Note: Descriptions are shown in the official language in which they were submitted.
. ~181~1
WO 95/19952 PCT/EP95/00167
Nitroxy group-containing bellzylamine derivativef- and
their use for treatinc cardiovascular 1; -~A~q as well as
increased intraocular pressure
Area of a~lication o~ the inventiQ~
The invention relates to benzylamine derivativea
which are used in the rh~ eu~i~Al industry for produc-
ing ~~';. ts.
Known techniçal backqround
Ni troxy _ ' ~ which are substituted in
various ways and are aaid to be suitable, for example for
the treatment of cardiovascular di3eases are described in
the prior art.
DescriPtion gf the in.rention
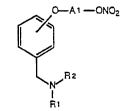
The invention relates to ,- a~ Of the formula
I (see Arp~na~d sheet of ~ormulae), in which
Al is l-15C-alkylene, 5-7C-cycloalkylene or di-
1-4C-alkylene-5-'7C-cyt~ An-~, and in which
Rl is 11YdL~ 1-7C-alkyl or 3-8C-cycloalkyl and
R2 is 11YdL~ 1-7C-alkyl, 3-8C-cycloalkyl or A2-Y,
2 0 or in which
Rl and R2 represent, together and with ;nrl~ ;nn of the
nitrogen atQm tQ which both are bonded, an unsub-
stituted or substituted 5-, 6- or 7- ' -~ ed hetero-
cycle which is s~lected from the group consisting o~
pyrrolidine, pipl~ridine, piperazine, morpholine and
homopiperazine,
where
A2 is 1-15C-alkylene, 5-7C-cycloalkylene or di-
1-4C-alkylene-5-7C-cy.~lgAl kAn~-,
Y is R3, NEI2, NEI-R4 or g-R5,
- a substituted pyrrolidino radical is
substituted by one or two identical or
different substituents selected frQm the group
Replacement sheet (Rule 26)
21~
Wo 95/19952 - 2 - PCT/EP95/00167
consisting of 1-4C-alkyl, 1-4C-alkoxy and
hydroxyl,
- a substituted piperidino radical is substituted
by one or two identical or dif~erent sub-
3tituents selected from the group consisting of
1-4C-alkyl, 1-4C-alkoxy and hydroxyl,
- a substituted piperazino radical can be substi-
tuted in po~ition 2, 3, 5 or 6 by a 1-4C-alkyl
radical and i8 substituted in position 4 by a
substituent seleeted from the group consisting
of 1-4C-alk~rl, 1-4C-alkoxycarbonyl, 1-4C-alkyl-
carbonyl, p]lenyl which is substituted by R6, R7
and R8, phe:Dyl-1-4C-alkyl which is substituted
in the phenyl radical by R6, R7 and R8, benzoyl
which is substituted in the phenyl radical by
R6, R7 and R8, pieolinoyl, nieotinoyl, iso-
nieotinoyl, benzhydryl whieh is, if required,
substituted by halogen or 1-4C-alkyl, and the
radieal R4,
- a substitutEd morpholino radieal is substituted
by one or t~o identical or different 1-4C-alkyl
radicals and
- a substitutEd homopiperazino radieal is substi-
tuted in position 4 by a substituent selected
from the group consisting of 1-4C-alkyl,
1-4C-alkoxycarbonyl, 1-4C-alkylearbonyl, phenyl
whieh is substituted by R6, R7 and R8, phenyl-
1-4C-alkyl whieh is substituted in the phenyl
radieal by R6, R7 and R8, and benzoyl whieh is
substitutedl in the phenyl radieal by R6, R7 and
R8,
where fur~h~ - r~
R3 is furyl, n.aphthyl, tetrahydronaphthyl, phenyl
whieh is sllbstituted by -O-Al-ONO2, or phenyl
whieh is substituted by R6, R7 and R8,
R4 is 1-7C-alkyl or the substituent
-CH2 -CE (OH) - (CH2O) p-Ar and
Rep~ t gheet (Rule 26)
WO 95/19952 - 3 - PCT/E:P95/00167
R5 is phenyl which i5 substituted by R6, R7 and
R8, phenyl-1-4C-alkyl which i~ substituted by
R6, R7 and R8, if required halogen- or
1-4C-alkyl-lsubstituted benzhydryl, dibenzo-
5-7C-cyrl~s~lk~nyl, dibenzocycloheptenyl or
benzo-pyrido-5-7C-cyrl ~ 11 k:~nyl,
and where in addition
R6 is hydL~ l, 1-4C-alkyl, 1-4C-alkoxy,
1-4C-alkylci~rbonyl, halogen, amino, mono- or
di- (1-4C-alkyl) amino or nitro,
R7 i~s hydrogen, :L-4C-alkyl, 1-4C-alkoxy, halogen or
ni tro,
R8 is I~YdL~Y~j~ or trifluoromethyl,
p i~ the number O or 1, and
Ar is a hydrocarbon ring ~sylstem which is complete-
ly or partly unsaturated, which is monocyclic
(with 5 to 6) or bicyclic (with 9 to 10 ring
atom~s), in which 1, 2 or 3 carbon atoms can be
replaced by heteroatoms ~rom the group of
nitrogen (~), oxygen (O) or sulfur (S), and
which can be sub3tituted by one or two
nt;r~l c~r different ~ubstituents from the
group of 1-4C-alkyl, 1-4C-alkoxy,
1-4C-alkylthio, 1-4C-alkoxy-1-4C-alkyl,
1-4C-alko~cy-1-4C-alkoxy, 3-4C-alkenyl,
3-4C-alk~nyloxy, 3-8C-cycloalkyl,
5-lOC-cycl,~alkylalkoxyalkyl, 1-4C-alkyl-
carbonyl, :L-4C-alkylcarbonylamino, ~ r'
~.r--' yl-1-4C-alkyl, halogen, hydroxyl, oxo,
nitro, cyano, 1-4C-alkyll3ulfonamido, amino,
mono- or di- (1-4C-alkyl) amino, ureido, mono- or
di- (1-4C-alkyl)ureido, mono- or di-
(3-8C-cycloalkyl)ureido, trifluoromethyl,
1-4C-alkoxy which i~s completely or partially
sub~stitutecl by fluorine, or 1-4C-alkoxy-
carbonyl, tetrahydrofurfuryloxy or morpholino,
and the ~salt~3 of theEse -, u--d~.
~erl~c-~_ t ~3heet (Rule 26)
a ~ ~
WO 95/19952 - 4 - PCT/3P95/00167
1-15C-Alkylene represents straight-chain or
branched alkylene radicals with 1 to 15 carbon atoms.
Examples which may be - t; ~nF~d are the radicals methy-
lene (-CH2-), ethylene (-CH2CH2-), trimethylene
(-CH2CH2CH2-), tetramethylene (-CH2CH2CH2CH2-), penta-
methylene (-cH2cEI2cH2cH2cH2-) ~ hexamethylene
( - CH2 - ( CH2 ) 4 - CH2 ) ~ oc tame thyl ene ( - CH2 - ( CH2 ) 6 - CH2 - ),
decamethylene (-CH2- (CH2) 8-CH2-) ~ tetradecamethylene
(-CH2- (CH2) l2-CH2-) ~ 1, 2-dimethylethylene
[-CH(CH3)-CH(CH3)-], l,,l-dimethylethylene [-C(CH3)2-CH2-],
i ~opropylidene [ - C (CH3 ) 2 - ] ~ 2, 2 -dimethylpropylene
[-CH2-C(CH3)z-CH2-], 2-methylpropylene [-CH2-CH(CH3)-CH2-]
- and 2-methylethylene [-CH2-CH(CH3)-].
5-7C-Cycloalkylene represents cycloalkylene
radicals with 5 to 7 carbon atoms. Cyclohexylene r~dicals
are preferred, exampl~s which may be mentioned being the
1,2- and the 1,4-cyclohexylene radical.
Di-1-4C-alkylene-5-7C-cyclo~lk~ne repreaents
cyclic hydroc~rh~nQ with 5 to 7 carbon atoms which are
substituted by two (i~l~nt;~l or different) alkylene
r-~dicals with 1 to 4 carbon atoms. A preferred di-
1-4C-alkylene-5-7C-c~cloalkane radical i9 the
1, 4-dimethylenecycloh~xane radical .
1-7C-alkyl re}~resents straight-chain and branched
alkyl radicals with 1 to 7 carbon atoms. Examples which
may be mentioned a~ e the heptyl, hexyl, neopentyl,
iQopentyl, pentyl, butyl, i~30-butyl, sec-butyl, tert-
butyl, propyl, iQopropyl, ethyl and the methyl radical.
3-8C-Cycloalkyl represents the cyclopropyl,
3 0 cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and
cyclooctyl radical.
1-4C-Alkyl represents straight-chain or branched
alkyl radicals with 1 to 4 carbon atoms. Example3 which
may be mentioned are the butyl, iso-butyl, sec-butyl,
3 5 tert -butyl, propyl, isopropyl, ethyl and the methyl
radical .
1-4C-Alkoxy represents a radical which, besides
~r~ t sheet (Rule 26)
r
` 2L~
WO 95/19952 - 5 - PCT/EP95/00167
the oxygen atom, containa one of the ab.,v ~ n~d
1-4C-alkyl radicals. Examples which may be mentioned are
the methoxy and the e~;hoxy radical.
~ Ialogen for t}le purpo3e of the present invention
5 i3 bromine, chlorine ,md $1uorine.
1-4C-l~lkoxycarbonyl L~:~Leae~.ts a radical which,
be3ides the carbonyl group, contains one of the above-
mentioned 1-4C-alkoxy radical3. Examples which may be
mentioned are the metlloxycarbonyl and the ethoxycarbonyl
10 radical.
1-4C-Alkylcarbonyl I~:~Leaelltg a radical which,
beEIides the carbonyl group, c~ntA;n~ one of the above-
mentioned 1-4C-alkyl r~dicals. An example which may be
mentioned ia the acetyl radical.
Dibenzo-5-7C-cy~loalkAnyl radicals which may be
mentioned are the ~ ^n ~ocyclopentyl, the dibenzocyclo-
hexyl and, in particular, the ~;hPn ~ocycloheptyl radical.
Benzo-pyrido-5-7C-cy~ 1 k~nyl radicals which may
be mentioned are the benzo-pyridocyclopentyl, the benzo-
20 pyridocyclohexyl andl, in particular, the benzo-pyrido-
cycloheptyl radical.
Mono- or di- (1-4C-alkyl) amino repre3ent3 an amino
radical which i3 subatituted by one or two identical or
different ab~,v~ t;~nPd 1-4C-alkyl radical3. Example3
25 which may be mentioned are the methylamino, the ethyl-
amino, the dimethylamino, the diethylamino and the
dii30propylamino radi cal.
1-4C-Alkylthi o represents a radical which,
beaides the 3ulfur atom, contains one of the above-
30 mentioned 1-4C-alkyl radicala. The methylthio radical i~
pref erred .
1-4C-Alkoxy-1-4C-alkyl repre3ent3 one of the
abovementioned 1-4C- 11kyl radical3 which i3 sub3tituted
by one of the abovementioned 1-4C-alkoxy radicalr..
35 Examples which may be mentioned are the methoxymethyl,
methoxyethyl radical and the butoxyethyl radical.
1-4C-~lkoxy-1-4C-alkoxy represents one of the
R~pl~ t 3heet (Rule 26)
~ 5 ~ ~
WO 95/19952 - 6 - PCT/EP95/00167
abovementioned 1-4C-alkoxy radicals which is substituted
by another 1-4C-alkox~ radical. An example which may be
mentioned is the meth~xyethoxy radical.
3-4C-Alkenyl is, for example, 2-butenyl a~d, in
particular, allyl.
3-4C-Alkenyloxy c~nt~;nc~ besides the oxygen
atom, a 3-4C-alke~yl radical. The allyloxy radical may be
t;r~n~d as an example of a 3-4C-alkerLyloxy radical.
5-lOC-Cycloalkylalkoxyalkyl represeIlts an alkoxy-
alkyl radical which is substituted by a cycloalkyl
radical. An example which may be mentioned is the cyclo-
propylmethoxyethyl radical.
An example of a 1-4C-alkylcarbonylami~o radical
which may be t i ~nPd ig the acetamido radical
1 5 ( - N~I - CO - C~3 ) .
C~rh~ yl represe~ts the radical N~2-CO-.
C~ ' ~1-1-4C-alkyl represents one of the
abovementioned 1-4C-alkyl radicals which is substituted
by ~ 1. The ~ lmethyl radical may be men-
tioned as an example of a ~ rh- ~.1-1-4C-alkyl radical.
1-4C-~lkylsulfonamido ~ ,r~ tc a 5111 e~)n:~mi ~
radical to which one of the ab~,v ti~n ~d 1-4C-alkyl
radicals is bonded. An exa~ple which may be mentioned is
the methylsulfonamido radical.
IJreido represents the radical -N~-CO-N~2. An
example of mono-1-4C-alkylureido which may be mentioned
is 3-methylureido and of di-1-4C-alkylureido i8
3,3-dimethylureido. Examples of mOAO- or di-3-8C-cyclo-
alkylureido radicals which may be -- t; r~ned are, for
example, the 3-cyclohexylureido and the 3,3-dicyclohexyl-
ureido radical.
r 1 ~c which may be mentioned of 1-4C-alkoxy
which is completely or partially substituted by fluorine
are the 1,2,2-trifluoroethoxy, the 2,2,3,3,3-pentafluoro-
propoxy, the perfluoroethoxy and, in particular, the
1,1,2,2-tetrafluoroethoxy, the trifluoromethoxy, the
2,2,2-trifluoroethox~r and the difluoromethoxy radical.
Repl~ t sheet (Rule 26)
t~ ~3 ~
WO 95/19952 - 7 - PCT/EP95/00167
Examples o~ substituted pyrrolidino radicals
which may be -- tin~n~d are the 2-methylpyrrolidino,
2, 5-dimethylpyrrolidino and the 3 _LYd1~)~LY~YL ~ ~Jlidino
radical .
Examples of substituted piperidino rl~i;c~l~ which
may be mentioned are the 3-Lyd. v~y~iperidino, 2-n-propyl-
piperidino, 5-ethyl-2-methylpiperidino, 4-n-propyl-
piperidino, 4, 4-dimethylpiperidino, 2, 6-dimethyl-
piperidino, 4-l~ydl~y~iperidino~ 2-ethyl-2-methyl-
piperidino, 2-methyl]?iperidino, 2, 6-dimethylpiperidino
and the 2-ethylpiperidino radical.
Examples o~ substituted piperazino radicals which
may be ~nn~d are the 4-methylpiperazino, 4- [2- (2-tri-
~luoromethylphenyl)ethyl]piperazino, 4-phenylpiperazino,
4-(2-methylphenyl)piperl~zino, 4-(2,3-dimethylphenyl)-
piperazino, 4- (2-chlorophenyl)piperazino, 4- (2-methoxy-
phenyl) piper~zino, 4- (2 -ethoxyphenyl) piperazino,
4- (3 -chlorophenyl) piperazino, 4- (4- Eluorophenyl) -
piperazino, 4- (4-chlorophenyl)piperazino, 4- (4-methoxy-
20 phenyl)piperazino, 3-methyl-4- (4-chluL~h~yl)pir~r7 ~ ;nn,
3 -methyl-4- (4-metho~yphenyl) piperazino, 3 -methyl-
4- (4-methylphenyl)piperazino, 4- (2,4-dimethylphenyl) -
piperazino, 4-acetylpiperazino, 4- (3, 4-dichlorophenyl) -
piperazino, 4- (3, 4-dimethylphenyl) piperazino,
25 4- (3-pyri~lin ~ll - yl)piperazino, 3-methyl-4-phenyl-
piperaz ino, 3 -methyl - 4 - ( 3 - chlorophenyl ) p iperaz ino,
4-benzylpiperazino, 4-propylpiperazino, 4- (3-methyl-
phenyl)piperazino, 4- (3-methoxyphenyl)piperazino,
4 - (4 -methylphenyl) piperazino, 4 - (2, 5-dimethylphenyl) -
30 piperazino, 4-benzhydrylpiperazino, 4-n-butylpiperazino,
4-iso-butylpiperazino, 4-tert-butylpiperazino, 4- (3-tri-
fluoromethylphenyl)piperazino, 4- (l-phenylethyl) -
piperazino, 4- (2-pheaylethyl)piperazino, 4- (2-hydroxy-
phenyl)piperazino, 4-(3~4-dimethoxyphenyl)pip~rlsin~"
35 4-isopropylpiperazino, 3-methyl-4- (3-methoxyphenyl) -
piperazino, 4- (4-l~y~l- oxy~,henyl)piperazino, 3-methyl-
4- (3-methylphenyl)piperazino, 4- (3-hydroxyphenyl) -
~r~ t ~3heet (Rule 26)
2~
WO 95/19952 - 8 - PCT/EP95/00167
piperazino, 4-(2,6-dinitro-4-trifluoromethylphenyl)-
piperazino, 4- (2-hydroxy-3-phen~.~y~L~ y1)piperazino,
4- (4-nitrophenyl)piperazino, 4- (4-acetylphenyl) -
piperazino, 4-ethoxycarbonylpiperazino and the
5 4- (4-chlorobenzhydryl)piperazino radical.
An example of a substituted morpholino radical
which may be mentiorled i~3 the 3, 5-dimethylmorpholino
radical .
Examples of srLbstituted homopiperazino radicals
10 which may be ~ n~d are the 4-methyl-, the 4-ethoxy-
carbonyl-, the 4-acetyl-, the 4- (2-methoxyphenyl) - and
the 4 -benzoyl h~ ~, r~lrazino radical .
- Examples which may be t; ~n~3 of benzhydryl
rA~ which are, if required, substituted by halogen
15 or 1-4C-alkyl are the benzhydryl, the bis-
4,4'-fluorobenzhydryl, thebi~l-4,4'-chlorobenzhydryl, the
4 - chlorobenzhydryl and the 4 -methylbenzhydryl radical .
Examples which may be - t; ~ned of phenyl radi-
cals which are substituted by R6, R7 and R8 are the20 radicals 3,4-dihydroxy-, 3-hydroxy-4-methoxy-,
3,4-dimethoxy-, 2-m~thoxy-, 2-ethoxy-, 3-methoxy-,
4-methoxy-, 2-hydroxy-, 3-hydroxy-, 4-hydroxy-,
3, 4 - dihydroxy-, 4 - ac e tyl -, 4 - f luoro -, 4 - chloro,
2-chloro-, 3-chloro-, 3,4-dichloro-, 3-trifluoromethyl-,
25 2-trifluoromethyl-, 2-methyl-, 3-methyl-, 4-methyl-,
2,3-dimethyl-, 2,4-dimethyl-, 3,4-dimethyl-,
2, 5-dimethyl-, 4-rLit~o-, 2, 6-dinitro-4-trifluoromethyl-
and 5 -chloro-2 -methy~ ~m; n~ph~nyl .
Selected ~ of Ar substituents which may be
30 mentioned are the fol~lowing radicals:
pherlyl, 4- (2-~ethoxyethoxy) phenyl, 2-allylphenyl,
2-acetyl-4-butyr~m;c~ h-~nyl, 4-~ lmethylphenyl,
4-methylphenyl, 2-tetrahydrofurfuryloxyphenyl, 2-chloro-
5-methylphenyl, 2-acetyl-4- (3,3-diethylureido)phenyl,
35 2-cyclohexylphenyl, 4-hydroxy-3-carbamoylphenyl,
4- (2-methoxyethyl) phe!nyl, 2 -methoxyphenyl, 4-niLl~,~h~ . ~yl,
2 - al lyloxyphenyl, 2 - cyclopentylphenyl, 2 - cyanophenyl,
Replacement sheet (Rule 26)
WO 95/19952 - 9 - PCT/3P95/00167
4-ace~Am;-1-phPnyl, 4-l~y~L~,~yt,henyl, 2-cyclopropylphenyl,
4-methAn~ 1f~nAm;rlrlphl~nyl, 4-(3-cyclohexylureido)phenyl,
2-methylth;rphPnyl, .4_~'A 1 ~lphenyl, 4-cyclopropyl-
methoxyethylphenyl, 2, 5-dichlor~J~hel~y1, 2 -butyryl-
5 4-fluorophenyl, 2-tri~luoromethylphenyl, 2-chlorophenyl,
2-fluorophenyl, 2--methylphenyl, 2-acetylphenyl,
5,6,7,8-tetrahydro-2-Ilaphthyl, 4_~A~h~olyl~ 1-naphthyl,
5,8-dihydro-1-naphthyl, 5,6-dihydro-1-naphthyl, 1-inden-
4-yl, 1-inden-7-yl, 2-methyl-4-indolyl, 6, 7-dihydroxy-
5,6,7,8-tetrahydro-1-llaphthyl, 4-indolyl, 3,4-dihydro-
2-l yd~o,.y~tuinolin-5-yl (= 3,4-dihydrocarbostyril-5-yl),
8-hydroxycarbo~styri1-5-yl, 2-naphthyl, 2-thiazolyl,
- 4-morpholino-1,2,5-thiA~ 7Ol-3-yl, 7-ethyl-2-benzo-
furanyl, 2-acetyl-7-benzofuranyl, 5-methyl-2~I-benzopyron-
8-yl, 1,4-b~n ~Q~ An-5-yl, 4-indanyl and 5,6,7,8-tetra-
hydro-5-oxo-1-naphthy:L .
Suitable saltls for the ~ ~ o~ the formula
I are all acid addition salts. Particular mention may be
made of the rhArr~--ologically acceptable ~salts of the
20 inorganic and organic acids customarily used in pharma-
ceutical tel-hn~llogy. phA~^l ologically unacceptable salts
which may, for example, be the initial products of the
process for the preparation of the compounds according to
the invention on the industrial scale are converted by
25 processes known to the skilled worker into rhArr-~olo~i-
cally acceptable salts. Suitable as Isuch are water-
soluble and water-;n~olllhle acid addition salts with
acid~s such als, for example, hydrochloric acid, hydrobro-
mic acid, rh~srhoric acid, nitric acid, sulfuric acid,
30 acetic acid, citric acid, D-gluconic acid, benzoic acid,
2-(4-l~ydL~ ybenzoyl)b~nzoic acid, butyric acid, sulfosal-
icylic acid, maleic acid, lauric acid, malic acid,
fumaric acid, ~succinic acid, oxalic acid, tartaric acid,
embonic acid, stea~^ic acid, toll~An~ulfonic acid,
35 methAn~ l fonic acid or 3-hydroxy-2-nArh~h~ acid, the
acids being employed in the preparation of the salts in
a ratio of amounts which is equimolar or different
Replace~ nt sheet (Rule 26)
15~:~
Wo 95/19952 - 10 - PCT/EP95/00167
therefrom, ~l~p~n~l~ng on whether the acid is mono- or
polybaaic and ~ r~n~q;n~ on which salt i8 required.
Compounds of the f ormula I which arQ to be
hA~i~ed are those in which
5 A1 is 2-lOC-alkylen~ or dimethylenecyt l~h~ n~, and in
which
R1 is hydLosle~l and
R2 i8 hydL~g~- or A2-Y,
or in which
10 R1 and R2 represent, together and with ;n~ ion of the
nitrogen atom to which both are bonded, an unsub-
stituted or subs~ituted piperazine radical,
where
A2 is l-lOC-al:kylene,
Y i5 R3, NH2, NH-R4 or S-R5,
- a substitut~d piperazino radical is substituted
in positio~. 4 by a substituent selected from
the group ,consisting of 1-4C-alko,-y~ -~yl,
l-4C-alkylcarbonyl, pi~ol; noyl~ nicotinoyl,
isonicotinoyl, benzhydryl and the radical R4
and
where fur~h- e
R3 is phenyl or phenyl which is substituted by
- O -A1- ONO2,
R4 is the subEtituent -CH2-CH(OH) - (CH20)p-Ar and
R5 is if required halogen- or 1-4C-alkyl-
substitute~ benzhydryl, dibenzocycloheptanyl,
dibenzocycloheptenyl or benzo-pyrido-
cycloheptarlyl,
30 and where in addition
p is the number 1 and
Ar is phenyl, 4- (2-me~hoxyethoxy)phenyl, 2-allyl-
phenyl, 2-chloro-5-methylphenyl, 2-allyloxy-
phenyl, 2-cyclopentylphenyl, 2-cyanophenyl or
1-naphthyl,
and the salts of the~3e -
C _ '~ of the formula I which are to be
R-Qpl :~C t sheet (Rule 26)
WO 95/19952 - ll - PCT/EP95/00167
particularly ~ R; 7~3d are those in which
Al is 2 -lOC-alkylen~ or dimethylenecyrl ~h~T~n~ and in
which
Rl is llydL~ l and
5 R2 is hyd~ ~l or A2-Y,
or in which
Rl and R2 represent, together and with ;n- 3llR;~m of the
nitrogen atom to which hoth are bonded, an un8ub-
stituted or subs :ituted piperazine radical,
10 where
A2 is l-lOC-al!cylene,
Y is R3, NEI2, NEI-R4 or S-R5,
- a substitut~d piperazino radical i5 substituted
in position 4 by a substituent selected from
the group consisting of 1-4C-alkylcarbonyl,
nicotinoyl, benzhydryl and the radical R4 and
where furTh- e
R3 is phenyl or phenyl which i8 substituted by
-O-Al -ON02,
R4 is the substituent -CH2-CE(OE) - (CE20)p-Ar and
R5 is benzhydryl which is substituted by
1-4C-alkyl, or benzhydryl, dibenzocyclo-
heptanyl, rl;h~n7ocycloheptenyl, or benzo-
pyrido-cycloheptanyl which is substituted by
chlorine,
and where in addition
p i5 the number l and
Ar is phenyl, 2-allylphenyl or l-naphthyl,
and the salts of these ~ _ 'R.
The inventiorL furTh- ~ relates to a process
for the preparation Oe the r ~ R of the for~Lula I and
their salta. The process comprises aldehydes of the
~ormula II (see ~rp~T~ sheet of formulae) in which Al
has the aboverLenti~ned meaning being reacted with
35 compounds of the formula III (see ~rp~n~3~fl sheet of
~ormulae) which are in the ~orm of ;llm galtg and in
which R1 and R2 have the abovementioned meanings, in the
11
Replacement sheet (Rule 26)
W0 95/19952 - 12 - PCT/EP95/00167
presence of sodium c~ranoborohydride, and, if required,
subse~uently the resulting - __ 'R being converted into
the salta, or reRulting salts being converted into the
free ~- ~1R,
The process i3 carried out in a manner known per
E~e to the skilled worker, for example a~3 described in the
following general preparation method.
In the following examples, which are intended to
explain the invention in detail, m.p. stands for melting
point, RT for room temperature and h for hour(8).
Exam~l es
General preparation m~thods
Variant A
10 mmol of the aldehyde II and 10 mmol of the
amino r~ "_ ' III (ill the form of an illm galt) are
dissolved in a suitable solvent (such as, for example,
methanol, ethanol or l:etrahydrofuran), 10 mmol of Rodium
cyanoborohydride are ~dded, and the mixture is stirred at
RT for one h. After addition of a further 10 mmol of
sodium ~;y~ oboz~liydLide~ the mixture is stirred for a
further 20 h. The solvent is stripped off, and the
residue is dissolved in a mixture of water and ethyl
acetate . The organic phase is separated of f, dried over
magnesium sulfate a~ld concentrated. The residue is
purified by chromatography and/or recrystallization.
Variant B
40 mmol are employed in place of 10 mmol of the
amino ~ _ ' III (in the form of an ;ll~n salt) .
Variant C
100 mmol are ,employed in place of 10 mmol of the
amino compound III (in the form of an ammonium salt).
12
Replace~ment sheet (Rule 26)
~ ~. 8 1 3 ~ ~
Wo 95/19952 - 13 - PCT/EP95/00167
1. 2- (2-NitroxYethoxY) -N- (2-PhenYlethYl)benzYlamine
Prepared from 2- (2-nitroxyethoxy)b~n7Al~hyde and
2-phenylethyla~Dmonium chloride by process variant A.
Purified by chromatography on silica gel (ethyl acetate) .
5 The title ,_ ' was isola~ed as tosylate and recrys-
tallized from diethyl ether. M.p. of the tosylate:
147-149C.
2. N-{2- [ (4-MethYl -alPha-PhenYlbenzyl) thio] -ethYl~-
4- (2-nitroxYethoxY) -benzYlamine
Prepared from 4 - (2 -nitroxyethoxy) b~n7Al ~hyde and
2- [ (4-methyl-alpha--phenylbenzyl) thio] ethylammonium
chloride in tetrahydro~uran by process variant A. Puri-
~ied by chromatography on silica gel (diChlc,L, - thAn~) .
M.p. of the hydrochloride: 98-103C.
3 . N- [2- (7-Chloro-10 ,11-dihYdro-5H-benzo [4, 5] cYclo-
hepta [1, 2b] ~Yridine- 5 - thio) -ethYl] -4 - (2 -nitroxY-
ethoxy) benzYlamine
Prepared from 4- (2-nitroxyethoxy)bc~n7~ hyde and
N-[2-(7-chloro-lO,ll-dihydro-5~-benzo[4,5]cyclohepta-
20 [1,2b]pyridine-5-thio)ethyl]amine x 2 }ICl in tetrahydro-
furan by process variant A. Purified by chromatography on
~ilica gel (ethyl acetate/methanol 7:1). Recrystallized
as dihydrochloride from ethyl acetate. M.p. of the
dihydrochloride: 148--151C.
25 4. N-{2-[(5~-Dibenzo~a, d~cYclohePten-5-yl)thiol-eth-
yl}-2- (2,2-dime~hYl-3-niLL~"~y"~",u..y)benzYlamine
Prepared froDI 2-(2,2-dimethyl-3-niLL~,-y~L~ y)-
bc~n7Al d~hyde and N- {2 - [ (5~-dibenzo [a, d] cyclohepten-
5-yl) thio] ethyl}amine in tetrahydrofuran by process
30 variant A. Purified by chromatography on silica gel
(ethyl acetate/petro~ eum ether 60-80/1:2) . M.p.: 97-99C.
13
R^rlAc~ t sheet (Rule 26)
WO 95/19952 - 14 - PCT/EP95/00167
5. N- [2 - (2, 2 -Dimel:hyl -3 -nitroxypropoxy) benzYl] -
piPerazine
Prepared from 2- (2,2-dimethyl-3-~iLl~"Ly~L~u~y)-
b~n~ hyde and piE)erazine x 2 HC1 in methanol by
process variant B . Af ter the reaction solution has been
cr~n<~-.n~rated, the residue was taken up in aqueous sodium
carbonate solution am~ extracted with ethyl acetate. The
organic phase was dried over potas3ium carbonate. The
title ~ __ ' was l?recipitated as hydrochloride and
recrys~ ecl from methanol/diethyl ether. M.p. of the
hydrochloride: 200C (rl~ itior.).
6 . 3 - ( 2 -Nitroxyethoxy) benzYlamine
Prepared from 3- (2-nitroxyethoxy)b~n~ hyde and
~llm acetate ir. ethanol by process variant C. The
title _ __ ' was precipitated as hydrochloride Erom
diethyl ether. N.p. oE the hydrochloride: 131.8-132.5C.
7 . N-AcetYl-N' - [2 - (2, 2 -dimethyl-3 -nitroxYProPoxY) -
benzyl] PiPeraZine
Prepared from N-acetylpiperazine x HC1 and
2-(2,2-dimethyl-3-~itroxy)b~n7~ Phyde in methanol by
process variant A. Recrystallized as tosylate from ethyl
acetate. M.p. of the tosylate: 123-126C.
8 . 2 - ( 2 -Ni troxYe thoxY ) benzYl amine
Prepared from 2- (2-ni~roxyethoxy)b~n~ hyde and
;llm acetate in methanol by process variant C.
Purified by chromatography on silica gel (methanol/ethyl
acetate 1:1) . The hydrochloride of the title - _~vulld was
precipitated from diethyl ether. M.p. of the hydrochlo-
ride: 124.1-125.7C.
9 . N- [2 - ( 2, 2 -Dimethyl - 3 -ni troxYProPoxY) benzYl ] -
N'- (3-pYri~l;ner:~rh~nyl)piperazine
Prepared from 2-(2,2-dimethyl-3-nit~."y~L~,yo~y)-
bDn~ hydeandN- (3-pyri~1;nF~ rh~nyl)piperazine x 2 HCl
14
R~pl~c~ t sheet (Rule 26)
2~
Wo 95/19952 - 15 - PCT/3P95/00167
in methanol by procesç~ variant A . Purif ied by chromatog-
raphy on silica gel (ethyl acetate/methanol 5 :1) . The
dihydrochloride of th~ title compound was recrystallized
from methanol/diethyl ether. M.p. of the dihydrochloride:
127-129C.
10. N- [3- (2-NitroxYe'~hoxY)benzY1]Piperazine
Prepared from 3- (2-nitroxyethoxy)b~n~ld~hyde and
piperazine diacetate in methanol by process variant s.
The dihydrochloride o the title cl _ ' was recrystal-
lized from methanol/diethyl ether. M.p. of the dihydro-
chloride: 165-167C.
11. 1- (2-~ydroxy-3-pl.e~ .Y~ JYl) -4- [3- (2-nitroxvethYl) -
- benzYl] PiPerazine
Prepared from 3- (2-nitroxyethoxy)bQrl7~ hyde and
N- (2-hydroxy-3-phenoxypropyl)piperazine x 2 llCl by
process vari nt A. The dihydrochloride of the title
c , _ ' wa3 recrystS~ l 1; ''9d from is~ ,y~ Ol. M.p. of the
dihydrochloride: 167-169C.
12 . D i - ~ 2 - [ ( 4 - ni troxYme thYl [ trans ] cYclohexYl ) me thoxY] -
benzYl}amine
Prepared f rom 2 - [ ( 4 - ni troxymethyl [ trans] cyclo-
hexyl)methoxy]~n~ hyde and ammonium acetate by
process vari~nt C . Purif ied by chromatography on silica
gel (ethyl acetate/'petroleum ether 60-80/1:1). M.p.
92-97C.
13 . N-DiPhenYlmethYl -N ' - [4 - ( 2 -nitroxYethoxY) benzYl] -
piPerazine
Prepared fro~l N-diphenylmethylpiperazine x 2 ~Cl
and 4- (2-nitroxyethoxy)b~n~ hyde in methanol by
30 process variant A. Purified by chromatography on silica
gel (ethyl acetate,lpetroleum ether 60-80/1:2). M.p.
127 -129C .
Replacement sheet (Rule 26)
~18~
WO 95/19952 - 16 - PCT/~3P95/00167
14 . N- [4- (2 -NitroxYethoxY) benzYl] homopiperazine
Prepared from ~ - (2-nitroxyethoxy)b~n7~ hyde and
homopiperazine diacetate in methanol by process variant
B. The oxalate of the title c~ ,_ 1 was recrystallized
5 from methanol. M.p. ~f the oxalate: 179C (decomposi-
tion) .
15. N- [4- (2-Nitrox~rethoxY)benzYl] -N' - (2-hYdroxY-
3-Ph=~ LY~ yl) -1,6-hexyl~n~ m;n~
Prepared from N- (2-hydroxy-3-phenc"~y~L~yl) -
1,6-hexyl~neA;~m;n~ x 2 ~Cl and 4-(2-nitroxyethoxy)-
b~n 7~ hyde in methanol by process variant A . The
dihydrochloride of th~ title _ _ ' was ~ y~ ~11 i 7ed
from methanol. M.p. o~ the dihydrochloride: 193-196C.
16. N- [3- (2-AllYlPhenoxY) -2-hYdroxYProPyl] -
N'- [4- (2-nitroxy,~thoxy)benzyl-1,8-octyl~n~ m1n~-
Prepared from N- [3- (2-allylphenoxy) -2-hydroxy-
propyl] -1, 8-octyl~n~ m;n and 4- (2-nitroxyethoxy) -
~r n 7 ~ Phyde in methanol by process variant A. The
hydrochloride of the title ~ 3 waa recrystallized
from methanol/ethanol/diethyl ether m.p. of the hydro-
chloride: 151.1-151. 7 C .
17. N- [3- (2-AllYlT~henoxY) -2-hydroxYpropYl] -
N' - [2- (2-nitroxYethoxy)benzyl] -1, 8-octYl~ne~ m;n~-
Prepared from N- [3- (2-allylphenoxy) -2-hydroxy-
propyl]-1,8-octyl~nr~rl;~m;nP x 2 BCl and 2-(2-nitroxy-
ethoxy) ~-~n7~ hyde in methanol by process variant A.
Purified by chromatc,graphy on silica gel (ethyl ace-
tate/methanol/triethylamine 16:4:1). The oxalate of the
title c _~o~n~ was recrystallized from ethanol/diethyl
ether. M.p. of the oxalate: 157-158C.
18. N- [3- (2-AllYlPhenoxY) -2-hYdroxYpropYl] -
N ' - [3 - (2 -nitroxY ethoxy) benzyl] -1, 8 -octYlene~ m; ne
Prepared from 3- (2-nitroxyethoxy)b~n7r~ hyde and
16
Replacement sheet (Rule 26)
-
~lg~ 58~
WO 95/19952 - 17 - PCT/EP95/00167
N- [3- (2-allylphenoxy) -2-hydroxYpropyl] -1, 8-octylene-
diamine x 2 ~ICl in me thanol by process variant A . The
oxalate of the title c _ _ ' was recrystsl l; 7Pr~ from
ethanol/diethyl ether. M.p. of the oxalate: 148-149C.
19. N- (2-~Ydroxy-3-naPhthylv~y~rrvvyl) -N' - [4- (2-nitroxy-
ethoxy)benzyl] -1, 4-butYlenPr~ m;n~s
Prepared fro~ N- [2-hydroxy-3- (l-naphthyloxy) -
propyl]-1,4-butylPn,9,ii9m;nP and 4-(2-nitroxyethoxy)-
benzaldehyde in -~h 9nr1 by process Yariant A. The
dihydrochloride of th~a title r _ ' was ~_Lyr~LdlliZed
by is~crplv~r~l~ol. M.p. ~f the dihydrochloride 150-152C.
20. N- [3- (1-Na~hthyloxy) -2-hYdroxY~ro-vYl] -
N' - [3- (2-nitroxY~thoxY)benzyl] -1,4-butYlPn~ m;ne
Prepared from N- [2-hydroxy-3- (l-naphthyloxy) -
propyl]-1,4-butylPns~;s~m;n~ and 3-(2-nitroxyethoxy)-
bPn~ Phyde in methanol by process variant A. The
dihydrochloride of thr5~ title - _ ' was ~ yDtallized
from isopropanol. M.p. of the dihydrochloride: 145-147C.
21. N- [2- (10-NitroxYdecyloxY)benzYl] -1, 6-hexYl~9nPr~; 9m;n~
Prepared from 2- (lO-nitroxydecyloxy)bPn~ Phyde
and 1,6-hexylPner~;~m;ne diacetate in methanol by process
variant B. The dioxalate of the title - _ ~ was
recrystallized from ethanol. M.p. of the dioxalate:
122 -127 C .
17
, t sheet (Rule 26)
8 ~
W0 95/19952 - 18 - PCT/EP95/00167
Industrial aPPlicatio3l
The compounds of the ~ormula I have valuable
properties which malce them capable of industrial exploi-
tation. They are, in particular, highly effective
5 substances for treat:ing cardiovascular diaeases and
diseases of the eye based on an increased intraocular
pressure .
The ~ ~,ou--dEl of the f ormula I represent a
desired enric_ment of the prior art in their excellent
10 efficacy, which is cl inF~ with a low toxicity and the
absence of substantial side effects. Because of the
nitrate groups in the molecule, the ~ of the
- formula I are aultable in principle for preventing and
treating those pathological states in humans which are
known to be treatable by organic nitrates (such as, for
example, glycerol tri3l~itrate, isosorbide 5-mononitrate or
isosorbide dinitrate) or by ~ able to eliminate
nitrogen 3i nY;~ (such as, for example, - lc~ n~).
In particular, the c __ ~c of the formula I can
be u8ed to prevent and treat ; ~rhe~Tn; C heart diseases
(angina pectoris, myocardial infarct), cardiac ~
sation, (pulmonary) hypertension, (c~rebral) thl ~08~R
and atheroscleroses, narrowing of (peripheral) vessels,
arrhythmias, certain disorders of the gastrointestinal
tract (such as, for example, ?"h~l lC;a, irritable colon)
and of increased intraocular presaure. The _ ___ '~ of
the ~ormula I are furth~- e distinguished by throm-
boxane-~nt~nn; ~tic and antiviral activity and by
~rnn~hnap~ lytic properties.
The invention there~ore furthr ~d relates to a
method for the treatment of mammals, in particular
humans, suffering from one of the abovementioned disor-
ders. The method is characterized in that a therapeuti-
cally effective and rh~ Ql~gically acceptable amount
of one or more c _ ,lc of the ~ormula I is administered
to the individual with the di80rder.
The inventio3l additionally relate8 to the com-
18
Replacement sheet (Rule 26)
~81~t
Wo 95/19952 - 19 - PCT/EP95/00167
pounds of the formula I for use for treating said disor-
ders .
The inventioll likewise embr~ces the use of
c~ ~lq of the formllla I for producing rhArr-~eut;cals
employed to control 3~id disorders.
The invention furth~ ~ relates to pharma-
ceuticals which comp~i3e one or more - _ .,u--ds of the
f ormula I .
The pharmaceuticals are produced by processes
which are known per ~e and are fA~; l; Ar to the skilled
worker. As rhA~-~eut;rAl~, the rhAr~ ol~ically active
r. __ ~ oE the fol-mula I (= active substances) are
employed either as s1lch or, preferably, in combination
with suitable phAr~ P~tir~l AnrillAry aubstances in the
~orm of tablets, coat~d tablets, capsules, suppositories,
plasters (for exampl~ as TTS), 1 ~ nCl~ 5ll~pAn~inn~,
aProsol~, sprays, oiILtments, creamg, gelg or g~ t;onl~,
with the active substance content advantageoualy being
between 0.1 and 95%.
2 0 The An r; 1 1 A r~ substances sui table f or the re-
~uired rhArrA-~eutica~ formulations are fA-n; l; Ar to the
skilled worker on tlle basis of his expert knowledge.
Besides solvents, gel-formers, suppository bases, tablet-
ting aids and other active substance vehicles, it is
possible to use, for example, antinY; ~An~, dipersants,
1 ~; f; ers, An~; f onT~ masking flavors, preservatives,
solubili~ers, colorants or, in particular, permeation
promoters and - _ 1~Y; nrJ agents (for example cyclo-
dextrins ) .
The active substances can be administered orally,
rectally or parente~-ally (in particular perlingually,
buccally, inLLc.v~ u~ly or percutAn~ol-~ly).
In general, it has proven advantageous in human
medicine to administer the active substance or substances
in the case of oral administration in a daily dose of
about 0.01 to about 10, preferably 0.05 to 5, mg/kg of
body weight, if required in the form of several, prefer-
19
Repl Ar ~ sheet (Rule 26)
WO 95/19952 - 20 - PCT/EP95/00167
ably from 1 to 4, individual doses to achieve the desired
result. For parenteral treatment it is possible to use
similar or (especiall~ in the case of intravenous admin-
istration of the active substances) as a rule lower
5 dosages. If the dosa~e ia gradually increa3ed, a lower
dose i~3 administered at the start of the treatment and
then slowly changed to a higher dose . Af ter the desired
result o~ treatment has been achieved, the dose is
returned to a lower one.
Establ i~ t of the optimal dosage and mode of
administration of th~ active aubstances which are re-
guired in each case can easily be carried out by every
skilled worker on the basis of his expert knowledge.
If the c _ ~q of the formula I are to be
15 employed to treat said disorders, it is also poEIsible for
the rh~r~ eu~; cal preparations to comprise one or re
other pharmacologically active ingr~dients from other
rhAr~--~e~;cal groups, such a5 other antihypertensives,
vasodilators, alpha-1 receptor blockers, alpha-2 receptor
20 stimulator~, beta-1 receptor blockers, beta-2 receptor
stimulators, ACE inhibitors, diuretics, saluretics,
A~ l oi~, analgesics, lipid-lowering agents, anticoagu-
lants, anti~ hr~l ;n~rgics~ methylxanthineEI, anti-
arrhythmics, antihi~tamines, dopamine stimulators,
25 serotonin receptor blockers etc. such as nifedipine,
dihydralazine, prazosin, t~ n;rl;n~ atenolol, labetalol,
fenoterol, captopril, digoxin, milrinone, mefruside,
clops~m;d~, spironolactone, chlor~h ~ n~, furosemide,
poly~h;~l~id~, hydroc]lloroth;~ 7; de, reserpine, dihydro-
30 ergocristine, r~;nn~m;n~" rauwolfia total ~lk5.1~acetylsalicylic acid,, b~- If ~hrate~ warfarin, atropine,
theophylline, 1 ;~ r~;nF~ astemizole, bL. - yyti
ketanserin etc.
Replaq t sheet (Rule 26)
. ~ 2~$:~5~l
WO 95/19952 - 21 - PCT/EP95/00167
PharmacoloA~Y
The phArm-Aol,~gical effect of the compounds of
the formula I was d~t~rmined in vivo on anesthetized
rabbits and in vitro :in the so-called rat aorta test.
The percentage decrease in the arterial blood
preasure and the effect on heart rate (percentage change)
after infusion of th~ compounds to be investigated was
det~rm;n~d on anesthel:ized rabbits.
In the rat aorta test, the relaxing effect of the
0 AI _ _ ~R to be inveRtigated wag det~; n~d on spiral
strips o~ the rat p~ ry artery. By cumulative addi-
tion, the do3e which i nhibits the contraction on average
by 50% (= ECso) was det~rm;n~d from the Ar~nAAntration
ef f ect plot .
The i~vestiga~:ed _ ~ are identified in the
following tables by numbers which co~ ~ e~v~d to the
numbers of the examples.
Determination of the blood pressure and of the heart rate
in anesthetized rabbi ts
The test wa~ carried out in analogy to the
procedure described i~l the international patent applica-
tion W092/04337. The result o~ the investigation is shown
in Table 1.
Table 1
Percentage reduction in the arterial blood pressure (BP)
and percentage change in the heart rate (~IR) in N anes-
thetized rabbits (wit]l N ~ 1 averages) by administration
of compounds of the formula I
21
~"rlA~ t sheet (Rule 26)
~ ~$~
WO 95/19952 - 22 - PCT/EP95/00167
Compound % decrease 96 change N
No. BP ~IR
3 39.8 - 6.2 2
7 17.9 - 0.3 2
511 17.8 - 5.2
13 54.6 -18.4 2
Rat aorta test
The test was carried out in analogy to the
procedure described in the international patent applica-
tion W092/04337. The result of the investigation i3 shown
in Table 2.
Table 2
Y~n~ effect of compounds of the formula I on the rat
aorta
15 C , ,.lld ECso Standard Variation N
No. [~M] de~riation
3 0.0055 0.0068 0.001 -0.02 6
6 0.0015 0.0012 0.0002-0.003 6
10 0 . 0067 0 . 0017 0 . 004 -0 . 009 6
20 11 0.0045 0.0026 0.0004-0.01 9
EC50 = concentration which i~hibit3 contraction by 50%
N ~ =u~b-r ~f ~t~lp~ of t ore~ te~t-d
R~pl~q t sheet (Rule 26)
2~ 81
WO 95/19952 - 23 - PCT/EP95/00167
S~ T OF FORMULAE
O--At--ONO2
(I)
R2
N~
Rl
O--A ~ N02
( I I )
CHO
N~ (Rl) R2 (III)
Replacement ~heet (Rule 26)