Note: Descriptions are shown in the official language in which they were submitted.
2181~22
METHOD OF COVERING A STENT WITH ACELLULARMATRIX
FIELD OF INVENTION
This invention relates to a method of covering a stent with biomaterial. In
particular, this invention relates to a method of covering a stent with acellular matrix.
S BACKGROUND OF INVENTION
Stents, concicting of an "open" metal scaffolding, are now widely used for
supporting l~lvwed or stenotic blood vessels that have been opened or e~r~nrled
by balloon angioplasty. The stent is deployed to its target location within a vessel by
threading the stent-c~, j~g c~ r through the vessel from an irlcision or
10 p~l~;uL~euus l~u~ ule some ~ e away. The stent isthen exrqn~led either on its
own accord or by ballooning the c.~ll.r~ l for supportive engagement with the interior
of the vessel wall to mqintqin vessel enl~eme~
Balloon çxp~qn-lqble stents are typically metal mesh that are mounted on
balloon c~thPt~rs and delivered to the target location. When the balloon is expqntl~d,
15 the stent ~ AI~rlc to the desired ~ , to support the interior of the vessel.
Examples of such stents are described in United States Patent Nos.: 5,059,211;
5,282,824; 5,306,286; and 5,334,201.
Self-~l.An~ stents are made of an alloy having a "memory" that expand to
the desired size after being placed at the target site of the vessel. Examples of such
20 stents are described in United States Patent Nos.: 4,800,882; 5,282,824; and
5,342,387.
In United States Patent No. 5,342,387,Summers, a wire double helix stent
design is illustrated. The double helix is advantageous because by l~luwing and
widening the gaps bc;L~en the parallel struts, it can be contracted and e~r-qn-le-l in
21~52~
tii~ml-ter willwul chA .~gi 1~ its length.
~lthou~h balloon s~Al~A~ Ahle stents of the prior art have been very successful
in treating ~ ow~d or occl~lded blood vessels, these stents still suffer from a serious
drawback.
All intravascular stents consist of an open metal scaffolding. The ratio of open
space to stent material varies from 80t20 to about 90/10. When the vessel is stretched
by balloon angioplasty and a stent is e~pan~f d in place across a now dilated lesion,
a healing response is triggered. The healing response is a proliferation of smooth
ml-scles cells from the area of vessel wall which has been injured by the procedure.
10 Although the scaffolding effect of the stent serves to restrict the build up of scar
tissue (smooth muscle sell proliferation) and subseq~ent ,~ whlg, the gaps in the
metal provide an OppOlLul~ily for en~l~ing smooth muscle cell proliferation to grow
through the open spaces of the stent. As a result, about 30% of p,qtifnt~ will
e~e~;f .re resten~ si~ of the vessel. The stent and the expqn~ion of the vessel ;~ t~
15 a reaction which causes tissue hlglowlll (intimal hyperplasia) which eventually leads
to ~~ whlg or l~s~ si~, which may l~oces-~ te a revascularization prosedure to
reopen the llallvwcd area inside the stent. This additional h~l~t;lllion is costly and,
more iu~ol~llly, exposes the patient to further risk.
Attempts have been made to ...i.~ these complications. In United States
20 Patent No. 5,282,824,C~idllLulco, a stent assembly is disclosed which has a flexible
nylon sleeve Attq-rll~od to the outside cil.;~llfel.~ ial surface of a stent. On
implantation of the stent, the sleeved stent is allowed to e~pq-n~ ~tl~ssing the flexible
sleeve against the walls of the blood vessel. The sleeve is intf n-lf d to pl. ~ tissue
growth belWc;ell the gaps defined by the stent. However, nylon and other ~ylllll~lic
2~ s~
m~tPri~l~ probably will not provide a long term solution as such materials can cause
massive infl~.. ~lol~ or thrombogenic lca.;Lions.
Recently, investigators have developed materials which are not associated with
thrombosis or infl~.. ~lc,ly reactions. Acell~ r matrix is a biolllatelial derived from
5 tissue extracted from -~ n.~ which isprocessed to remove all cells and soluble
proteins. This bio..l~ l has been shown to be non-thrombogenic and non-
infl~.. ~o~ rPllul~r matrix cc,lll~lises a framework of largely insoluble collagen
and elastin, which are very stable pl'Ot~inS. EA~lhllellL~l studies with this matrix have
been sllGces~rul in a variety of cardiovascular applications. (CoulLIllan et al.:
10 "Development of ~licaç~ial .Are~ r Matrix Bio...~ Biochpnlir~l and
Mech~nir~l Effects of Cell Extraction" Journal of Biom~o~ir~l Materials Research,
Vol. 28, 655-666 (1994), and Wilson et al. ".Are~ r Matrix Allograft Small Caliber
Vascular Protheses", Vol. XXXVI Trans. AM. Soc. Artif. Intern Organs, (1990), and
see also United States Patent nos. 4,776,853and 4,801,299)
Hel~lofole, acellular .. -l. ;res have been surgically implanted during
eAl,clillltll~l studies. ~rPlllll~r matrix prothesis have not been incorporated as an
integral part of stents.
Summary of the Invention
The disadvantages of the prior art may be o~._lcollle by providing a method
20 of pr~aling a stent for impl~nt~tion by hs~ ulg an open ended tube of acellular
matrix through a stent when in a co..l.~çted or collapsed condition, and rolling the
open ends of the tube back over itself. The combination tube and stent is capable
of being tr~n~lllmin~lly or surgically insell~d to a target site.
It is desirable to provide a bi-....~t~ l CO~'~.li~ for a stent, whel~ul the
- 2181522
- 4 -
covering is non-thrombogenic and inhibits tissue i~ wl~- when deployed inside a
blood vessel, duct, or conduit.
It is desirable to provide a biom~t~n~l covering which can form a barrier
b~lween an implanted stent and the wall of the host blood vessel, duct, or conduit.
It is further desirable to provide a biomaterial covering which provides a
smooth inner surface through which fluid flows.
It is further desirable to provide a biollla~lial co~"hl~ to encourage o~ rd
gro-wth from the an&slolllosis sites inward.
It is further desirable to provide a plurality of stents covered with biomaterial
10 on a single c,,~ t- I for multiple deployment of the stents.
It is still further desirable to provide a stent co.rel~,d with biomaterial for use
as a vascular graft for bylJassillg stenotic or occh~clecl blood vessels.
It is still further desirable to provide a stent covered with acellular matrix or
biomaterial for use as a stent or graft for other ducts or col~luils within a living body.
It is still a further object of the invention to provide a stent lined with ~rPllnl~r
matrix that resllic~ tissue ingrowth for co~gP~ l vascular defects such as pulmonary
artery stenosis, portacaval shunts, arterio-venous shunts, deterioration of sll.h- .~o~s
vein grafts for colul~y artery by-pass grafts and peripheral arteries and endohlmin~
grafting.
According to one aspect of the invention, a method of l,~a~illg occlusion and
stenosis of a blood vessel, duct, or conduit is provided. The method colll~lises the
steps of: providing a c~th~ter having a distal end; mrllntin~ a tube of ~cell~ r matrix
or other biolllat,lial on the distal end of the c~thrtrr; sliding a stent over the
bi.~ t~ l; rolling,l~ e~;lively,thedistalandpl(~ullalendsofthebiGllla~lial over
-- 2181522
- S -
the distal and proximal ends of the stent; delivering the stent and bi~ at~lial to a
target site; ç~ n~ g the stent; and withdlawiilg the c~ ter.
According to one aspect of the invention, a mPthorl of treating occlusion and
stenosis of a blood vessel is provided. The method c~ p~ises the steps of: providing
5 a c"l-~l~,r having a distal end and an internal release wire; muulllhlg a tubular
acellular matrix on the distal end of the c~ , sliding a self-exp~n-lin~ stent over
the matrix, the self-e~ "~ stent having a ~ûlt:~;live sheath; ~ the distal
and proximal ends of the stent through the acellular matrix to engage the release
wire, contr~^tin~ the stent into an implantable condition; willl~awhlg the sheath;
10 rolling, l~,s~e~;lively, the distal and proximal ends of the tubular acellular matrix over
the distal and l"o~il,lal ends of the stent; joining the distal and proximal ends of the
tubular acellular matrix to the matrix, illse.lillg the c~l.rl~l distal end into the blood
vessel; guiding the c~thf~tçr distal end to a targeted portion of the blood vessel;
withdlawing the release wire, allowing the stent to çxp~nf1 and withdlawhlg the
C~7~llf tl from the blood vessel.
According to another aspect of the invention, a stent with an inner tubular
lining of acelhll~r matrix or other bio"la~lial is provided. The inner lining has open
ends for rolling about the ends of the stent. The ends of the tube enclose the ends
of the stent and are ~tt~rhf d to the tube.
Acco,ding to another aspect of the invention, a stent with an inner tubular
lining of acellular matrix or other biGllldl~lial is provided. The inner lining has open
ends for rolling about ends of the stent. The open ends of the tube are ~tt^^hf~A to
each other.
2~81522
Description of the D. ,.~. il~ iJ
In dlawing~ which illustrate embo ~ of the invention:
Figure 1 is a pc.~ecliv~ view of the present invention in an ullvvla~ed
condition and mounted on a dual release wire CAI~ tt~
Figure 2 is a side sectional view of the self~ stent and acellular
matrix mounted on a single release wire c~thPter;
Figure 3 is a section~l view of the self~ p stent partially covered
with an ~ r matrix;
Figure 4 is a sectional view of the self~ n~ stent fully covered with
an ac~lllll~r matrix; and
Figure 5 is a pc,~c.,li~.~e view of al~ller self-ç~ u~ g stent which can
be incol~lal~d into the present invention.
D~h;le~ Description of the Invention
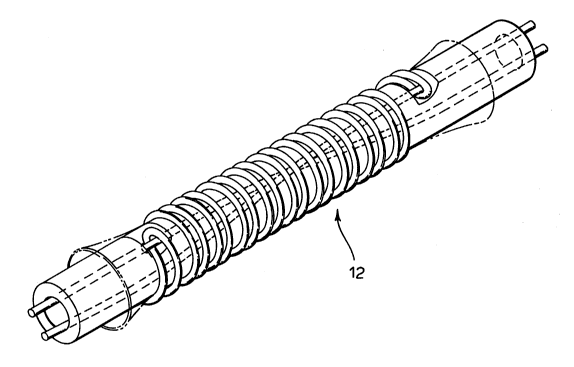
The pl~f~,.l.d stent to be used with the present invention is illu~lla~d in
15 Figure 1. Stent 12 is more particularly described in United States Patent no.
S,342,387, the contents of which are illcol~olaled herein by l~fc.~.lce. In the
pler~,.l~ embotlim~nt~ stent 12 is self-~ an~il-g. However, the present invention
also conLellll)lates lltili7in_ any self-e~l-AI-~lAble or balloon ~"l-A~-A~ble stent.
Ref~.ling to Figure 2, the stent 12 is illllstrAt~l mounted on a CA~ t~l 14.
20 Acellular matrix 16 is mounted belweell the c~th~ter 14 and the stent 12, pre~..l;.
an inner lining for the stent 12.
Acellular matrix 16 of the ple~ ,d embodiment is derived from ",~...,..~liAn
preferably a human vessel, in~ j~ blood vessels, namely arteries and veins, ducts,
or conlui~, and are ~ .erol~, tubular in shape having open ends. The size of the
2~8~522
vessel to be hal~csled is r1irtq-t~d by the size and type of stent to be implanted in the
patient. Preferably, acellular matrix 16 is t~ c~d from human sources. However,
bovine, ~ ciuc, canine or similar ~ lqliqn sources may also be suitable. Further,
cryo-preserved human veins or other ducts or col~ui~ are contemplated as being a
5 suitable source for the bio...~ q-l .
The method of e~llacli~g and ~le~h~ the matrix 16 is fully described in
Courtman et al.: "Development of Pelical.lial ~relllllqr Matrix Biomaterial:
Bioch.omirql and Mech~nir~l Effects of Cell F.~ l;on" Journal of Biom~lirq-l
l~qt~riql~ Research, Vol. 28, 655-666 (1994), and Wilson et al. "Acellular Matrix
10 Allograft Small Caliber Vascular ~ollleses", Vol. XXXVI Trans. AM. Soc. Artif.
Intern Organs, (1990), and United States Patent nos. 4,776,853and 4,801,299,all of
which are incorporated herein by ler~,lellce.
Stent 12 may have an int~rnql plolcclive sleeve 18. Sleeve 18 has a
longit~ inqlly ek~ slot 20. Slot 20 allows access for distal end 22 and proximal
15 end 24 of the stent to be inserted into notches 26 and 28 in the cqth~ter 14. Notches
26 and 28 receive, lc~c~,lively, the distal end 22 and proximal end 24 of stent 12 to
retain the stent for deployment. Proximal end 24 is first engaged with the release
wire 30 and then the distal end 22 is wound down to compact the stent 12. The distal
end 22 is then looped through with the release wire 30. Release wire 30 e.~lr~ s
20 intçrnqlly within the c~lh~ l 14 through loops formed in each the distal end 22 and
proximal end 24 of the stent 12 to retain the stent 12 on the c-q-th~ter 14 in a
compq-cte~ condition.
Once the stent 12 engdges the release wire 30, the protective sleeve 18 can be
withdrawn by sliding it along the c~ e, to~alds the proximal end thereof. After
2181~22
the protective sleeve 18 is withdrawn, the distal end 32 of acellular matrix 16 is rolled
back over itself to cover the distal end of stent 22. Similarly, the proximal end 34 is
rolled over itself to cover the proximal end 24 of stent 12.
Referring to Figure 3, the distal end 32 is rolled back to cover only a portion
5 of the distal end region of the stent 12. Similarly, the proximal end 34 is rolled back
a portion of the length of stent 12 to cover the pl~hl~al end region thereof.
The distal end 32 and the p~ illlal end 34 are Att~h~d to the inner tubular
body of the acellular matrix 16 by sululill~, surgical stapling, gluing, taping, or any
other method for qttqrhin~ biolllat~ ~ial to itself.
The stent 12 and acellular matrix 16 can now be deployed using t~chniques
and m~thotl~ well known in the art.
~ lths)ugh the ~lcf~,l.,d embodiment has desclil,ed the acellular matrix 16
being mounted an a cath~ter for cov. .hlg the stent 12, it is now readily understood
that similar cylin~rir-q-l a~alus could be used. The stent 12 and acellular matrix 16
15 of the present invention could be mounted on such cylinder and later llal~Ç~ d to
a stent for imp!qntqtion.
Refe.li~ to Figure 4, the distal end 32 and the ~ hllâl end 34 of acellular
matrix 16 are fully retracted until the ends 32 and 34 abut. A continuous suture line
may be used around the ch.;ulllfe~.llial seam for joining the ends 32 and 34 together.
20 In this embo~lim~ont the stent 12 is fully covered, both int~rnqlly and ext~rnqlly and
may be deployed using techni~ es and m.oth~s well known in the art.
It is noted that the distal end 22 and proximal end 24 of stent 12 extend
through the acellular matrix 16 when in the ready for deployment condition. Once
2181522
the release wire 30 is retracted, the distal end 22 and the proximal end 24 of stent 12
will retract back through the pul~ ulcd ~c,~ing in ace~ r matrix 16 which will
close, fully covering stent 12.
The stent 12 and acell~ r matrix 16 are also useful in grafting. The stent 12
5 and acellular matrix 16 may be implanted on ends of a blood vessel which are to be
joined. The stent 12 will provide ~rovcd structural support for the vessel over
conventional prior art grafts. This i~ loved support will reduce the risk of
al~cu~y~llls.
Additionally, the stent 12 and acelllll~r matrix 16 can be made of a larger
10 ~ m~ter to operate as a graft for larger ducts within the human body. For example,
the stent 12 and acellular matrix 16 of the present invention has applications as a
proll,esis for the trachea, oesophagus, ali,.,~ canal, geniluuli-~y or other similar
bodily ducts.
Referring to Figure 5, a second embodiment of a self-e~ stent 112
15 which could be covered and implanted by the present invention is illustrated.
It will be obvious to those skilled in the art that various mo-lifir~tions and
changes can be made to the method wi~,uul dcp~~ g from the spirit and scope of
this invention.