Note: Descriptions are shown in the official language in which they were submitted.
68087
21 831 1 2
FILTER ~Y~
Technical Field
This invention relates to systems for leukocyte depletion that are gravity primable
with biological fluids such as blood or blood components.
5 Back~round of the Invention
A wide variety of blood product proces~ing protocols include filtering to removeundesirable materials such as leukocytes from blood or at least one blood component
before use. However, some of these protocols are time co,.~v~..i..g, labor intensive
and/or o~ ;lwise inefficient. For example, some protocols for filtering blood
10 components require priming the filter with a fluid other than the blood component, e.g.,
a non-blood component cont~inin~ fluid such saline, or a preservative solution. This can
be undesirable, particularly for those protocols wherein the volume of fluid in the system
should be minimi7~
Alternatively, or additionally, some protocols require "squeeze priming" the
15 filter, which includes colll~)lessil~g a cont~in~r of fluid, e.g., the blood component
container, to force the fluid into the filter. Typically, the teC1-ni~ n carrying out the
filtration protocol colllplesses the bag by hand. After the blood component sufficiently
wets the filter, the colllplession is released and the bag is hung at a sufficient ~ t~n-~e
above the filter to allow the rçrn~ining contents of the colll~ l to drain into, and
20 through, the filter. Squee~ priming can be undesirably labor inlel sive, since, for
example, the technician must have sufficient hand strength to colllpless the bag and
m~int~in the colllpression until the filter is primed. Alternatively, the technician must
operate a culllpression device, e.g., a pres~ule cuff, for each priming. Since the
technician may filter numerous units of blood over the course of the day, the cllmlll~tive
25 time and energy devoted to squeeze priming can be significant.
Accordingly, there is a need in the art for an easily primable blood filtration
system, that can be used in a variety of filtration protocols. In particular, there is a
need for a leukocyte depletion filtration system that can be primed at a gravity head of
blood or a blood component, without "squeeze priming", and without plilllhlg with
68087
21 831 1 2
saline.
The present invention provides for ameliorating at least some of the
disadvantages of the prior art. These and other advantages of the present invention will
be appalell~ from the description as set forth below.
5 Summary of the Invention
Systems according to the present invention provide for leukocyte depleting a
biological fluid and are arranged to prime a filter assembly including a synthetic,
polymeric leukocyte depletion m~lillm by a gravity head of biological fluid. Forexample, one embodiment of a system according to the invention includes a leukocyte
10 depletion filter assembly interposed between a first conduit and a second conduit,
wller~ the first conduit provides fluid co~ ir~tion belweell a coll~i~l of
leukocyte-co--1;~ biological fluid and the filter assembly, and the system provides a
head height or priming height of about 14 inches of biological fluid or more.
The system provides for p~ g the filter assembly with a biological fluid,
lS without using a non-blood component cont~inin~ fluid, e.g., saline, to prime the
assembly. Thus, embodiments of the system are especially desirable for those blood
filtering protocols wl~leill the volume of fluid in the system should be ...;..;...i~A.
Additionally, the system provides for priming the filter assembly with biological
fluid without squeeze priming. Thus, embodiments of the system are especially
20 desirable for use in some ellvirol~ ents (e.g., blood banks) wherein a plurality of units
of biological fluid can be filtered contemporaneously, and/or wherein ll.~ ous units are
filtered over the course of the day.
In some embodiments, the system provides for plillmlg the filter in about 60
seconds or less. In an embodiment, the system is a closed system, and includes a25 container for collecting the leukocyte-depleted biological fluid in fluid co------~ ic~tion
with the second conduit.
Methods accordillg to the invention provide for priming a leukocyte depletion
filter assembly with a biological fluid at a gravity head of biological fluid, and depleting
leukocytes from the fluid. In an embodiment, the biological fluid is a red blood30 cell-co.~ g biological fluid such as whole blood. In some embodiments, the
biological fluid is leukocyte-depleted in a closed system.
68087 21 831 1 2
Brief Description of the Drawin~s
Figure 1 illustrates an embodiment of a system according to the present
invention, that has been placed in fluid collullunication with a source container of
biological fluid.
S Figure 2 is a side view of a filter assembly that can be utilized in an embodiment
of a system according to the present invention.
Figure 3 illustrates another embodiment of a system according to the present
invention.
Specific Description of the Invention
In accordance with the invention, a system for processing a biological fluid
colll~lises a filter assembly COlll~)liSillg a housing having an inlet and an outlet and
defining a fluid flow path between the inlet and the outlet, and having a synthetic
polymeric leukocyte depletion medium disposed within the housing and across the fluid
flow path; and a conduit having a first end and a second end, wherein the first end is
15 capable of providing fluid co~uui-ic~tion with a first CO~ suitable for holding a
leukocyte-cont~ining biological fluid, and the second end is in fluid c~ ic~tion with
the inlet of the housing; wll~lein the system is arranged to prime the filter assembly with
the biological fluid by gravity. Typically, the system has a biological fluid priming
height of about 14 inches or more gravity head of biological fluid, more typically, a
priming height of about 17 inches or more gravity head. P~fel~lbly, the system has a
priming height of about 22 inches or more gravity head of biological fluid.
In another embodiment, a system for processing a biological fluid colu~lises a
filter assembly co~ isillg a housing having an inlet and an outlet and defining a fluid
flow path between the inlet and the outlet, and having a synthetic polymeric leukocyte
depletion medium disposed within the housing and across the fluid flow path; and a first
conduit having a first end and a second end, wherein the first end is capable ofproviding fluid colllulullication with a first container suitable for holding a
leukocyte-cont~ining biological fluid, and the second end is in fluid co~ ir~tion with
the inlet of the housing; wherein, once the first end of the conduit is placed in fluid
colll,llunication with the first container of leukocyte-cont~ining biological fluid, the
system is capable of ~lilllhlg the filter assembly with the biological fluid by gravity
68087 2~ 831 1 2
without colll~icssillg the first container of leukocyte-cont~inin~ biological fluid to prime
the filter assembly.
In some embodiments, the system provides for priming the filter assembly with
biological fluid in about 60 seconds or less.
Typically, the system also includes a second conduit and a container for receiving
leukocyte-depleted biological fluid, wllclchl the second conduit is interposed between the
outlet of the housing and the container for l~eivh~g the leukocyte-depleted biological
fluid. In some embodiments, the system is a closed system. The system can include
additional containers and COlh~ i, such as at least one container and conduit
dowl~ll~ll of, and in fluid co.. i-;ration with, the cont~in.or for lcceiving the
leukocyte-depleted biological fluid. The system can also include one or more flow
control devices such as clamps, as well as one or more cormectors.
The system can also include at least one additional device, including, but not
limited to one or more filter assemblies; gas venting devices such as gas storage bags,
gas collection and displacement loops, gas inlets, and gas outlets. Suitable gas venting
devices include those disclosed in, for example, U.S. Patent Nos. 5,126,054; 5,217,627;
and 5,451,321. Typical gas venting devices, that can be utilized in closed or open
systems, include at least one porous me~ m, such as a liquophobic membrane, thatallows gas to pass therethrough.
The present invention also provides a method for proces.sing a biological fluid
com~lisillg creating a gravity head between a first coll~iller of biological fluid and an
unprimed leukocyte depletion filter assembly, the assembly including a porous tn~illm
collll~rishlg a ~yll~ ic, polymeric me~ m; and passing the biological fluid through the
assembly to prime the assembly and leukocyte deplete the biological fluid.
In accordance with the invention, a method for plocessing a biological fluid
comprises placing a first container of leukocyte-cont~ining biological fluid in fluid
co..~ ication with a leukocyte depletion filter assembly including an ull~vel~d
synthetic, polymeric leukocyte depletion mlq linm; creating a gravity head of biological
fluid between the first container and the filter assembly; and, without colll~l~sillg the
30 first container to prime the filter assembly, passing the biological fluid through the filter
assembly to prime the assembly and to deplete leukocytes from the biological fluid.
In accordance with the invention, a method for processing a biological fluid
68087 2 1 83 1 1 2
comprises creating a gravity head between a first container of leukocyte-cont~ining
biological fluid and a leukocyte depletion filter assembly without co",l~lessillg the first
container to prime the filter assembly, the assembly including an unwetted ~yll~letic~
polymeric leukocyte depletion m~Aillm; wetting the m~ lm with the biological fluid;
S and passing the biological fluid through the assembly.
In some embodilllell~, a method according to the invention provides for gravity
imillg the filter assembly with biological fluid in about 60 secon-ls or less.
In accordance with the invention, a biological fluid includes any treated or
ullLIea~d fluid associated with living olg~ , particularly blood, including whole
10 blood, warm or cold blood, and stored or fresh blood; treated blood, such as blood
diluted with at least one physiological solution, in~l~ldin~ but not limited to saline,
nutrient, and/or anticoagulant solutions; blood components, such as platelet concentrate
(PC), platelet-rich plasma (PRP), platelet-poor plasma (PPP), platelet-free plasma,
plasma, fresh frozen plasma (FFP), components obtained from plasma, packed red cells
15 (PRC), transition zone material or buffy coat (BC); analogous blood products derived
from blood or a blood component or derived from bone marrow; red cells separatedfrom plasma and resuspended in physiological fluid; and platelets se~a,aled from plasma
and resuspended in physiological fluid. The biological fluid may have been treated to
remove some of the leukocytes before being processed according to the invention. As
20 used herein, blood product or biological fluid refers to the components described above,
and to similar blood products or biological fluids obtained by other means and with
similar ~lopellies.
A "unit" is the quantity of biological fluid from a donor or derived from one unit
of whole blood. It may also refer to the quantity drawn during a single donation.
25 Typically, the volume of a unit varies, the amount dirr~ g from patient to patient and
from donation to donation. Multiple units of some blood components, particularlyplatelets and buffy coat, may be pooled or combined, typically by combining four or
more units.
Each of the components of the invention will now be described in more detail
30 below.
In accordance with the invention, a biological fluid proces~ing system includes a
leukocyte depletion filter assembly, a plurality of conduits, and at least one container for
68087 21 83t 1 2
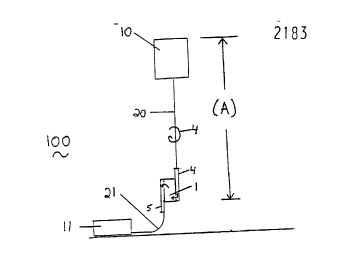
receiving leukocyte depleted biological fluid. For example, as illustrated in Figures 1
and 3, biological fluid processing system 100 includes a leukocyte depletion filter
assembly 1 interposed between first conduit 20 and second conduit 21, with second
conduit 21 providing fluid colllululfication between the assembly and the receiving
S container 11. Figure 1 also illustrates a source container 10, that is suitable for holding
a unit of leukocyte-cont~ining biological fluid. First conduit 20 provides fluidco.,.. ,.. i-~tion b~lween source container 10 and filter assembly 1.
Typically, as illustrated in Figure 1, the system 100 includes at least one flowcontrol device 4 such as a clamp, seal, valve, ~ rer leg closure, or the like, located
within or on at least one of the conduits and/or the collt~
The system can also include additional components such as at least one
connector, at least one conlaillel, and at least one conduit. For e~le, Figure 3illustrates an additional conduit 22, and an additional container 13. Additionalcolllault;l~ can be receiving and/or L~ rer col-~ille.~ for biological fluid, and/or
containers for additive, preservative, or storage solutions.
As illustrated in Figure 2, leukocyte depletion filter 1 assembly comprises a
housing 2 having an inlet 4 and an outlet 5 and defining a fluid flow path between the
inlet and the outlet, and a porous, synthetic, polymeric leukocyte depletion m.oAillm 3
disposed in the housing and across the fluid flow path.
The col~ s which are used in the biological fluid proces~ing system can be
constructed of any material compatible with biological fluids. A wide variety of these
containers are already known in the art. For example, blood collection and satellite bags
are typically made from plasticized PVC, e.g. PVC plasticized with dioctylphth~l~te,
diethylhexylphth~l~te, or trioc;lylllilllellitate. The bags may also be formed from a
polyolefin, polyulelhalle, polyester, or a polycarbonate.
As used herein, fluid collllllul~ication may be established by any structure which
allows the biological fluid to pass from one location to another, such as by at least one
conduit or tube. The conduits 20, 21, and 22 used in the instant invention may be
constructed of any material compatible with biological fluid. Preferably, they may be
composed of a flexible material, such as polyvinyl chloride (PVC), or plasticized PVC,
e.g., PVC plasticized with dioctylphth~l~te, diethylhexylphth~l~t~, or trio~;lyl~ llit~te.
Preferably, using Figure 1 for reference, the head height is the ~ t~nr~ (A)
68087 21 831 1 2
between the top of the source bag (after the bag is hung) and the point at which the
blood contacts the medium. In other embodiments, the head height can be, for example,
the ~ t~nre between the uppermost surface of the blood in the source bag and the point
at which the blood contacts the medium.
Typically, first conduit 20 has a sufficient length to allow the creation of a
priming height (also referred to as a static height or a head height) of at least about 14
inches of biological fluid once it is interposed between the source container ofleukocyte-cont~inin~ biological fluid and the inlet of the leukocyte depletion filter
assembly. Preferably, first conduit 20 has a sufficient length to allow the creation of a
plilllhlg height of at least about 22 inches once it is interposed between the source
container and the filter assembly.
Using Figure 3 for r~felellce, in one p,efelled embodiment of a system accordingto the invention, first conduit 20 should be of sl-ffil~iPnt length to allow the system to
provide a ~ g height of at least about 26 inches after the first conduit is sterile
docked to the source container, to m~int~in a closed system.
Typically, using Figure 1 for reference, once first conduit 20 is interposed
between the source container 10 and the filter assembly 1, the system provides a head
height of about 14 inches or more, e.g., about 18 inches or more. Illustrative head
heights include the range of from about 26 inches to about 65 inches; about 27 inches to
about 58 inches; about 30 to about 54 inches; about 33 inches to about 48 inches; about
35 inches to about 45 inches; about 40 inches to about 44 inches.
A variety of materials can be used, inrll~l(lin~ any porous synthetic polymeric
material, for the leukocyte depletion medium 3 of the filter assembly 1. Suitable
synthetic polymeric material includes, for example, polybutylene terephth~l~te (PBT),
polyethylene, polyethylene terephth~l~te (PET), polymethylpentene, polypropylene, and
nylon 6.
In one pr~felled embodiment, the medium 3 comprises a fibrous medium,
typically a medium prepared from melt-blown fibers, as disclosed in, for example, U.S.
Patent Nos. 4,880,548; 4,925,572; 5,152,905; 5,258,127, and 5,443,743; and
InlelllaLional Publication WO 96/03194. The me~ m can include a plurality of layers,
as disclosed in the U.S. Patents and the International Publication listed above.Surface characteristics of the medium can be modified by chemical reaction
68087 2 1 83 1 1 2
including wet or dry oxidation, by coating or depositing a polymer on the surface, or by
a grafting reaction. Grafting reactions may be activated by exposure to an energy source
such as gas plasma, heat, a Van der Graff generator, ultraviolet light, electron beam, or
to various other forms of radiation, or by surface etching or deposition using a gas
5 plasma treatment.
The porous medium is preferably treated for increased efficiency in proces~in~ abiological fluid. For example, the m~ m may be surface modified to affect the critical
wetting surface tension (CWST) of the medium, as described in, for example, U.S.Patent Nos. 4,880,548; 4,925,572; 5,152,905; 5,258,127, and 5,443,743; and
Illlel~lional Publication WO 96/03194.
Preferably, the porous m~Aium according to the invention, which is, more
preferably, a porous fibrous m~ m, has a CWST of greater than about 58 dynes/cm.For example, a fibrous medium may have a CWST in the range from about 60 dynes/cm
to about 115 dynes/cm, e.g., in the range of about 61 to about 105 dynes/cm, or about
78 to about 98 dynes/cm. In some embo~ , the fibrous medium has a CWST of
about 62 dynes/cm, or greater, e.g., in the range from about 63 to about 70 dynes/cm,
or in the range from about 85 to about 97 dynes/cm.
The housing 2 can be fabricated from any suitable rigid impervious material,
including any impervious thermoplastic material, which is compatible with the biological
fluid being processed. For example, the housing can be fabricated from a metal, such as
stainless steel, or from a polymer. In a plefellcd embodiment, the housing is a
polymer, more preferably a transparent or tr~n~lnr,ent polymer, such as an acrylic,
poly~ropylene, poly~lyleile, or a polycarbonated resin. Such a housing is easily and
economically fabricated, and allows observation of the passage of the biological fluid
through the housing.
The surfaces of the housing cont~cting the fluid may be treated or u~ ,aled. Forexample, the surfaces of the housing cont~cting the fluid may be rendered liquophilic for
better priming. Methods for treating the surface of the housing include but are not
limited to radiation grafting and gas plasma tre~tm.ont
Any housing of suitable shape to provide an inlet, an outlet, and an adequate flow
area may be employed. The filter assembly in accordance with this invention may be
fashioned in a variety of configurations including, but not limited to, those described in
68087 21 831 1 2
U.S. Patent Nos. 4,880,548; 4,925,572; and 4,923,620. In other configurations, the
inlet and outlet can be arranged so that, for example, biological fluid contacts the
m~Aium near the top of the housing and passes through the m~Aillm near the bottom.
As used herein, the term "closed" refers to a system that allows the collection,5 processing, filtration, storage, and preservation of donor blood or blood components
without the need to enter the system (and risk cont~min~tion of the system). A closed
system can be as originally made, or result from the connection of the individual (or
partially conn~ted) components of such a system using what are known as "steriledoc~ing" devices. Illu~LIaliv~ sterile docking devices are disclosed in U.S. Patent No.
4,507,119.
All of the l~fel~ces cited herein, including publications, patents, and patent
applications, are hereby incorporated in their entireties by reference.
Examples
The Examples are set up as described below. The leukocyte depletion filter
assembly contains a filter element of polybutylene terephth~l~te fibers. The elem~ nt,
that has a Critical Wetting Surface Tension (CWST) of about 66 dynes/cm, is arranged
in a housing generally in accordance with U.S. Patent No. 4,925,572. As illustrated in
Figure 2, the filter assembly is arranged to allow blood to contact the element near the
bottom of the housing, and to exit from the element near the top of the housing.A system including two blood bags, a clamp, first and second plastic conduits,
and the leukocyte depletion filter assembly is set up in a l~ l~r shown in Figure 1. A
blood bag 10 (the first bag) cont~inin~ a unit of whole blood and anticoagulant is hung
vertically above the filter assembly 1, and the clamp 4 associated with the first conduit
20 between the bag 10 and the filter assembly 1 is initially closed. The blood fills first
bag 10 so that the uppermost surface of the blood reaches the top inner surface of the
bag when hung.
An empty blood bag 11 (the second bag) is placed on the level surface of the test
bench, with second conduit 21 interposed between the filter assembly 1 and the second
bag 11. In Example 1, the ~i~t~nre from the top of the first bag 10 to the test bench
surface is 48 inches. For Examples 2-4, the ~lict~n~e from the top of the first bag to the
bench surface is 58 inches.
68087
21 831 1 2
The head height, that is the ~ t~nre (A) between the top of the hung first bag 10
and the point at which the blood initially contacts the me linm 3 in the assembly 1 is set
out below. Different head heights are utilized in the four Examples, i.e., 48 inches,
47.5 inches, 42.5 inches, and 25.5 inches.
The clamp is opened, and the blood is passed from the first bag, through the
assembly, and into the second bag. The first bag is not squeezed or colllplessed to
prime the filter assembly. The time required for blood to begin to pass through the
outlet of the housing, and the time for subst~nti~lly all of the residual air to pass through
the outlet, are recorded. The sum of those two times is the plilllil~g time. Once the
filter is primed, blood passes through the outlet in a steady stream, ess~l~ lly without
air bubbles. Each filter assembly filters a unit of blood.
For each filtration, the leukocyte depletion efficiency is about 99.9% or greater.
Example 1.
Distance (A) is 48 inches. Blood passes into the outlet of filter #1 in 21 seconds.
Bubbles pass into the outlet for 10 seconds, followed by a steam stream of blood. Thus,
Filter #1 primes in 31 seconds (21 seconds plus 10 seconds).
The values for filters #2 through #8 are plt;sell~d in the following format:
plilllillg time, followed by, in parenth~ses, the time for initial blood passage, and air
clearance.
Filter #2 primes in 25 seconds (18 seconds plus 7 seconds).
Example 2.
Distance (A) is 47.5 inches. Filter #3 primes in 27 seconds (17 seconds plus 10
seconds). Filter #4 primes in 30 seconds (19 seconds plus 11 seconds).
Example 3.
Distance (A) is 42.5 inches. Filter #5 primes in 36 seconds (23 seconds plus 13
seconds). Filter #6 primes in 26 seconds (21 seconds plus 5 seconds).
Example 4.
Distance (A) is 25.5 inches. Filter #7 primes in 55 seconds (40 seconds plus 15
68087 21 831 1 2
seconds). Filter #8 primes in 57 seconds (42 seconds plus 15 seconds).
The Examples show that filters can be primed about 1 minute or less using a
gravity head of biological fluid according to the invention. In some embodiments, the
priming is about 30 seconds.
S While the invention has been described in some detail by way of illustration and
example, it should be understood that the invention is susceptible to various
m~lifir~tions and alle,i,AIive forms, and is not lc;~ ie~l to the specific embo-1imr-ntc set
forth. It should be understood that the~ specific embo~limrnt~ are not intended to limit
the invention but, on the contrary, the intention is to cover all mo lifir~tions,
10 equivalents, and all~ lives falling within the spirit and scope of the invention.