Note: Descriptions are shown in the official language in which they were submitted.
~ wo gSrl9~13 2 1 8 0 3 2 6 PCTICA95100025
T.TT~ ~7Tr~
Field of the Inventiorl
The present inverltion relates to lubricant
compositions, and mor~! specifically to lubricant
5 compositions for use iLn lubricating the tracks which
convey bottles, cans and similar containers and pAr~A~q
for b~vt~ yuS from one station to another in a bottling
plant .
Backaround of the Invention
Beverage6 are so]Ld in a variety of containers such
as glass bottles, plastics bottles, plastics containers,
cans, or waxed carton packs. These containers are
cu..v~yed through a number of stations in a plant where
they are filled with t:he desired L~ L~e:; the containers
15 are c~ --v~:ye:d from one station to another by a track which
is usually of stA;nl-~ steel when the containers are
glass bottles, or of z~ plastics material such as an
acetal resin (sold uncler the name Delrin) when the
containers are other than glass bottles. Such tracks
20 will hereinafter be referred to as ~ICUllVt:y~L track".
When the containerq are being f illed with beverage
at a filling station ~n the bottling plant, they are kept
at a fixed position ullder the filling station while the
C-~llv~:yOI track contimles to move forwards below the
25 container. In addition, blockage of the path along which
the containers are travelling can occur if a container
falls over or gets jar~med. In C:uch instances it is
important that the CL ~IVe~ track is properly lubricated
so that the track can continue to move even though the
30 containers on the tra~k are t~ ~lily prevented from
advancing .
In order to ensu]-e smooth operation of the f illing
process, it is imperative to ensure that the C~llVt:YUL
track is properly lub]-icated and cleaned. If the
35 Cullvt:yOI track is not properly lubricated, the containers
can easily fall over or fail to stop moving when they
reach the appropriate station in the plant. This can
Wo 9S/19413 2 1 8 0 3 2 6 PCT/CA95/00025
cau6e serious disruption to the efficient operation of
the f illing process .
Lubricant compositions which are currently u6ed for
lubricating and cleaning ~ v~y~ I track are generally of
5 three main types:
(i) eomposition6 based on fatty acids,
(ii) eompositions based on fatty amines,
and
(iii) eompositions based on phosphate esters.
Aqueous solutions of fatty aeids are not suitable
f or use in areas of hard water, unless they are
stabilized by the inCuL~u~ation of a complexing agent
such as ethylPnP~liAm;np tetra-acetic acid (EDTA).
A further problem whieh we have eneountered with
eompositions based on fatty acids or amines or on
phosphate esters is that these known formulations are
very aggressive to the coloring pigments used to label
the surfaces of the containers, particularly steel and
mi nllm cans used in the beverage industry. We have
found that these known lubricating eompositions have a
~arked tendency to leach the printed matter printed an
the surf ace of the containers .
U. 5 . Patent 3, 574, lOO describes a c~ r track
lubricant eomposition whieh eompri~aes an aqueous solution
of (a) a phosphate ester of an oleyl alcohol ethoxylate
~nd (b) a water 60luble amphoteric ~
We have now ~)L ~duced a new lubricating co~po6ition
for use in lubricating CullVt:yU~ traek which has the
advantage that it does not eause fading of the eolors in
the printed matter on the containers which are being
proeessed through the f illing plant . The lubrieant
eompositions of the present invention ean also be used in
areas of hard water.
S~lml-qry of the Invention
Aeeording to one aspeet of the present invention,
there i6 provided a high pH col.c~l.L~ ~.te whieh upon
dilution with water forms a print eompatible lubrieant
21 80326
WO 95/19413 PCT/CA95/00025
composition for use as a lubricant for COIIVe:yOI track,
said c~ te comprising the following . L~:-
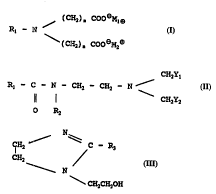
ta) (i) an alkylamine dicarboxylate _ ' Of
general f ormula ( I )
(CH2~ ~ CooeM,~3
Rl -- N (I)
\ ( CH2 ) " COO eM2(~
10wherein R~ is a C~ to Cl8 saturated or
unsaturated alkyl groUE\, inr~ in~ mixture6 of such alkyl
groups, n is an integer of from 1 to 12, preferably n is
2, and each of M~ and M2, which may be the same or
different, in~ y r~, ~s~ s ~ L~el- or a Group I
15 metal, typically sodium or potassium, and with the
proviso that the distribution of alkyl chain lengths of
the group R~ is such that: c (a) and (b) remain in
solution;
and/or
2 0 ( i i ) a a f general f ormu la ( I I )
CH2Y,
R~ -- C -- N -- CH2 -- CH2 -- N / (II)
ll I CH2Y2
O R2
wherein R~ is a C~ to C~8 alkyl group as def ined
above, R2 I~:~Lc:sellls llyd.co~el~ or -(CH2)~, -OH wherein m is
an integer of from 2 to 12, and each of Y~ and Y2 which
may be the same or different, is in~lP~PnrlPntly sPlected
from -CH2 OH, -COOeN3- and -CH2COOeM3~, wherein M3 is
hy~lr~,~ell or a Group I metal, typically sodium or
potasium;
21 80326
wo 95/19413 PCT/CA95/00025
(b) a cyclic imidazoline of the general formula
(III)
CH2 ~ C -- R3 (III)
CH2 N
\CH2CH20H
wherein R3 i8 a C7 to C20 saturated or u~.~.&tuLated
lO alkyl group;
(c) a C12 to C1~ saturated or unsaturated alkyl
51l1rhnn~te anionic surfactant, and
(d) optionally, a pH reducing agent to providQ said
pH in the range of 7 to ll.
15 uES~-n~ ON OF T~ K~ MRr)nIMFNTS OE' ~ TNVl;~NTION
In the group R1 of _ ^ t (a), the distribution of
zllkyl chain lengths must be such that the product is
stable and - -nts (a) and (b) do not come out of
solution. This is achieved, for example, if the alkyl
20 chain of the group R~ is ~L~ ~7 ;nF~ntly a C~2 alkyl group,
e.g., a mixture of alkyl groups as obtained from coconut
acid, or a mixture of an u~ Lu~c~ted C~ alkyl group (ie
oleyl) with said IJL~-~' ;ns-nt-ly C~2 alkyl mixture.
Thus, when _ (a) is an alkylamine
25 dicarboxylate of formula (I), it is preferably a salt of
coco-amine dipropionate, or a mixture of said coco-amine
salt, with a salt of oleyl-amine dipropionate. These
salts are typically the mono sodium salts.
These alkylamine dicarboxylate ~ '~ may often
30 be referred to in the art as alkyl betaine o~nA~ and
are intended to be interrhAnq~hle insofar as describing
various aspects of the invention.
In a particularly preferred .~mhotl;r-~t of the
concentrate of the present invention, ' L (a)
35 comprises a mixture of the said oleyl- and coco-amine
dipropionate salts, preferably in a 1:2 weight ratio of
oleyl-amine dipropionate: coco-amine dipropionate.
,
O wO 95119413 2 1 8 0 3 2 6 PCT/CA9StO0025
The coco-amine dipropionate salt is derived from
coconut acid, which acid is a mixture of long chain fatty
acids having chain lengths varying from C~ to C~, with a
p~ u.,d~nce of C10, C~2 and Cl4.
~7hen _ (a) i8 a _ ' of formula (II), it
is preferably a, _ ' of said formula in which R1 is an
oleyl-group or a coco-g:roup as defined above, R2 is a
-CH2CH20H group and Y~ arld Y2 are as def ined above . A
particularly preferred ' of formula (II) is a
_ ' of the formula:
CH2CH2COOeNa~
(Coco) - C -- N -- CH2CH2 - N
ll I ~ CH2CH2COOeNa~
O CH2CH2OH
C ~olln~lc of general formula (I) and general formula
(II) are commercially alvailable. For example, the oleyl-
amine dipropionate and coco-amine dipropionate salts
mentioned above are sold by T.AkPIAnrl Laboratories Ltd of
Nanchester, England as oleyl betaine - T.AkPl An~l ODA -
(--roso~lium salt of oleyl amine dipropionate) and coca
betaine - T~AkelAnA AMA - (- --;';llm salt of coca amine
dipropionate) . These materials are sold as 3 0% active
solutions.
C '- of general formula (II) are available from
Rhone Poulenc and sold 11nder-the MIRANOL trade name.
These materials are sold as solutions which are 37-45%
active .
Typically, c nt (a) is uged in an active amount
of from 0 . 4 to 18 wt%, based on the total weight of the
concc:.lLLe,te. Nore preferably, the active amount of
component (a) used to form the cu.,~,L~te of the present
invention ranges from 3 . 8 to 13 wt%. These ranges apply
whether ~ ^nt (a) i8 an alkylamine dicarboxylate of
general formula (I) or a __ ' of general formula
(II) .
_ _ _ _ _ _ _ _ _ _
wo 95/19~13 2 1 8 0 3 2 6 PCT/CA95/00025
C -~nt (b) i8 a cyclic imidazoline of general
formula (III) a6 defined above. In these ~ R3 is
preferably a saturated or Ull ~uL,~ted C7 to C~ alkyl
group, more preferably an ul.sa~uLc,ted C17 alkyl group.
Typically, the cyclic ;m;tal~701 ;n~ is used in an
active amount of from 0.35 to 14 wt%, based on the total
weight of the UI~ G~LCLte. ~ore preferably, the active
amount of the cyclic imidazoline ~ _ ' used to form
the concentrate ranges from 3 to 10 wt~c, based on the
total weight of the Cu.l~ LL-te.
Materials of general formula (III) are also
commercially available. For example, an imidazoline of
this type in which R3 i8 an un6aturated Cl7 alkyl group is
supplied by T.;-k~ nrl Laboratories Ltd as Imidazoline
180H. This material is sold as a liquid which is
typically 65-71% active, with an average activity of 68%.
C - ~ (c) is an alkyl s~l~rhnn~te anionic
surfactant and is preferably an oleyl slllrhnn~te anionic
surf actant . Surf actant6 of this general type are
commercially available materials. For example, the oleyl
sulphonate anionic surfactant mentioned above is sold by
Hoechst AG as Hostapur OS and is sold as a 40% active
solution .
Typically, the alkyl ~llrhnn~te anicnic surfactant
is present in the CUI~ te in an active amount of rrOm
0. 2 to 5 wt%, based on the total weight of the
concentrate. In preferred compositions the active amount
of the alkyl sulphonate anionic surfactant ranges from
0 . 2 to 1 wt%, based on the total weight of the
3 0 CUI~ LL ~te .
Ls (a) and (b) are incorporated in the
present compositions to provide the desired lubrication
properties. Preferably the active weight ratio of
-nt (a) to ~ ~rL (b) is 0.9-1.4:1.
The alkyl slllrht~nAte surfactant - _ ~nt (c) - is
1nr~ ecl in the present compositions as a IIYdLUL~ U~e,
i. e . it acts to st:~h; 1; ~e the formulation thereby to
O WO 95/19413 2 1 8 0 3 2 6 PCTIC~95/00025
prevent phase separation, particularly of the dilute
solutions which are produced when the C ollc~:"LLate is
diluted with water. The alkyl ulrh~n~te surfactant does
not play any active role in the lubrication process nor
5 does it appear to have ~ny function in preventing
leaching of pigment fro,~ the printing i~nks used on
containers which are cu,~ .=d through bottling plants.
We have found that it i6 possible to include a
further _ in th~ c-~,~ . al es of the present
10 invention. We have foulnd that if one in~,uL~uLateS up to
3 wt%, based on the total weight of the ~;ol~ Late, of
(d) a non-ionic surfact~nt which is a linear or branched
alkoxylated alcohol or alkoxylated phenol, each having
from 5 to 20 units of el hoxylation, in the _u..~el.~Late of
the invention, the soil hs~n~l in7 characteristics of the
resultant lubricant compositions are; vvt:d. This
surfactant appears to play no part in lubrication, in the
stability of the composition, nor in protecting the
pigment on the printed surface of the container.
A preferred such nc)n-ionic surfactant is an iso-C~3-
Cl5 alcohol which has 12 units of ethoxylation. These
surfactants are availab~ e commercially; for example a
material of this type is sold by BASF AG as Lutensol
T0129 and is said to be 88% active.
When a non-ionic surfactant of this type i6
inCc~L~uL~ted as ~ t (d) i~n the cv..c6~ La~es of the
present invention, it i8 usually present in an active
amount of from 0.5 to lC wt%, preferably from 1 to 5 wt%,
based on the total weigh~t of the cullce-.LLate.
3 0 The ~ul~c~ L ates o~ the present invention are
typically ~~ aled by dissolving the cyclic imidazoline
(_ -nt b) in a mixture of water and isopropanol. The
solution is stirred until the imidazoline has dissolved,
whereupon _ - L (a) is then introduced.
When ~ - -nt (a) comprises a mixture of oleylamine
dicarboxylate and coco-amine dicarboxylate, the ~LU''I~:dULt:
is that the oleylamine dicarboxylate is i,-LLodu- ed first
W0 95/19413 2 1 ~ 0 3 2 6 PCT/CAgSIooo~S
and the solution i5 6tirred gently and heated up to a
temper7~ture of about 60C until a viscous slurry is
obtained (usually nbout l/2 hour) .
Finally the oleyl E-~lrhr~n~te surfactant ( ,--,t
5 c) is added to the formulation.
The pH of the formulation can be varied from 7 to 11
d~ren~ i n~ on the properties required . We have observed
that at lower p~ values for the cullc "L-~.te good
lubrication with drag coefficients of 0.15 - 0.16 are
lO realized. However, better pigment compatibility is
observed at the high pH values. A pH value of about 9 to
ll is preferred for optimum pigment comp~tibility.
To lower the pH of the formulation, as needed, a
suitable acid is added. We have found that addition of a
15 simple acid such as acetic acid results in an unstable
product. We have found that acids which have llydLOLLU~Jic
properties are suitable. EYamples of such 1IYdLULLU~iC
acids are caprylic acid and n~nrlP~ Anr it acid.
Neo~lPc~noic acid is preferred; surprisingly, the
20 resultant product remains stable in hard water. Use of
caprylic acid results in a product that is not stable to
hard water.
According to a further aspect of the present
invention there is provided a lubricant compo6ition for
25 u6e as a lubricant for CUI~ L track, 6aid lubricant
composition comprising a ~iu..~ ~..LLate as defined above
diluted with from 80 . 00 to 99 . 99 parts by volume of
water. More typically, the cu~,cenLr~tes of the present
invention are diluted with from 99 . 0 to 99 . 9 parts by
30 volu~e of water.
The compositions of the present invention are
usually sold as conc~--LL-tes and are diluted for use as
conveyor track lubricants.
Typical use uu~luellLL~ltions of the formulation would
35 be from 0.1 to 1% vol/vol made up in water. The eYaCt
~_ullC~I-LL~tion depends on factors such as the speed of the
:yul track, the type of package or container being
WO 95~19413 2 1 8 0 3 2 6 PCT/CA95/00025
carried by the track, the total loading on the CV--V~:YUr
track and the amount of soiling caused by spillage.
Dilution of the lubricant ..o~.~el.LL~te is normally
performed at a central ,1; FrQn~Dr~ and the diluted
5 lubricant composition is then pumped to spray nozzles at
the point of use. There are some areas of the ~ V~:yvI
track that require very little lubricant. Typically
these are the zones before the riller and before the
pasteurizer. In these regions secondary dilution is
10 often employed. Lubricant is likely to be at its highest
use concentration at thl~ f iller .
The lubricant solll~; i f)n~ are typically sprayed onto
the cu..v-:yo~ from fan jet nozzles placed at the start of
each length of track. E'or particularly long runs,
15 seron~Ary spray jets ma~ be positioned along the length
of the track.
In areas of heavy E;oiling it may be n~-rDCCAry to
continually spray lubricant onto the ~rack. However, in
mo6t instances timers are employed to vary the dosing
20 rate. Typically on and o~f times will be between 10 and
go seconds. Off times will not always egual on times.
Also i~ is likely that throughout a plant timer settings
wi l l vary .
In some application~6, A final water wash jet will be
25 placed at the end of a ~ottle/can filling track. This
will wash residues of lubricant from the package before
crating and dispatching.
Excess lubricant will be allowed to fall from the
track either to the floor or suitable drip trays. In
30 either event it will eventually enter the drainage and
water LL e~ ' L systems .
A6 has been stated above, we have found that the
lubricant compositions of the present invention have the
particular adv~ a~ that they 2Lre compatible with can
35 print, i.e. they do not rapidly leach print from the
surface of containers being carried by CVIIV~VL track
which is being lubricated by the said lubricant
2 1 ~0326
Wo 95/19413 PCT/CA9510002
compositions. ~urthermore the lubricant compositions of
the invention can also be used in areas of hard water
without any apparent ~dverse effects.
The present invention is illustrated by the
5 following Examples:
EXAMPLE 1
A ~ e~ a~e suitable for use upon dilution with
water as a COII~ L track lubricant waE formulated in the
lO following manner from the ~ 8 set out in the Table
below: -
Raw %wt/wt 8ulk 96 Active
Material f.. rc.. ~ tion in formulation
Imidazoline 180H 5 . 4 3 . 7
TAkPlAn~l ODA 5-7 1.7
T.AkPlAnt9 A15A 10 . 4 3 .1
Hostapur OS 1. O 0 . 4
20 Isopropyl Alcohol 5.7 5.7
Soft Water t<5ppm 71. 8 71. 8
CaC03)
Referring to the Table, TT~ O1 ;nP 18011 i8 a
, _ ' of general formula (III) in which R3 is an
unsaturated Cl7 alkyl group and ig gl~rpl; Pc~ by T.~kPl Anl
Laboratories Ltd as a liquid which is 65-71% active.
T AkPl An~ ODA ig an oleylan~ine dipropionate (mono
sodium salt), i.e. a - ,_ ' of general formula (I),
sold by T.Akpl Antl Laboratories Ltd as a 30% active
solution .
T Akf-l An~l AMA is a coco-amine dipropionate (mono
sodium salt), i.e. a ~ , ' of general formula (I),
sold by T AkP~ An~l Laboratories Ltd as a 30% active
solution.
Ilor~dyu~ OS is an oleyl E~llrht~nAte anionic
surfactant i.e. ~ (c) - sold by Hoechst AG as a
40% active material.
WO 95/19413 2 ~ 8 0 3 2 6 PCT~C~9s/0002s
Imidazoline 180H (component c) was added to a
mixture of the isopropyl alcohol and soft water. The
resultant solution was stirred until the imidazoline was
fully dissolved.
T.AkPl An~l ODA (~~ - - L (a): oleyl amine
dipropionate) was then added to the solution and stirred
for up to 30 minutes with gentle heating at up to 60C.
During this mixing proc~as6, the mixing vessel was sealed
to prevent loss of volatile material. The resultant
product was a viscous slurry.
1AkPlAn~ A ( -nt (a): coco-amine
dipropionate) was add~d to the slurry and stirred until
the mixture had cooled ~o room temperature. The mixture
at this stage was a clear solution.
Finally, llo~La~uL OS ( ~ --L (b): anionic
surfactant) wa6 added to the solution.
The resultant formulation had a pH between 10 and
11. The product was folmd to be stable when diluted to
1% in hard water and dill not cause fading of the printing
inks used on the surfac~ag of printed Al~lm;mlm cans, i.e.
the formulation is comp21tible with the printing on
aluminum cans.
The product formul.ltion shown in the Table above i8
typically diluted in water for use as a Cu~ r track
lubricant. Typical use aollc~-.LL~tions are from 0.1 to 1%
vol/vol .
EXAl~LE 2
Following the ~Loae~uL.: described in Example 1, an
alternative aclueous cc,~c~ LLc-Le formulation was prepared
f rom the ~ lts set out in the Table below: -
Wo 95119~13 2 1 8 0 3 2 6 PCT~CA95/00025
Raw %wt/wt Bulk %Active
Material Cu~ L~tion in formulation
Tm~rlA~Qllne 180H 5.4 3-7
5 T~kPl~n~ ODA 5.4 1.7
Miranol C2MSF lO . 0 3 . 9
HOD l,~UL 05 1. 0 0 . 4
Isopropyl Alcohol 5 . 7 5 . 7
Soft Water 72 . 5 72 . 5
Apart from Miranol C2MSP, the ~_, 1,5 are the
same as those described in Example l. Miranol C2MSF is a
' of ge~eral formula (II) of the ~LLu~,l,ur~
CH2CH2COOeNa~3
(Coco) -- C -- N -- CH2CH2 -- N ~
Il I ~ s~ oOeNa~
O CH2CH20H
and is supplied by Rhone Poulenc as a solution which is
said to be 39% active.
As will be seen, this formulation i8 similar to that
described in Example 1, except that the T. IkPl ~n-l AMA
material ( i . e . the coco-amine dipropionate) is replaced
by the Miranol C2MSF material described above.
The properties of the resultant formulation were very
similar to those of the formulation o~ Example l.
l~xample 3
3 0 Tests were condueted to evaluate the ink
compatibility of the various lubrieant compositions of
this invention as well as their lubrieity at various pH
levels .
The lubricity of the test eomposition was measured
using an apparatus whieh eomprised a moving COIlve:yur on
top of whieh a stationary load eell is mounted. A
dynamie load is positioned upstream of the load eell and
a statie load is positioned upstream of the dynamie load.
O wo sst19413 2 1 8 0 3 2 6 PCT/CA95/0002s
In order to test the lubricity or drag coefficient ~, a
four kilogram glass bottle was positioned on the CU~IVCYUL
and ~ cse..Led the dynamic load, whereas the static load
consisted of ten 600 gr~m glasE; bottles. The drag
5 coefficient was calculated on the basis of;
= draa (~)
weight of dynamic load (g)
lO For Alumin~m cans, the dynamic load was replaced with
cans to give a load Or 3 . 6 kilograms and the static load
consisted of ten Al-1m;nl~m/steel cans. The drag
coefficient was calculated in accordance with the above
formula .
The durability of the lubricant composition was also
evaluated. Durability is measured as the time taken for
the drag coefficient to increase to 0.25 after
di~c. )d?~iOrl of the lubricant gupply to the CullV~yuL
whilst continuous dosing of the cc--veyur with water is
continued over that peri~d of monitoring. Both the drag
coefficient and durability are a measure of lubricant
effectiveness. A good lubricant has a low drag
coefficient, a high dura]~ility rating and quickly reaches
equilibrium lubrication.
In order to test the print compatibility of the
various lubricants of this invention with ink that is
used in marking Alllm;nl1m cans or steel cans, the
following ink compatibility test was conducted.
This is an aggressi~e test method that we believe
3 0 mimics the conditions that a ~e ~ _L .~ge can would
experience in a pasteuri:~er. In detail, test strips were
cut from Alllm;mlm b~cL~e cans printed pr~d~ ;nAntly
with red ink. Each strip had dimensions of approximately
20 mm by 80 mm. Contact of the strips with lubricant was
achieved by placing strips into lubricant solutions at
65OC. Furth- e, these solutions were made up to a
concentration of approxinately ten times the expected
maximum use concentration. For this type of formulation,
we expect the maximum use. ~u..~e..LLi-tion to be about 0.596
21 80326
WO 95/19413 PCTICA9S/00025
14
wt/wt, hence test 601utions were made to 5. 09i wt/wt in
soft water (< 10 ppm calcium carbonate). Contact time
was taken between 1 and 24 hours during which time the
t~ ~ItUl-' was maintained at 65C. At the end of the
5 duration, the strip was removed from the solut ;nn and
compared qualitatively against a control formulation that
was known to be benign towards the ink. Arbitrary units
were Aasiqn~d to reflect the degree of aggressive removal
of print pigment. A lower unit value indicates mild, if
10 any, pigment removal, whereas a higher unit value
indicates an aggressive pigment removal. The control
standard es~hl; qh~-q that a score of 1 indicates a benign
action, whereas a score of 4 indicates an unacceptable
aggressive action by the lubricant.
Formulations of Example 1 were tested and identif ied
by the composition numbers D853; D854; D855 and D856.
The only difference in each composition is that the pH
was adjusted to the level noted in the following Table 1.
O WO 95/19413 2 1 ~ 0 3 2 6 PCTICA95100025
f
f l
,
N O
I C~ f.~ f'
f'l 1~ f'l
~ . t
a o
u~ O
a O
N H
C~ O
1~ ..
o O
U~ f'~
a o
~ I
r f
- ~
WO 95/19413 2 1 8 0 3 2 6 PCTICA95/00025
From the above Table, it is apparent that acceptable
print compatibility was ,~chieved with formulations D856
and D855 . The formulation of D854 with a pH of 7 . 2 iB
understood to be at the outermost edge of print
5 compatibility acceptability, although from Table 1, it is
apparent that a pH of about 9.5 and about 10.2 for the
formulation are far superior. As is understood by those
skilled in the ~-rt, evaluation of lubrication performance
depends on the drag coefficient, durability and time to
lO reach equilibrium value of ~. From Table 1, we can see
that as pH decreases, lubrication performance decreases.
The drag coefficient is reasonably consiætent for the
variou6 selected pH with a slight increase being noted at
a pH of 9.5, but dropping back to a level cfJLL~ ;nfJ
15 to the lower pHs when the pH is increased above 9 . 5 . It
is understood, however, that, in measuring drag
coef f icient, the slight increase at A pH of 9 . 5 may be
due to a particular ~Luce-lu-~ with that composition and
should not be interpreted as indicating any special
20 circumstance for the pH lubricity value at 9.5. However,
in c~~ncid~ring the values for durability and time to
react equilibrium value for ,~L, Table 1 fl LL,~te6 the
above increase in lubrication performance with decreasing
pH, but it is ~ r~..L that the higher pH composition
25 still constitutes good lubricants.
Example 4
In order to further evaluate print compatibility of
the lubricant compositions of this invention, a second
technique f or testing print compatibility was undertaken .
30 The revised method required a - ~a;fi-~ation of the method
def ined in Example 3 to provide a more quantitative way
of ~qsPssi n~ print compatibility. This was achieved by
using the same Al ;ml~^ beverage cans used in the
previous method, filling them with water and placing them
35 completely in a ten times working ~ u~C~ LL~tion of the
lubricant. Excess fluid was allowed to drain from the
paper and then carefully wrapped around the hot can. The
O WO 95/19413 2 1 8 0 3 2 6 PCT/CA95/00025
17
can was maintained at 65~C by placing in an insulated box
for a contact time of ~l5 minutes. The blotting paper was
then allowed to air dr~ and compared qualitatively
against the eontrol formulation, As with the previous
5 test method, the seoring of the lubricant's aggressive
nature f or removing print pigment was evaluated in a
similar manner.
The results of the test i nc~ i n~ also measuring of
drag coeffieient were u~ndertaken and summarized in the
10 following Table 2.
TABLE 2
D610 D600 Control
pH 9.5 10,5
~L 0.13 - 0.14 0.15 - 0.18
15 Durability/ 156 . 0 24 . 0
Seconds
Time to reach 5 13
equilibrium minul:es minutes
lubrication
20 Qualitative 1 1 1 - 2
Ink
Compatibility
Rating
pH INCR `ASING --
Lubrieant Effieacy Il -~F~gI~
Ink Compatibility ACCEPTABLE
From the above Tab]Le 2, it is apparent that
preferred compositions of the invention having a pH of
9 . 5 and 10 . 5 have aceeptable ink eompatibility with very
high durabilities and very aeeeptable drag eoefficients.
35 From the results of Tables 1 and 2, it is apparent that a
suitable lubricant, in a,ccordance with the composition of
this invention, can be provided with a pH preferably from
9 to 11 to yield ink co~patibility in accordance with the
test techniques.