Note: Descriptions are shown in the official language in which they were submitted.
218`4146
Le A 31290-Forei~ Countries / Bu / AB/S-P
- 1 -
New het~ .lically su~s'd~d ~
Ihe present invention relates to new heterocyclically sllksth~ pseudopeptides, aprocess for their ~ lion and their use as ~ iral agents.
Trifluoromethyl~., ~ il-g pseudopeptides are described as ~~ c~ iral agents in the
publication EP 528 242.
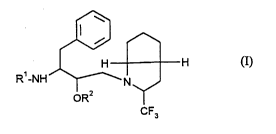
The present invention relates to new heterocyclically substituted pseudopeptides of the
general formula (I)
~W ^
H H (I)
R'-NH--~--N~
CF3
in which
R' represents a radical of the formula
R3-o ~NH 1 CO--
CH2 or R4--O CO-
0~ NH2
in which
R3 and R4 are identical or di~r~ and denote a heterocyclic radical of the
21~4146
- Le A 31290-Foreign Countries - 2 -
formula
L~, ~ or [~
R2 represents hydrogen or straight-chain or branched acyl having up to 20 carbonatoms, which is optionally substituted by carboxyl, or by straight-chain or
5branched alkoxycarbonyl having up to 6 carbon atoms,
and their salts.
The compounds of the general formula (I) according to the invention have a plurality
of asymmetric carbon atoms.
The representative radical of the general formula (Ia)
--co--NH~ H (Ia)
oR2 CF3
10 has 5 asymmetric carbon atoms ( ), which are present independently of one another in
the R- or S-configuration. The l(R), 2(R), 3(S), 4(S), 5(S) and 6(S) configurations are
preferred.
The amino acids employed in the compounds of the general formula (~) according to
the invention can be present either in the L- or in the ~form.
15 Physiologically acceptable salts of the heterocyclically substituted pseudopeptides
having a trifluoromethyl-substituted 2-azabicyclooctane can be salts of the substances
according to the invention with mineral acids, carboxylic acids or sulphonic acids.
21 841 46
- Le A 31290-Foreign Countries - 3 -
Particularly pl~r~ d salts are, for example, those with hydrochloric acid, hydrobromic
acid, sulphuric acid, phosphc)ric acid, m~th~n~lllph~nic acid, ethanesulphonic acid,
tol~ llrh- nic acid, b~n7~n~ rhonic acid, naphthalenedisulphonic acid, acetic acid,
propionic acid, lactic acid, tartaric acid, citric acid, fumaric acid, maleic acid or ben_oic
5 acid.
I~er~ d compounds of the general formula (I) according to the invention are those
in which
Rl represents a radical of the formula
R3-o ,NH ~ co
n ~' R4-o co-
o CH2 or
0~ NH2
in which
R3 and R4 are identical or different and denote a heterocyclic radical of the
formula
~, or ~,
R2 represents hydrogen or straight-chain or branched acyl having up to 6 carbon
atoms,
and their salts.
Particularly preferred compounds of the general formula (I) according to the invention
are those
21841`~6
~~ Le A 31290-Foreign Countries - 4 -
in which
R' represents a radical of the forrnula
R3-o ~rNH ~ co--
~ R4--O--CO-
o CH2 or
0~ NH2
in which
S R3 and R4 are identical or diff~l~.. l and denote a heterocyclic radical of the
forrnula
~ L~, or [~,
R2 represents hydrogen or straight-chain or branched acyl having up to 5 carbon
atoms,
10 and their salts.
Very particularly plefelled compounds of the general formula (I) according to the
invention are those
in which
R2 represents hydrogen or straight-chain or branched acyl having up to 4 carbon
15 atoms.
A process for the ~rep~ion of the compounds of the general formula (I) according to
the invention has additionally been found, characterized in that
21~4146
Le A 3129~Foreign Countries - 5 -
compounds of the formula (II) or (III)
~ ~\
H H
H2N,~----N~/ (Il)
OH CF3
H7NCO--NH ~ N~/ (111)
~' OH CF3
NH2
are reacted ~,vith compounds of the general formula (IV)
R'-OH (~V)
in which
5 R' has the meaning indicated above,
if appropriate with prior activation of the carboxylic acid~ in inert solvents and in the
presence of a base and/or of an auxiliary, and in a second step if R2 ~ H an acylation
is carried out in inert solvents, if ap~lol,liate in the presence of a base and/or of an
auxiliary.
10 One process according to the invention is illustrated by way of example by the
following reaction scheme:
Suitable solveIlts for all process steps are the customary inert solvents which do not
change under the reaction conditions. These preferably include organic solvent~s such
as ethers, e.g. diethyl ether, glycol mono- or dimethyl ether, dioxane or tetrahydrofuran,
23189-7981
21~4146
- Le A 3129~Foreign Countries - 6 -
o ~ H ~ H
+ H2N CO--NH--~N
O-CO2H ~- OH CF,
NH2
O--CO--NHyCO--NH~--'N~lH
OH
o _ CF3
NH2
or hydrocarbons such as bPn7PnP, toluene, xylene, cyclohexane or petroleum fractions
or halogenohydrocarbons such as methylene chloride, chloroform, carbon tetrachloride,
or dimethyl sulphoxide, dimethylforrnamide, hexamethylphosphoramide, ethyl acetate,
pyridine, triethylamine or picoline. It is also possible to use mixtures of the solvents
S mentioned. Dichloro",~lh~nP dimethylro~ ~ide or tetrahydrofuran are particularly
~l~r~ d.
Depending on the individual process step, suitable bases are the customary inorganic
or organic bases. These preferably include alkali metal hydroxides such as sodium or
potassium hydroxide, or alkali metal carbonates such as sodium or potassium carbonate,
10 or alkali metal alkoxides such as sodium or potassium methoxide, or sodium orpotassium ethoxide, or organic amines such as ethyldiisopropylamine, triethylamine,
picoline, pyridines, dimethylaminopyridine or N-methylpiperidine, or amides such as
sodium amide or lithium diisopropylamide, or lithium N-silylalkylamides, such aslithium N-(bis)triphenylsilylamide, or lithium alkyls such as n-butyllithium.
15 The base is employed in an amount from 1 mol to 10 mol, preferably from 1 mol to
3 mol, relative to 1 mol of the compounds of the formula (II) or (III).
2184146
- Le A 31290-Foreigrl Countries - 7 -
Suitable auxiliaries are ~ rel~bly con~n~inE agents, which can also be bases, inparticular if the carboxyl group is present in activated form as an anhydride. Ihe
customary con(~ ing agents are pler~lled here, such as carbodiimides, e.g.
N,N'-diethyl-, N,N'-diisopropyl- or N,N'-dicyclohexylcarbodiimide,
5 N{3-diethylaminoisopropyl~N'-ethyl-carbodiimide hydrochloride, N-cyclohexyl-
N'-(2-morpholinoethyl)-carbodiimide metho-p-toluenesulphonate (CMCT or
morpho-CDI) or carbonyl compounds such as carbonyldiimidazole, or 1,2-oxazolium
compounds such as 2-ethyl-5-phenyl-1,2-oxazolium-3-sulphate or 2-tert-butyl-5-methyl-
oxazolium perchlorate, or acylamino compounds such as 2-ethoxy-1-ethoxyc~ul~llyl-
10 1,2-dihydroquinoline, or prop~n~hosI h-)nic anhydride, or isobutyl chloroformate, or
benzotriazolyloxy-tri(dimethylamino)phosphonium hexafluorophosphate,
l-hydroxyl~ iazole or dimethylaminopyridine.
The reactions can be carried out either at ~trnn~ph~ic pressure or at elevated or
reduced pressure (for example 0.5 to 5 bar), plcr~l~ly at atmospheric pressure.
15 The reaction is in general carried out in a temperature range from -20C to +40C,
pl~r~lably from 0C to room t~nl~l~ re.
The acylation is in general carried out in one of the abovementioned ethers or
halogenohydrocarbons, pl~relably tetrahydrofuran or methylene chloride, in a
temperature range from -30C to +50C, pl~rel~bly from -10C to room temperature.
20 Suitable bases and/or auxiliaries for the acylation are in general the abovementioned
organic amines and pyridines. Triethylamine and dimethylaminopyridine are pl~r~lled.
The base is employed in an amount of from 1 mol to 10 mol, preferably from 1 molto 3 mol, relative to 1 mol of the respective alcohol.
The compound of the general formula (II) is known.
25 The compound of the forrnula (III) is new and can be prepared, for exarnple, by
converting
21 841 46
`~ Le A 3129~Forei~ Countries - 8 -
compounds of the general formula (V)
C6Hs
W-NH CO--NH
Oq~
NH2
in which
W represents an amino protective group, pl~rt.~ly tert-butoxycarbonyl,
fLrst using an epoxidation reaction, if a~lo~.iate with the aid of a base or of a phase
S lLd~rer catalyst, into the compounds of the general formula (VI)
,~
W-NH CO--NH~7 (VI)
Oq~
NH2 ,.
in which
W has the abovementioned mP~ning,
and then reacting with 3-trifluoromethyl-2-azabicyclo[3.3.0]octane of the formula (VII)
/\
H H
(VII)
HN
CF3
21~4146
- Le A 31290-Forei~n Countries - 9 -
in solvents and removing the protective group by customary methods.
Suitable solvents are the Cl~O~ organic solvents which do not change under the
reaction conditions. Ihese ~ r~l~ly include organic solvents such æ alcohols, e.g
m~th~nol, ethanol or n-propanol, ethers, e.g diethyl ether, glycol mono- or dimethyl
S ether, dioxane or tetrahydrofuran, or hydrocarbons such æ benzene, toluene, xylene,
cyclohexane or petroleum fractions or halogenohydrocarbons such æ methylene
chloride, dichloroethane (DCE), chloroform, carbon tetrachloride, or dimethyl
sulphoxide, dimethylro",~ ide, hexarnethylIhosphoramide, ethyl acetate, pyridine,
triethylamine or picoline. It is also possible to use mixtures of the solvents mentioned.
10 Dichlorom~qth~n~ dichloroethane, dimethylr~n,l~,de or n-propanol are particularly
r~ d.
Suitable reagents for the epoxidation are the compounds known from the literature, for
examplem-chloroperbenzoicacid,m~gn~sillrnmonoperoxyrhth~l~te dimethyldioxirane
or methyl(trifluoromethyl)dioxirane. m-Chloroperbenzoic acid and m~g,n~sium
monoperoxyphth~l~te are pl~relled (cf. P. Br n~h~m et al., Synthesis (1987), 1015; W.
Adam et al., J. Org. Chem. ~, 2800 (1987) and R Curci et al., J. Org. Chem. 53, 3890
(1988)].
If the epoxidation is carried out with the aid of a phæe transfer catalyst, the auxiliaries
employed are, for example, organic ~mmonium chlorides or bromides, for example
20 benzyltriethylammonium chloride or bromide, methyltrioctylammonium chloride,
tetrabutylammonium bromide or tricaprylmethyl~mmcnium chloride (Aliquat 336).
Benzyltriethylammonium chloride and bromide are plefel,ed.
The epoxidation is carried out in a temperature range from -10C to +90C, preferably
from 0C to +60C.
25 Ihe reactions can be carried out either at atmospheric pressure or at elevated or
reduced pressure (for example 0.5 to 5 bar), pl~r~l~ly at atmospheric pressure.
The compounds of the general formula (IV) are known per se or can be prepared by
21~4146
Le A 31290-Foreign Countries - 10 -
customary methods.
The compounds of the general formulae (V) and ~Vl) are in the main known
[cf. EP 528 242].
The compound of the general forrnula (VII) is new and can be prepared, for example,
5 by fluorination of 2-azabicyclo[3.3.0]octane-3-carboxylic acid in the respective
configuration with ~ and SF4.
It has surprisingly been found that the compounds of the general formula (I) have an
extremely strong action against retroviruses. This is co~ A using an H[V-specific
protease enzyme test.
10 The results of the exarnples mentioned below were determinPA according to the HIV
test system described in the following reference [cf. Hansen, J., Billich, S., Schulze, T.,
Sukrow, S. and Molling, K (1988), EMBO Journal, Vol. 7, No. 6, pp. 1785 - 1791]:purified HIV protease was inc~lb~ted with synthetic peptide which imit~tçs a cleavage
site in the gag precursor protein and is an in vivo cleavage site of ~V protease. The
15 resulting cleavage products of the synthetic peptide were analyzed by means of
reversed phase high p~lrolll~lce liquid cLI~nl~ography (RP-HPLC). The ICso values
indicated relate to the substance concentration which, under the abovementioned test
conditions, causes a 50 % inhibition of the protease activity.
2 1 84 1 46
~ Le A 31290-Foreif~n Countries - 11 -
Lnzyme assay, HrV-1 -
Table I
No. ICsOImol/ll IC;,5[mol/l]
3.0 x 10-8 3.8 x 10-7
2 2.7 x 10-l 3.6 x 10-9
3 9.5 x 10-9 n.t.
4 2.1 x 10-9 3.9 x 10-9
4.1 x 10-9 4.7 x 10-8
2.5 x 10-8 5.0 x 10-8
7 2.5 x 10-8 n.t.
8 3.2 x 10-9 4.1 x 10-8
Ihe compounds according to the invention additionally showed action in lentivirus-
infected cell cultures. It was possible to show this using the HIV virus as an
example.
HIV infection in cell culture
The HlV test was carried out with slight modifications according to the method of
Pauwels et al. [cf. Journal of Virological Methods 20, (1988), 309-321].
Normal human blood Iymphocytes (PBLs) were concentrated by means of Ficoll-
Hypaque and stim~ te(l with phytoh~tom~lutinin (90 ~g/ml) and interleukin-2
(40 U/ml) in RPMl 1640, 20 % foetal calf serum. For infection with the infectious
HIV, PBLs were pelleted and the cell pellet was then suspended in 1 ml of HlV virus
adsorption solution and incub~t~l at 37C for 1 hour.
2184146
Le A 31290-Foreign Countries - 12 -
The virus adsorption solution was centrifuged and the infected cell pellet taken up in
growth mediurn so that a concentration of 1 x 105 cells per ml wæ established. Ihe
cells infected in this way were pipetted at 1 x 104 cells/well into the wells of 9~well
microtitre plates.
S The first vertical row of the microtitre plate c nt~in~l only growth medium and cells
which had not been infected, but otherwise had been treated exactly as described above
(cell control). The second vertical row of the microtitre plates cont~in~ only H~V-
infected cells (virus control) in growth mediu~ The other wells contained the
compounds according to the invention at di~ concentrations, starting from the
10 wells of the 3rd vertical row of the microtiter plate, from which the test sl1bst~nces
were diluted 21 tirnes in steps of 2.
The test batches were inalb~tecl at 37C until in the untreated virus control the
syncytia formation typical of HlV occurred (between day 3 and 6 after infection),
which was then ~se~se~l microscopically. In the untreated virus control about 2015 syncytia resulted under these test conditions, while the untreated cell control showed
no syncytia
The IC50 values were (let~nnined as the co~ct;~ lion of the treated and infected cells
at which 50 % (about 10 syncytia) of the virus-in~-cecl syncytia were suppressed by
the tre~tm~nt with the compound according to the invention.
20 It has now been found that the compounds according to the invention protect
H~V-infected cells from virus-in~hlc~d cell destruction.
- 2184146
Le A 31290-Foreign Countries - 13 -
Cell culture assay, PBL
Table II:
PBL
~le No. ICsO[mol/l] I~,5[moUIl
2.2 x 10 3.5 x 10
2 2.0 x 10 3.5 x 10
3 1.2xlO~ l.9xlO~
4 >1.6 x 10-5 n.t.
1.4 x 10-5 2.2 x 10-5
6 2.8 x 10 1.3 x 10
7 6.2 x 10~ n.t.
8 1.7 x 10-5 >2 x 10-5
The compounds according to the invention are useful active compounds in human
and veterinary medicine for the tre~tm~nt and prophylaxis of disorders caused byretroviruses.
Indication areas in human medicine which can be mentioned, for example, are:
1.) The tre~tm~nt and prophylaxis of human retrovirus infections.
2.) The tre~1m~nt or prophylaxis of disorders (AIDS) caused by HIV I (hurnan
immlm(ldeficiency virus; formerly called HTLV III/LAV) and HIV II and the
stages associated therewith such as ARC (AIDS-related complex) and LAS
(Iymphadenophathy syndrome) and also the immllnodeficiency and
encephalopathy caused by this virus.
218ql46
- Le A 31290-Foreign Countries - 14 -
3.) The tre~tm~nS or the prophylaxis of an HILV-I or HTLV-II infection.
4.) Ihe tre~tm~nt or the prophylaxis of the AlDS-carrier state (AlDS-ll~ls~ ler
state).
Indications in v~ ~y medicine which can be mentioned are, for example:
5 Infections with
a) Maedi-visna (in sheep and goats)
b) progressive pnYlmoni~ virus (PPV) (in sheep and goats)
c) caprine arthritis ~n~h~liti~ virus (in sheep and goats)
d) zwoegersiete virus (in sheep)
10 e) infectious ~n~mi~ virus (equine)
f) infections caused by the feline le~ virus
g) infections caused by the feline irnmunodeficiency virus (FIV)
h) infections caused by the sirnian immlmodeficiency virus (SIV)
From the indication area in human medicine, the abovementioned iterns 2, 3 and 4 are
15 ~ r~ d.
The present invention includes ph~ ,eutical preparations which, in addition to
non-toxic, inert pharm~ ically suitable excipients, contain one or more compounds
of the formula (I) or which consist of one or more active compounds of the formula
(I), and processes for the production of these p~ lions.
- 2184146
Le A 31290-Foreign Countries - 15 -
Ihe active compounds of the formula (I) should bc present in the abovementioned
ph~ re~ltical plc~al~ions in a concentration of al)ploxill~ely 0.1 to 99.5 % by
weight, plcrc~bly of approximately 0.5 to 95 % by weight, of the total rnixture.
In addition to the compounds of the formula (I), the abovementioned ph~rm~ce~tical
S ~lc~lions can also contain other ph~rrn~r~ltic~l active compounds.
Ihe abovementioned ph~rm~celltical plc~lions are prepared in a Cl~ilOn~y manner
by known methods, e.g. by mixing the active compound or compounds with the
excipient or excipients.
In general, it has proved advantageous both in human and in ~/cl~il~y medicine to
10 a~rnini~t~r the active compound or compounds in total amounts of approximately 0.5
to ~uloxi~ cly 500, ~lcrcl~bly 1 to 100, m~kg of body weight every 24 hours, if
a~r~liate in the form of a plurality of individual doses, to achieve the desired results.
An individual dose c~nt~in~ the active compound or compounds, plcrcl~ly in amounts
of approximately 1 to approximately 80, in particular 1 to 30, mg/kg of body weight.
15 However, it may be n~ss~ry to depart from the doses mentioned, narnely depending
on the nature and the body weight of the subject to be treated, the type and the severity
of the disorder, the manner of ~lc~ion and ~(lmini~tration of the medir~m~nt andthe time or interval within which ~mini~tration takes place.
The compounds according to the invention are enzyme inhibitors and can be employed
20 as such for all purposes for which enzyme inhibitors can be used. An example to be
mentioned here is use as a label for affinity cl~ro~ ography, use as an aid for the
elucidation of enzyme structures and reaction m~l~ni~m~, and use as a reagent for
diagnostics.
21 ~41 46
Le A 31290-Foreign Countries - 16 -
F~mDle 1
(2S,3S)-3-(Tetrahydrofuran-3-yl-oxycarbonyl-L-asparaginyl)amino- 1 - {(S,S,S)-
3-trifluoromethyl-2-azabicyclo[3.3.0]octan-2yl} -2-hydroxy~phenylbutane
o ~ ~
C~ 1 H ~ H
O--CO--NH ,CO--NH ~N
- OH
CF3
NH2
0.1 g (0.22 mmol) of (2R,3S~3-(L-asparaginyl)amino-1-~(S,S,S)-3-trifluoromethyl-2-
azabicyclo[3.3.0]octan-2yl}-2-hydroxy~phenylbutane and 0.11 g (0.44 mmol) of
tetrahydrofuran-3-yl 4-lliL~ enyl~l~l.~l~ (analogously to Daniel F. Veber et al., J.
Org. Chem., Vol. 42, No. 20, 1977, pp. 3286-8) are dissolved in 3 ml of THF and
stirred overnight at RT under an argon ~tmosph~e. The mixture is then evaporated to
dryness, and the residue is separated by column cllroll~lography (silica gel 60,dichlorom~th~n~lm~th~n-)l = 100:5, Rf = 0.4).
Yield: 120 mg (96.0 % of theory) of colourless foam
The compounds listed in Table 1 are prepared in analogy to the procedure of Example
1:
- 2184146
- Le A 31290-Foreign Countries - 17 -
Table 1:
~W /\
H 1.~ H
R3--O ~=CO--NH~
NH2
E~L No. ~ R, /m~ (~) Yield (% of
~lY)
2 o Dichloro~ e/MeOH 89.6
= 100:5
0.4 (colourless foam)
3 so2 Dichlolu~ h~nelMeOH 92.3
4 =100:5
0.2 (colourless foam)
4 so2 Dichlorom~th~n~MeOH 74.0
(~ = 100:7
0.5 (colourless foam)
h~ -npe 5
(2R,3S)-3-(Tetrahydrofuran-3-yl-oxycarbonyl)-amino-1-{(S,S,S)-3-trifluoromethyl-2-a~abicyclo[3.3 Ø]octan-2-yl}-2-hydroxy~phenylbutane
2184146
--Le A 31290-Foreign Countries - 18 -
o
,~ H ~ H
--0--CO--NH ~Ny
OH CF3
0.1 g (0.29 mmol) of (2R,3S)-3-amino-1-{(S,S,S)-3-trifluoromethyl-
2-azabicyclo[3.3Ø]octan-2-yl}-2-hydroxy~phenylbutane and 0.11 g (0.44 mmol) oftetrahydrofuran-3-yl 4-nitrophenylca~ (analogously to Daniel F. Veber et al., J.Org. Chem., Vol. 42, No. 20, pp. 328~8) are dissolved in 3 ml of abs. THF and stirred
S overnight at RT under an argon atmosphere. The mi~ure is then evaporated to dryness
and the residue is separated by column cl~o,l~lography (silica gel 60;
dichlorometll~n~lethyl acetate = 4:1).
Rf = 0.5 (dichloromethane/ethyl acetate = 1:1)
Yleld: 50 mg (37.5 % of theory) of colourless foam
10 The compounds listed in Table 2 are prepared in analogy to the procedure of
Example 5.
2184146
Le A 31290-Foreign Countries - 19 -
Table 2: -
~^
H ' ~ H
R4 0--CO--NH~ N~
HO
CF3
E~. No. 1~ Rr /m~ (C) Yield (% of
~eoly)
6 0 0.4 (Dichloro- 75.0
C~ methane/ethyl acetate =
9:1)
colourless cIystals
125C
7 so2 0.5 (Dichloro- 67.8
4 methane/ethyl acetate =
4 1)
colourless foam
8 so2 0.5 (Dichloro- 95.4
methane/ethyl acetate =
4 1)
colourless glass
~le 9
(2S,3S)-3-(Tetrahydrofuran-3-yl-oxycarbonyl-L-asparaginyl)amino-1-~(S,S,S)-
3-trifluoromethyl-2-azabicyclo[3 .3 .O]octan-2-yl-2-acetoxy~phenylbutane
- 2184146
~~ Le A 3129~Forei~ Countries - 20 -
O--CO--NHycO--NH~N~?' H
0~ CO CF3
NH2 CH3
50 mg (0.0876 mmol) of the compound of Example 1, 25 mg (0.035 ml) of
triethylamine and 13 mg (0.13 mmol) of acetic anhydride are dissolved in 2 ml of dry
tetrahydrofuran (THF) under an argon ~trnosph~re~ treated with a spatula tipful of
dimethylaminopyridine (DMAP) and stirred overnight.
S The mixture is evaporated to dryness in vacuo and stirred with 5 ml of water and
15 ml of ethyl acetate. Ihe organic phase is separated off, dried wi~ sodium slllph~te
and separated by column cl~ro~ ography (silica gel 60, eluent: dichlorometll~n~lethyl
acetate = 4:1).
Colourless crystals, m.p. = 191C; Rf = 0.4 (dichloromethane/ethyl acetate = 4:1),
yield: 35 mg (65.2 % of theory).
~le 10
(2S,3S}3-[Tetrahydrofuran~3S}3-yl-oxycarbonyl]-amino-1-{(S,S,S}3-trifluoromethyl-
2-azabicyclo-[3.3.0]octan-2-yl}-2-isobutyroxy-4-phenylbut~ne
O-CO-NH'f~,
In analogy to the procedure of Example 9, the title compound is prepared from 50 mg
(0.11 mmol) of the compound from Example 6, 20 mg (0.027 ml/0.2 mmol) of
` 2184146
Le A 31290-Foreign Countries - 21 -
triethylamine and 15 mg (0.14 mmol) of butyric anhydride using a spatula tipful of
dimethylaminopyridine.
Colourless foam; Rf = 0.6 (dichloromethane/e~yl acetate 4:1)
Yield: 40 mg (69.3 % of theory)