Note: Descriptions are shown in the official language in which they were submitted.
2 1 832 1 4
BALLOON DILATION ~AT~ ~ WITH ~Y~OIu~
RA~:K(j~O~U OF TH~ INV~NIION
1. Field of the Tnvention
The present invention relates to balloon dilation
catheters, and more particularly concerns a catheter
having i ~v~d p~lch~h;l;ty, tr~r~hil;ty and time of
inflation.
2. Descri~tion of Relevant Art
Therapeutic balloon dilation catheters, used for
balloon angioplasty, are known in the art which are of the
so-called "monorail" configuration. Such monorail
catheters generally have a comparatively short distal
monorail portion of the catheter slidably received over a
guide wire. This distal portion inr~ s an ~p~n~;hle
dilation balloon. Other than the comparatively short
distal portion of the guide wire received within the
catheter, the rr--in~rr of the guide wire is exposed
externally of the catheter. The monorail portion is
coupled with a catheter body tube formed of a flexible
material that allows the catheter to be pushed through
curved vascular pathways and also enables passage of a
balloon inflation fluid. Some typical balloon dilation
catheters are shown in U.S. Patent Nos. 5,217,482 to
Reith; 4,762,129 to Bonzel; 5,061,273 to Yock; and
4,748,982 to Horzewski et al.
During angioplasty a guide catheter with a guide wire
therein is initially inserted into a patient and serves
both to guide a therapeutic catheter along the vascular
pathway to a location close to the heart and to protect
the patient's blood vessels from trauma which could be
caused by contact with the therapeutic catheter.
_ _ _ _
~ 2 2l 832l4
The guide catheter, with the guide wire in place, is
pushed along the vascular pathway until the distal end of
the guide catheter reaches a position adjacent but
somewhat short of the position at which the angioplasty is
to take place. Beyond the distal end of the guide
catheter the guide wire is extended through the stenosis
of the vascular area that is to be treated. A theIa~uLic
catheter, having the expansible dilation balloon on a
distal extremity, is inserted into the guide catheter and
over the guide wire, which extends through the monorail
section along the guide wire of the therapeutic catheter,
so that the catheter is guided beyond the end of the guide
catheter to the treatment location. While the balloon is
positioned at the treatment location, a dilation fluid is
injected into the therapeutic catheter body tube and
inflates the thin wall expansible dilation balloon to
enlarge the stenosis.
The therapeutic catheter is formed of a body tube
that performs a number of functions. It provides the
axial drive or push that moves the collapsed and folded
balloon at the distal end through the vascular pathways.
~or this reason certain proximal portions must be
relatively stiff and have a high ~pllch~hility~ although
the catheter bends in the vascular pathway. ~he catheter
body tube also forms a lumen that provides a flow path for
dilation fluid. In order to decrease inflation and
dilation time, the lumen cross-section must be as large as
possible and still fit within the guide catheter, which in
turn must fit within the vascular pathway.
In certain types of angioplasty it is desirable to
provide a positive purge of ~luid within the dilation
balloon to onsure that the latter is free of air before
using the therapeutic catheter. To facilitate positive
purging, the body tubes of some balloon dilation catheters
are formed of a single plastic extrusion having a triad of
~ 3 2183214
three side-by-side integral lumens, one to flow fluid
distally, a second to flow Pluid proximally, and a third
to accept an axially insertable stiffening wire to stiffen
and strengthen the integral extruded plastic 5ide-by-side
tubes. Such an arrangement is expensive and of relatively
large diameter, limiting its use to larger vascular
pathways. Its pushability is relatively low, requiring a
stiffening wire which uses a significant part of the
cross-section of the total flow area available. U.S.
Patent No. 5,061,273 to Yock shows a catheter having a
multi-lumen body tube.
If the conventional therapeutic catheter body tube is
made of a flexible plastic, it loses pushability and may
not be able to drive the balloon to its proper position.
1~ If made of a stiffer plastic, it may not readily follow
the curvature of vascular pathways. If it is stiffened
with an integral wire, flow area is lost and inflation and
deflation time is increased. Therefore, prior catheters
have involved a compromise among several conflicting
requirements.
In the monorail type of aatheter, both the guide tube
that extends through the inflation balloon and the guide
wire itself exit the therapeutic catheter body at a guide
wire port in the side wall of the body tube. This area of
the catheter is weakened by the guide wire port and has a
tendency to kink severely as the catheter is moved along
the vascular pathway, and as the wire traverses the guide
wire port.
Accordingly, it is an object of the present invention
to provide a balloon dilation catheter that avoids or
minimi~P~ above r ' ;nnPd problems.
STTMMARY OF 'rTTF: ,TNVENTION
In carrying out principles of the present invention,
according to a preferred Prho~;~~nt thereof, elongated
~ 4 2~ ~321~
proximal and distal body tube sections are fused to one
another with an PYp~ncihle dilation balloon having a
proximal end bonded to the distal end of the distal body
tube. The two body tube sections collectively form a
therapeutic catheter body tube and have a guide wire port
therein proximally of the proximal end of the expansible
dilation balloon. A guide tube extends through the
balloon and through the port of the body fuse area, having
a distal end opening at and bonded to the distal end of
the balloon and having a proximal end opening at and
sealed to the guide wire port. Within the body tube is a
relatively stiff hypotube, having an open proximal end
adjacent the proximal end of the proximal body tube
section and an open distal end adjacent the guide wire
port. The hypotube, together with the catheter body tube,
form inner and outer nested flow passages for flow of
fluid therethrough. The hypotube is provided with a
stiffening distal solid taper wire section extending
within the catheter body tube from a proximal portion to
a distal portion of the guide wire port. The hypotube may
be fixedly secured relative to the body tube, or it may be
removable. In one embodiment, the inner and outer flow
passages are sealed separately from one another so as to
provide for a positive purging operation, flowing fluid
distally toward the balloon through one of the passages
and flowing fluid proximally from the balloon through the
other of the passages. The hypotube provides stiffening
for the proximal body tube section without any significant
decrease in flow passage area. This provides ; ~ ~v~d
pushability, improved trackability, and decreased time of
inflation and deflation.
~RTFF DES~RTPTION OF T~ DR~WTNGS
FIG. 1 schematically represents a guide catheter
which is introduced into a patient's femoral artery and
5 21832l4
which extends at its distal end around the aortic arch to
terminate at a location adjacent the patient's heart. A
therapeutic catheter extends through the guide catheter to
the patient's heart.
FIG. 2 is a longitudinal section of a therapeutic
catheter embodying principles of the present invention.
FIG. 3 is an enlarged section of the distal end of
the catheter of FIG. 2.
FIG. 4 is an enlarged fragmentary view of a distal
end of the hypotube used in the catheter of FIGS. 2 and 3.
FIG. 5 is a section taken on lines 5-5 of FIG. 4.
FIG. 6 is a longitudinal section of the proximal end
of the therapeutic catheter of FIGS 2 and 3.
FIGS. 7 and 8 show details of the attachment of the
proximal end of the hypotube.
FIG. 9 is an enlarged sectional view of a portion of
the proximal end of a modified therapeutic catheter.
FIG. 10 is a view like that of FIG. 9 showing still
another rmho~; r -nt.
DFTATr,Fn D~scRTpTIoN OF r~r~MPr.~Ry ~MR~DTM~TS
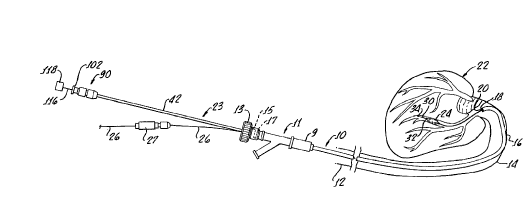
As illustrated in FIG. l, a tubular guide catheter 10
is introduced into a patient's femoral artery 12 and
caused to extend upwardly to terminate at the aortic arch
14. The guide catheter 10 inrln~s a distal ~ ULV~
section 16 which allows a distal end opening 20 of the
guide catheter to be disposed toward the patient's heart
22. At the proximal end of the guide catheter 10 is
mounted a rotatable hemostatic adapter 9 and a Y or two-
arm connector or adapter 11 which is mounted on the
rotatable adapter. The Y connector 11 is provided with a
knurled knob 13 which carries a threaded valve member 15
that carries an o-ring 17, which is adapted to be urged
into sealing engagement with the exterior of a therapeutic
balloon dilation catheter 23 that is inserted into the
~ ' 6 2 1 832 ~ 4
guide catheter 10. Therapeutic balloon dilation catheter
23 has an expansible dilation balloon 24 on its distal
end. Catheter 23 is introduced into the guide catheter 10
along a guide wire 26 that has a proximal end secured to
a manually r-nipl~lAted and removable torque membér 27.
Initially the guide wire 26 and the guide catheter 10 are
inserted into the femoral artery, with the distal end of
the guide catheter stopping short of the location of the
stenosis to be treated. The guide wire, which extends
through the guide catheter, then is pushed distally beyond
the end of the guide catheter and is r~niplllAted by
rotation of torque 27 to enter the selected artery and to
pass through the stenosis or area to be treated. The
guide wire has a very flexible distal end portion 30 that
extends into the area to be treated. This distal end
portion 30 projects distally from the open end of the
guide catheter and carries a radiopaque marker 34 on its
tip, by which a physician can vi~ li7~ the guide wire
location and steer the guide wire to the LLea, -~t site.
20With the guide catheter and guide wire in place, the
therapeutic catheter 23 is then slid over the proximal end
of the guide wire (with torquer 27 removed) which projects
axially from the proximal end of the guide catheter lo.
The therapeutic catheter 23 is axially pushed into and
along the length of the interior of the guide catheter 10
until its dilation balloon 24 extends distally beyond the
end 18 of the guide catheter and into the desired
treatment location to which it is guided by guide wire 26.
The dilation balloon also includes a radiopaque marker or
band, as will be described more particularly below.
FIG. 2 shows the therapeutic catheter and guide wire,
both of which are inserted into and through the lumen of
the guide catheter interior. The guide catheter is not
shown in FIGS. 2-8. FIG. 2 shows the entire therapeutic
catheter. FIG. 3 shows the distal end of the therapeutic
7 2~83214
catheter. The catheter is formed of a distal body tube
section 40 made of, for example, a 70/30 polyethylene
tubing (employing 70% high density polyethylene and 30%
low density polyethylene) to provide a relatively more
flexible distal body tube section. To the proximal end of
the distal body tube section 40 is fused a proximal body
tube section 42, with the two being fused together at a
fuse area 44 (FIG. 3). The proximal body tube seotion 42
is stiffer, being made of a 90/10 polyethylene tubing (90%
lo high density polyethylene and 10% low density
polyethylene).
To the distal end of the distal body tube 40 is
bonded, as at 46, an expansible dilation balloon 48,
having a ~YpAnc;hle wall 50 and a distal end 52. A slit
60 is formed in the fused area 44 between the distal and
proximal body tubes. A flexible guide tube 62 extends
axially within the dilation balloon and has a distal end
64 bonded to and sealing the distal end 52 of the
inflation balloon. The guide tube 62 extends proximally
through the proximal end of the dilation balloon and
through the distal body tube 40 to the slit 60. This slit
defines a guide wire port in the wall of the fused area
between the distal and proximal tube sections. The
proximal open end of the guide tube is sealed to the guide
wire port 60.
When the therapeutic catheter is used, guide wire 26
(shown in FIGS. 1 and 2, but not in other figures) extends
along the exterior of the proximal body tube 42, through
the guide wire port 60, through the guide tube 62, and
through the distal open end 64 of the guide tube to
provide a guide wire distal section 30 (FIG. 1) that
extends outwardly beyond the distal end of the dilation
balloon. A radiopaque band 70 encircles an int~ -~iAte
area of the guide tube 62, within the balloon, and is
secured thereto by any suitable means, such as a sleeve
8 2 1 832 ~ 4
(not shown) that extends over a portion of the guide tube
60 and over the radiopaque band 70.
Mounted within the proximal body tube 42 and
extending from a point near the proximal end thereof
distally toward the fused area 44 is a hypotube 76 formed
of thin wall, hollow, stainless steel, relatively stiff
tubing. The hypotube has a distal end 78 (FIG. 3) that is
formed with a distal opening 80 and has a distal
stiffening section 84 fixed thereto. The distal end of
the hypotube is cut off at a slant, leaving a portion of
the end of the hypotube of a height of about 0.016 inches
at the distal tip and a distal opening 80 in its distal
end. A solid tapered stainless steel wire 84, having a
small distal end of about 0.003 inches and a larger
proximal end of about 0.016 inches, has its proximal end
positioned in and welded to the distal end 78 of the
hypotube (see FIGS. 4 and 5) to provide a tapered solid
catheter stiffening extension for the hypotube. Thus the
distal end portion of the hypotube includes both an
opening 80 communicating with the interior of the hypotube
(and the interior of the distal body tube section 40) and
a solid stiffening tapered wire section 84. The tapered
wire section 84 extends from the distal end of the
hypotube distally across the entire length of the fused
area 44 to a point distally of the fused area. As shown
in FIG. 5, the tapered solid wire 84 i5 seated in the
upwardly open distal end 78 of the hypotube and is welded
thereto. The tapered stiffening wire 84 extends
completely across and under the guide wire port 60, beyond
both sides thereof, and provides an increased rigidity to
the portion of the catheter in which the guide wire port
is formed. This m~n;mi7~s a tendency of the weakened (by
port 60) area of the catheter to kink as the therapeutic
catheter is moved in and out of the guide catheter along
21 832 1 4
the guide wire, as will be described more particularly
below.
As illustrated in FIG. 6, the proximal body tube 42
section extends proximally to a proximal connection
fixture 90, wherein the proximal end 92 of the proximal
body tube section 42 is securely bonded. The proximal
connection fixture, in some embodiments, includes an
angulated fitting (as shown in FIGS. 9 and 10). However,
the fixture of the ~mho~; r ~nt of FIGS. 2 through 8 has a
straight through configuration, as best seen in FIGS. 2
and 6. The fixture 90 of FIGS. 2 and 6 extends axially
for ronnPc~inn to a luer fitting 102, having a proximal
port 104 that opens axially to the proximal end of the
fixture.
Hypotube 76 ;nrlv~c a proximal end 106 that is
fixedly connected to the proximal body tube section 76 by
a hook 94 which extends radially outwardly from the
hypotube proximal end and is bent back around the end 92
of the proximal body tube section 76, as best seen in
2Q FIGS. 7 and 8. Hook 94 is formed as an integral part of
the proximal end of the hypotube and bent to its hook
shape. A short length of heat shrink tube 96 is bonded to
and around the proximal end of the body tube section 76
and the bent end of the hook is clamped between the heat
shrink tube and the body tube section.
The hypotube 76 and the proximal body tube section 42
divide the interior of the therapeutic catheter body tube
into inner and outer nested fluid flow passages 110,112
(FIG. 7). The inner passage is formed by the interior of
the hypotube, whereas the outer passage is an annular
passage formed between the exterior of the hypotube and
the interior of the proximal body tube section. These two
passages, in the arrangement of FIGS. 2-8, are in fluid
c~ ;c~tion with one another at both proximal and distal
ends of the hypotube, since the connecting hook 94 will
lo 218321~
allow fluid injected through the axial opening 104 to flow
into proximal ends of both the inner and outer passages
110, 112. A stiffening stylet wire 116 (FIGS. 2 and 3,
but not shown in FIGS. 6 and 7) has a proximal end fixedly
secured to an end cap 118 and extends through the axial
opening 104, through the fixture 90 and through the length
of the hypotube 76 to its distal end. The cap 118 is
detachably engaged with the luer fitting 102, and thus the
stiffening stylet may be removably inserted into the
fixture and into the interior of the hypotube. With the
stylet wire removed, the axial opening 104 may be used for
inflation and deflation. Application of inflation fluid
to the fixture 90 will cause fluid to flow through both
the inner and outer flow passages 110,112. Similarly,
1~ deflation may occur by flow of fluid through both the
inner and outer passages upon application of a negative
p~es~uLe to the axial opening 104.
In operation of the device, the guide catheter 10,
with a guide wire positioned therein, is inserted into the
femoral artery and pushed along the vascular pathway until
it reaches the position illustrated in FIG. 1 with the
distal end of the guide catheter positioned at the inner
end of the aortic arch. The guide catheter remains in
this position and the guide wire is then pushed further
into the heart and maneuvered until it enters the desired
artery. This maneuvering is assisted by the radiopaque
tip 34, which helps the physician steer the flexible tip
of the guide wire to the LLeai -~t site. Now the distal
end of the therapeutic catheter, via its distal opening
64, is inserted over the proximal end of the guide wire
(which is outside of the patient's body and has torque 27
removed) and pushed along the guide wire, within the guide
catheter, causing the guide wire to traverse the inner
guide tube 62 and the guide port as the distal end of the
therapeutic catheter and the expansible dilation balloon
~ 11 2183214
are pushed along the guide wire within the interior of the
guide catheter. At this time the PYpAnq;hle dilation
balloon, which is illustrated in FIGS. 1, 2 and 3 in
inflated condition, is in collapsed folded condition so as
to present an exterior size that is not significantly
larger than the exterior size of the distal body tube 40.
As the therapeutic catheter is pushed along the guide wire
inside of the guide catheter, its distal end emerges from
the distal opening end 18 of the guide catheter, and its
position can be effectively observed by the physician with
the help of the radiopaque band 70 on the guide tube 62.
The cql1arqP~ dilation balloon rnntinuPq to pass along the
guide wire until it enters the treatment area where its
motion is stopped. During this part of the procedure the
presence of the hypotube greatly PnhAnC~C the pllchAh; 1; ty
of the therapeutic catheter. Pushability may be further
PnhRnrPd by the insertion of stylet wire 116 into the
interior of the hypotube to extend to the fused area 44.
The fact that the stiffened proximal body tube section has
a distal end which is relatively close to the proximal end
of the dilation balloon further PnhAnreq trackability of
the apparatus, since the axial -~ essive force exerted
along the stiffened proximal body tube section is
transferred to the more flexible distal body tube section,
and thence to the dilation balloon, at a point that is not
far from the proximal end of the balloon.
At this point in the pLo~eduLe, the balloon will be
inflated. If a stylet has been inserted, this will be
removed. Inflation fluid is applied via opening 104 in
fixture 9o to effectively fill the interior of the
proximal connection fixture 90 and force fluid through
both inner and outer flow passages 110,112. The presence
of the thin wall hypotube blocks very little of the
~Loss-scctional area of the total flow passage within the
therapeutic catheter body tube, and inflation may occur at
2 1 832 1 4
12
a relatively rapid rate. So tcor deflation will occur at
a relatively rapid rate as a negative pressure is applied
to fixture 90.
If necessary, the entire therapeutic catheter may be
readily and rapidly, withdrawn after deflation of the
balloon, by pulling it proximally to cause the balloon and
guide tube to ride along the guide wire. If a larger or
different dilation balloon is required, a second
therapeutic catheter with an G~l UpL iate dilation balloon
then has its most distal end inserted over the free
proximal end of the guide wire and the second therapeutic
catheter is inserted just as is the first, with the prior
procedure then repeated. The tapered wire distal end
section 84 of the hypotube extends completely across the
fused area of the body tubes and across the guide wire
port to stiffen this weakened section and body tube and
minimize the potential for kinking of the therapeutic
catheter during insertion and withdrawal thereof.
FIG. 3 illustrates the proximal end of a modified
form of therapeutic catheter. In this embodiment the
distal end of the therapeutic catheter (not shown in FIG.
5) may be identical to the distal e~d of the catheter
illustrated in FIGS. 2 through 8. In the arrangement of
FIG. 9, however, the hypotube is made removable rather
than being fixed to and within the proximal connection
fixture. In FIG. 9 parts that correspond to similar parts
of FIG. 2 through 8 are designated by the same reference
numerals, having a prefix "2" added, so that, for example,
proximal body tube section 242 of FIG. 9 oorresponds to
proximal body tube section 42 of FIG. 2. The proximal
body tube section 242 has its proximal end secured to and
within a Y type proximal connection fixture 290, having an
angulated branch 291 and an axial brancn 293. Angulated
branch 291 communicates with the interior of the
connection fixture. The latter has secured to the
13 2~33214
proximal end of its axial branch a luer fitting 202 that
has a threadably engageable and detachable sealing cap
218. In this embodiment hypotube 276, which has a distal
end identical to the distal end of the hypotube 76 of FIG,
2, extends at its proximal end entirely through the
proximal connection fixture 290 and through the luer
fitting 202 to a fixed connection to detachable cap 218.
The proximal end portion 275 (within fixture 290~ of
hypotube 276, in this arrangement, is formed with a
plurality of apertures 277 that provide for fluid
communication between the interior of the proximal
connection fixture 290 and the interior of the hypotube.
Thus, these apertures 277 effectively provide fluid
communication between the inner and outer flow passages
210, 212 at the proximal end of the catheter. A stylet
216 secured to cap 218 extends through the hypotube to
provide further stiffening, if deemed nPcpc~ry or
desirable..
The arrangement of FIG. 9 is used substantially in
the same manner as is the embodiment of FIGS. 2 through 8.
With the hypotube in place and cap 218 secured to luer
fitting 202, inflation and deflation can be accomplished
rapidly in the same manner as it is accomplished with the
~ of FIGS. 2 through 8. Inflation fluid is
passed through the branch 291 into the outer annular
passage within the fixture and flows through holes 277 to
the inner passage 210. However, in the arrangement of
FIG. 9 the hypotube may be entirely removed and the luer
fitting 202 sealed by a second cap (not shown) so that an
even greater rate of inflation and deflation can be
accomplished, when the even very small amount of
cross-sectional obstruction of the hypotube and stylet are
removed. The arrangement of FIG. 9 has, in addition, all
of the advantages of the arrangement of FIGS. 2 through 8,
in~ ;ng ~nh~nrP~ pl~h~hility and trackability and faster
?1832~4
14
inflation and deflation time. With the hypotube and
stylet wire within the body tube, pn~hAh;l;ty is ~nhAn~P~
for insertion of the catheter, and rapid inflation and
deflation can be accomplished. After insertion, the
hypotube and stylet can be removed for even faster
inflation and deflation.
Illustrated in FIG. 10 is a modification of the
arrangement of FIGS. 2 through 8, configured to provide
for positive purging. FIG. 10 again illustrates only the
proximal portion of the therapeutic catheter, which has a
distal portion identical to the distal portion of the
catheter of FIGS. 2 through 8. Only the proximal end of
the catheter is changed. In the arrangement of FIG. 10
corresponding parts are designated by corr~sp~n~; ng
reference numerals with the prefix "4" added thereto, so
that, for example, proximal body tube section 42 of FIG.
5 corresponds to proximal body tube section 442 of FIG.
10. So, too, a hypotube 476 and radiopaque markers 477,
corresponding to hypotube 76 and markers 77, are mounted
in proximal body tube section 442. The hypotube,
positioned within the proximal body tube section 442, has
a distal end (not shown in FIG. 10) that is identical to
the hypotube distal end illustrated in FIGS. 2 through 5,
having the same relation to the other distal portions of
the therapeutic catheter as is illustrated in FIGS. 2
through 5. In the arrangement of FIG. 10 a proximal Y
connection fixture 490 is fixed to the proximal end of the
proximal body tube section 442 and is provided with an
angulated fitting branch 491 and an axial branch 493.
3Q Fixture 490 has a proximal end fixedly carrying an axial
luer fitting 402, having an interior bore 403. The
proximal end 406 of the hypotube 442 is fixed to and
sealed to a mounting collar 407 fixed in the proximal end
of the fixture 490. This seals inner passage 410 from
outer passage 412. The hypotube has an open proximal end
~t ~21 4
opening into the bore 403 of fitting 402 and thus is open
to the atmosphere via the fitting 402. In this
arrangement there are no holes in the proximal end of the
hypotube and the inner passage 410 of the therapeutic
catheter (as within the hypotube) is isolated and sealed
from the outer annular passage 412 at the proximal portion
of the instrument.
In this arrAn-, L pushability and tr~o~hil;ty are
~nh~nced just as in the arrangements previously described.
In fact, if deemed n~c~s~ry or desirable, a stiffening
stylet wire (not shown in FIG. 10) may be inserted into
the hypotube to further enhance pushability. Inflation
and deflation time may be increased by providing f4r
inflation fluid to be applied simultaneously through both
the inner and outer passages via fittings 491 and 402,
respectively. Similarly, deflation time is decreased by
applying a negative pressure to both of the fittings 491
and 402, respectively.
A significant advantage of the arrangement of FIG. 10
is the fact that there are provided in a simple, easily
manufactured therapeutic catheter structure, a pair of
nested parallel and mutually isolated, and mutually
sealed, fluid flow passages that may be used for positive
purging of air from the interior of the expansible
dilation balloon. Either of the inner or outer passages
410,412 may be used to flow fluid distally into the
catheter toward the balloon, while the other is used
simultaneously to extract fluid flowing out of the
catheter from the balloon. For example, axial fitting 402
may have a ~e~uLized purging fluid applied thereto to
flow axially through the hypotube to the dilation balloon.
Air or other fluid within the balloon will then flow
axially outwardly toward the proximal end of the
therapeutic catheter through the outer annular f low
passage 412 to flow outwardly through angulated fitting
~ 16 2183214
490. Thus, the arrangement of FIG. 10 makes available a
number of alternative procedures in a structure that is
easily manufactured and has a compact external
configuration. For example, the inner path may be used to
flow purging fluid into the catheter while purqed fluid is
flowed out through the angulated fitting 491.
Alternatively, inflation fluid may be caused to flow in
through one or both of the inner and outer passages to
enhance inflation and deflation time. In still a third
mode, a stiffener wire or stylet ~not shown in FIG. 10)
may be inserted into the hypotube to increase therapeutic
catheter stiffness during insertion of the catheter.
In all of the embodiments described herein additional
radiopaque marking bands may be applied to the hypotube
itself so as to better enable location of the hypotube
relative to the therapeutic catheter body tube.
As a typical but non-limiting example of a device
constructed according to FIGS. 2 through 8, proximal body
tube 42, formed of a 90/lo polyethylene, may have a wall
2Q th;~kn~c~ of .005 inches, an external diameter of .040
inches, and a length of 41.0 inches. Distal body tube 40,
formed of a softer 70/30 polyethylene ~ubing, may have a
wall thickness of .004 inches, an external diameter of
.040 inches, and a length of 4.0 inches. The ~p~n~;hle
inflation balloon has a length of l cm, with the guide
tube 62 therein having a wall thickness of .004 inches, an
outer diameter of .024 inches and a total length from its
distal opening 64 to its proximal end at the guide wire
opening 60 of 4.5 inches.
In a typical example the hypotube of FIGS. 2 through
8 may have an outer diameter of .023 inches, a wall
thickness of .002 inches and a total length to its distal
end (not ;nClud;ng the solid tapered stiffening wire 84
thereof) of 45.0 inches. The solid tapered stiffening
21 832 1 4
17
.
wire 84 may have a total length of 8.0 inches (not
including the hypotube).
There have been shown several arrangements of an
i ov~d balloon dilation catheter in which a hypotube is
positioned within the proximal body tube section, and
within part of the distal body tube section, and is
arranged to provide a number of advantages, including
~nh~nced pn~h~h;llty, ~nh~nced trackability, faster
inflation and deflation time, and, in one arrangement, a
positive purging.