15 mM
KH2PO4, 5 mM MgSO4, 30,000 units/l superoxide dismutase and 5
mM nicotinamide.
The present invention is also directed at a chamber for
performing warm digestion of pancreas (see FIG. 1). The
chamber 10 is manufactured from a transparent material such as
plastic or glass. It is preferable that the material is
sterilizable by autoclaving or other suitable sterilization
procedures well known to those skilled in the art. The
chamber 10 comprises a lower chamber 12, which in a preferred
embodiment of the present invention, is a cylinder which is
closed at a first end 14 and open at a second end 16. In use
pancreas pieces are added to the lower chamber along with
collagenase solution described above.
In one embodiment of the present invention a lid 18 fits
into and seals the second end of the lower chamber. The lid
may also attach to the lower chamber by a screw fit, well
_ g_
21791~1
.
known to those skilled in the art. Lid 18 comprises a cap 20
to seal the lower chamber and to prevent the contents of the
lower chamber from being contaminated. In a preferred
embodiment the cap is dimensioned to fit within a channel 15
positioned around the interior circumference of the lower
chamber, adjacent to the second end. Cap 20, when inserted
into the lower chamber fits into the channel and is held in
place at the second end of the lower chamber.
Attached to the underside, the side which is inserted
into the lower chambçr, is a filter 22. The filter is
attached to and spaced from the cap by posts 24. The filter
is dimensioned to fit closely to the interior wall of the
lower chamber, adjacent to channel 15, when the lid is placed
on the lower chamber.
The filter includes perforations 26 to allow digested
material to pass from the lower chamber, through the filter
and out port 28. In a preferred embodiment of the present
invention the perforations are about 0.8 mm in diameter and
allow smaller digested material to be decanted from the lower
chamber. Port 28 is provided with a cover 30 which is
dimensioned to slidably mate with the exterior of port 28 to
seal the port to prevent contamination of the interior of the
chamber. The cover may also attach to the port by a screw
fit, well known to those skilled in the art.
ExamPle 1
Isolation of Pancreatic Islets
and Comparison of Storaqe Solutions
Ten consecutively harvested human pancreas (7 male and 3
female) were used in this study. All pancreata were
harvested, after obtaining appropriate consent, from cadaveric
organ donors and transported in cold solution of 30 mM
raffinose, 100 mM K-lactobionate, 15 mM KH2PO4, 5 mM MgSO4, 5
mM adenosine, 3 mM glutathione, 1 mM allopurinol, 8 mM
--10--
2179101
dexamthazone and 5~ (w/v) hydroxyethyl starch (HES), pH 7.4
(UW solution). The age of donors ranged from 17 to 63 years
(40.8+4.7 years) and cold ischemic time ranged from 3 to 11
hours (8.4+1.0 hours).
After intraductal injection of 150-250 ml of a solution
of 5.6 mM glucose, 15 mM KH2PO4, 0.33 mM Na2HPO4, 0.82 mM MgSO4,
5.4 mM KCl, 137 mM NaCl and 1.3 mM CaCl2, pH 7.2 (HBSS)
containing 2.5 mg/ml collagenase (Collagenase P, lot
#13494022-50, Boehringer Mannheim Co., Indianapolis IN) at
37~C, the pancreata was,cut into approximately 8-10 pieces (2-
3 cm3) and placed in a disposable clear plastic container (500
ml in size), such as that shown in FIG. 1, with three glass
marbles (1 cm in diameter). First ! the pancreata was gently
agitated with a shaker (Wrist Action Shaker Model 175, Burrell
Co. Pittsburgh, PA) in a 37~C water bath for 15-20 minutes
(Step 1).
The collagenase solution containing the digested tissue
was filtered through a 8 mm screen and transferred to a second
clear plastic container to which 1/3 of the total amount of
cold preservation solution, 30 mM D-mannitol, 100 mM
K-lactobionate, 15 mM KH2PO4, 5 mM MgSO4, 30,000 units/l
superoxide dismutase, 5 mM nicotinamide, pH 7.3 (LAP-l) was
added. This container was then hand-shaken on ice to further
digest the tissues. Every 5 minutes, shaking was stopped, 1/3
of the solution containing digested tissues was removed though
a 800 ~m screen, the same volume of fresh cold LAP-1 solution
was added to the container and the digestion continued
(Step 2).
This process was repeated several times until most of the
islets were freed from the tissue. The undigested tissue that
remained on the 8 mm screen was further digested in a 37~C
water bath for an additional 5-10 minutes until most of the
remaining tissue consisted of pancreatic ducts. Usually, this
second warm digestion was sufficient to release the remaining
acinar and endocrine components from the larger ductal
--11--
2179101
-
structures. This digested tissue was also transferred into
the cold digestion container, for further cold digestion. The
entire procedure was completed within 60 minutes.
The following four solutions were used to store digested
pancreatic tissue in the cold: HBSS, UW solution, modified UW
solution which consisted of the same components as UW
solution, but omitting HES, and LAP-1 solution. The
components of these solutions are compared in Table I.
iTable I
N l:.L~ ~ S
SOLUTION HBSS mUW UW LAP-1
T -rm;AntS5.6 mM glucose 30 mM raffinose 30 mM raffinose 30 mM D-
mannitol
100 mM K- 100 mM K- 100 mM K-
lactobionate lactobionate lact~h;~nAte
H- buffers 15 mM KH2PO, 15 mM KH,PO, 15 mM KH2PO, 15 mM KH,PO~
0.33 mM Na2HPO,
Metabolites 0.82 mM MgSO, 5 mM MgSO~ 5 mM MgSO, 5 mM MgSO~
and others
5.4 mM KCl 5 mM A~n~in~ 30,000 U/l SOD
137 mM NaCl 3 mM 5 mM
glutathione nicotinamide
1.3 mM CaCl2 1 mM
allopurinol
8 mM 8 mM
Cl~'YAmthA7.~ne ~ hA7~nf~
HES (5~ w/v)
Na- (mEq)1 138 30 30 30
K (mEq) 5.8 120 120 120
mOsm/12 284 320 320 320
pH 7.2 7.4 7.4 7.3
1 mEq = milliequivalents
2 mOsm/l = milliosmolar/l
LAP-1 (100 mM K-lactobionate, 30 mM D-mannitol, 15 mM
KH2PO4, 5 mM MgSO4, 30,000 U/l superoxide dismutase-SOD from
bovine erythrocytes, Sigma Chemical Co., St. Louis, MO-and 5
mM nicotinamide-Sigma Chemical Co., St. Louis, MO) was
adjusted to a pH of 7.3 at room temperature. Newborn calf
-12-
2i79101
-
serum was added to each solution at 20~ (v/v) concentration to
store digested tissues.
Four separate 50 ml plastic tubes (Fischer Scientific,
Pittsburgh, PA), each containing 25 ml of either HBSS, UW,
mUW, or LAP-1 were placed on ice. Each time tissue was
removed from the container during the cold digestion process,
the tissue was dispensed equally between the four tubes until
an equal amount of digested tissue (a total of 1.5 to 2 ml)
was placed in each tube. The tubes were then stored for
90 minutes on ice. ,The remaining digested tissues were
processed by a bulk islet isolation procedure, as described
below.
After 90 minutes preservation at 4~C, digested tissue
from each tube was separately suspended in Euro-Ficoll
solution (density of 1.100 g/cm3) and purified by a
discontinuous gradient centrifugation on Euro-Ficoll solutions
(d=1.100, 1.087, 1.056) in HBSS at 450 x g for 20 minutes at
4~C. After centrifugation, cells were taken from each density
interface to identify the islet layer. Cells in the second
layer, the interface between 1.087 and 1.056 and the third
layer, the interface between 1.100 and 1.087, contained
islets. The islets, from the interfaces were collected and
washed twice with the corresponding cold solution at 4~C,
followed by one wash with RPMI1640 culture medium (see below)
at room temperature. Yield and purity of islet preparations
were evaluated by dithizone (DTH) staining. The islet number
was expressed as 150 ~m islet equivalents (IEQ).
Islets resuspended in RPMI1640 culture medium were
distributed equally into six plastic Petri dishes (Falcon
#1008, Becton Dickinson Co., Franklin Lakes, NJ) per group to
be used for counting the islet numbers on days 0, 1 and 3
following preparation. Additionally, three dishes of each
group were prepared for evaluation of islet viability and for
assessment of glucose stimulated insulin release in a static
incubation assay and a perfusion system and the measurement of
-13-
2179101
islet insulin content. The medium used for culture was
RPMI1640 supplemented with 10~ (v/v) fetal bovine serum (FBS),
10 mM nicotinamide, 25 mM HEPES, 24 mM NaHCO3, 100 units/ml
penicillin G, 100 ~g/ml streptomycin and 0.25 ~g/ml
amphotericin B. All culture dishes were placed in a tissue
culture incubator in a 5% (v/v) CO2/air environment, at 37~C.
Islet Yield
Immediately after isolation, the total islet yield in
each group was evaluated, in duplicate, using DTH staining.
The islet number was converted to 150 ~m islet equivalents
(IEQ). The result of each group was expressed as a percentage
of the islet number yielded with HBSS in each series.
Islet Purity After Isolation
The sample used for counting islet yield was also used to
evaluate the purity of islet preparations. The purity was
estimated from the proportion of DTH stained and unstained
cells. The result was expressed as a percentage of the DTH
stained cell number to the total cell number.
Islet Numbers Durinq Culture
Islet numbers in two randomly selected dishes were
counted on days 0, 1 and 3 (day 0 is the day of islet
2S isolation) using DTH staining.
ViabilitY of the Islets
On days 0, 1 and 3 of culture, the viability of islets
was assessed using supravital staining with fluorescein
diacetate and ethidium bromide as described by Gray et al.,
Stain Technology 62 373-381 (1987). The viability of each
islet was scored as 0 (0%: dead islet), 1 (25% viable islets),
2 (50% viable islets), 3 (75% viable islets) and 4 (100%:
fully viable islet) and the percent viability was calculated
from the following formula:
-14-
' 2179101
% viability = (0.25 x number of islets scored as 1 + 0.5
x number of islets scored as 2 + 0.75 x number of islets
scored as 3 + number of islets scored as 4) divided by total
islet number x 100.
Insulin Release Assay
To assess ~-cell function, static incubation tests were
performed on each group between day 3 and day 8. One thousand
islets (IEQ) were incubated in 1 ml RPMI1640 containing 60
mg/dl glucose (basal m,edium) for 45 minutes and then in 1 ml
RPMI1640 containing 300 mg/dl glucose (high glucose
stimulation medium) for 45 minutes. The islets were again
incubated in 1 ml basal medium for 45 minutes. Insulin levels
released into each medium were measured by solid-phase
radioimmunoassay (Autopak insulin kit, ICN Biomedicals
Inc., Costa Mesa, CA). A stimulation index was calculated as
the insulin released into the stimulation medium divided by
insulin released into the basal medium. After the static
incubation test, insulin was extracted from the islets by
overnight incubation in acid alcohol to measure islet insulin
content.
In addition, dynamic insulin release in response to
glucose stimulation was evaluated by a perfusion system with
the islets isolated using LAP-l cold preservation solution.
Five hundred islets, placed in a cytodex gel (Cytodex 2,
Pharmacia Inc, Piscataway, NJ) column, were first perfused at
37~C with Krebs buffer solution containing 60 mg/dl glucose
and 2% (v/v) heat-inactivated newborn bovine serum for 100
minutes to stabilize the baseline. During the next 15
minutes, samples were collected every one minutes (basal
insulin release). The medium was then changed to Krebs buffer
containing 300 mg/dl glucose and perfused for 30 minutes
before changing to the basal buffer to observe prompt shut-off
of insulin release. Insulin levels in each sample collected
during the perfusion (1 minute/tube) were measured by solid
-15-
2179101
phase radioimmunoassay.
Electron Microscopy of Islets
Immediately after isolation, islets were fixed with 1.25~
(w/v) glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, at
room temperature for the ultrathin sectioning method. Islets
were washed in 0.1 M phosphate buffer containing 5~ (w/v)
sucrose (pH 7.4, 360 mOsm) and suspended in 70~ (v/v) calf
serum in 0.1 M phosphate buffer, pH 7.4. Subsequently, cells
were centrifuged at 450 g for 15 minutes to form a pellet
which was immersed in 2~ (w/v) glutaraldehyde in 0.1 M
phosphate buffer (pH 7.4, 440 mOsm) for 2 hours. Each pellet
was cut into small blocks and washed with 0.1 M phosphate
buffer containing 8~ (w/v3 sucrose (pH 7.4, 440 mOsm) followed
by post-fixation in 1~ (w/v) osmium tetroxide in 0.1 M
phosphate buffer containing 7~ (w/v) sucrose (pH 7.4,
440 mOsm) for 2 hours. The fixed specimens were dehydrated in
a graded series of ethanol and embedded in epoxy resin. The
thin sections were stained with uranyl acetate and lead
citrate. Observations were performed with a Hitachi H-7000
transmission electron microscope.
Data Analyses
All data were expressed as mean+standard error unless
otherwise specified. Differences between groups were compared
using an unpaired, two-tailed Student's t-test and were
considered statistically significant if p value ~0.05.
-
Results
Immediately after islet isolation, groups mUW, UW and
LAP-1 yielded higher numbers of islets than the HBSS group.
The difference was statistically significant in groups mUW and
LAP-1. There was a wide range of islet yields from one set of
experiments to another, depending on the volume of digested
tissue used. For this reason, islet yields were expressed as
-16-
217~10~
percent of the value obtained with HBSS. Islet yields in
group LAP-1, in particular, were twice as high as those in the
HBSS group (192 + 92 ~ ), see Table II.
Table II
Raw data expressed as IsletData expresses as a percentage
Equivalents (IEQ) of the HBSS values
Sample HBSS mUW UW LAP HBSS mUW UW LAP
Hu148 2675 8267 2492 6750 100 309.05 93.17 262.34
Hu153 14775 17883 16168 18467 100 121.04 109.36 124.99
Hu154 17608 31641 18667 19825 100 180.72 106.62 113.23
Hu166 31592 22017 46625 40160 100 69.69 147.68 127.09
Hu167 41808 69475 38450 46367 100 142.26 91.97 110.9
Hu169 13442 19292 11392 22625 100 143.62 84.75 168.32
Hu164 4050 8533 13167 14000 100 210.69 325.11 346.68
Hu165 4930 8160 7310 12910 100 165.62 148.28 261.87
Hu167-1 13687 22313 25533 42900 100 163.02 186.66 313.44
Hu167-2 19380 21573 23307 20673 100 111.32 120.26 106.67
Mean 16385 21916 20310 24467 100 161.68 141.36 192.46
SD' 12372 15214 13723 13734 0 64.87 71.91 92.02
SD= standard deviation
The purity of islet preparations in the HBSS group was
41.5+14.8~ . In contrast, the purity in groups mUW, UW and
LAP-1 was 71.5+8.9%, 74.9+10.7%, 75.8+7.4%, respectively,
25 which were significantly higher than that of the HBSS group
(p<0.001). Differences between these three groups, however,
were not significant. Highly purified islets (as high as 95%
pure) were isolated with LAP-1. In contrast, islets in the
HBSS group were contaminated with exocrine tissue and the
purity was as low as 25%.
In the HBSS group, the islet numbers decreased to
55.6+14.4% on day 1 and 27.4+16.5% on day 3 as compared to
that on day 0 (100%). In contrast, significantly higher
2179101
numbers of islets were counted in all other groups on both
days 1 and 3 as compared to those in the HBSS group.
Especially in group LAP-1, the islet number was maintained at
the highest level of 94~6i20~4~ on day 1 and 78.5+24.2~ on day
3, but a significant difference was not obtained between
groups mUW and UW.
At the time of islet isolation, the percent viability
estimated by staining with fluorescein diacetate and ethidium
bromide was already lowest in the HBSS group (HBSS vs UW, HBSS
vs LAP-1; p<0.05). Islet viability in this group continuously
decreased to 37.4+19.5~ on day 1 and 16.8+13.2~ on day 3,
whereas the other three groups maintained significantly higher
islet viability. As shown by a electromicrograph of a
representative case, islet damage was clearly less in the LAP-
1 group than that in HBSS.
Contamination with cell debris and acinar cells was more
prominent in the HBSS group as compared to LAP-1. In
addition, islet cells in the HBSS group displayed a decreased
density of cytoplasm, mitochondrial swelling and unclear or
ruptured cell membranes. By contrast, these findings were
consistently less frequent in the LAP-1 group.
The highest insulin content was found in islets isolated
with LAP-1. However, no statistically significant differences
were detected between any of the groups.
In groups mUW, UW and LAP-1, the stimulation indices were
higher than that of the HBSS group. In particular,-in groups
mUW and LAP, the indices were significantly higher than that
of the HBSS group.
Islets isolated in LAP-1 released insulin immediately
after glucose stimulation with a two phase insulin release,
which promptly ceased when the high glucose buffer was
replaced by the basal buffer. The curve demonstrated the
normal function of ~-cells.
The two-step digestion technique for the human pancreata
has been used successfully to isolate a large number of viable
-18-
217~101
islets. The technique involves two phases of digestion
processes, first at 37~C and then on ice. In cold digestion,
the collagenase solution was gradually replaced by cold
preservation solution. The tissue is broken down into smaller
fragments by warm collagenase digestion, it continues to
digest on ice into fine fragments and finally to free islets.
Islets prepared by the two-step procedure maintained a well
preserved capsule and an excellent three-dimensional
structure. This method prevents overexposure of islets to
collagenase and thus a,voids over-digestion of islets as well
as acinar cells.
UW solution is the most advanced and widely used cold
preservation solution for storing organs awaiting
transplantation. UW solution contains a low concentration of
Na+ and high concentration of K+, similar to the composition
of intracellular fluid. In addition, UW solution contains the
lactobionate anion and raffinose as impermeable components and
HES to maintain the colloid pressure. This composition
prevents cell swelling that occurs when cells are stored in
conventional physiological solutions (i.e. HBSS) in the cold,
a condition in which the cell membrane sodium-potassium pump
ceases to function. Although HES is an important component
for maintaining vascular networks when an organ is flushed out
with cold preservation solution, it may be omitted, with-no
deleterious effect on cell viability, in cold storage of small
organs or tissues. We therefore modified the UW solution to
contain only lactobionate-K+, raffinose, KH2PO4 and MgSO4 and
compared the effectiveness of both mUW and UW in this study.
Along with these tests, we also developed a new cold
preservation solution, LAP-1, for islets with special
attention to their vulnerability. LAP-1 solution contains
lactobionate and D-mannitol as impermiants, to inhibit cell
swelling by maintaining extracellular osmotic pressure. D-
mannitol is also known to have a scavenging activity for
hydroxy radicals (OH-) to which ~-cells are highly
--19--
- 2179101
susceptible. As with UW solution, LAP-l solution contains
KH2PO4 as a component of the buffering system. In addition,
the solution contains SOD (30,000 units/l) and nicotinamide (5
mM/l). Our expectation is that SOD and nicotinamide would
enter islet cells, that may have been damaged during the
isolation process, to protect them from further damage and to
support their recovery in culture or after being transplanted.
In this study, the performance of four solutions for
storing digested pancreatic tissue at low temperature have
been compared by evaluating the yield, the purity of the
recovered islets and the viability of islets maintained in
culture. Cell viability was also assessed by electron
microscopic ex~m;~tion of islets and by ~-cell function. The
yield and purity of isolated islets were markedly improved by
using solutions especially designed for cold storage of organs
(UW, mUW, LAP-l), instead of using cold HBSS. Although HBSS
has been widely used for human islet isolation, the first
interface between HBSS and 1.056 g/cm3 Euro-Ficoll contained
many fragmented islets and the second interface between 1.056
and 1.088 g/cm3 Euro-Ficoll contained most islets and a
considerable number of exocrine cells. This indicated that
the density of both islet and acinar cells had changed
significantly, probably due to cell swelling during cold
storage. This resulted in more islet destruction (thus, lower
yield) and lower islet purity. In contrast, with other
solutions, almost all islets were found in the second
interface with far fewer acinar cells, indicating that UW, mUW
and LAP-l solutions prevented cell swelling and had maintained
the density of both islet and exocrine tissues. Electron
microscopic examination of islets isolated with LAP-l solution
revealed no cell swelling and a normal structure of islet
cells. Since the islet yield and purity were not
significantly different between these three solutions, HES and
raffinose may not be essential components of the cold
preservation solution for preventing cell swelling under the
-20-
2179101
,
conditions used in the islet isolation.
Islet numbers were also counted during culture for three
days. The islet number on day 1 was especially important,
since it probably reflects an actual islet number that may
survive following transplantation. On day 1, the islet
numbers in the HBSS group had already decreased by nearly 50
and declined to only 25~ of the initial count on day 3. This
decline indicated severe and often irreversible damage to
islets that occurred during the cold storage of pancreatic
digests. Islet numb~rs in the other three groups were
significantly higher at all points, ranging between 95~ and
75~ on day 1 and between 78~ and 60~ on day 3, as compared to
those on day 0. The decreased islet numbers and viability on
day 3 in this study may have been influenced by the culture
conditions, since only a small volume of culture medium was
used in order to accommodate islet counting. Although no
significant differences were found between these three groups,
islet viability was higher in groups UW and LAP-1. These
results show that the prevention of cell swelling during the
islet isolation process contributes not only to the
improvement of islet yield and purity, but also to greater
islet viability. The results of ~-cell functional tests
correlated with these viability results. The stimulation
index was highest in the LAP-1 group. However, islet insulin
content, as expressed by ~U/islet (IEQ), did not show any
differences between the groups. This is not surprising, since
an equal number of islets, that survived for more than 3 days
in culture, was used for these tests. This also indicates
that islets recover fully in culture by day 3 and survive.
The beneficial effects of UW, mUW and LAP-1 solutions
over HBSS have been clearly demonstrated in the preparation of
human pancreatic islets. Among these, LAP-1 solution was most
effective, providing the best yield, purity and viability of
isolated islets. Also, the high viscosity of the UW solution
made it undesirable for use in the isolation of islets as
-21-
2179101
manipulations with the viscous solution were difficult. In a
perfusion system to test dynamic insulin release in response
to high glucose stimulation, islets in the LAP-l group
exhibited excellent functional responsiveness.
Pancreatic islets have two different characteristics: one
as a cell and another as an organ. The islet acts as a
miniature organ, since it consists of several different cell
types which interact functionally with one another and has its
own vascular system. In order to maintain normal islet
function, islet struc~ure needs to be preserved carefully.
The collagenase digestion process is a procurement process of
islets from the larger organ, the pancreata, under warm
ischemic conditions. The subsequent processes, that include
cold storage, purification and washing of islets, correspond
to the cold anaerobic preservation of a donor organ. Based on
the well documented theory for organ harvesting, shortening of
the warm ischemic process is the first critical issue and the
use of an appropriate preservation solution for cold storage
is the second critical issue in the procurement of highly
viable donor organs. The intermittent two-step digestion
procedure to minimize warm anaerobic ischemia and the use of
LAP-l solution for cold preservation of digested pancreatic
tissue, both follow this basic theory. Their use resulted in
higher yield, purity and viability of islets from the human
pancreata.
Human islets were isolated by an intermittent two-step
collagenase digestion procedure with warm collagenase
digestion of the pancreata in less than 20 minutes. The cold
digestion process and the cold storage of digested tissue
(containing islets) should use an appropriate cold
preservation solution. LAP-l was designed as a cold
preservation solution especially for islets; it showed clear
advantages in the preparation of high quality islets and is
also economical for use in a large quantity.
-22-
2179101
. ~
Example 2
Preparation of Donor Pancreas
A total of 46 human pancreata were processed
consecutively for islets using the new isolation procedures,
described above in Example 1 (group 1). Islet preparations
from 46 pancreata processed by a conventional isolation
procedure served as the control group (group 2).
All pancreata were harvested with appropriate consent
from cadaveric organ donors through the two organ procurement
agencies of Southern California. Procurement was performed by
the standard techniques, by either the members of the UCLA
Liver Transplant Program or surgeons from other institutions.
All organs used in this study were a part of the pancreas
containing the body and tail. The mean donor age of group 1
was 33+3.2 years and ranged from 5 to 64 years; in group 2,
the mean age was 36+2.7 years, ranging from 11 to 69 years and
there were no significant differences between two groups. The
pancreata was transported to the laboratory in either UW
solution or Euro-Collins solution. Cold ischemic time in
groups 1 and 2 was 8.1+0.4 hours and 6.6+0.5 hours,
respectively, and not significantly different. The organ was
trimmed of surrounding membranes, vessels, fat and lymph
nodes, cannulated with an angiocatheter and weighed. The
organ was expanded by injecting 150-250 ml of warm collagenase
solution using a 30 ml syringe and sectioned into
approximately 8-10 pieces. The collagenase solution contained
Collagenase P (Boehringer Mannheim CO., Indianapolis, IN), 2~
(vlv) heat-inactivated newborn bovine serum (NBS, Sigma
Chemical Co., St Louis, MO), 1 mg/ml calcium chloride and
DNase in HBSS. The concentration of collagenase was optimized
for each lot and ranged from 1.5 to 2.5 mg/ml.
For group 2, pancreatic tissues were enzymatically
digested by a one-step procedure in a 37~C water bath. The
procedure has been described in detail by Benhamou et al.,
Transplantation 57 1804-1810 (1994). Briefly, the sectioned
-23-
2179101
.
pancreas was placed in a 5x7 cm stainless-steel, enclosed mesh
container containing three 1.5 cm glass marbles. The
container was then placed into a disposable clear plastic
chamber and the tissue was covered by collagenase solution.
The total collagenase volume, used both for expansion of the
organ and solution placed in the container, was approximately
200 ml. The chamber was agitated in a 37~C water bath via a
swing-arm shaker (Wrist Action Shaker, Model 75, Burrell Co.,
Pittsburgh, PA). Samples were taken at regular intervals.
When free islets were noted, usually within 15 minutes after
the start of digestion, the collagenase and digested tissue
mixture were removed from the chamber. Sufficient fresh warm
collagenase solution was added to the chamber to cover the
remaining tissues and the digestion was continued. This
process was repeated every 5 minutes until no more free islets
were detectable, or until all tissues were digested. The
total process required 45 to 60 minutes and the total
collagenase solution was up to 600 ml. The
collagenase/digested tissue mixture decanted from the
container was immediately diluted with cold HBSS containing
20~ (v/v) NBS and stored on ice until digestion was completed.
For group 1, tissues were digested by a two-step
procedure, first in a 37~C water bath, then followed by cold
digestion of the partially digested tissues on ice. Pieces of
the pancreas were placed in a disposable, clear plastic
container containing three glass marbles. The chamber was
gently agitated in a 37~C water bath via a swing-arm shaker as
above, for 15 minutes, until the pancreas was partially
digested (Step 1: warm digestion phase). Collagenase solution
containing partially digested tissues was filtered through a
sheet of large-mesh (8 mm) screen into a second clear plastic
container placed on ice. Cold LAP-l preservation solution was
added to the second container at a volume approximately 1/3 of
the decanted volume. In the first container, warm collagenase
was again added to cover the remaining tissue and warm
-24-
2i79101
digestion was repeated. After 5 minutes, all
collagenase/digested tissues in the first container were
transferred into the second container. The second container
was connected to a collection bottle and the cold preservation
solution via two separate side ports. The second container
was gently hand-shaken on ice. Every 5 minutes, 1/3 of the
supernatant containing digested tissues was decanted, through
an out-flow port, into a collection bottle containing cold
LAP-l solution supplemented with 20~ (v/v) NBS. LAP-l
solution was added to ,the second container, via another port,
at a volume equal to that decanted and the container was
shaken again. This cold digestion process was repeated until
all islets were freed (Step 2: cold digestion phase). If
necessary, warm digestion was repeated a third time, so that
only undigested, larger ductal structures remained.
To avoid harmful effects on digested tissues, collagenase
and other enzymes released from pancreatic acinar cells must
be inactivated as soon as possible. For this purpose, finely
digested pancreatic tissues containing free islets were
collected at 5-10 minute intervals and stored on ice in a
large amount of cold storage solution. In group 1, pancreatic
digests were stored in cold LAP-l solution supplemented with
20~ (v/v) NBS, whereas in group 2, tissues were stored in cold
HBSS containing 20~ (v/v) NBS.
Pancreatic digests in both groups were suspended in Euro-
Ficoll solution with a density of 1.100 g/cm3 and islets were
separated from acinar cells by discontinuous gradient
centrifugation on three layers of Euro-Ficoll solutions
(densities of: 1.100, 1.087 and 1.056) using a COBE2991 cell
processor (Cobe Laboratories, Inc., Lakewood, CO). After 10
minutes centrifugation, islet fractions were identified and
collected. Islets were washed twice with cold LAP-l solution
and once with RPMI1640 medium containing 10~ (v/v) fetal
bovine serum ( FBS) at room temperature.
Isolated islets were cultured in plastic Petri dishes
-25-
2179101
(Falcon #1005, Becton Dickinson Co., Franklin Lakes, NJ) at
37~C with RPMI1640 containing 20~ (v/v) FBS, 10 mM
nicotinamide, 25 mM HEPES, 24 mM NaHCO3, 100 units/ml
penicillin G, 100 mg/ml streptomycin and 0.25 mg/ml
amphotericin B.
Temperature
The temperature of the digestive medium during the cold
digestion phase in the intermittent two step digestion process
(group 1) was monitor~d every 5 minutes in five isolation
cases.
Islet Yield
Immediately after isolation, the total islet yield was
evaluated in duplicate by taking a known volume of sample and
staining with dithizone (DTH). The islet number was counted,
each size separately and the total islet number converted to
that equivalent to islets 150 ~m in size (IEQ). The islet
yield was expressed as thè total islet number isolated from a
given pancreas and the number of islets per gram of pancreatic
tissue.
Islet Purity
The purity of the islet preparation was evaluated on the
same sample as that used for islet counting and was calculated
as the ratio between DTH-stained and total cells, expressed as
a percentage.
Assessment of Viable Islets After 48 Hours in Culture
Islets that remained viable in culture for 2 days were
taken as "viable" islets that would survive, in vivo, after
transplantation. This was evaluated by placing islets in
culture and assessing their viability 48 hours after
isolation. Islet viability was determined using DTH for
counting and fluorescein diacetate and ethidium bromide (FDEB)
-26-
2179101
-
staining for viability assessment as described by Gray et al.,
Stain Technology 62 373-381 (1987).
In Vitro Insulin Release AssaYs
s To assess the ~-cell function of cultured islets, two
glucose stimulation tests, static incubation and a dynamic
insulin release assay in a perfusion system, were performed on
several randomly selected preparations in group 1.
i) Static incubation assay:
One thousa~d islets (IEQ) were incubated
successively in basal, stimulation and basal medium as
described above. RPMI1640 medium was used with the glucose
level reduced to 60 mg/dl for the basal medium and increased
to 300 mg/dl for the stimulation medium. Insulin released
during each incubation was measured by a solid-phase
radioimmunoassay using human insulin as standard (Autopak
Insulin Kit, ICN Biomedicals Inc., Costa Mesa, CA).
The stimulation index was calculated as: insulin released
into the stimulation medium divided by insulin released into
the basal medium. ii) Dynamic insulin release assay in a
perfusion system: Five hundred islets (IEQ) were layered in a
cytodex bead column, which was placed in a 37~C water bath and
perfused successively in the following order: basal,
stimulation and basal medium as described previously by
Benhamou et al. (Hormone & Metab. Res. 27 113-120, 1995).
Media used in the perfusion system were Krebs Ringer
bicarbonate buffer solutions containing 60 mg/dl (basal) and
300 mg/dl (stimulation) glucose, respectively. Insulin was
assayed as for static incubation.
In Vivo Islet Function Test
The ability of islets to reverse diabetes was assessed,
in vivo, by transplanting them into athymic mice made diabetic
with an intravenous injection of 165 mg/kg streptozotocin.
Islets (1,000-1,200 IEQ) from group 1, cultured for 3 days,
~-27-
21 79101
were transplanted under the left renal capsule of diabetic
mice. After transplantation, levels of non-fasting blood
glucose, urine glucose and body weight were monitored three
times a week. Glucose tolerance tests were also performed on
day 14 by injecting 0.5 g/kg glucose into the tail vein and a
K-value was calculated using blood glucose levels at 5, 10, 30
and 60 minutes after glucose challenge. On day 20 or 21, mice
underwent a left nephrectomy to confirm the recurrence of
diabetes and grafts were examined histologically.
Statistical Studies
All data were expressed as mean value+standard error of
the mean and the difference between groups 1 and 2 was
considered significant if the p-value was less than 0.05,
using a two-tailed unpaired student t-test. The significance
of differences in viable islet recovery was examined using a
chi-square test.
There were no significant differences between groups 1
and 2 with regard to the donor age, the cold ischemia time and
harvesting conditions. The pancreas weight was also similar
in both groups; 55i2-7g in group 1 as compared to 56i3.4 in
group 2.
Table III
Group n~ Pancreas Digestion Time
Weight (g)
Warm Cold Total
4655i2.7 21.5i0-7 41.7il.2 59.3i2.5
2 4656i3.4 56.li2.2 - 56.li2.3
p-value N.S2 p~O.OO1 - N.S.
1 n = number of isolations
2 N.S. = Not significant
As shown in Table III, the duration of warm digestion was
significantly less in group 1 (21.5+0.7 minute) compared to
-28-
21791 01
that in group 2 (56.1i2.3 minute). In group 1, warm digestion
was followed by cold digestion for 41.7il.2 minute, so that
the total time required to digest the pancreas was 59.3i2.5
minute, similar to that of group 2.
Temperature of the medium was measured during the cold
digestion procedure (n=4) Table IV.
Table IV
Time Average Time Average
(min.) Temp. (~C) (min.)Temp. (~C)
o1 B7 30 15
37 35 12
37 40 10
37 45 9
202 30 50 7
1 Time 0-15 min. : Warm digestion process
2 Time 20-55 min.: Cold digestion process
Also, the change in the collagenase concentration during
this process was calculated based on the amount of LAP-1
solution added each time, see Table V.
Table V
TimeCollagenase Time Collagenase
(min.) (mg/ml) (min.) (mg/ml)
ol 2.5 30 0.75
2.5 35 0.50
2.5 40 0.30
2.5 4S 0.20
202 1.6 50 0.15
1.2 55 0.10
1 Time 0-15 min. : Warm digestion process
2 Time 20-55 min.: Cold digestion process
-29-
2179101
.
By the repeated replacement of warm collagenase solution
with cold LAP-1 solution during the cold digestion process,
the temperature of the solution rapidly declined from the
initial 37~C to 15~C in less than 30 minutes, then to c10~C by
540 minutes. By dilution calculations, the collagenase
concentration also decreased from 2.5 mg/ml to 0.1 mg/ml by
the end of the digestion period.
Islets were successfully isolated from 44 of the
46 pancreata in group 1 (success rate: 95.6~) and from 33 of
10the 46 pancreata in group 2 (71.7~; p<0.01). In 9 of the 13
failures in group 2, the isolation process was terminated
before the islet purification step with the COBE2991 cell
processor due to an insufficient number of islets and their
- poor appearance during digestion. In the remaining 4 cases,
15most of the islets disappeared or disintegrated after the
purification process.
In group 1, the total islet yield was also significantly
higher than that of group 2 as shown in Table VI.
20Table VI
Total Yield1 Yield/g Pancreasl
Group Cells x 10 3 Group Cells x 10 3
1 (n2=44) 336 + 36 1 (n=44) 6.2 + 0.7
2 (n=33) 196 + 25 2 (n=33) 3.6 + 0.3
1 Islet numbers shown by IEQ
2 n = number of isolations
The islet yield in group 1 was 335,739+36,244 IEQ from
the body and tail of the pancreas (pancreas weight: 33+3.2 g)
and 6,233+681 IEQ/g of tissue, while that in group 2 was
195,587+25,242 IEQ per pancreas (pancreas weight: 36+2.7 g)
and 3,763+509 IEQ/g (p~0.01). The purity of isolated islets
was 83.6+2.5~ in group 1 and 69.2+4.7~ in group 2 (p<0.05),
see Table VII.
-30-
217~101
Table VII
Group Purity
1 (nl=44) 84 ~ 3
2 (n=33) 69 + 5
l n = number of isolations
Islets placed in culture were viable, as assessed by FDEB
staining, after 48 hour!s in 43 of the 44 successful isolations
in group 1, see Table VIII.
Table VIII
Group Viable- Non-Viable
1 (nl=44) 43 (97.7~) 1 (2.3~)
2 (n=33) 26 (78.8~) 7 (21.8~)
1 n=number of isolations
Thus, overall, 43 of a total of 46 pancreata prepared by
our new procedure provided viable islets as assessed by highly
strict criteria. With these criteria, the success rate in
group 1 was 93.5~. In contrast, islets in group 2 were viable
after 2 days in culture in 26 of the 33 successful isolations
(see Table VIII). Thus, the overall success rate was 56.5~
t26 of the 46) in this series of isolations. The difference
between groups 1 and 2 was significant (p<0.001).
Static incubation assays were performed on six different
islet preparations in group 1. Stimulation indices calculated
from these experiments are shown in Table IX.
-31-
~179101
Table IX
Exp. No. Stimulation
Index
Hu153 1.91
Hu154 1.91
Hu164 - 3.03
Hu166 1.09
Hu167 2.65
Hu168 1.15
Mean+SE1.96~0.32
- Islets in all preparations responded to high glucose
stimulation, although the stimulation indices were lower in
#166 and #168. As represented by preparation #196 (see Table
X), dynamic perfusion studies also demonstrated normal ~cell
function of islets prepared in group 1 as shown by immediate
and two phase insulin release after a high glucose challenge
and the prompt increase in insulin release when the medium was
changed to basal.
-32-
2179101
Table X
Tl G2 IRI3 Tl G~ IRI3 Tl G2 IRI3 Tl G' IRI3
2 60 48 22 300 170 42 60 108 62 60 45
4 60 50 24 300 164 44 60 100 64 60 65
6 60 40 26 300 160 46 60 85 66 60 45
8 60 55 28 300 155 48 60 70 68 60 50
60 54 30 300 160 50 60 68 70 60 30
12 60 45 32 300 164 52 60 68 72 60 28
14 300 160 34 300 12554 60 55 74 60 32
0 16 300 145 36 300 13056 60 52 76 60 30
18 300 155 38 300 12558 60 45 78 60 30
300 165 40 300 11060 60 52 80 60 30
T = Time ~minutes). .
2 G = Glucose (mg/dl)
3 IRI = Immunoreactive insulin (~U/ml)
Reversal of diabetes in athymic mice was achieved by
transplantation of 1,000-1,200 islets from group 1, see Table
XI.
Table XI
NFBGl NFBGl
Day Mouse 1 Mouse 2 Day Mouse 1 Mouse 2
-5 100 - 6 140 140
-3 - 110 10 - 60
o2 360 350 15 60 95
- 195 21 10 03
2 195 - 22 280 12 03
3 - 120 23 - 400
4 120 - 24 390
1 NFBG=non-fasting blood glucose
2 day of transplant
3 day of nephrectomy (graft removal)
-33-
2179~01
.
Mice became normoglycemic (~150 mg/dl) with negative
urine glucose within 5 days after grafting. K-values of the
IVGTT were 0.30+0.02~/minutes. Histological examination with
hematoxylin-eosin staining revealed well-formed islets and
well-preserved ~-cells as indicated by insulin staining.
These in vivo results demonstrated survival and function of
islets after transplantation.
Islet transplantation has not yet become a routine
procedure for diabetic kidney recipients, due to a low success
rate and the relatively short period of insulin independence.
Islet isolation from donor pancreas is the initial process
crucial to realizing successful islet transplantation. At
present, simultaneous kidney and islet transplantation for
type I diabetic patients with end stage renal failure would be
the most reasonable form of islet transplantation because of
the need for immunosuppression. In these patients, the use of
islets isolated solely from the pancreas of the kidney donor
would be the best approach to minimizing rejection by avoiding
additional antigenic disparities. To make this possible, a
large number of islets need to be isolated from a single human
pancreas. Another important issue for successful islet
transplantation is the ability to obtain high quality islets
which survive and function well after grafting.
There are several factors that affect islet viability
during islet preparation. Donor factors, the surgical
techniques for the removal of the pancreas and the
preservation solution used for transporting the procured
pancreas can influence islet viability and recovery.
Hospitalization period of the donor also affects the isolation
outcome. In the islet isolation process, the most critical
procedure is that of the collagenase digestion. This process
is essential for separation of islets from the surrounding
tissues, but, at the same time, islets can be damaged by both
warm ischemic injury and enzymatic digestion mediated by
collagenase and possibly enzymes released from acinar cells.
-34-
2179101
Another critical procedure is the handling of islets during
the cold phase of the isolation process, which involves cold
storage of pancreatic digests, the islet purification process
and cell washing. If not properly processed, islets may
suffer cell swelling and cold ischemic injury. Additionally,
islet survival can be affected by mechanical damage induced by
pipetting and cell washing.
Collagenase distention of the pancreas and the digestion
of tissues are an essential process for freeing islets, but
are also harmful for both islets and acinar cells. In order
to minimize the toxic effect of enzymes, the following three
modifications are necessary: i) to reduce warm digestion time,
ii) to minimize the exposure of freed islets to warm enzyme
solution and iii) to stop enzyme activities by immediately
storing digested tissues and freed islets in a suitable cold
preservation solution. In this study, we have demonstrated
that prolonged warm collagenase digestion is not necessary in
order to free islets. The exposure of pancreatic tissues to
warm collagenase can be reduced to less than 25 minutes.
Tissue digestion continues on ice and releases islets. With
the isolation technique of the present invention, the warm
digestion time was significantly reduced from 50 minutes,
required by earlier methods, to approximately 20 minutes.
Since most of the islets were freed during the cold digestion
process, the intermittent two-step procedure minimized the
direct exposure of islets to warm, concentrated enzyme
solution. Moreover, during cold digestion the enzyme solution
was gradually replaced by the cold preservation solution and
thus, islets were released into cold, diluted enzyme solution,
which was further diluted by the cold preservation solution
when digested tissues were stored.
The temperature of the digestion medium was rapidly
lowered to 5-7~C during the cold digestion phase. Also, the
concentration of collagenase in the digestion medium decreased
to 0.1 mg/ml based on the dilution calculations. The results
-35-
2~79101
indicate that free islets may disintegrate by enzyme action
during the warm digestion process. It was also noted that the
amount of collagenase used in the two-step digestion was less
than that used in the one-step procedure (up to 300 ml vs.
500-600 ml). By combining the two-step digestion procedure
and the use of LAP-l solution as a cold preservation solution,
the success rate for obtaining viable islets markedly improved
to 93.5% (43 of the 46 consecutive isolations) from 56.5% (26
of 46) prepared by our old method (as assessed after 48 hours
in culture). Prior to this study, these isolation failures
were attributed to various conditions including the donor's
physical condition, medications, procurement techniques and/or
prolonged cold ischemia time. Although these factors
influence the outcome of islet isolation, the results in the
present study suggest that the failure of isolation, poor
viability of islets and lower islet yields can also result
from prolonged warm collagenase digestion and cold ischemic
injury during cold preservation in HBSS. It would also appear
that the new isolation technique can isolate islets from a
damaged pancreas by providing conditions that avoid further
islet damage and preserve viable islets, whereas islet damage
may be accelerated by the conventional technique.
Both islet yield and purity were improved in group 1.
After density gradient centrifugation, islets were located in
a confined area of the Euro-Ficoll, separate from the acinar
cells. In contrast, islets and acinar cells in group 2 were
distributed in a broader range of Euro-Ficoll layers. The
prevention of cell swelling also increases islet yields. All
pancreata used in both groups 1 and 2 were partial, containing
only the body and tail. On separate occasions, islets were
isolated from the whole pancreas using the newly developed
technique. In all cases greater than 500,000 IEQ were
isolated. This number of islets fulfills the requirement for
transplantation currently recommended for type I diabetic
patients by providing >6,000 IEQ/kg of recipient body weight,
-36-
-- 2~79101
>90~ purity and >95~ viability. The recovery of viable islets
after 48 hours in culture is an important index for the
prediction of i~let survival following transplantation. In
group 2, 20~ of successfully isolated islet preparations (as
assessed immediately after isolation) died after being placed
in culture, suggesting that irreversible islet damage occurred
during the isolation process. In contrast, islets in only one
of the 44 successful isolations in group 1 were lost in
culture and islets were fully viable in the remaining 43
cases. These islets rçsponded with normal insulin release in
both in vi tro and in vivo assays.
Although the two-step digestion procedure appears
complex, the cold digestion process can be performed in a
totally enclosed system, similar to that designed for our old
method. The process is economical in regard to both time and
personnel. The entire procedure can be carried out by two or
three trained individuals within 4 to 5 hours, from the start
of cleaning of the pancreas to either placing isolated islets
in culture or completing the packaging for transplantation.
Thus, simultaneous islet and kidney transplantation is
possible. With this technique, a sufficient number of islets
to fulfill the transplantation requirement can be isolated
from one pancreas and simultaneous kidney and islet
transplantation is possible from a single donor.
All references cited above are hereby incorporated herein
by reference in their entirety.
The above descriptions of exemplary embodiments of are
for illustrative purposes. Because of variations which will
be apparent to those skilled in the art, the present invention
is not intended to be limited to the particular embodiments
described above. The present invention may also be practiced
in the absence of any element not specifically disclosed. The
scope of the invention is defined by the following claims.