Note: Descriptions are shown in the official language in which they were submitted.
WO 95/24207 PCT/US95/02938
218438~
- TITLE OF THE INVhNTION
COMPOSITION FOR THE TREATMENT OF LUNG DISEASE
BACKGROUND OF THE INVENTION
This invention is directed to ph~rm~ceutical compositions
comprising an elastase inhibitor and an (F)-actin shortening protein such
as gelsolin or vitamin D binding protein (DBP), for use in the treatment
of Cystic Fibrosis and related diseases. In particular, this invention is
directed to ph~rm~ceutical compositions comprising elastase inhibitors
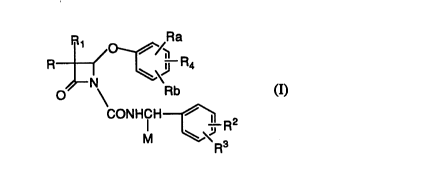
Of Formula I
Ra
R1 o~/~
O CONHCH ~--R2
M R3
and (F)-actin shortening proteins, Gelsolin being preferred.
We have found that a group of new substituted azetidinones
are potent elastase inhibitors and therefore are useful anti-infl~mm~tory
and antidegenerative agents.
Proteases from granulocytes and macrophages have been
reported to be responsible for the chronic tissue destruction mech~ni~m~
25 associated with infl~mm~tion, including rheumatoid al~llilis and
emphysema. Accordingly, specific and selective inhibitors of these
proteases are candidates for potent anti-infl~mm~tory agents useful in
the treatment of infl~mm~tory conditions resulting in connective tissue
destruction, e.g., rheumatoid arthritis, emphysema, bronchial
30 infl~mm~tion, chronic bronchitis, glomerulonephritis, osteoallhlilis,
spondylitis, lupus, psoriasis, atherosclerosis, sepsis, septicemia, shock,
myocardial infarction, re~elrusion injury, periodontitis, cystic fibrosis
and acute respiratory distress syndrome.
W O 95/24207 PC~r~US95/02938
21843~5
The role of proteases from granulocytes, leukocytes or
macrophages are related to a rapid series of events which occurs during
the progression of an infl~mm~tory condition:-
(1) There is a rapid production of prostaglandins (PG)
and related compounds synthesized from arachidonic acid. This PG
synthesis has been shown to be inhibited by aspirin-related nonsteroidal
anti-i~fl~mm~tory agents including indomethacin and phenylbutazone.
There is some evidence that protease inhibitors prevent PG production;
(2) There is also a change in vascular permeability which
causes a leakage of fluid into the infl~med site and the resulting edema is
generally used as a marker for measuring the degree of infl~mm~tion.
This process has been found to be induced by the proteolytic or peptide
cleaving activity of proteases, especially those contained in the
granulocyte, and thereby can be inhibited by various synthetic protease
inhibitors, for example, N-acyl benzisothiazolones and the respective
1,1-dioxides. Morris Zimmerman et al., J. Biol. Chem., 255, 9848
(1980); and
(3) There is an appearance and/or presence of lymphoid
cells, especially macrophages and polymorphonuclear leukocytes
(PMN). It has been known that a variety of proteases are released from
the macrophages and PMN, further indicating that the proteases do play
an important role in infl~mm~tion.
In general, proteases are an important family of enzymes
within the peptide bond cleaving enzymes whose members are essential
to a variety of normal biological activities, such as digestion, formation
and dissolution of blood clots, the formation of active forms of
hormones, the immune reaction to foreign cells and org~ni~m~, etc., and
in pathological conditions such as the degradation of structural proteins
30 at the articular cartilage/pannus junction in rheumatoid arthritis etc.
Elastase is one of the proteases. It is an enzyme capable of
hydrolyzing the connective tissue component elastin, a property not
contained by the bulk of the proteases present in m~mm~ . It acts on a
protein's nonterminal bonds which are adjacent to an aliphatic amino
acid. Neutrophil elastase is of particular interest because it has the
WO 95/24207 PCT/US95/02938
- 2184385
broadest spectrum of activity against natural connective tissue
substrates. In particular, the elastase of the granulocyte is important
because, as described above, granulocytes participate in acute
infl~mm~tion and in acute exacerbation of chronic forms of
infl~mm~tion which characterize many clinically important
infl~mm~tory diseases.
Proteases may be inactivated by inhibitors which block the
active site of the enzyme by binding tightly thereto. Naturally
occurring protease inhibitors form part of the control or defense
mech~ni~m~ that are crucial to the well-being of an org~ni~m. Without
these control mech~ni~m~, the proteases would destroy any protein
within reach. The naturally occurring enzyme inhibitors have been
shown to have a~lo~riate configurations which allow them to bind
tightly to the enzyme. This configuration is part of the reason that
inhibitors bind to the enzyme so tightly (see Stroud, "A Family of
Protein-Cutting Proteins" Sci. Am., July 1974, pp. 74-88). For
example, one of the natural inhibitors, a1-Antitrypsin, is a glycoprotein
contained in hllm~n serum that has a wide inhibitory spectrum covering,
among other enzymes, elastase both from the pancreas and the PMN.
This inhibitor is hydrolyzed by the proteases to form a stable acyl
enzyme in which the active site is no longer available. Marked
reduction in serum al-antitrypsin, either genetic or due to oxidants, has
been associated with pulmonary emphysema which is a disease
characterized by a progressive loss of lung elasticity and resulting
respiratory difficulty. It has been reported that this loss of lung
elasticity is caused by the progressive, uncontrolled proteolysis or
destruction of the structure of lung tissue by proteases such as elastase
released from leukocytes. J. C. Powers, TIBS, 21 l (1976).
Rheumatoid arthritis is characterized by a progressive
destruction of articular cartilage both on the free surface bordering the
joint space and at the erosion front built up by synovial tissue toward
the cartilage. This destruction process, in turn, is attributed to the
protein-cutting enzyme elastase which is a neutral protease present in
WO 95/24207 PCT/US95/02938
~21 84385
human granulocytes. This conclusion has been supported by the
following observations:
(1) Recent histochemical investigations showed the
5 accumulation of granulocytes at the cartilage/pannus junction in
rheumatoid arthritis; and
(2) a recent investigation of mechanical behavior of
cartilage in response to attack by purified elastase demonstrated the
direct participation of granulocyte enzymes, especially elastase, in
0 rheumatoid cartilage destruction. H. Menninger et al., in Biological
Functions of Proteinases, H. Holzer and H. Tschesche, eds. Springer-
Verlag, Berlin, Heidelberg, New York, pp. 196-206, 1979.
(F)-actin shortening proteins including gelsolin, plasma
gelsolin (brevin), vitamin D binding protein (DBP), villin, fr~gmin, and
15 severin, and their use in shortening (F)-actin and treating (F)-actin
mediated diseases is described in U.S. 5,260,224, issued to Stossel et al.,
on November 9, 1993, which patent is hereby incorporated by
reference.
As discussed therein, Gelsolin has been cloned
20 (Kwiatkowski, D. J. et al., Nature, 323:455-458 (1986); Kwiatkowski,
D. J. et al., J. Cell Biol., 106:375-384 (1988)) and fragments of the
native protein which retain the ability to bind actin have been identified
(Bryan, J., J. Cell Biol., 106:1553-1562 (1988); Yin, H. L. et al., J. Cell
Biol., 107:465a (1988), abst. no. 2616); Kwiatkowski, D. J. et al., J.
25 Cell Biol., 108:1717-1726 (1989); Way, M. et al., J. Cell Biol.,
109:593-605 (1989)).
Gelsolin's primary function in the plasma is to sever actin
filaments. If gelsolin is present in excess of actin, only gelsolin-actin
complexes result; if actin is in excess, there are free actin oligomers and
30 gelsolin-actin complexes. The actin severing occurs by way of a non-
proteolytic cleavage of the noncovalent bond between adjacent actin
molecules.
Efficacious levels of actin-binding compounds may be
7~11mini~tered so as to provide therapeutic benefits against the secondary
toxic effects of excessive extracellular actin. By "efficacious levels" of
WO 95/24207 PCT/US95/02938
- 21843~S
actin-binding compounds is meant levels in which the toxic effects of
free extracellular actin are, at a ...i.lil.l-llll, ameliorated. By "excessive"
extracellular actin is meant an amount of extracellular actin which
exceeds the ability of the plasma l~roteills to bind and clear the actin
from extracellular fluids without secondary tissue (l~m~ge or toxic
effects. By "secondary" tissue ~l~m~ge or toxic effects is meant the
tissue damage or toxic effects which occur to otherwise healthy tissues,
organs, and the cells therein, due to the presence of excessive
extracellular actin in the plasma, usually as a result of a "primary"
tissue injury elsewhere in the body.
Stossel et al., recently presented a paper and abstract
entitled Filamentous (F)-Actin Is Abundant In Cf Sputum, And F-Actin-
Shortening Proteins Dimini.~h Sputum Viscosity In Vitro. Stossel et al.,
found actin in CF s~ululll by i~ oblotting, and CF s~u~um samples
accelerated the nucleation of monumeric actin, consistent with the
presence of F-actin. Stossel et al., speculated that F-actin therefore
might contribute importantly to the mechanics of CF ~ululn. In a test
model F-actin severing proteins in~ eously and substoichio-
metrically shorten actin filaments, and nM concentrations of one such
protein, hllm~n plasma gelsolin, rapidly reduced the viscosity of CF
sputum by up to 70% in a concentration-dependent manner. Gelsolin
also ~limini~hed the elastic modulus of CF sputum by 50% in shear
deformation over a frequency range of 0.001-10 Hz. Over ten times
more bovine pancreatic DNAse I (which binds actin subunits in addition
to its DNA hydrolyzing activity) was required to ~limini~h CF S~UlUlll
viscosity by the same extent as gelsolin.
More recently, Vasconcellos et al., have reported that
concentrations of 100 to 500 nM, gelsolin, purified from human plasma,
rapidly tlimini~hed the viscosity of spulul-- form cystic fibrosis patients
an average of 64% from 332~199 Pa-s over a 60 mimlte incubation
period. On the other hand, at a concentration of 250 nM, bovine
pancreatic DNase I and Gc golbulin had no effect on viscosity in this
time period, however, they did appear to enhance gelsolin activity. See
Science, Vol. 263, pp 969-971 (February 18, 1994).
WO 95/24207 PCTIUS95102938
21 84385
BRIEF DESCRIPTION OF THE INVENTION
This invention relates to ph~rm~ceutical compositions and
methods for the treatment of lung disease, and in particular Cystic
Fibrosis comprising the a non-toxic effective amount of an elastase
inhibitor such as the compounds of Formula (I),
Ra
R O~ ~ R
R~ ~ 4
~N Rb
O CONHCH ~ R2
~\~
R3
a non-toxic effective amount of a (F)-actin shortening protein, such as
5 gelsolin, and a pharmaceutically acceptable carrier.
DETAILED DESCRIPTION OF THE INVENTION
This invention relates to ph~m~.eutical compositions and
methods for the treatment of lung disease, and in particular Cystic
20 Fibrosis comprising the a non-toxic effective amount of an elastase
inhibitor such as the compounds of Formula (I),
Ra
1 o~/
CONHCH ~~ R2
R3
3 a non-toxic effective amount of a (F)-actin shortening protein, such as
gelsolin, and a pharmaceutically acceptable carrier.
With regard to the compounds of Formula I, said compounds
being further detailed in the Schemes, Fx~mples and Claims, the instant
invention is more particularly directed to the compounds of the
WO 95/24207 PCT/US95/02938
218438~
Ra
R~ ~\
~N Rb
CONHCH ~ R2
~J
o and pharmaceutically acceptable salts thereof wherein:
R is C1 6alkyl;
Rl is C1 6alkyl or C1 6alkoxy-C1 6alkyl;
Mis
(1) hydrogen,
(2) Cl 6alkyl,
(3) hydroxy C1 6alkyl,
(4) halo C1 6alkyl,
(S) C2 6alkenyl, or
(6) C1 -6alkoxy-cl -6alkYl;
Ra and Rb are each individually
(1) hydrogen,
(2) C1 6alkyl,
(3) halo,
(4) carboxy,
(S) C1 6alkoxy,
(6) phenyl,
(7) C1 6alkylcarbonyl,
(8) di-(C1 6alkyl)amino,
(9) hydroxy;
3 0 R2 and R3 are each independently
(1) hydrogen,
(2) C1 6alkyl,
(3) halo,
(4) carboxy,
(5) C1 6alkoxy,
WO 95124207 PCT/US95/02938
21 84385
(6) phenyl,
(7) C1 6alkylc~1,v.lyl,
(g) ~oC2 3alkyIoxy carbonyl wherein the
am~no is optd~nally mono or di sub~ti~te~
~1-6slkyl,
(9) ~oC2 3al1yl~milw c~l,~lyl wll~ the
amino is opnonally m~no or di ~ul~s~ te~
C1 6alkyl,
(10) hydroxy,
(11) ~mino~rbonyI whe~ein the amino is op~o~lly
mono or d~ s~bsti~lteA uli~ Cl 6alkyl,
(12) hydroxyme~yl,
(13) ~m;nc,c~onyloxy Cl.3a~floxy wL.~ . ~e
amino is Opei~n~l1y mono ar di substitllt~A with
1~ C~ cyl,
(1~) cyano,
(15) mo~ho~ o&~l~o~ phenyl~
(16) amino wherein ~c ~m;no is op~onally mono
or di sub~it~lted wi~ C1 6alkyl,
wi~h thc prolriso ~at R2 a~d R3 may be joined
cl to fo~m a methylr~ liosy group or a furan
ring,
(1~) morphol;.loc~ yl;
;~ R?
R" is(a) a-~,-Y-N~ , or
RE~
(b) Q-C-OR" where R,~ i5 carboxy
G~ ~; alky!.
2~
WO 95t24207 2 1~ 4 3 8 5 PCT/US95/02938
benzyloxycarbonylCl 3alkyl, or
t-butoxycarbonylC1 3alkyl,
wherem
Q is a covalent bond or
R6
wherein Rs and R6 are each individually Cl 3alkyl or hydrogen,
/ R12 \ ~10
Y is -N- C n--C--
or / ~12 \ /R10
o I n-C--
\ H /
or a covalent bond;
R12 is hydrogen or Cl 3alkyl;
R7 and R8 are each individually
(a) hydrogen,
(b) C1 6alkyl,
(c) Cl 6alkyloxy C2 3alkyl,
(d) hydroxy C2 6alkyl,
(e) polyhydroxyC2 6alkyl,
WO 95/24207 PCT/US95/02938
21 84385
- 10-
(f) carboxamido C1 -6alkYl,
(g) polyacyloxyC2 6alkyl,
(h) C1 6alkanoyl,
(i) substituted phenyl or phenyl C1 6alkyl,
wherein the substituent is X1 as defined
immediately below,
(j) C2 6alkenyl,
(k) C6 10cycloalkenyl,
o (l) heteroaryl C1 6alkyl wherein the hetero aryl
includes pyridinyl, imidazolyl, triazolyl,
benzylimidazolyl, and furyl,
(m) carboxy C1 6alkyl,
(n) carbo C1 6alkoxy Cl 3alkyl,
(O) phenylsulfonyl,
(p) Cl 6alkylsulfonyl,
(q) benzyloxy,
(r) morpholinyl C1 3alkylsulfonyl,
(s) tetrahydropyranyl,
(t) aminoC1 3alkylsulfonyl wherein the amino is
optionally mono or di substituted with
Cl 6alkyl,
(u) aminocarbonyl wherein the amino is optionally
mono or di substituted with C1 6alkyl,
(V) aminocarbonyloxyC2 6alkyl wherein the
amino is optionally mono or di substituted with
Cl -6alkyl,
(w) azabicyclo of 7 to 12 atoms,
(x) di Cl 3alkylamino C2 6alkyl wherein the
amino is optionally mono or di substituted with
Cl 6alkyl,
(y) bicycloalkyl of 7 to 12 atoms,
(z) C3 10cycloalkyl optionally substituted with
Cl 6alkyl,
(aa) pyrazolidinyl,
WO 95/24207 PCT/US95102938
21843~S
(bb) substituted piperidinyl or prrolidinyl wherein
the substituent is hydrogen, C1 3alkyl,
hydroxyCl 3alkylbenzyl, carboxamido or
amino wherein the amino is optionally mono
or di substituted with C1 6alkyl,
(cc) substituted pyrrolidinyl wherein the substituent
is carboxamido or amino wherein the amino is
optionally mono or di substituted with C
o 6alkyl,
(dd) pyrimidinyl,
(ee) N-cyano-N'-phenylamidino,
(ff) phosphonoCl -6alkyl, or
(gg) a-C1 3alkyl benzyl or mono or di substituted
benzyl or mono or di substituted pyridyl-
methyl, wherein the substituents are X1 and
x2
wherein
Xl is
(1) hydrogen,
(2) halo,
(3) Cl 6alkyl,
(4) halo-C1 6alkyl,
(5) C2 6alkenyl,
(6) hydroxy-C1 6alkyl,
(7) Cl 6alkylcarbonyl,
(8) C1 6alkylcarbonylamino,
(9) CN,
(10) CF3,
(11 ) CH30,
(12) amino wherein the amino is optionally mono
or di substituted with Cl 6alkyl,
(13) carboxy, or
(14) phenylsulfonylaminocarbonyl;
X2 is hydrogen, halo or C1 6alkyl;
WO 95/24207 PCT/US95/02938
21 84385
- 12-
n is 1, 2, 3, 4 or 5;
Rg is selected from hydrogen, Cl 4 alkyl, and
C1 3alkoxyCl 3alkyl; orphenyl, phenyl Cl 3alkyl,
pyridyl, and pyridyl C1 3alkyl;
R1o and R1 1 are each independently selected from
hydrogen, C1 4alkyl, and C1 3alkoxy C1 3alkyl, or aryl as
defined above, or are together O=; or
wherein R7 and R8 are joined together to form mono or di substituted
ring of 4, 5, 6, or 7 atoms or 7 to 12 atoms such as
( 1 ) piperidinyl or homopiperdinyl,
(2) piperazinyl,
(3) morpholinyl, thiomorpholinyl or 1,1-dioxo-4-
thiomorpholinyl,
(4) pyrroylidinyl,
(S) pyrryl,
(6) imidazolyl,
2 0 (7) triazolyl,
(8) saturated azabicyclo of 7 to 12 atoms,
(9) azaspiro having 3 to 9 carbon atoms, said ring being
saturated,
(10) tetrazolyl,
(1 l) pyrazolidinyl,
(12) dihydodimethoxyisoquinolyl,
(13) azetidinyl, or
(14) diazabicyclo ring of 7-12 atoms,
wherein the substituents are each selected from the group consisting of
hydrogen and C1 3alkyl, benzyloxycarbonyl, carboxy, phenyl C1 3alkyl
amino carbonyl, pyrrolidinylmethyl, hydroxy C1 3alkyl, C1 6alkyloxy,
Cl 4alkyloxy carbonyl, aminocarbonyl wherein the amino is optionally
mono or di substituted with C1 6alkyl, and oxo; or
-N(R7)R8 may be an amino acid residue including natural amino acids
such as lysine; or
WO 95/24207 PCT/US9~/02938
218 138~
R8 and Rg are joined together to form a mono or di substituted
saturated monocyclic ring of 6 to 7 atoms and having two hetero atoms
which are the nitrogens to which R8 and Rg are attached; said rings to
include piperazinyl and homopiperazinyl; or Rg and Rlo are joined
together to form a mono or di substituted monocyclic saturated ring of
S to 7 atoms and having one hetero atom which is the nitrogen to which
Rg is attached; or
wherein Rg and R12 are joined together to form a mono or di
o substituted saturated monocyclic ring of 5, 6, or 7 atoms, said ring
having one hetero atom which is the nitrogen to which Rg is attached;
or
wherein R1o and R12 are joined together to form a mono or di
substituted saturated monocyclic ring of 5, 6, or 7 carbon atoms; or
wherein R8 and R1 1 are joined together to form a mono or di
substituted saturated monocyclic ring of 5, 6, or 7 atoms, said ring
having one hetero atom which is the nitrogen to which R8 is attached;
and the substituents are independently selected from Hydrogen and
C1 3alkyl.
As appreciated by those of skill in the art the term "alkyl"
such as in C1 6alkyl, includes, methyl, ethyl, propyl, butyl, pentyl, and
hexyl, and where a~ro~riate, branched chained forms including
isopropyl and tert-butyl.
As may also be appreciated by those of skill
in the art, the (-CR12-)n spacer in definition Y, may, in the alternative
be placed to the right of CR1oR11-
~ R7
As may also be appreciated, the group -N,
R8
,R7
may also be oxidized to the corresponding oxide -N ~ ~ O
R8
In one Class the instant invention is directed to the
compounds of the Formula (I)
WO 95/24207 PCTIUS95/02938
21 84385
- 14 -
Ra
R1 0~/~
R~ ~ R4
~N Rb
CONHCH ~ R2
~\~
o and pharmaceutically acceptable salts thereof wherein:
R is C1 6alkyl;
R1 is C1 6alkyl or C1 6alkoxy-Cl 6alkyl;
Mis
( 1 ) hydrogen,
(2) C1 6alkyl,
(3) hydroxy C1 6alkyl,
(4) halo C1 6alkyl,
(5) C2 6alkenyl, or
(6) C1 6alkoxy-C1 6alkyl;
Ra is
(1) hydrogen,
(2) C1 6alkyl,
(3) halo,
(4) carboxy,
(5) C1 6alkoxy,
(6) phenyl,
(7) C1 6alkylcarbonyl,
(8) amino wherein the amino is optionally mono
or di substituted with C1 6alkyl;
3 0 Rb is hydrogen, or C1 -6alkyl,
R2 and R3 are each independently
(1) hydrogen,
(2) C1 6alkyl,
(3) halo,
(4) carboxy,
WO 95/24207 2 1 8 ~ ~ 8 5 PCT/US95/02938
(5) Cl_6alkoxy,
(6) phenyl,
(7) Cl 6alkylcarbonyl,
(8) amino wherein the amino is optionally mono
or di substituted with C1 6alkyl, or with the
proviso that R2 and R3 may be joined together
to form a methylenedioxy group or a furan
ring;
o 0 7
Il ~R
R4 is (a)~Q~c~Y-N~R , or
ll
(b) -Q-C-ORX where Rx is carboxy-C~ 6alkyl,
benzyloxycarbonylC1 3alkyl, or
t-butoxycarbonylCl 3aLkyl,
wherem
Q is a covalent bond or
IR5
2 5 C -
I
R6
30 wherein R5 and R6 are each individually C1 3alkyl or hydrogen
WO 95/24207 PCT/US95/02938
21 84385
- 16 -
Y is / ~12 \ ~(10
g \ / R "
/ ~12 \ / 10
o C ~tC--
\ H / R~
or a covalent bond;
R12 is hydrogen or Cl 3aLkyl;
R7 and R8 are each individually
(a) hydrogen,
(b) C1 6alkyl,
(c) C1 6alkyloxy C2 3alkyl,
(d) hydroxy C2 6alkyl,
(e) carboxamido Cl -6alkYl,
(f) C1 6alkanoyl,
(g) substituted phenyl or phenyl Cl -6alkyl
wherein the substituents are Xl, and X2,
(h) C2 6alkenyl,
(i) C6 10cycloalkenyl,
(j) heteroaryl C1 6aLkyl wherein the hetero aryl
includes pyridinyl, imidazolyl, triazolyl,
benzylimidazolyl, and furyl,
(k) carboxy C1 6alkyl,
(1) Cl 6alkylsulfonyl,
(m) carboCl 6alkyloxyC2 3alkyl,
(n) morpholinyl C1 3alkylsulfonyl,
(o) aminoC1 3alkylsulfonyl wherein the amino is
WO 95/24207 PCT/US95/02938
- 218438~
- 17 -
optionally mono or di substituted with
Cl -6alkyl,
(p) aminocarbonyl wherein the amino is optionally
mono or di substituted with Cl 6alkyl,
(q) aminocarbonyloxyCl 6alkyl wherein the
amino is optionally mono or di substituted with
C1 6alkyl,
(r) di C1 3alkylamino C1 6alkyl wherein the
amino is optionally mono or di substituted with
Cl -6alkyl,
(s) pyrazolidinyl,
(t) substituted piperidinyl as defined above,
(u) substituted pyrrolidinyl as defined above,
(v) pyrimidinyl,
(w) benzyloxy,
(x) C3 10cycloalkyl,
(z) a-C1 3alkyl benzyl or mono or di substituted
benzyl or mono or di substituted
pyridylmethyl, wherein the substituents are X
and X2,
wherein
Xl is
(1) hydrogen,
(2) halo,
(3) Cl 6alkyl,
(4) halo-C1 6alkyl,
(5) C2 6alkenyl,
(6) hydroxy-C1 6alkyl,
0 (7) C1 6alkylcarbonyl,
(8) C1 6alkylcarbonylamino,
(9) di-Cl 3alkylamino, or
(10) carboxy;
X2 is hydrogen, halo or C1-6alkYI;
WO 95/24207 PCT/US95/02938
21 84385
- 18 -
n is 1 , 2, 3, 4 or 5;
R9 is selected from hydrogen, C1 4 alkyl, and
C1 3alkoxyC1 3alkyl;
Rlo and R1 1 are each independently selected from
hydrogen, C1 4alkyl, and C1 3alkoxy C1 3alkyl; or
wherein R7 and R8 are joined together to form mono or di substituted
o ring of 4, 5, 6, or 7 atoms such as
( 1 ) plperldinyl,
(2) piperazinyl,
(3) morpholinyl,
(4) pyrroylidinyl,
(5) pyrryl,
(6) imidazolyl,
(7) triazolyl,
(8) tetrazolyl,
(9) pyrazolidinyl,
(10) azetidinyl,
wherein the substituents are each selected from the group consisting of
hydrogen and C1 3alkyl, beniyloxycarbonyl, carboxy, phenyl C1 3alkyl
amino carbonyl, pyrrolidinyl, methyl, hydroxy C1 3alkyl,
C1 6alkyloxy, C1 4alkyloxy carbonyl, and oxo; or
R8 and Rg are joined together to form a saturated ring of 5 to 7 atoms
and having two hetero atoms; or
Rg and R1o are joined together to form a saturated ring of 5 to 7 atoms
and having one hetero atom; or
wherein Rg and R12 are joined together to form a ring of 5, 6, or 7
atoms, said ring being saturated; or wherein R1o and R12 are joined
together to form a ring of 5, 6, or 7 atoms, said ring being saturated;
or
wherein R8 and R1 1 are joined together to form a ring of 5, 6, or 7
atoms, said ring being saturated and having one hetero atom.
WO 95/24207 PCT/US95/02938
-
218 138~
- 19 -
In one subclass, the invention concerns compounds of
Formula I
wherein
R is C1 3alkyl;
Rl is Cl 3alkyl;
Mis
(a) C 1 -6alkyl, or
(b) C2-6alkenyl;
R2 is
(a) hydrogen,
(b) C1 6alkyl, or C1 6alkoxy, and
R3 is hydrogen, or
R2 and R3 are joined together to form a methylenedioxy
15group or a furan ring;
R5 and R6 are each individually hydrogen or C1 3alkyl;
R7 and R8 are each independently selected from
(a) hydrogen,
(b) C1 3alkyl,
(c) C1 3alkoxy C2 3alkyl,
(d) C3 7cycloalkyl,
(e) hydroxyC2 3alkyl,
(d) carbo C1 4alkyloxymethyl,
(g) substituted benzyl wherein the substituents are
X1 and X2
25wherein X1 is hydrogen and X2 is
(1) hydrogen,
(2) halo, or
(3) C~1 3alkyl;
nis 1,2or3,and
Rg, R1o and R1 1 are each independently selected
from hydrogen, C1 4aIkyl, and C1 3alkoxy
C1 3alkyl; or
WO 95/24207 PCT/US95/02938
2 1 84385
- 20 -
R7 and R8 are joined together to form a substituted ring
selected from
(a) piperidinyl,
(b) piperazinyl, and
(c) morpholinyl;
or
R8 and Rg are joined together to form a ring of 6 to 7 atoms and having
two hetero atoms;
Rg and Rlo are joined together to form a saturated ring of 5 to 7 atoms
and having one hetero atom; or
wherein Rg and R12 are joined together to form a ring of S, 6, or 7
atoms, said ring being saturated; or
wherein Rlo and R12 are joined together to form a ring of S, 6, or 7
atoms, said ring being saturated;
or
wherein R8 and Rl 1 are joined together to form a ring of S, 6, or 7
atoms, said ring being saturated and having one hetero atom.
In a narrower sub-class are the compounds wherein
Q is a covalent bond;
R is methyl or ethyl;
Rl is methyl or ethyl;
- Mis
(a) Cl 4alkyl, or
(b) C2 3alkenyl;
R2 is
(a) hydrogen,
O (b) Cl 3alkyl, or Cl 3alkoxy, and
R3 is hydrogen, or
R2 and R3 are joined together to form a furan or
dioxacyclopentane ring;
nis 1 or2;
Rg and Rlo are each independently selected from
WO 95/24207 PCT/US95/02938
- 218~8~
(a) Cl 3alkyl,
(b) Cl 3alkoxy Cl 3alkyl,
(c) hydrogen,
R7 and R8 are each independently selected from
(a) Cl 3alkyl,
(b) Cl 3alkoxy C2 3alkyl,
(c) hydrogen,
(d) hydroxyethyl,
o (e) carboethoxymethyl,
(f) cyclopropyl,
or
R7 and R8 are joined together to form a substituted ring
selected from
(a) piperidinyl, and
(b) morpholinyl, or
R8 and Rg are joined together to form a piperazine ring.
As is defined above, various rings are formed when R8,
20 Rg, R1o and R12 are joined. The following is a non-limiting
description of some of the preferred rings that are formed when these
various substituents are joined.
R~ and R9 are joined
R,~ R" ~) ,R~
WO 95/24207 PCT/US95/02938
21 84385
Rg and Rlo are joined
s ~R7R~ 12~'~NR7R~ [~NR7R~
~<NR7Rs ~ R11
~NJ 1' 12 ~N) ~N! R12
R 12 ~< R 11 1~ N R7R8 /~ N R7R8
~N NR7R8 INJ ~NJ 12
W O 95/24207 PCTrUS95/02938
- 2184385
- 23 -
Rg and Rl~ are joined
R10 R11
~ ~ N R7R8
--- R10 R11 -I- R10
R10 R" R ~NR7R8
NR7RB ~ C~,O.RN"
Rlo R11
R10 R11 >~ NR7R8
~ N R7R8
_ _ _ . _ _ .
WO 95/24207 PCT/US95/02938
2l 84385
- 24 -
Rlo and Rl ~ are joined
NR7R8
PCNR7R8 5~ 1' Ç<R"
R[~R7R8
~ ~ NRg ~ ~ NRg ~ ~ NRg
~R11 ' /~NR7R8
1~ NR7R8
~ ~NRg ~ ~NRg
WO 95/24207 2 18 4 ~ ~ ~ PCT/US95/02938
- 25 -
Rlo and Rl ~ are joined
R12
;-- Rg I ~ RgN~
R12 R7 R7 R12 R7
Rg
RgN~ R7
R9!N~ "' ~)
WO 95124207 PCT/US95/02938
21 84385
- 26 -
As discussed above, (F)-actin shortening proteins are
effective in reducing the viscosity of sputum, particularly cystic Fibrosis
SpUIulll. As shown by the Vasconcellos et al., reference cited above,
liquefaction is not instantaneous. However, once a suff1cient degree of
viscosity reduction is achieved, a patient can more easily expectorate or
otherwise rid himself of excess sputum.
One problem that has not been appreciated is that
liquefaction, considered alone, may affect the patient adversely. For
example, viscosity reduction may provide the destructive proteases
within the sputum greater access to lung and related tissues. Thus, the
applicant has found it to be of surprising importance to inhibit these
destructive proteases during the transition period from the onset of
viscosity reduction until the ~l~u~unl is expectorated. Moreover, the
15 high proportion of protease destruction caused by the elastase in the
sputum has also gone unappreciated.
The compounds of Formula I are surprisingly, highly
active in viscous ~yulunl as well as liquified ~Ululll.
As a result of the above factors, treatment with applicants
2 composition is capable of returning a patent with lung disease to
substantially normal lung function, as measured, for example, by FEV
(forced expiration volume). In particular, treatment with applicants
composition is capable of retllrning a patent with lung disease to at least
60-75% of normal lung function, as measured, for example, by FEV1.
Moreover, treatment with applicants composition is capable of returning
a patent with lung disease to 75-90% of normal lung function or
greater, as measured, for example, by FEV1.
Accordingly, in one aspect the invention encompasses a
method of treating a patient with a lung disease, comprising:
~(lmini.~tration to a patient in need of sputum viscosity reduction, a
therapeutically effective non-toxic amount of an (F)-actin shortening
protein and a therapeutically effective non-toxic amount of compound
of Formula I as described herein.
Within this aspect the invention encompasses a method of
treating a patient with a lung disease, comprising:
WO 95/24207 PCT/US95/02938
-- 218~8~
- 27 -
~tlmini~tration to a patient in need of sputum viscosity reduction, a
therapeutically effective non-toxic amount of an (F)-actin shortening
protein and a therapeutically effective non-toxic amount of compound
5 of Formula I as described herein, said amounts effective to return the
lung function of said patients to at least 60-75% of normal as measured
by FEV1.
Within this class the invention encompasses a method of
treating a patient with a lung disease, comprising:
atlmini.~tration to a patient in need of sputum viscosity reduction, a
therapeutically effective non-toxic amount of an (F)-actin shortening
protein and a therapeutically effective non-toxic amount of compound
of Formula I as described herein, said amounts effective to return the
lung function of said patients to 75 to 90% of normal as measured by
FEV 1 .
The compounds of the invention are prepared by known
methods or are prepared among other methods by the following
representative schemes. For example, methods for making such
compounds are disclosed in EP O 337 549, published October 18, 1989,
20 which is hereby incorporated by reference.
This invention also relates to a method of treating
infl~mm~tion in patients using a compound of Formula (I), particularly
a preferred compound as the active constituent.
It has been found that the following compound are effective
2 inhibitors of the proteolytic function of human neutrophil elastase as
shown below in Table 1 to 10.
WO 95/24207 PCT/US9StO2938
21 84385
- 28 -
TABLE 1
Et "" ~11 O A
CH3
NH
Pr
No. A Kobs/rIl
-cH2cH2N(cH3)2 1,566,000
2 -CH2CO2H 1,667,000
3 -CH2-C(O)N(CH2CH2OH)2 3,428,000
4 -CH2-C(O)N(CH3)CH2C(O)NH2 4,293,000
-CH2C(O)NH-C(CH2OH)3 4,448,000
6 -cH2c(o)N(cH3)2 2,997,000
7 -CH2CH2N(CH3)Ac 1,558,000
8 -CH2C(O)-Pro-OCH2Ph 12,501,000
9 -CH2C(O)-Pro-OH 1,571,000
-CH(CH3)CO2CH2Ph 2,891,000
11 -CH(CH3)CO2H 1,132,000
12 -CH(CH3)C(O)N(Et)2 2,815,000
13 -CH(CH3)CH2N(CH3)2 2,472,000
14 -CH2CH2CH2N(CH3)2 2,855,000
-CH2CH2N(O)(CH3)2 2,162,000
16 -CH2CH2N(Et)2 2,291,000
17 -CH2CH2(4-morpholinyl) 4,733,000
18 -CH2CH2CH2CH2N(CH3)2 1,934,000
19 -CH2C(O)-Pro-NHCH2Ph 4,956,000
-CH2C(CH3)2N(CH3)2 1,470,000
21 -CH2CH2N(i-Pr)2 1,671,000
22 -CH2CH2(4-carbobenzyloxy- 1 -piperazinyl) 4,115,000
WO 95/24207 PCT/US95/02938
218~8~
- 29 -
No. A Kobs/rIl
23 -CH2CH2N(n-Bu)2 992,000
24 -cH2cH2cH2cH2cH2cH2N(cH3)2 1,988,000
-CH2CH2(1 -piperazinyl) 1,709,000
26 -CH2CH2(4-methyl-1-piperazinyl) 4,685,000
27 -CH2CH2(4-acetyl- 1 -piperazinyl) 3,262,000
28 -CH2CH2N(Ph)2 188,000
29 -CH2CH2N(CH2CH=CH2)2 891,000
-cH2cH(ph)N(cH3)2 656,000
31 -CH2CH2N(CH3)CH2Ph 1,180,000
TABLE 2
Et `~" ~CH2-C-O-A
\~ CH3
- NH
Pr
No. A Kobs/lIl
32 -CH2CH2N(CH3)2 1,993,000
33 -CH2CH2CH2N(CH3)2 1,151,000
34 -CH2CH2N(Et)2 1,339,000
-CH2CH2-(4-morpholinyl) 1,725,000
36 -CH(CH3)CH2N(CH3)2 1,688,000
37 -CH2-C(CH3)2N(CH3)2 2,100,000
38 -CH2CO2H 1,008,000
39 -CH2CH2N(CH3)CH2Ph 751,000
WO 9~/24207 PCT/US95/02938
21 84385
- 30 -
TABLE 3
Et ` ~
o.~N O CH2-C-A
CH3
NH
o Pr
No. A Kobs/rIl
-N(CH2CH2OH)2 1,241,000
41 4-methyl-1-piperazinyl 974,000
42 4-morpholinyl 1,088,000
43 -NHcH2cH2N(cH3)2 1,211,000
44 -N(CH3)CH2CH2N(CH3)2 1,243,000
-NHCH2CH2CH2N(CH3)2 1,118,000
46 -NHCH2CH2-(4-pyridyl) 2,254,000
47 -NHCH2CO2H 876,000
48 -NHCH(CH3)CO2H 676,000
49 -NHcH2c(o)N(cH2cH2oH)2 1,295,000
-N(CH3)CH2CO2H 989,000
51 -NHCH(CH3)C(O)N(CH2cH2OH)2 939,000
52 -N(cH3)cH2c(o)N(cH2cH2oH)2 273,000
53 -N(CH3)CH2CH2-(4-morpholinyl) 2,511,000
54 -N(CH3)CH2CH2N(cH2cH2OcH3)2 1,388,000
SS -N(CH3)CH2CH2N(Et)2 1,316,000
56 -N(CH3)CH2CH2CH2N(CH3)2 1,047,000
57 -NHCH2CH(CH3)N(CH3)2 1,344,000
58 -N(CH3)CH2CH2N(i-Pr)2 1,634,000
59 -N(n-pr)2 1,144,000
WO 95/24207 2 1 8 4 3 8 ~ PCT/US95/02938
- 31 -
No. A Kobs/rIl
-N(Et)2 1,079,000
61 3-chloroanilino- 733,000
62 3-methoxyanilino- l ,621,000
63 4-fluoroanilino-
64 -N(CH3)CH2CH2CH2CO2H 917,000
-N(CH3)CH2CH2CH2C(O)NHSO2Ph 1,335,000
66 -N(CH3)CH2CH2CH2N(cH3)cH2Ph 1,355,000
67 -N(CH3)2 942,000
o 68 -N(CH3)CH2Ph 1,897,000
69 -N(CH3)CH2CH2N(CH3)CH2Ph 2,792,000
-NH-O-CH2Ph 2,371,000
71 -N(CH3)(4-carboxyphenyl) 1,508,000
72 -N(CH3)(4-benzenesulfonylaminocarbonyl-)
phenyl) 3,284,000
TABLE 4
Et~`~" ~C-A
\~ CH3
NH~
2s Pr
No. A Kobs/rIl
73 -NHCH2CH2N(CH3)2 968,000
74 -NH-CH2CO2H 1,434,000
-N(CH3)CH2CH2N(CH3)2 1,916,000
76 -N(Et)CH2CH2N(CH3)2 1,436,000
77 -NHCH2CH2N(Et)2 1,187,000
78 -NHCH2CH2-(4-morpholinyl) 1,841,000
79 -N(CH3)CH2CH2-(4-morpholinyl) 2,118,000
WO 95/24207 PCT/US95/02938
21 8~385
- 32 -
No. A Kobs/rIl
-N(CH3)CH2CH2N(CH2CH2OCH3)2 2,078,000
81 -N(CH3)CH2CH2N(Et)2 2,191,000
82 -N(Ph)CH2CH2N(CH3)2 2,504,000
83 -N(cH3)cH2cH2cH2N(cH3)2 1,797,000
84 -NHCH2CH2N(i-Pr)2 2,100,000
-N(CH3)CH2CH2N(O)(CH3)2 1,589,000
86 -N(CH3)CH2CH2N(i-Pr)2 2,449,000
87 -NH-SO2CH2CH2-(4-morpholinyl) 775,000
88 -NH-SO2CH2CH2N(CH3)2 788,000
89 -NHCH2CH2-(4-imidazolyl) 2,092,000
-NHCH2CH2-(1 -piperidinyl) 941,000
91 -N(CH3)CH2CH2-(1-piperidinyl) 892,000
92 -N(CH3)CH2CH2NHCH3 1,453,000
93 -N(CH3)CH2CH2N(CH3)Ac 1,960,000
94 -NHCH2CH2-(1-pyrrolidinyl) 1,239,000
-N(CH3)CH2CH2-(1 -pyrrolidinyl) 1,005,000
96 -NHCH2CH2-(1 H- 1,2,4-triazol- 1 -yl) 1,397,000
97 -NH-CH2CH2-(1-imidazolyl) 1,070,000
98 -NH-CH2CH2-(3-azabicyclo-[3.2.2-non-3-yl) 3,043,000
99 -NH-CH2CH2-(3-azaspiro[5.5]-undec-3-yl) 2,583,000
100 -NH-CH2CH2-(2H-tetrazol-2-yl) 2,006,000
101 -NH-CH2CH2-(lH-tetrazol-1 -yl) 2,053,000
102 -NHCH2C(O)-Pro-NHCH2Ph 2,747,000
103 -N(CH3)CH2CH2-(3-azabicyclo-[3.2.2]non-
3-yl) 2,996,000
104 -N(CH3)CH2CH2-(4-imidazolyl) 2,389,000
105 -N(CH3)CH2CH2N(CH3)Ac 2,398,000
106 -N(CH3)CH2CH2N(CH3)C(O)NHCH3 2,486,000
107 -N(CH3)CH2CH2N(CH3)SO2CH3 2,530,000
108 -N(CH3)CH2CH2(3-azabicyclo-[3.2.2]non-
3-yl) 2,953,000
109 -NHCH2CH2-(1,1 -dioxo-4-thiamorpholinyl)1,275,000
110 4-dimethylaminobenzylamino 5,598,000
WO 95/24207 PCT/US95/02938
218~
No. A Kobs/rIl
111 3-dimethylaminoanilino 2,286,000
112 -N(CH3)CH2CH2-(1,1 -dioxo-4-thia-
morpholinyl) 1,596,000
113 4-dimethylaminoanilino 2,591,000
114 -NHCH2CH2-(l-benzyl-lH-imidazol-2-yl) 3,853,000
115 -N(CH3)CH2CH2(2-pyridyl) 2,272,000
116 -N(CH3)(1-azabicyclo[2.2.2]oct-3-yl 3,480,000
117 -NHCH2CH2(4-benzyloxycarbonyl- 1 -
piperazinyl) 6,231,000
118 1,2-diethylpyrazolidin-4-ylamino 1,001,000
119 2-(1-S-pyrrolidinylmethyl)-1-pyrrolidinyl 2,692,000
120 -NHCH2CH2(4-hydroxy- 1 -piperidinyl) 1,728,000
121 -NHCH2CH2(1 -homopiperidinyl) 2,069,000
122 -N(CH3)CH2CH2(1-homopiperidinyl) 2,899,000
123 -NHCH2CH2(3-hydroxy- 1 -piperidinyl)1,534,000
124 -N(CH3)CH2CH2(3-hydroxy- 1 -piperidinyl) 1,963,000
125 -N(CH3)CH2CH2N(CH3)CH2Ph 2,054,000
126 -N(CH3)CH2CH2(4-benzyloxy-1-piperidinyl) 3,476,000
127 -N(n-Pr)2
128 -N(Et)2 1,454,000
129 -N(CH3)CH2CH2(4-hydroxy- 1 -piperidinyl) 1,994,000
130 -N(CH3)CH2CH2(4-oxo- 1 -piperidinyl)2,297,000
131 -NHCH2CH2(3-hydroxy-1-pyrrolidinyl) 1,111,000
132 -N(Et)CH2CH2(1-piperidinyl) 1,244,000
133 -N(CH2Ph)CH2CH2(1-piperidinyl) 1,521,000
134 4-fluoroanilino- 724,000
135 3-chloroanilino- 201,000
136 3-methoxyanilino
3 137 -N(CH2Ph)CH2CH2N(CH3)2 1,380,000
138 -N(CH3)CH2CH2(3-hydroxy-l-pyrrolidinyl)960,000
139 -N(3-picolyl)CH2CH2(1 -piperidinyl)1,189,000
140 -NHCH(CH3)CH2CH2CH2N(Et)2 1,361,000
WO 95/24207 PCT/US95/02938
21 84385
- 34 -
No. A Kobs/rIl
141 -NHCH2CH2(2-S-hydroxymethyl-1-
pyrrolidinyl 1,507,000
142 -N(CH3)CH2CH2(4-t-butoxycarbonyl- 1 -
piperazinyl) 3,471,000
143 -N[CH2CH2N(CH3)2]2 1,878,000
144 -N[CH2CH2N(Et)2]2 1,508,000
145 -N(CH3)CH2CH2N(CH3)(3-picolyl) 2,877,000
146 3,5-dimethyl-1 -piperazinyl 1,518,000
147 -N(CH3)CH2CH2N(O)(CH3)CH2Ph 2,493,000
148 -N(CH3)CH2CH2N(CH3)(4-picolyl) 2,389,000
149 2-S-(N-benzyl-N-methylaminomethyl)-1-
pyrrolidinyl 3,268,000
150 -N(CH3)CH2CH2N(CH3)(2-picolyl) 2,165,000
151 -N(CH3)CH2CH2(1-piperazinyl) 1,191,000
152 1-homopiperazinyl 1,951,000
153 -N(CH3)CH2CH2N(CH3)cH2cH2Ph 2,797,000
154 2-(1 -R-pyrrolidinylmethyl)- 1 -pyrrolidinyl 1,666,000
155 4-benzyl-1-homopiperazinyl 1,979,000
156 -N(cH3)cH2-[cH(oH)]4cH2oH 1,198,000
157 -N(CH3)CH2-[CH(OAc)]4CH2OAc 1,171,000
158 -N(CH3)CH2CH2N(CH3)(1-Naphthalenyl-
methyl) 1,075,000
159 -N(CH3)CH2CH2N(CH3)(2-Naphthalenyl-
methyl) 1,337,000
160 -N(CH3)CH2CH2N(CH3)CH(CH3)Ph 1,569,000
161 -N(CH3)CH2CH2N(CH2Ph)2 1,021,000
162 1-ethyl-3-piperidinylamino 949,000
163 -N(CH3)CH2CH2N(CH3)(2-furfuryl) 1,818,000
3 164 -N(CH3)CH2CH2CH2CO2H 1,064,000
165 -N(CH3)CH2CH2CH2C(O)NHSO2Ph 1,550,000
166 -N(CH3)CH2CH2N(CH3)CH2CH=CH2 1,359,000
167 -N(CH3)CH2CH2CH2N(CH3)CH2Ph 1,293,000
168 -N(cH3)-(cH2)6-N(cH3)cH2ph 2,157,000
WO 95124207 PCT/US95/02938
218~3~
- 35 -
No. A Kobs/rll
169 -N(CH3)CH2CH2OH 1,457,000
170 -N(cH3)cH2cH2oc(o)N(cH3)2 1,518,000
171 -N(CH3)CH2CH2N(CH3)CH2CO2-t-Bu 1,831,000
172 -N(CH3)(1-ethyl-3-piperidinyl) 1,545,000
173 -N(CH3)CH2CH2N(CH3)(tetrahydro-2H-
pyran-2-yl-methyl) 2,943,000
174 2,2,6,6-tetramethylpiperidin-4-ylamino869,000
175 -N(CH3)(4-carboxyphenyl) 1,055,000
176 -N(CH3)(4-benzenesulfonylaminocarbonyl-
phenyl 3,231,000
177 -N(CH3)CH2CH2N(CH3)(4-cyanobenzyl)2,201,000
178 -N(CH3)CH2CH2N(CH3)(4-methylbenzyl)1,870,000
179 -N(CH3)CH2CH2N(CH3)(3-cyanobenzyl)2,448,000
180 -N(CH3)CH2CH2N(CH3)(4-trifluoromethyl-
benzyl 905,000
181 -N(CH3)CH2CH2N(CH3)(3-trifluoromethyl-
benzyl 564,000
182 -N(CH3)CH2CH2(CH3)(4-fluorobenzyl)2,137,000
183 -NHCH(CH3)PH(O)OH 977,000
184 L-lysine (a-N) 755,000
185 -N(CH3)CH2CH2N(CH3)(cyclopropylmethyl) 1,787,000
186 -N(CH3)CH2CH(Ph)N(CH3)2 1,053,000
187 -N(CH3)2 1,749,000
188 -N(CH3)CH2Ph 1,837,000
189 -N(CH3)(1-benzyl-3-piperidinyl) 1,879,000
190 -NH-O-CH2Ph 1,797,000
191 -N(3 -picolyl)CH2CH2N(CH3)CH2PH 2,538,000
192 -N(CH3)CH2CH2N(CH3)(4-me~oxybenzyl)1,785,000
193 -N(4-picolyl)CH2CH2N(CH3)CH2Ph 2,243,000
194 -N(2-picolyl)CH2CH2N(CH3)CH2Ph 2,473,000
195 -N(CH3)CH2CH2N(CH3)(2,4-dimethylbenzyl) 1,119,000
WO 95/24207 PCT/US95102938
21 84385
- 36 -
No. A Kobs/rIl
196 -N(CH3)CH2CH2(2,6-dimethyl-4-
morpholinyl) 1,530,000
197 -NH2 1,638,000
198 -NHCH3 1,825,000
199 4-morpholinyl 2,376,000
200 cis-2,6-dimethyl-4-morpholinyl 1,837,000
201 -NH-CH2CH2CH2CH3 2,460,000
202 -N(CH3)CH2CH2N(CH3)C(=N-CN)NHPh1,763,000
203 -N(CH3)CH2CH2N(CH3)(3-fluorobenzyl) 1,262,000
204 -N(CH3)CH2CH2N(CH3)(2-chlorobenzyl) 1,591,000
205 -N(CH3)CH2CH2N(CH3)(3-methoxybenzyl) 1,911,000
206 -N(CH3)CH2CH2N(CH3)(3,5-dimethoxy-
benzyl) 1,735,000
207 3,4-dihydro-6,7-dimethoxy-2-(1 H)iso-
quinolinyl 2,698,000
208 -N(CH3)(1-benzyl4-piperidinyl) 1,948,000
209 L-lysine (-N) 929,000
210 -N(CH3)CH2CH2N(CH3)(2-~d~m~ntyl)2,132,000
211 -N(CH3)(4-piperidinyl) 85,000
212 S-Methyl-2,5-diazabicyclo[2.2.1]hept-2-yl 860,000
213 -N(CH3)CH2CO2H 984,000
214 -N(CH3)CH2CH2CH2N(CH3)CH2CH3 1,099,000
215 -N(CH3)(1 -methyl-4-piperidinyl.)1,283,000
216 -N(CH3)(1 -propyl-4-piperidinyl)1,312,000
217 -N(CH3)(1-ethyl-4-piperidinyl) 1,422,000
218 -N(CH3)CH2CH(CH3)N(cH3)cH2Ph 2,123,000
219 -N(CH3)CH2CH(CH3)N(CH3)2 1,588,000
220 -N(CH3)CH2CH2N(CH3)(bicyclo[2.2.1]-hept-
2-yl) 1,874,000
221 -N(CH3)CH2CH2NH(2-~m~ntyl) 3,010,000
222 -N(CH3)CH2CH2N(CH3)(6,6-dimethylbicyclo-
[3.1.1]hept-2-yl 2,288,000
WO 95/24207 PCTIUS95/02938
- 2184385
- 37 -
No. A Kobs/rIl
223 -N(CH3)CH2CH2N(CH3)(bicyclo[3.2.1]-oct-
2-yl) 2,584,000
224 -NH(t-Bu)
225 -N(CH3)CH2CH2N(CH3)(1-cyclohexen-1-yl) 1,839,000
226 -N(cH3)cH2cH2NHc(cH3)2cH=cH2 1,309,000
227 2-S-carboxamido- 1 -pyrrolidinyl 931,000
228 2-hydroxymethyl- 1 -piperidinyl 50,000
229 3-dimethylamino-1-pyrrolidinyl 1,336,000
230 -N(CH3)CH2CH2N(CH3)(cyclohexylmethyl)
231 -N(CH3)CH2CH2N(CH2CH=CH2)C(CH3)2-
CH=CH2 925,000
232 -N(CH3)CH2CH2N(CH3)(4-ethylcyclohexyl) 2,476,000
233 -N(CH3)CH2CH2N(CH3)(2-ethylcyclohexyl) 2,030,000
234 -N(CH3)CH2CH2N(CH3)(4-methylcyclohexyl) 2,166,000
235 -N(CH3)CH2CH2N(CH3)(cyclohexyl) 1,952,000
236 -N(CH3)CH2CH2N(CH3)CH2CO2H-TFA 31,000
237 -N(CH3)CH2CH2N(CH3)CH2C(O)N(CH3)2 2,679,000
238 3-dimethylamino- 1 -azetidinyl
239 1-diphenylmethyl-3-azetidinyl
240 -N(CH)CH2CH2N(CH3)(cyclohexylmethyl) 3,003,000
241 -NHCH2CH2N(Et)CH2CH2OCH3 1,090,000
WO 95/24207 2 1 8 4 3 8 5 PCT/US95/02938
- 38 -
TABLE 5
E t ""` ~3\
o~N~O ll N N-R
~CH3
NH~
o Pr
No. R Kobs/rIl
242 -CH3 1,700,000
243 4-fluorophenyl 7,486,000
244 3-chlorophenyl 2,453,000
245 phenyl 5,276,000
246 benzyl 5,171,000
247 H 1,100,000
248 i-Pr 2,392,000
249 i-Bu 2,476,000
250 -CH2CO2Et 1,571,000
251 -CH2CO2H 1,947,000
252 Et 2,324,000
253 Pr 1,768,000
254 2-pyrimidinyl 2,142,000
255 -CH2CH2OC(O)NHCH3 2,548,000
256 cyclopropyl 3,587,000
256a -CH2CH2OH 2,000,000
PCT/US95/02938
WO 95/24207
218~3~5
- 39 -
TABLE 6
Et "` ~l
~ N (cH2)n-GA
O \~
NH~ [ >
o Pr
No. n A Kobs/rIl
257 1 NH2 2,342,000
258 1 4-morpholinyl 1,785,000
259 1 -N(CH3)CH2CH2N(CH3)2 2,522,000
260 0 -N(CH3)CH2CH2N(CH3)2 3,317,000
261 0 -N(Et)2 3,207,000
262 0 -N(CH3)(n-Bu) 3,125,000
263 0 4-methyl-1-piperazinyl 3,805,000
264 0 -N(CH3)CH2CH2N(CH3)CH2Ph 3,427,000
265 0 4-cyclopropyl- 1 -piperazinyl 4,500,000
265a 0 1-piperazinyl 3,250,000
265c 0 4-(2-hydroxyethyl)- 1 -piperazinyl 4,800,000
265d 0 4-morpholinyl 3,700,000
WO 95/24207 2 1 8 4 3 8 5 PCT/US95/02938
- 40 -
TABLE 7
Et ""` ~3` 1l
.~ N~o (C H2)n-C-A
~R4
NH~`CO N O
o Pr \ J
No. n B4 A Kobs/~Il
266 1 H 4-morpholinyl 169,000
267 1 H -N(Et)2 33
268 1 H -N(CH3)CH2CH2N(CH3)2 142,000
269 1 CH3 NH2 637,000
270 1 CH3 N(Et)2 740'000
271 1 CH3 N(n-Pr)2 826,000
272 0 Et -N(CH3)CH2CH2N(CH3)2 2,423,000
273 0 Et -N(CH3)(n-Bu) 3,258,000
WO 95/24207 PCT/I~S95/02938
2 1 84385
- 41 -
TABLE 8
E ""` ~
N (CH2)n-GA
O' \f~O
N~ ~CRON~O
o Pr
No. n R3 A Kobs/~Il
274 1 H NH2 430,000
275 1 H -N(CH3)CH2CH2N(CH3)2 290,000
276 1 H -OCH3 440,000
277 0 H -N(CH3)CH2CH2N(CH3)2 548,000
278 0 H -OCH2CH2N(CH3)2 135,000
279 0 H -N(Et)2 566,000
280 0 H 4-moIpholinyl 577,000
TABLE 9
2 s Et "`~ ~l(CH2)n-C-A
~R2
Nl~ R3
Pr
WO 9S/24207 PCT/US95102938
21 84385
-- 42 --
G o o o o o o o 8 8 o 8 8 8 8
8 C 8 8 C 8 C o d~ ~ r~
C ~C~ O ~ ~ ~-- ~ ~ d
X 5: ~ ~ ~ X X ~ ~
V V V V V V V V V
Z Z Z Z Z Z Z Z Z
T ~ ~I T
V V V V V V V V V
o o o V V V V V V ~ V o o V
~_ ~V ~ ~V ~ V V ~V ~ ~o V
d- d d I I I I I I I I d- I d
V
~ Z~
C"
C C
V V
m ~
~ '' " o '' '' '' o o X ~ X V V
~L~
z
x ~ x :c x ~ ~ x ~ O m
_ ~ o o o ~ o o ~ ~ ~ o
o~ 1 ~ d- ~ ~0 r-- oo CJ~ O ~ C` ~') d-
,_ oo oo oo x x x oo x oo cr~ ct~ C~ Cl~ cr~ ct~
WO 95/24207 2 1 8 4 3 8 5 PCT/US95/02938
-- 43 --
o o o o o o
o o o o o o
~ 8 o o ~ o Q
D 1~ Y 0~ ~C
O ~ O 00 ~ ~
_ _ _ _ _ _
G
5: ~ X
~ V
V ~
Z Z .~i Z Z Z Z Z
X
~ ~ V
I ~ ~ ~ ~ ~ X 5
V
X
V ~
~:1 Z Z ~ Z Z Z Z Z
~ ~ ~l o ~ ~ v ~ ~
~l ~
:~ x o
~1oooooooo
ol ~O 1~ x c~ O ~
~ ~ o~ o o o o
WO 95/24207 PCTIUS95/02938
843a5
- 44 -
TABLE 10
Et ~C-A
~,CH3
NH~
o Pr
No. R~ B6 A Kobs/~II
304 CH3 CH3 -OCH2CH2N(CH3)2563,000
305 CH3 CH3 -OCH2CH2N(Et)2 749~000
306 CH3 CH3 -OCH2CH2N(i-Pr)2 612,000
307 CH3 CH3 -N(CH3)CH2CH2N(CH3)2 352,000
308 CH3 CH3 -N(cH3)cH2cH2N(Et)2 377,000
309 CH3 CH3 -N(Et)CH2CH2N(CH3)2 398,000
310 H OH -N(CH3)CH2CH2N(CH3)2 838,000
Enzyme Assays for the Inhibition of Human Polymorphonuclear
Leukocyte Elastase Via Hydrolysis of N-t-Boc-alanyl-alanyl-
prolyl~l~nine-p-nitroanilide (Boc-AAPAN) or N-t-Boc-alanyl-
25 prolylvaline-p-nitro-anilide (Boc-AAPVN) Reagent:
0.05M TES (N-tris[hydroxymethyl]methyl-2-mino-
ethanesulfonic acid) Buffer, pH 7.5.
0.2 mM Boc-AAPAN or Boc-AAPVN.
To prepare substrate, the solid was first dissolved in 10.0
30 ml DMSO. Buffer at pH 7.5 was then added to a final volume of 100
ml.
Crude extract of human polymorphonuclear leukocytes
(PM:N) cont~inin~ elastase activity.
WO 95124207 2 1 8 4 3 8 5 PCT/US95/02938
- 45 -
Inhibitors (azetidinones) to be tested dissolved in DMSO
just before use.
To 1.0 ml of 0.2 mM Boc-AAPAN in a cuvette, 0.01-0.1
5 ml of DMSO with or without inhibitor was added. After mixing, a
measurement was taken at 410 m,u to detect any spontaneous hydrolysis
due to presence of test compound. 0.05 ~illiliters of PMN extract was
then added and the ~OD/min at 410 m~l was measured and recorded.
Beckman model 35 spectrophotometer was used.
Results were expressed to the nearest thousand Kobs/I
which is the second order rate constant in per mole per second for
inactivation of the enzyme.
The elastase activity in the crude PMN extract may vary
from one preparation to another. A control of each new batch is run,
15 and the volume added in the assay procedure is adjusted according to
actlvity.
This invention also relates to a method of treating
infl~mm~tion in patients using a compound of Formula (I), particularly
a preferred compound as the active constituent.
It has been found that the compounds of Formula (I) are
effective inhibitors of the proteolytic function of hllm~n neutrophil
elastase.
Accordingly, the compounds of Formula (I), can be used to
reduce infl~mm~tion and/or relieve pain in diseases such as emphysema,
rheumatoid arthritis, osteoarthritis, gout, bronchial i~Lfl~mm~tion,
chronic or acute bronchitis, cystic fibrosis, adult respiratory distress
syndrome, atherosclerosis, sepsis, septicemia, shock, periodontitis,
glomerular nephritis or nephosis, myocardial infarction, reperfusion
injury, infectious a~ ;Lis, rheumatic fever and the like, and may
- 30 reduce hemorrhage in acute promyelocytic leukemia and the like.
For each of the uses, the compounds of Formula (I) and
(F)-actin shortening ~ro~ s, may be ~tlmini.stered orally, topically,
parenterally, by inh~l~tion spray or rectally in dosage unit Formulations
cont~ining conventional non-toxic ph~rm~ceutically acceptable carriers,
adjuvants and vehicles. The term parenteral as used herein includes
WO 95/24207 PCT/US95/02938
2 ~ 84 385
- 46 -
subcutaneous injections, intravenous, intramuscular, intrasternal
injection or infusion techniques. In addition to the treatment of warm-
blooded ~nim~l~ such as mice, rats, horses, dogs, cats, etc., the
compounds of the invention are effective in the treatment of hllm~n~.
The ph~ ceutical compositions cont~ining the active
ingredient may be in a form suitable for oral use, for example, as
tablets, troches, lozenges, aqueous or oily suspensions, dispersible
powders or granules, emulsions, hard or soft capsules, or syrups or
elixirs. Compositions intended for oral use may be prepared according
to any method known to the art for the manufacture of pharmaceutical
compositions and such compositions may contain one or more agents
selected from the group consisting of sweetening agents, flavoring
agents, coloring agents and preserving agents in order to provide
ph~rm~ceutically elegant and palatable preparation. Tablets contain the
active ingredient in admixture with non-toxic ph~ ceutically
acceptable excipients which are suitable for the manufacture of tablets.
These excipients may be, for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; gran~ ting and disintegrating agents, for example, corn
starch, or alginic acid; binding agents, for example starch, gelatin or
acacia, and lubricating agents, for example, magnesium stearate, stearic
acid or talc. The tablets may be uncoated or they may be coated by
known techniques to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a
longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed.
Form~ tions for oral use may also be presented as hard
gelatin capsules wherein the act*e ingredient is mixed with an inert
solid diluent, for example, calcium carbonate, calcium phosphate or
kaolin, or as soft gelatin capsules wherein the active ingredient is mixed
with water or an oil medium, for example, peanut oil, liquid paraffin,
or olive oil.
Aqueous suspensions contain the active materials in
admixture with excipients suitable for the m~mlf~cture of aqueous
WO 95/24207 PCTtUS95/02938
- 2184385
- 47 -
suspensions. Such excipients are suspending agents, for example,
sodium carboxymethylcellulose, methylcellulose, hydroxypropylmethyl-
cellulose, sodium ~lgin~te, polyvinylpyrrolidone, gum tragacanth and
gum acacia; dispersing or wetting agents may be a naturally-occurring
phosphatide, for example, lecithin, or condensation products of an
alkylene oxide with fatty acids, for example, polyoxyethylene stearate,
or condensation products of ethylene oxide with long chain aliphatic
alcohols, for example, heptadecaethyleneoxycetanol, or condensation
products of ethylene oxide with partial esters derived from fatty acids
and a hexitol such as polyoxyethylene sorbitol monooleate, or
condensation products of ethylene oxide with partial esters derived from
fatty acids and hexitol anhydrides, for example, polyoxyethylene
sorbitan monooleate. The said aqueous suspensions may also contain
one or more preservatives, for example, ethyl, or n-propyl, p-hydroxy-
benzoate, one or more coloring agents, one or more flavoring agents,
and one or more sweetening agents, such as sucrose or saccharin.
Oily suspension may be for T ~ ted by suspending the
active ingredient in a vegetable oil, for example, arachis oil, olive oil,
sesame oil or coconut oil, or in a mineral oil such as liquid paraffin.
The oily suspensions may contain a thickening agent, for example,
beeswax, hard paraffin or cetyl alcohol. Sweetening agents such as
those set forth above, and flavoring agents may be added to provide a
palatable oral l~r~aration. These compositions may be preserved by the
addition of an antioxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation
of an aqueous suspension by the addition of water provide the active
ingredient in ~lmixtllre with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending agents are exemplified by those already
mentioned above. Additional excipients, for example, sweetening,
flavoring and coloring agents, may also be present.
The ph~rm~ceutical compositions of the invention may also
be in the form of oil-in-water emulsions. The oily phase may be a
vegetable oil, for example, olive oil or arachis oils, or a mineral oil, for
WO 95/24207 PCT/US95/02938
2l 84385 --
- 48 -
example, liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally-occurring gums, for example, gum acacia or
gum tragacanth, naturally-occurring phosphatides, for example, soy
bean, lecithin, and esters or partial esters derived from fatty acids and
hexitol anhydrides, for example, sorbitan monooleate, and condensation
products of the said partial esters with ethylene oxide, for example,
polyoxyethylene soll,i~l monooleate. The emulsions may also contain
sweetening and flavoring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example, glycerol, propylene glycol, sorbitol or sucrose.
Such formulations may also contain a demulcent, a preservative and
flavoring and coloring agents. The ph~rm~ceutical compositions may
be in the form of a sterile injectable aqueous or oleagenous suspension.
This suspension may be formulated according to the known art using
those suitable dispersing or wetting agents and suspending agents which
have been mentioned above. The sterile injectable preparation may also
be a sterile injectable solution or suspension in a non-toxic parenterally-
acceptable diluent or solvent, for example, as a solution in 1,3-butane
diol. Among the acceptable vehicles and solvents that may be employed
are water, Ringer's solution glucose in water and isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or suspending medium. For this purpose any
bland fixed oil may be employed including synthetic mono- or
diglycerides. In addition, fatty acids such as oleic acid find use in the
preparation of injectables.
The ph~rm~ceutical compositions of this invention may also
be ~tlmini~tered in the form of suppositories for rectal ~lmini.ctration of
the drug. These compositions can be prepared by mixin~ the drug with
a suitable non-irritating excipient which is solid at ordhlaly
temperatures but liquid at the rectal temperature and will therefore melt
in the rectum to release the drug. Such materials are cocoa butter and
polyethylene glycols.
For topical use, creams, ointments, jellies, solutions or
suspensions, etc., cont~inin~ the anti-infl~mm~tory agents are employed.
WO 95/24207 PCTtUS95/02938
- 21 8~385
- 49 -
The amount of active ingredient(s) that may be combined
with the carrier materials to produce a single dosage form will vary
depending upon the host treated and the particular mode of
~1mini~tration. For example, a formulation intended for the oral
~lmini~tration of hllm~n~ may contain from S mg to 2000 mg or 5000
mg of each active agent(s) compounded with an appropriate and
convenient amount of carrier material which may vary from about 5 to
about 95 percent of the total composition. For purposes of this
o specification, this broad dosage range is specif1cally intended to include,
but is not limited to, range of 5 mg to 2000 mg; 25 mg to 2000 mg; 5
mg to 1000 mg; 25 mg to 1000 mg; 5 mg to 500 mg; and 25 mg to 500
mg. Dosage unit forms will generally contain between from about 25
mg to about 500 mg of active ingredient(s).
Furthelll.ore, it is also possible that most effective
treatment may warrant ~lmini~tration of an initial dosage of one range
(e.g., 1-5 mg of active agent per kg of patient weight) followed by
~tlmini~tration of a second range (e.g., 0.1 to 1 mg of active agent per
kg of patient weight).
It will be understood, however, that the specific dose level
for any particular patient will depend upon a variety of factors
including the activity of the specific compound employed, the age, body
weight, general health, sex, diet, time of ~lmini~tration, route of
~lmini~tration, rate of excretion, drug combination and the severity of
25 the particular disease undergoing therapy.
With specific regard to (F)-actin shortening proteins, in
particular gelsolin, the dosage can be calculated in the following
manner. The normal blood gelsolin concentration is 2.4 ,uM (2.4
~moVL), and the normal blood DBP concentration is 5 ,uM (5 ,umol/L).
30 Thus, the total blood actin-binding capacity (ABC) is approxim~tely 7.5
~lmoVL. The blood volume if 6% of the body weight, hence a 70 Kg
person has 4.2 liters of blood and thus (4.2 L x 7.5 ~mol/L) 31.5 ,umols
ABC. Since DBP and gelsolin are distributed throughout the
extracellular space (which is 10% of the body weight, the body contains
(7.5 x 7) 52.5 ,umols ABC.
WO 95/24207 PCT/US95/02938
2 1 843~5
- so -
It may be desired to ~lmini.~ter between 32 and 53 llmols
of an actin binding (herein actin shortening) compound (or 0.46
~mol/kg body weight) to cover total complexing or depletion of
endogenous ABC. Since 0.425 mg of actin is equal to 1 ,umol, and since
there is 4.86 mg actin per gram of skeletal muscle, each gram of muscle
contains 11.3 ,umol actin or 4.6 grams of muscle destruction could
neutralize total body ABC. However, because the toxic effects of actin
are presumably local (e.g., inhibition of clot lysis), sequestered or
kinetically determined (e.g., actin permeates an organ faster than
binding proteins neutralize it), it is likely that a theoretically minimum
dose will have to be adjusted upward in order to achieve kinetically
favorable therapeutic effects. The kinetic effect can be important, for
example, since hemolysis of about half of erythron, which should
liberate only 4.2 ,umol of actin, reduces the pl~mi~ gelsolin
concentration by half acutely (Smith, et al., Blood 72:214-2181 (1988)),
suggesting slow equilibration between extravascular and blood
compartments. Conversely, a therapeutically effective state, capable of
breaking up local deposits of actin, may be achievable only by a
transient pulse of a high concentration of actin-binding molecules.
The compounds of the invention can be ~(lmini~tered in any
ayyroyliate ph~ cological carrier for a(lmini~tration. They can be
~lmini~tered in any form that effects prophylactic, palliative,
preventative or curing conditions of tissue injury in h~lm~n~ and
~mm~l~
The following example illustrates the preparation of the
compounds of Formula I useful in the method of treatment of the
present invention, but does not limit the scope of the invention. Starting
materials may be optionally prepared as disclosed in EPO 337 549
published.
October 18, 1989 which is hereby incorporated by
reference. Where ayyloyliate, compounds may be produced and used
in the form of ph~rm~ceutically acceptable salts. For example, the basic
compounds may be used in the form of a hydrochloride or mesylate or
WO 95/24207 PCT/US95/02938
- 21 84385
other acceptable salt. See Preformulation in Remington's
Pharmaceutical Sciences, Mack Publi~hin~, Easton PA.
SCHEME 1
F
~o R1
~\~ R2
NH ~,~
M R3
o N~\Q-C-CI
~ R2
NH~
2 R3
- M
R7
H-Y-N~
R8
~N O~ \O-C-Y-N~R
,~R2
NH ~,~
- 3 M R3
WO 95/24207 PCI~/US95/02938
2 1 84385
- 52 -
SCHEME 2
R ~ ~Q IOI OH
/ ~ \
M R3
oxalyl Br2CH2c0
chloride base
Ex 3a Ex 1 A
CH3
Ex 3B HO-CH-CO2Bnb-C-O-CH2-C02-
TFA Ex 1B
anisole
R CH3
\Q-C-O-CH-CO2Bn ,~
7 `Q-C-O-CH2-cO2H
, R7
Ex 4 H2/cat R8
~ CDI
~1l 1l ,R7
-C-O-CH2-C-N~ R
WO 95/24207 PCT/US95/02938
21 84385
- 53 -
- SCHEME 2 CONrr'D
s 1) oxalyl chloride
,R7
2) HN\ Ex 5
R8
8 ICH3
-C-O-CH-CO2H
s5~ 1l ICH3 1l R7
-C-O-CH-C-N
The glycolic acid derivatives described herein can be
prepared according to the following scheme. The starting acid (as
2s carboxylate anion) may be alkylated (Ex lA) with a suitably protected
a-halo acetic acid derivative to give the glycolate ester _ which can be
deprotected (Ex lB) to the glycolic acid ester 5. Treatment of 5 with an
amine lltili7.in~ a condensing agent such as dicyclohexylcarbodiimide or
carbomyldiinidazole (Ex 2) affords the deserved amide 6. Alternately,
30 the starting acid 1 may be converted to its acid chloride 2 (Ex 3A) and
- treated with a suitably protected a-hydroxyalkanoic acid (Ex 3B) in the
presence of base to give the protected ester 7. Deprotection (Ex 4),
followed by conversion to the acid chloride and treatment with the
ayyroyliate amine (Ex 5) affords the desired amide 9.
WO 95/24207 PCT/US9S/02938
21 ~4385
- 54 -
EXAMPLE 1
A. t-Butoxycarbonylmethyl [S-(R*, S*)]4-((3,3-diethyl-1-
(((1-(4-methylphenyl)butyl)amino)-carbonyl)-4-oxo-2-
azetidinyl)-oxy)benzoate
To a solution of 0.806 gm of [S-(R*, S*)] 4-((3,3-diethyl-1-
((( 1 -(4-methylphenyl)butyl)amino)-carbonyl)-4-oxo-2-azetidinyl)oxy)-
benzoic acid in ~3ml DMF is added 0.23 gm. triethylamine followed by
0.50 gm of t-butyl bromoacetate and the mixture stirred overnight at
room temperature. Ethyl acetate (25 ml) is then added and the resultant
mixture is washed with 2 x 10 ml water, 10 ml saturated sodium
bicarbonate, and 20 ml brine. The organic layer is dried through
sodium sulfate and concentrated in vacuo. Chromatography on Silica
gel 60 (350 ml column) and elution with 10% ethyl acetate in hexanes
gave 0.67 gm of the t-Butoxycarbonylmethyl [S-(R*, S*)]4-((3,3-
diethyl- 1-(((1 -(4-methylphenyl)butyl)amino)carbonyl)-4-oxo-2-
azetidinyl)oxy)benzoate .
In a similar m~nner can be prepared 2-(dimethylamino)-2-
oxoethyl, (S-(R* ,S*))-4-((3 ,3 -Diethyl- 1-(((1 -(4-methylphenyl)butyl)-
amino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoate (Compound 6), and
2-(N-methylacetamido)ethyl. ~2-(R*,S*)}- 4-{ {3,3-Diethyl-1-{ { { 1-(4-
methylphenyl)butyl } amino ) carbonyl } -4-oxo-2-azetidinyl } oxy } -
benzoate, (Compound 7).
B. Carboxymethyl [S-(R*,S*)]4-((3,3-diethyl-1- (((1-(4-
methyl-phenyl)butyl)amino)carbonyl)-4-oxo-2-
azetidinyl)-oxy)benzoate
To the above ester is added 2 ml of anisole and the
resulting mixture is cooled in an ice bath and 5 ml of ice cold
trifluoroacetic acid is added. The reaction mixture is stirred cold for
three hours then allowed to come to room temperature. After 30
minlltes, the reaction mixture is concentrated in vacuo and the residue
chromatographed on silica gel 60. Elution with 20% ethyl acetate in
hexanes cont~ining 1% acetic acid gives 0.53 gm of desired
WO 95/24207 2 1 8 4 3 8 5 PCT/US95/02938
- 55 -
carboxymethyl [S-(R* ,S *)]4-((3,3 -diethyl- 1 -(((1 -(4-methyl-
phenyl)butyl)amino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoate.
Compound 2
Analysis: C28H34N2o7+o.3H2o
Calc: C,65.19;H,6.75;N,5.43
Found: C, 65.30; H, 6.76; N, 5.23.
Compound 6
o Analysis: C30H39N306+0.5H20
Calc: C,65.91;H,7.38;N,7.60
Found: C, 65.71; H, 7.63; N, 7.50.
Compound 7
Analysis: C31H41N306+0.6EtOAc
Calc: C, 66.35; H, 7.64; N, 6.95
Found: C, 66.52; H, 7.89; N, 6.83.
EXAMPLE 2
2-(bis(2-hydroxyethyl)amino)-2-oxoethyl(S-(R*,S*))-4-((3,3-
diethyl- 1 -(((1 -(4-methyl-phenyl)butyl)amino)-carbonyl)-4-oxo-2-
azetidinyl)oxy) benzoate
To a solution of 0.125 gm of the acid from lB in 2-3 ml of
methylene chloride is added 0.050 gm of carbonyldiimidazole. The
mixt~lre is stirred for 30 minutes at room temperature at which time
0.060 gm of diethanol~mine is added along with 1 ml of DMF and 2 ml
of methylene chloride. The resulting mixture is stirred overnight at
room temperature then concentrated in vacuo. Silica gel
chromatography of the residue using 2.5 to 5.0% methanol in methylene
chloride gives 0.123 gm of the desired Compound 3, 2-(bis(2-hydroxy-
ethyl)amino)-2-oxoethyl(S-(R*,S*))-4-((3,3-diethyl-1-(((1-(4-methyl-
phenyl)butyl)amino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoate .
WO 9S/24207 PCT/US95/02938
21 843~5
- 56 -
Compound 3
Analysis: C32H43N3O8~ +0.4H20
Calc: C, 63.53; H, 7.30; N, 6.95
Found: C, 63,51; H, 7.45; N, 6.95.
Similarly were prepared
Compound 4
Analysis: C31H40N4O7
Calc: C, 64.12; H, 6.94; N, 9.65
Found: C, 64.12; H, 7.18; N, 9.44.
Compound 5
Analysis: C32H43N309 +0.3H20
Calc: C, 62.08; H, 7.09; N, 6.79
Found: C, 61.89; H, 7.39; N, 6.88.
Compound 8
Analysis: C40H47N3o8
Calc: C, 68.85; H, 6.79; N, 6.02
Found: C, 68.79; H, 7.06, N, 5.88.
EXAMPLE 3
Preparation of 1 -Methyl-2-oxo-2-(phenylmethoxy)ethyl(2S-( l (S *) ,R* ,-
(R)))-4-((3,3-diethyl- 1 -(((1 -(4-methylphenyl)butyl)amino)carbonyl)-4-
oxo-2-azetidinyl~oxy) benzoate. Compound 10
To a solution of 1.0 gm [S-(R*, S*)]4-((3,3-diethyl-1-(((1-
(4-methylphenyl)butyl)amino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoic
acid in 10 ml methylene chloride is added 2 ml of oxalyl chloride
followed by a catalytic amount of DMF. The reaction is stirred 1 hour
at room temperature then concentrated in vacuo to yield the acid
chloride which is used as is in the next step.
WO 95/24207 PCT/US9S/02938
21 84385
A solution of the above acid chloride in 10 ml of methylene
chloride is cooled in an ice bath and a solution of 1.25 gm benzyl L-
lactate and 2.0 gm of triethyl~mine in 10 ml of methylene chloride is
added. The mixture is stirred at room temperature overnight then
concentrated in vacuo. Chromatography of the residue on silica gel
using methylene chloride as the eluent yields 0.795 of the desired 1-
Methyl-2-oxo-2-(phenylmethoxy)ethyl (2S-(l(S*),R*,(R)))-4-((3,3-
diethyl- 1-(((1 -(4-methylphenyl)butyl)amino)carbonyl)-4-oxo-2-
azetidinyl)oxy)benzoate, Compound 10.
Analysis: C36H42N207
Calc: C, 70.34; H, 6.89; N, 4.56
Found: C, 70.45; H, 7.05; N, 4.48.
EXAMPLE 4
Preparation of 1-carboxyethyl [S-(R*,S*)] 4-((3,3-diethyl-1-(((1-(4-
methyl-phenyl)butyl)amino)carbonyl)-4-oxo-2-azetidin-yl)oxy)benzoate
A mixture of 0.69 gm of the benzylester prepared in
Example 3 and 0.2 gm 10% Pd/C in 10 ml of EtOAc is treated with
hydrogen at 40 psi. When the reaction is complete the n~ ule is
filtered and concentrated in vacuo to yield 0.56 gm of l-carboxyethyl
[S-(R*,S*)]4-((3,3-diethyl-1 -(((1 -(4-methyl-phenyl)butyl)amino)-
carbonyl)-4-oxo-2-azetidinyl)oxy)benzoate, Compound 11.
Analysis: C29H36N2o7
Calc: C, 66.40; H, 6.92; N, 5.34
Found: C, 66.66; H, 7.26; N, 5.05.
WO 95/24207 PCTIUS95/02938
21 84385
- 58 -
EXAMPLE 5
2-(diethylamino)- 1 -methyl-2-oxoethyl[S-(R* ,S*)] -4-((3 ,3-diethyl- 1-
5 (((1-(4-methyl-phenyl)butyl)amino)-carbonyl)-4-oxo-2-azetidinyl)oxy)
benzoate
The acid (.250 gm) from Example 4 is treated with oxalyl
chloride according to the procedure of Example 3A and the
corresponding acid chloride is obtained. This material is dissolved in 5
o ml methylene chloride and 0.4 ml of diethylamine added. After 1 hour
the reaction mixture is concentrated in vacuo and the residue taken in
ethyl acetate and washed with saturated sodium bicarbonate solution.
The organic layer is dried through sodium sulfate, concentrated and the
residue chromatographed on silica gel. Elution with 5% of ethyl acetate
in methylene chloride gives Compound 12.
Analysis: C33H45N306
Calc: C, 68.37; H, 7.82, N, 7.25
Found: C, 68.40; H, 7.93, N, 7.40.
EXAMPLE 6
(S(R*,S*))-l -(((4-((3,3-diethyl-1-(((1 -(4-methyl-phenyl)butyl)amino)-
carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl)oxy)acetyl) L-proline
2s ---
When benzyl L-lactate is replaced by L-proline benzyl ester
hydrochloride and triethyl~mine in the procedure of Example 3 the
corresponding amide with L-proline benzyl ester, Compound 8, is
obtained.
Analysis: C40H47N308
Calc: C, 68.85; H, 6.79, N, 6.02
Found: C, 68.79; H, 7.06, N, 5.88.
WO 95/24207 2 1 8 4 3 8 5 PCT/US~5 ~2g38
- 59 -
Reduction of the material obtained in Fx~mple 6A
according to the procedure of Example 4 affords Compound 9.
Analysis: C33H41N308+0.5H20
Calc: C, 64.27; H, 6.86; N, 6.81
Found: C, 64.49; H, 6.90; N, 6.68.
EXAMPLE 7
[S-(R*,S*)] 1-(((4-((3,3-diethyl-1-(((1-(4-methyl- phenyl)butyl)amino)
carbonyl-4 -oxo-2-azetidinyl)oxy)benzoyl)oxy)acetyl-N -benzyl-L-
prolinamide
Treatment of the acid obtained in Example 6B, Compound-
9~ with oxalyl chloride according to Example 3A gives the
corresponding acid chloride which when treated with benzylamine gives
the desired benzyl amide, Compound 19.
Analysis: C40H4gN4O7
Calc: C, 68.95; H, 6.94; N, 8.04
Found: C, 68.93, H, 7.02; N, 7.96.
EXAMPLE 8
To a solution of the acid chloride (~clJarcd from 0.55 gm
of [S-(R*, S*)] 4-((3,3-diethyl-1-(((1-(4-methylphenyl)butyl)amino)-
carbonyl)-4-oxo-2-azetidinyl)oxy)benzoic acid according to the
procedure of Example 3A) in 3 ml of methylene chloride is added 0.15
gm of N,N-dimethylaminoethanol. The reaction mixture is stirred
overnight at room temperature, concentrated in vacuo, then taken up in
ethyl acetate (25 ml) and washed with saturated sodium bicarbonate
solution. The organic layer is dried through sodium sulfate and
concentrated in vacuo. Silica gel chromatography of the residue using
2.5% methanol in methylene chloride g*es 0.59 gm of Compound 1,
2-(dimethylamino)ethyl (S-(R*,S*))-4-((3,3-diethyl-1-(((1-(4-methyl-
phenyl)butyl)amino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoate
WO 95t24207 PCT/US95/02938
2 1 84385
- 60 -
Analysis: C30H41N3O5
Calc: C, 68.81; H, 7.89; N, 8.02
Found: C, 68.85; H, 8.09; N, 7.97.
When N,N-dimethylaminoethanol~mine is replaced by the
appropriate amino alcohols the corresponding esters are obtained.
Compound 13 1-Dimethylamino-2-propyl [S-(R*,S*)]-4-[[3,3-diethyl-
1 -[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl]-4-oxo-2-
azetidin-yl]oxy]benzoate
Analysis: C31H43N305
Calc: C, 69.25; H, 8.06; N, 7.82
Found: C, 68.97; H, 8.01; N, 7.80.
Compound 14 3-Dimethylamino-l-propyl [S-(R*,S*)]-4-[[3,3-diethyl-
1 -[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl]-4-oxo-2-
azetidin-yl]oxy]benzoate
Analysis: C31H43N3O5
Calc: C, 69.25; H, 8.06; N, 7.81
Found: C, 68.85; H, 8.19; N, 7.72.
Compound 16 2-Diethylaminoethyl [S-(R*,S*)]-4-[[3,3-diethyl- 1 -[[[1 -
(4-methyl-phenyl)butyl]amino]carbonyl]-4-oxo-2-
azetidinyl] -oxy]benzoate
Analysis: C32H45N306
Calc: C, 69.66; H, 8.22; N, 7.62
Found: C, 69.37; H, 8.41; N, 7.51.
Compound 17 2-(1-[4-morpholino]e~yl) [S-(R*,S*)]-4-[[3,3-diethyl-1-
[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl]-4-oxo-2-
azetidinyl]oxy]benzoate
WO 95/24207 PCTlU~5~ '93fi
- 21 a438s
- 61 -
Analysis: C32H43N306
Calc: C, 67.94; H, 7.66; N, 7.43
Found: C, 67.67; H, 7.90; N, 7.26.
Compound 18 4-dimethylaminobutyl [S-(R*,S*)]4-[[3,3-diethyl-1-[[[1-
(4-methyl-phenyl)butyl]amino]carbonyl]4-oxo-2-
azetidinyl]oxy]benzoate
Analysis: C32H45N305 +0.2 H20
Calc: C, 69.21; H, 8.24; N, 7.56
Found: C. 69.35; H, 8.24; N, 7.29.
Compound 20 2-dimethylamino-2-methyl-1-propyl [S-(R*,S*)]-4-[[3,3-
diethyl-1 -[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl]-
4-oxo-2-azetidinyl]oxy]benzoate
Analysis: C32H45N3O5
Calc: C, 69.66; H, 8.22; N, 7.62
Found: C, 69.52; H, 8.47; N, 7.59.
Compound 21 2-(diisopropylamino)ethyl [S-(R*,S*)]-4-[[3,3-diethyl-1-
[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl]4-oxo-2-
azetidinyl]oxy]benzoate
Analysis: C34H49N305
Calc: C, 70.44; H, 8.52; N, 7.25
Found: C, 70.28; H, 8.76; N, 7.13.
Compound 22 Benzyl [S-(R*,S*)]4-[2-[[4-[[3,3-diethyl-1-[[[1-(4-
methylphenyl)butyl]amino]carbonyl]4-oxo-2-
azetidinyl]oxy]-benzoyl]oxy]-ethyl]-l -Piperazine-
carboxylate
Analysis: C40HsoN407
Calc: C, 68.75; H, 7.21; N, 8.02
Found: C, 68.39; H, 7.30; N, 7.84.
WO 95/24207 PCT/US9S/02938
2 1 84385 --
- 62 -
Compound 23 2-(dibutylamino)ethyl [S-(R*,S*)]-4-[[3,3-diethyl-1-[[[1-
(4-methyl-phenyl)-butyl]amino]carbonyl]-4-oxo-2-
azetidin-yl]oxy]benzoate
Analysis: C36Hs3N305
Calc: C, 71.14; H, 8.79; N, 6.91
Found: C, 71.00; H, 9.03; N, 6.81.
Compound 24 [S-(R*,S*)]-6-(dimethylamino)hexyl-4-[[3,3-diethyl-1-
[[[1 -(4-methyl-phenyl)butyl]amino]carbonyl] -4-oxo-2-
azetidin-yl]oxy]-benzoate
Analysis: C34H49N305+1H20
Calc: C, 68.31; H, 8.60; N, 7.03
Found: C, 68.34; H, 8.29; N, 6.86.
Compound 26 2-(4-me~yl-1-piperazinyl)ethyl[S-(R*,S*)]-4-[[3,3-
diethyl-1-[[[1-(4-methyl-phenyl)butyl]amino]carbonyl]-
4-oxo-2-azetidinyl]oxy]benzoate,
Analysis: C33H46N405+0.8H20
Calc: C, 66.82; H, 8.09; N, 9.44
Found: C, 67.28; H, 8.10; N, 8.96.
Compound 28 2- (diphenylamino)ethyl [S -(R * ,S *)] -4- [ [3,3 -diethyl- 1 -
[[[1 -(4-methylphenyl)-butyl]amino]carbonyl] -4-oxo-2-
azetidin-yl]oxy]benzoate
Analysis: C40H4sN30s +1.4H20
Calc: C, 71.40; H, 7.16; N, 6.25
Found: C, 71.62; H, 6.99; N, 5.99.
Compound 29 2-(di-2-propenylamino)ethyl [S-(R*,S*)]-4-[~3,3-diethyl-
1 -[[[1 -(4-methylphenyl)-butyl]amino]carbonyl]-4-oxo-2-
azetidin-yl]oxy]benzoate,
Analysis: C34H45N305
Calc: C, 70.93; H, 7.88; N, 7.30
Found: C, 71.18; H, 8.06; N, 7.34.
WO 95/24207 PCT/US95/02938
- 21 84385
- 63 -
Compound 30 2-(dimethylamino)-2-phenylethyl [S-(R*,-S*)]-4-[[3,3-
diethyl-l-[[[l- (4-methyl- phenyl)butyl]amino]carbonyl]-
4-oxo-2-azetidinyl]oxy]benzoate
Analysis: C36H45N3O5
Calc. C, 72.09; H, 7.56; N, 7.00.
Found: C, 71.75; H, 7.67; N, 6.70.
Compound 31 2-[methyl(phenylmethyl)amino]ethyl [S-(R*,S*)]-4-
o [[3,3-diethyl-1-[[[1-(4-methyl-phenyl)butyl]-
amino]carbonyl]-4-oxo-2-azetidinyl]oxy]benzoate
When [S-(R*,S*)]-4-[[3,3-diethyl-1-[[[1-(4-methyl-
phenyl) butyl]amino]carbonyl]-4-oxo-2-azetidinyl]oxy]-2,6-dimethyl
benzoic acid is used in place of [S(R*,S*)]-4-[[3,3-diethyl-1-[[[1-(4-
methyl-phenyl)butyl]amino]carbonyl]-4-oxo-2-azetidinyl]oxy]benzoic
acid in the procedure of Example 3A and allowed to react with the
ayyroyliate amino alcohols the following esters are obtained.
Compound 304 2-(dimethylamino)ethyl [S,(R*,S*)]-4-[[3,3-diethyl-
1 -[[[1 -(4-methylphenyl)-butyl]amino]carbonyl]-4-
oxo-2-azetidin-yl]oxy]-2,6-dimethyl-benzoate
Compound 305 2-~diethylamino)ethyl [S-(R*,S*)]-4-[[3,3-diethyl-1 -
2 5 [ [ [1 -(4-methylphenyl)-butyl] amino] carbonyl] -4-oxo-
2-azetidin-yl]oxy]-2,6-dimethyl-benzoate
Compound 306 2-[bis(l-methylethyl)amino]-ethyl [S-(R*,S*)]-4-
[[3,3-diethyl-1 -[[[1 -(4-methylphenyl)butyl]amino]-
carbonyl] -4-oxo-2-azetidinyl]oxy] -2,6-dimethyl-
benzoate
Tre~trnen~ of the acid [S-(R*,S*)]-4-[[3,3-diethyl-1-[[[1-
(4-methylphenyl)butyl]amino]carbonyl]-4-oxo-2-azetidinyl]oxy]-
benzene-acetic acid with oxalyl chloride according to the procedure
WO 95/24207 PCT/US95/02938
21 84385
- 64 -
of Example 3A affords the corresponding acid chloride which when
allowed to react with the a~rol~liate amino alcohol according to the
procedure of Example 8 gives the following amino esters:
Compound 32
Analysis: C31H43N3O5
Calc: C, 69.25; H, 8.06; N, 7.82
Found: C, 69.02; H, 7.86; N, 7.74.
Compound 33
Analysis: C32H45N3O5
Calc: C, 69.66; H, 8.22; N, 7.62
Found: C, 69.10; H, 8.17; N, 7.50.
Compound 34
Analysis: C33H47N305
Calc: C, 70.06; H, 8.38; N, 7.43
Found: C, 69.70; H, 8.41; N, 7.05.
Compound 35
Analysis: C33H45N306
Calc: C, 68.37; H, 7.82; N, 7.25
Found: C, 68.55; H, 8.19; N, 7.08.
Compound 36
Analysis: C32H45N305
Calc: C, 69.66; H, 8.22; N, 7.62
Found: C, 69.60; H, 8.49; N, 7.55.
EXAMPLE 9
To a solution of 0.247 g of Compound 1 in 2 ml of ethyl
acetate is added 0.125 gm of m-chloroperoxy benzoic acid. After 30
es at room temperature the reaction mix~lre is concentrated in
WO 95/24207 2 1 8 4 3 8 5 PCT/US95/02938
- 65 -
vacuo. Chromatography of the residue on silica gel using methylene
chloride/methanol/water 85/15/1.5 gives the desired N-oxide Compound
15.
Analysis: C3oH4lN3o8+l.4H2o
Calc: 63.79; H, 7.82; N, 7.44
Found: 63.89; H, 7.85; N, 7.27.
EXAMPLE 10
[S-(R*,S*)] 2-[4-[[[2-(dimethylamino)-ethyl]amino]-carbonyl]phenoxy]-
3,3 -diethyl-N- [1 -(4-methylphenyl)-butyl] -4-oxo- 1 -azetidinecarbox -
amide
To a solution of 0.104 g carbonyldiimidazole in 2 ml
methylene chloride is added a solution of 0.227 g of [S-(R*,S*)]~-
((3,3-diethyl-1-((-1-(4-methylphenyl)butylamino)carbonyl)-4-oxo-2-
azetidinyl)-oxy)benzoic acid in 3 ml methylene chloride. The
mixture is stirred at ambient tempe.a~ule for 30 mim-tes at which
time 0.100 g of N,N-dimethyl-ethylene~ mine is added. After
stirring overnight at room temperature the reaction mixture is
poured into benzene (50 ml) and washed with water. The organic
layer is separated, dried through sodium sulfate and concentrated in
vacuo. Silica gel chromatography using 5% methanol in methylene
chloride yields 0.160 g of 2-[4-[[[2-(dimethylamino)ethyl]amino]-
carbonyl]phenoxy]-3,3-diethyl-N-[1-(4-methylphenyl)butyl]-4-oxo-1-
azetidinecarbox~mitle.
(Compound 73).
Analysis: C30H42N404+0.4H20
Calc: C, 68.00; H, 8.14; N, 10.57
Found: C, 68.01; H, 8.18; N, 10.62.
WO 95/24207 2 1 8 4 3 8 5 PCTtUS95/02938
- 66 -
EXAMPLE 11
A. (S-(R*,S*))-4-((3,3-diethyl-1-((1-(4-methylphenyl)butyl-
amino)carbonyl)-4-oxo-2-azetidinyl)-oxy)-benzoyl
chloride
To a solution of 0.150 g of [S-(R*,S*)] 4-((3,3-diethyl-
1-(((1 -(4-methylphenyl)butyl)amino)-carbonyl)-4-oxo-2-azetidinyl)-
oxy)benzoic acid in 5 ml methylene chloride cont~ining a catalytic
o amount of dimethylformamide is added 0.5 ml of oxalyl chloride.
The mixture is stirred at room temperature for 30 minutes and then
concentrated in vacuo to yield (S-(R*,S*))-4-((3,3-diethyl-1-((1-(4-
methylphenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)-benzoyl
chloride.
B [S-(R*,S*)]-2-[4-[[[2-(dimethylamino)ethyl]methyl-
amino]carbonyl]-phenoxy]-3,3-diethyl-N-[1-(4-
methylphenyllbutyll-4-oxo- 1 -azetidinecarboxamide
The above acid chloride is dissolved in 3 ml methylene
20 chloride and a solution of 0.20 gm of N,N,N'-trimethylethylene-
min~ in 2 ml methylene chloride is added. The mixture is stirred
overnight and then concentrated in vacuo. The residue is extracted
between ethyl acetate and saturated sodium bicarbonate solution.
The organic layer is dried through sodium sulfate and concentrated
25 in vacuo. Silica gel chromatography of the residue (5% methanol in
methylene chloride) affords 0.117 g of [S-(R*,S*)]-2-[4-[[[2-
(dimethylamino)ethyl]methylamino]carbonyl]phenoxy]-3,3-diethyl-
N-[1-(4-methylphenyl]butyl]-4-oxo-1-azetidinecarboxamide.
(Compound 75).
Analysis: C3 1 H44N404+0.5H20
Calc: C, 68.23; H, 8.31; N, 10.27
Found: C, 68.20; H, 8.41; N, 10.10.
WO 95/24207 PCT/US95/02938
2 1 84385
- 67 -
When N,N,N'-trimethylethylene~ min~ of Example 1 lb
is replaced by the a~prol~-iate amines, there is obtained the
corresponding amides.
1) Compound 76
Analysis: C32H46N4O4
Calc: C, 69.79; H, 8.42; N, 10.17
Found: C, 69.02; H, 8.60; N, 9.54.
2) Compound 77
Analysis: C32H46N404 +0.75 H20
Calc: C, 68.12; H, 8.49; N, 9.93
Found: C, 68.22; H, 8.48; N, 10.17.
3) Compound 78
Analysis: C32H44N405
Calc. C, 68.06; H, 7.85; N, 9.92
Found: C, 67.84; H, 8,07; N, 9.62.
4) Compound 79
Analysis: C33H46N4O5 +H2O
Calc: C, 66.42; H, 8.11; N, 9.39
Found: C, 66.73; H, 8.19; N, 9.23.
S) Compound 80
Analysis: C35H52N406
Calc: C, 67.28; H, 8.39; N, 8.97
Found: C, 67.08; H, 8.77; N, 8.41.
6) Compound 81
Analysis: C33H48N404 +H2O
Calc: C, 68.01; H, 8.65; N, 9.61
Found: C, 68.42; H, 8.59; N, 9.17.
WO 95/24207 PCT/US95/02938
21 843~5
- 68 -
7) Compound 82
Analysis: C36H46N4O4 +0.5H20
Calc: C, 71.14; H, 7.79; N, 9.21
Found: C, 71.41; H, 7.68; N, 9.10.
8) Compound 83
Analysis: C32H46N404 +1.2H20
Calc: C, 67.15; H, 8.52; N, 9.79
Found: C, 67.21; H, 8.26; N, 9.47.
9) Compound 84
15 10) Compound 86
Analysis: C35H52N4O4
Calc: C, 70.91; H, 8.84; N, 9.45
Found: C, 70.37; H, 8.84; N, 8.77.
20 11) Compound 89
Analysis: C31H39N504 +0.7H20
Calc: C, 66.71; H, 7.29; N, 12.54
Found: C, 66.91; H, 7.40; N, 12.14
12) Compound 90
Analysis: C33H46N404 +0.8H20
Calc: C, 68.67; H, 8.31; N, 9.70
Found: C, 68.82; H, 8.11; N, 9.70.
30 13) Compound 91
Analysis: C34H48N404 + 0.3H20
Calc: C, 70.11; H, 8.41; N, 9.61
Found: C, 70.17; H, 8.64; N, 9.33.
WO 95/24207 PCT/US95/02938
-
21 84385
- 69 -
14) Compound 92
Analysis: C30H42N404 + 1.2H20
C alc: C, 66.14; H, 8.22; N, 10.29
Found: C, 66.18; H, 8.12; N, 10.31.
15) Compound 93
Analysis: C32H44N406 + 0.3H20
C alc: C, 65.57; H, 7.67; N, 9.56
Found: C, 65.72; H, 7.50; N, 9.34.
16) Compound 94
Analysis: C32H44N4O4 + 0.3H20
Calc: C, 70.04; H, 8.08; N, 10.21
Found: C, 70.34; H, 8.90; N, 8.93.
17) Compound 9S
alysis: C 33 H 46 N 4 0 4 + .S H 20
Calc: C, 69.32; H, 8.28; N, 9.80
Found: C, 69.41; H, 8.25; N, 9.58.
18) Compound 99
Analysis: C3gH54N404 + l.SH20
C alc: C, 69.37; H, 8.72; N, 8.51
Found: C, 69.48; H, 8.44; N, 8.36.
19) Compound 102
alysis: C 40 H 49 N 5 0 6 + 1.0 H 2 0
C alc: C, 67.30; H, 7.20; N, 9.81
Found: C, 67.50; H, 7.24; N, 9.53.
20) Compound 104
alysis: C 3 2 H 41 N 5 0 4 + 0.75 H 2 0
C alc: C, 67.04; H, 7.47; N, 12.21
Found: C, 67.16; H, 7.56; N,ll.9S.
WO 95/24207 PCT/US95/02938
21 84385
- 70 -
21) Compound 105
~41lalysis: C 32 H 44 N 4 0 5 + 0.5 H 2 0
C alc: C, 66.99; H, 7.91; N, 9.77
Found: C, 67,00; H, 8.25; N, 9.50.
22) Compound 106
A nalysis: C 3 2 H 45 N 5 0 5 +0.8H20
C alc: C, 64.69; H, 7.90; N, 11.78
Found: C, 64.93; H, 8.25; N, 11.12.
23) Compound 107
Analysis: C31H44N306S +0.3H20
Calc: C, 61.42; H, 7.41; N, 9.24
Found: C, 61.43; H, 7.54; N, 9.05.
24) Compound 110
Analysis: C35H44N404
C alc: C, 71.89; H, 7.58; N, 9.58
Found: C, 71.65; H, 7.55; N, 9.34.
25) Compound 111
alysis: C 34 H 42 N 4 0 4 + 0.5 H 2 0
C alc: C, 70.44; H, 7.47, N, 9.66
Found: C, 70.82, H, 7.46; N, 9.20.
26) Compound 113
~41lalysis: C 34 H 42 N 4 0 4 + 0.3 H 2 0
Calc: C, 70.88; H, 7.45; N, 9.72
Found: C, 71.12; H, 7.44; N, 9.32.
27) Compound 115
A nalysis: C34H42N404 +0.7H20
C alc: C, 69.99; H, 7.50, N, 9.60
Found: C, 70.14, H, 7.63; N, 9.25.
WO 95124207 PCT/U$95/02938
2 1 84385
28) Compound 116
Analysis: C34H46N404 +1.4H20
Calc: C, 68.06; H, 8.19; N, 9.33
Found: C, 68.40; H, 8.14; N, 8.87.
29) Compound 117
Analysis: C40H51N5O6 +0.6H20
Calc: C, 67.79; H, 7.42; N, 9.88
Found: C, 67.81; H, 7.58; N, 9.76.
30) Compound 118
Analysis: C33H47N504 +0.7H20
Calc: C, 67.14; H, 8.26; N, 11.86
Found: C, 67.54; H, 8.51; N, 11.28.
31)Compound119
Analysis: C35H48N404 +1.25H20
Calc: C, 68.76; H, 8.32; N, 9.16
Found: C, 69.07; H, 8.19; N, 8.75.
32) Compound 121
Analysis: C34H4gN4O4 +lH20
Calc: C, 68.66; H, 8.47; N, 9.42
Found: C, 69.02; H, 8.32; N, 9.06.
2s
33) Compound 125
Analysis: C37H48N404 +0.5H20
Calc: C, 71.47; H, 7.94; N, 9.01
Found: C, 71.65; H, 7.91; N, 8.73.
34) Compound 126
Analysis: C41H54N405 +2H20
Calc: C, 68.83; H, 8.25; N, 7.64
Found: C, 69.03; H, 7.79; N, 7.50.
WO 95/24207 PCT/US95/02938
21 84385
35) Compound 132
alysis: C 35 H 50 N 4 0 4
C alc: C, 71.15; H, 8.53; N, 9.48
Found: C, 70.90; H, 8.74; N, 9.12.
36) Compound 133
alysis: C 4 0 H 52 N 4 0 4 + 0.9 H 2 0
C alc: C, 71.80; H, 8.10; N, 8.37
Found: C, 71.86; H, 8.17; N, 8.18.
37) Compound 137
A nalysis: C37H48N404 +0.8H20
C alc. C, 70.85; H, 7.97; H, 8.93
Found: C, 71.01; H, 7.97; N, 8.54.
38) Compound 139
A nalysis: C 39 H 51 N 5 0 4 +0.8H20
C alc: C, 70.09; H, 7.93; N, 10.48
Found: C, 70.18; H, 7.79; N, 10.42.
39) Compound 142
Analysis: C38H55N506
C alc: C, 67.33; H, 8.18; N, 10.33
Found: C, 67.02; H, 8.31; N, 9.89.
40) Compound 143
A nalysis: C34H51N504
C alc: C, 68.77; H, 8.66; N, 11.80
Found: C, 68.57; H, 8.50; N, 11.53.
41) Compound 144
~nalysis: C 38 H 59 N 5 0 4 + 0.4 H 2 0
C alc: C, 69.45; H, 9.17; N, 10.65
Found: C, 69.69; H, 9.02; N, 10 35.
WO 95/24207 PCT/US9~/02938
21 84385
42) Compound 145
~41lalysis: C 36 H 47 N 5 0 4 + H 2 0
C alc: C, 68.44; H, 7.82; N, 11.08
Found: C, 68.69; H, 7.74; N, 10.76.
43) Compound 148
~41lalysis: C 36 H 47 N 5 0 4 + 0.4 H 2 0
C alc: C, 69.62; H, 7.56; N, 11.27
Found: C, 69.79; H, 7.70; N, 11.12.
44) Compound 149
~ alysis: C 39 H 50 N 4 0 4 + 0.4 H 2 0
C alc: C, 72.50; H, 7.93; N, 8.67
Found: C, 72.44; H, 7.99; N, 8.87.
45) Compound 150
~41lalysis: C 36 H 47 N 5 0 4 + 0.3 H 2 0
C alc. C, 69.83; H, 7.74; N, 11.31
Found: C, 69.93; H, 7.65, N, 11.10.
46) Compound 151
A nalysis: C33H47N504
C alc: C, 68.60; H, 8.20; N, 12.12
2s Found: C, 68.40; H, 8.16; N, 11.90.
47) Compound 245
~41lalysis: C 36 H 44 N 4 0 4
Calc: C, 72.46; H, 7.43; N, 9.39
Found: C, 72.49; H, 7.49; N, 9.25.
48) Compound 246
Analysis: C37H46N404 +0.25H20
C alc: C, 72.26; H, 7.61; N, 9.10
Found: C, 72.35; H, 7.83; N, 8.73.
WO 95/24207 PCT/US95/02938
21 34385
- 74 -
49) Compound 154
Analysis: C35H4gN4O4 +0.8H20
Calc: C, 69.70; H, 8.29; N, 9.29
Found: C, 69.65; H, 8.27; N, 9.35.
50) Compound 158
Analysis: C35H48N404 +0.5H20
Calc: C, 73.29; H, 7.65; N, 8.33
Found: C, 73.71, H, 7.75, N, 7.75.
51) Compound 159
Analysis: C35H4gN4O4
Calc: C, 73.09; H, 7.66; N, 8.31
Found: C, 73.40; H, 7.75; N, 7.80.
52) Compound 160
Analysis: C38H48N404 +1.0H20
Calc: C, 70.78; H, 8.12; N, 8.68
Found: C, 71.00; H, 8.05; N, 8.59.
53) Compound 161
Analysis: C43H52N404 +lH20
Calc: C, 73.05; H, 7.70; N, 7.92
Found: C, 73.29; H, 7.95; N, 7.37.
54) Compound 166
Analysis: C33H46N404 +1.5H20
Calc. C, 67.20; H, 8.37; N, 9.50
Found: C, 67.38; H, 7.98; N, 9.41.
55) Compound 171
Analysis: C36H52N406 +1.6H20
Calc: C, 64.95; H, 8.36; N, 8.41
Found: C, 65.26; H, 8.15; N, 8.07.
WO 95/24207 PCT/US95/02938
2 1 84385
56) Compound 177
Analysis: C3gH47N5O4
Calc: C, 71.56; H, 7.43; N, 10.98
Found: C, 71.64; H, 7.62; N, 10.93.
57) Compound 178
Analysis: C3gH50N4O4
Calc: C, 72.81; H, 8.04; N, 8.94
Found: C, 72.96; H, 8.17; N, 8.83.
58) Compound 179
Analysis: C3gH47N5O4
Calc: C, 71.56; H, 7.43; N, 10.98
Found: C, 72.00; H, 7.55; N, 10.87.
59) Compound 180
Analysis: C3gH47F3N4O4
Calc: C, 67.04; H, 6.96; N, 8.23
Found: C, 67.02; H, 7.25; N, 8.23.
60) Compound 181
Analysis: C3gH47F3N4O4
Calc: C, 67.04; H, 6.96; N, 8.23
Found: C, 66.63; H, 6.98; N, 7.94.
61)Compoundl82
Analysis: C37H47F N404
Calc: C, 70.45; H, 7.51; N, 8.88
Found: C, 70.28; H, 7.74; N, 8.82.
62) Compound 185
Analysis: C34H4gN4O4
Calc: C, 70.80; H, 8.39; N, 9.71
Found: C, 70.44; H, 8.45; N, 9.51.
WO 95/24207 PCT/US95102938
2 1 84385 --
- 76 -
63) Compound 186
Analysis: C37H4gN4O4 +0.8H20
Calc: C, 70.85; H, 7.97; N, 8.93
Found: C, 71.12; H, 8.25; N, 8.45.
64) Compound 191
A nalysis: C 42 H 5l N 5 0 4 +.5CH2C12
C alc: C, 69.78; H, 7.16; N, 9.58
Found: C, 69.75; H, 7.31; N, 9.68.
65) Compound 203
A nalysis: C37H47N404 +l.lH20
C alc: C, 68.31; H, 7.62; N, 8.61
Found: C, 68.32; H, 7.57; N, 8.53.
66) Compound 204
A nalysis: C37H47ClN404
C alc: C, 68.66; H, 7.32; N, 8.66
Found: C, 68.32, H, 7.48; N, 8.42.
67) Compound 205
alysis: C 38 H 50 N 4 0 5 + 0.7 H 2 0
C alc: C, 69.63; H, 7.90; N, 8.54
Found: C, 69.72; N, 7.91; N, 8.54.
68) Compound 206
A nalysis: C 39 H 52 N 4 0 6 +0.8H20
C alc: C, 68.15; H, 7.86; N, 8.15
Found: C, 68.01; H, 8.02; N, 8.15.
W O 95/24207 PC~rrUS95102938
- 21 84385
- 77 -
EXAMPLE 12
A. [S-(R*,S*)]3,3-Diethyl-2-[4-[[[2-(4-hydroxy-1-
piperidinyl)ethyl]amino]carbonyl]phenoxy]-N-[ l -(4-
methylphenyl)butyll -4-oxo- 1 -azetidinecarboxamide
When 1-(2-aminoethyl)-4-benzyloxypiperidine is used in
place of N,N,N-trimethylethylene ~ mine in the procedure of Example
1 lb there is obtained [S-(R*,S*)] 3,3-diethyl-2-[4-[[[2-(4-benzyloxy-1 -
piperidinyl]ethyl]amino] carbonyl]phenoxy] -N- [1 -(4-methylphenyl)-
butyl]-4-oxo- 1 -azetidinecarboxamide.
B. [S-(R+,S*)]-3,3-diethyl-2-[4-[[[2-(4-hydroxy-1-
piperidinyl)ethyl]amino]carbonyl]phenoxy]-N-[ l -(4-methyl-
phenyl)butyll-4-oxo- 1 -azetidine-carboxamide
A solution of the amide from Step A above in 10 ml of
glacial acetic acid cont~ining 22 mg of 10% Pd/C is hydrogenated under
42 lb hydrogen pressure. When TLC indicate completion of the
reaction, the n~i~ is filtered and concentrated in vacuo after the
addition of 50 ml toluene. The residue is dissolved in ethyl acetate,
washed with saturated sodium bicarbonate solution. The organic layer
is dried with sodium sulfate and concentrated in vacuo. The residue is
chromatographed on 15 g silica gel using 5% methanol in methylene
chloride and yields 96 mg of [S-(R+,S*)]-3,3-diethyl-2-[4-[[[2-(4-
hydroxy- 1 -piperidinyl)ethyl]amino]carbonyl]phenoxy]-N-[ l -(4-methyl-
phenyl)butyl]-4-oxo- 1 -azetidine-carboxamide.
Analysis: C33H46N4O5 +1.3H20
Calc: C,65.83;H,8.13;N,9.30
Found: C, 66.10; H, 8.06; N, 8.91.
When 1-(2-~minoethyl)-4-benzyloxypiperidine is replaced
in the procedure of F.x~mple 12 by the appropriate amines, the
following compounds 123, 124, 129, 131 and 138 are obtained, for
example:
WO 9S/24207 PCT/US95/02938
21 84385
- 78 -
1) Compound 129
Analysis: C34H4gN4O5
Calc: C, 68.89; H, 8.16; N, 9.45
Found: C, 68.68; H, 8.18; N, 8.65.
2) Compound 131
Analysis: C32H44N405 +1H20
Calc: C, 65.92; H, 7.96; N, 9.61
Found: C, 66.07; H, 7.86; N, 9.45.
3) Compound 138
Analysis: C33H46N405 +0.5H20
Calc: C, 67.43; H, 8.06; N, 9.53
Found: C, 67.61; H, 8.06; N, 9.37.
Diamine Intermediates
The ~ mines used to prepare the amino amides
described herein were commercially available or prepared according
to the following routes
R, 1.) Cl-CH2CN R,~
R/ 2.) LAH ~N-C H2 C H2-N H2
EXAMPLE 13
A.N-cyanomethylhomopiperazine
To a solution of 1.98 g homopiperazine in 50 ml acetone is
added 4.25 g of powdered anhydrous sodium carbonate and 1.3 ml of
30 chloroacetonitrite. After 24 hrs the reaction mixture is filtered and the
filter cake washed with 100 ml acetone. The combined filtrates are
concentrated in vacuo and the residue chromatographed on silica gel
using methylene chloride as the eluent. The yield of N-cyanomethyl
homopiperazine is 2.69 g.
WO 95124207 PCT/US95/02938
- 21 84385
- 79 -
B.N-(2-aminoethyl)homopiperazine
To a suspension of 1.02 g li~hiulll alllmimlm hydride in 50
ml of ether is slowly added a solution of 2.65 gm N-cyanomethyl
homopiperazine in 25 ml ether. After the addition is complete the
mixture is heated at reflux for 1 hour, then cooled to room temperature
and quenched carefully with 1 ml water, 1 ml of 15% sodium hydroxide
solution and 3 ml water. The mixture is filtered through sodium
sulfate, the filter cake washed well with ether and the combined filtrates
concentrated in vacuo to yield 2.60 g. N-(2-aminoethyl)homopiper-
azine.
R~
/N-CH2CH2-NH2 1.) HCO2Et, NaOEt,
R2 2.) BH3 THF
R1~
~N-CH2CH2-NH-CH3
R2
EXAMPLE 14
N-(2-methylaminoethyl)homopiperazine
A. N-(2-forrn~midoethyl)homopiperazine
To a carefully prepared solution of 0.718 g of 60% sodium
hydride dispension in 75 ml of absolute ethanol which has been cooled
to 0C is added 7.3 ml of ethyl formate. After 5 minutes there is added
a solution of 2.55 gm of N-(2-aminoethyl)homopiperazine in 25 ml
- absolute ethanol. The mixture is stirred at room temperature overni~ht.
Saturated sodium bicarbonate solution (15 ml) is then added and the
reaction mixture is stirred with 150 ml ethyl acetate, filtered through
MgS04 and the filtrate concentrated in vacuo. Chromatography of the
residue on 150 gm silica gel using an eluent of methylene
chloride/methanol/conc. ammonium hydroxide (95/5/0.5) gives 2.78 g
of N-(2-fonn~midoethyl)homopiperazine.
WO 95t24207 PCT/US95/02938
21 84385
- 80 -
B. N-(2-methylaminoethyl)homopiperazine
To a solution of 2.75 gm of N-(2-form~midoethyl)-
homopiperazine in 20 ml of dry THF under N2 is added carefully 60 ml
of borane THF solution. After the addition is complete the reaction
mixture is heated to reflux for S hours then stirred at room temperature
overnight. The reaction mixture is quenched by the careful addition of
20 ml of 6 N HCl followed by refluxing for 1 hour. The reaction
mixture is cooled, 50 ml of water added and solid KOH added carefully
to ~lk~line pH. Extraction with ether gives the desired product N-(2-
methylamlno)homopiperazlne .
EXAMPLE 15
~CH2
CH3NH-CH2CH2-N\ 1.) R-X, Na2CO3
CH3 2.) H2lHoAcpd(oH)2c
CH3-N-CH2CH2-NH-CH3
A. N-benzyl-N,N'-dimethyl-N'-(2-phenylethyl)ethylene-
diamine
A mixture of 0.900 gm N-benzyl-N,N'-dimethylethyl-
ene(li~mine, 1.10 gm powdered sodium carbonate and 0.75 ml of 2-
25 phenylethylbromide is refluxed for 5 hours. An additional 0.25 ml ofbromide is added during this time. The reaction mixture is then cooled
and filtered. The filterate is concentrated in vacuo and the residue
chromatographed on silica gel using an eluent of CH2C12/CH30H/-
NH40H (97/3/0.3) to yield 0.875 gm of N-benzyl-N,N'-dimethyl-N'-(2-
3 phenylethyl)ethylene~ mine.
.
B. N N'-dimethyl-N-(2-phenylethyl)ethylene~ mine
To a solution 0.870 gm N-benzyl-N-N'-dimethyl-N'-(2-
phenylethyl)ethylene~ mine in 10 ml ethanol and 5 ml acetic acid is
added 0.18 gm Pd (OH)2/C. The mixtllre is hydrogenated at 40 psi for
WO 95/24207 PCT/US95~'~,Z538
2 1 84385
- 81 -
3.5 hours, then filtered and concentrated in vacuo. The residue is made
akaline with lN NaOH and extracted well with ethyl acetate (5 X 25
ml). The combined extracts are filtered through sodium sulfate and
5 concentratedto yield N,N'-dimethyl-N-(2-phenylethyl)ethylene~ mine.
EXAMPLE 16
1.) Ar-CHO
N-CH2CH2-NH2
o R ~ 2.) LAH
Rl~
N-CH2CH2-NH-CH2-Ar
R2~
A solution of 1.28 gm 1-(2-amino-ethyl)piperdine and 1.07
gm pyridine-3-carboxaldehyde in 40 ml of toluene is heated to reflux
under a Dean Stark trap. After 10 ml toluene distilled over the NMR of
an aliquot indicated no aldehyde left. The reaction mixture was
concentrated and the imine used directly in the next step.
B.
To a suspension of 0.380 gm of lithium ~ minum hydride
in 30 ml of dry THF which has been cooled to -10C is added dropwise
a solution of the above imine in 20 ml of dry THF. After about 1 hour
the cold reaction mixture is quenched by the addition of S ml of 5 N
NaOH, then diluted with 100 ml ether and 20 ml of water. The organic
layer is separated, washed with brine, filtered thru sodium sulfate and
concentrated to give 2.17 gm of 1-[2-(3-pyridylmethylamino)ethyl]-
piperidine suitable for use in subsequent reactions.
WO 9S/24207 PCT/US95/02938
21 84385
- 82 -
EXAMPLE 17
A,CH2CI
R-NH-CH2-CH2-NH-R
f H2 Ar
R-NH-CH2 CH2-N-R
To 7.50 gm of N,N'-dimethylethylene~ mine which has
been cooled in an ice-ethanol bath is added portionwise over a 30
minute period 1.40 gm of 3-picolyl chloride. After stirring cold for 1
hour after the addition is completed, the reaction mixture is
concentrated in vacuo and the residue partitioned between 50 ml of
5 ether and 10 ml of 5 N NaOH solution. The organic layer is separated
and the aqueous layer extracted 2 times with 50 ml of ether. The
combined organic extracts are dried through sodium sulfate and
concentrated in vacuo. Chromatography on 150 gm silica gel using
CH2C12/CH30H/NH40H (90/10/1) as eluent gives 0.930 g of N,N'-
2 dimethyl-N-(3-pyridylmethyl)ethylene~ mine.
EXAMPLE 18
Amino Acid ~ diamine
A. To an ice cooled solution of 2.29 N-CBZ-D-Proline in 50
ml of CHCl2 is added 1.35 gm 1-hydroxybenzotriazole hydrate
followed by 2.06 gm of dicyclohexylcarbodiimide. After 20 lllillU~t~S,
0.85 ml of pyrrolidine is added and the reaction mixture stirred
3 overnight after which time it is filtered and the filtrate concentrated in
vacuo. The residue is partitioned between 100 ml ethyl acetate and 50
ml of 2 N hydrochloric acid. The organic layer is separated, washed
with 50 ml of 1.0 N sodium hydroxide solution, dried through sodium
sulfate and concentrated in vacuo. Chromatography on 150 gm of silica
WO 95/24207 PCT/USÇ5/'~93~
-- 2 1 843&5
- 83 -
gel using ethylacetate in hexanes (30-100%) as eluent gives 2.04 gm of
the desired pyrrolidine amide
5 B- To a solution of 1.519 gm of the amide (prepared in A) in
20 ml absolute ethanol is added 75 mg of 10% Pd on carbon catalyst.
The mixture is hydrogenated at 40 psi for about an hour then filtrate
and the filtrate concentrated to yield D-proline pyrrolidine amide.
o C To a suspension of 0.380 gm of lithium ~hlminum hydride
in 15 ml of dry tetrahydrofuran is carefully added a solution of the D-
proline amide (prepared in B above) in 10 ml tetrahydrofuran. The
mixture is refluxed for 2 hrs then cooled and quenched with 2 ml of 2.5
N sodium hydroxide. The mi~ul~; is filtered through a pad of sodium
sulfate and the filter cake washed with 2 x 50 ml of ether. The
combined filtrates are concentrated in vacuo to yield 0.80 gm of desired
2-( 1 -pyrrolidinylmethyl)pyrrolidine.
EXAMPLE 19
[S-(R*,S*)]-2-[4-[[(4-Methyl)piperazin-1-yl]carbonyl] phenoxy]-
((3 ,3-diethyl-N-[ 1 -(methylphenyl)butyl] -4-oxo- 1 -azetidinecarbox-
amide
A solution of S-(R*,S*)]-4-(((3,3-diethyl-1- ((4-methyl-
2 5 phenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl chloride
(3.8 mmol), prepared as in Example 1 lA, in 50 ml of methylene
chloride was cooled in an ice bath and a solution of 0.70 gm of N-
methylpiperazine in 10 ml of methylene chloride was added over 5 min.
The reaction was stirred for 1 hr and was then poured into a mixture of
30 ice water and 10% potassium carbonate. The product was extracted
with two portions of methylene chloride and each methylene chloride
layer was washed with a portion of brine. The methylene chloride
layers were combined, dried over sodium sulfate and evaporated. The
residue was purified with flash chromatography using ethyl acetate, then
W O 95/24207 PCTrUS95/02938
21 84~85
- 84 -
2% triethyl~mine/10% methanoV88% ethyl acetate to afford 2.1 gm of
the title compound as a white solid.
Analysis: C30H42N4O4
Calc: C, 69.64; H, 7.92; N, 10.48
Found: C, 69.62; H, 8.23; N, 10.46.
EXAMPLE 20
[S-(R*,S*)]-2-[4-[[(4-Methyl)piperazin-1-yl]carbonyl] phenoxy]-
((3,3-diethyl-N- [1 -(3,4-methylenedioxyphenyl)butyl] -4-oxo- 1 -
azetidine-carboxamide
When [S-(R*,S*)]-4-(((3,3-diethyl-1-((3,4-methylene-
dioxyphenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl
chloride (3.1 mmol), prepared as in Example 1 lA, was reacted with N-
methylpiperazine as in Example 19, there was obtained 1.75 gm of the
title compound.
Analysis: C31H40N406
Calc: C, 65.94; H, 7.14; N, 9.92
Found: C, 65.80; H, 7.31; N, 10.05.
EXAMPLE 21
[S-(R*,S*)]-2-[4-[[(4-Hydroxyethyl)piperazin-1-yl] carbonyl]phenoxy]-
((3 3 -diethyl-N- r 1 -(methylphenyl) butyll -4-oxo- 1 -azetidinecarboxamide
When [S -(R*,S *)] -4-(((3,3 -diethyl- 1 -((4-methylphenyl)-
butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl chloride (3.8
mmol), ~r~lJaled as in F.x~mple 11A, was reacted with N-(2-hydroxy-
ethyl)piperazine (7.6 mmol) and diisopropylethyl~mine (3.8 mmol) as
in Fx~mrle 19, there was obtained 2.1 gm of the title compound.
Analysis: C32H44N4O5
Calc: C, 68.06; H, 7.85; N, 9.92
Found: C, 67.88; H, 7.87; N, 10.17.
WO 95/24207 PCT/US95/02938
21 843~5
- 85 -
EXAMPLE 22
[S-(R*,S*)]-2-[4-[[(4-Hydroxyethyl)pil~efazin-l-yl] carbonyl]phenoxy]-
((3 ,3 -diethyl-N- [ 1-(3 ,4-methylenedioxyphenyl)butyl] -4-oxo- 1-
azetidinecarboxamide
When [S-(R*,S*)]-4-(((3,3-diethyl-1-((3,4-methylenedioxy-
phenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl chloride
(3.1 mmol), prepared as in Fx~mple llA, was reacted with N-(2-
hydroxyethyl)piperazine (6.2 mmol) and diisopropylethyl~mine (3.1
mmol) as in Example 19, there was obtained 1.50 gm of the title
compound.
Analysis: C32H42N407-1.5H20
Calc: C, 61.94; H, 6.89; N, 9.06
Found: C, 61.95; H, 6.92; N, 8.96.
EXAMPLE 23
[S-(R*,S*)]-2-[4-[[(4-Cyclopropyl)~i~e~dzin-l-yl] carbonyl]phenoxy]-
((3 ,3-diethyl-N-[ 1 -(4-methylphenyl)butyl]-4-oxo- 1 -azetidine-
carboxamlde
To a solution of [S-(R*,S*)]-4-(((3,3-diethyl-1-((4-methyl-
phenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl chloride
(3.8 mmol), prepared as in Fx~mple 1 lA, in 50 ml of methylene
chlorine was added N-(cyclopropyl)piperazine dihydrochloride (5.7
mmol) and then a solution of diisopropylethyl~min~ (15.8 mmol) in 10
ml of methylene chloride was added over 5 min with ice-bath cooling.
The reaction was stirred for 1 hr at 0C and then poured into ice water.
The product was extracted with two portions of methylene chloride and
each methylene chloride layer was washed with a portion of brine. The
methylene chloride layers were combined, dried over sodium sulfate
and ethyl acetate/50% hexanes, then 70% ethyl acetate/30% hexanes to
afford 2.1 gm of the title compound as a white solid.
WO 95/24207 PCT/US95/02938
21 84385 --
- 86 -
Analysis: C32H42N404
Calc: C, 70.69; H, 7.91; N, 9.99
Found: C, 70.62; H, 8.04; N, 9.95.
EXAMPLE 24
[S-(R*,S*)]-2-[4-[[(4-Cyclopropyl)piperazin-1-yl] carbonyl]phenoxy]-
((3 ,3-diethyl-N- [ 1 -(3 ,4-methylenedioxyphenyl)butyl] -4-oxo- 1-
azetidinecarboxamide
o When [S-(R*,S*)]-4-(((3,3-diethyl-1 -((3,4-methylene-
dioxyphenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl
chloride (3.1 mmol), ~r~ared as in Example 1 lA, was reacted with N-
(cyclopropyl)piperazine (4.6 mmol) and diisopropylethylamine (9.3
mmol) as in Example 23, there was obtained 1.80 gm of the title
compound.
Analysis: C32H42N407
Calc: C, 67.10; H, 7.17; N, 9.49
Found: C, 67.03; H, 7.31; N, 9.47.
EXAMPLE 25
[S-(R*,S*)]-2-[4-[[(4-Piperazin-1 -yl)carbonyl]phenoxy]-((3,3-diethyl-
N-r 1 -(4-methylphenyl)butyll -4-oxo- 1 -azetidinecarboxamide
Step A: [S-R*,S*)]-2-[4-[[(4-(t-Butoxycarbonyl))piperazin-1-
yl]carbonyl]phenoxy] -((3 ,3-diethyl-N-[ 1 -(4-methyl-
phenyl)butyll -4-oxo- 1 -azetidinecarboxamide
[S-(R*,S *)] -4-(((3 ,3 -Diethyl- 1 -((4-methylphenyl)-
30 butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl chloride (0.4
mmol), prepared as in Fx~mple 1 lA, was reacted with N-(t-butoxy-
carbonyl)piperazine (0.6 mmol) and triethyl~mine (1.2 mmol) as in
Fx~mple 23. The crude, title product so obtained was used directly in
the following Step B.
WO 9~/24207 PCT/US95/02938
2 1 84385
- 87 -
Step B: [S-R*,S*)]-2-[4-[(Piperazin-1-yl)carbonyl]phenoxy]-
((3,3-diethyl-N-[1 -(4-methylphenyl)butyl-4-oxo- 1 -
azetidine-carboxamide
The product from Step A was dissolved in 0.5 ml of anisole
and 2 ml of cold TFA was added. The reaction was stirred at 0C for 1
hr and was then diluted with methylene chloride and evaporated. The
residue was taken up in methylene chloride, washed with 10% sodium
carbonate and brine, dried over sodium sulfate and concentrated. The
o residue was purified by flash chromatography using 5, then 10%
methanol/methylene chloride to afford 0.212 gm of title product.
Analysis: C30H40N4os-lH2o
Calc: C, 66.89; H, 7.85; N, 10.40
Found: C, 67.06; H, 7.55; N, 10.30.
EXAMPLE 26
[S-(R*,S *)]-2-[4-[ [(4-Piperazin- 1 -yl)carbonyl]phenoxy] -((3,3-diethyl-N-
~ 1 -(3.4-methylenedioxyphenyl)butyll-4-oxo-1 -azetidinecarboxamide
Step A: [S-R*,S*)]-2-[4-[[(4-Benzyloxycarbonyl) Piperazin-1-
yl]carbonyl]phenoxy]-((3,3-diethyl-N-[1 -3,4-methylene-
dioxyphenyl)butyll-4-oxo- 1 -azetidinecarboxamide
When [S-(R*,S*)]-4-(((3,3-diethyl-1-((3,4-methylene-
2 5 dioxyphenyl)butylamino)carbonyl)-4-oxo-2-azetidinyl)oxy)benzoyl
chloride (0.41 mmol), prepared as in Example llA, was reacted with
N-(benzyloxycarbonyl)piperazine (0.77 mmol) and diisopropyl-
ethyl~mine (1.6 mmol) as in Fx~mple 23, there was obtained 290 mg of
the title compound.
Step B: [S-R*,S*)]-2-[4-[(Piperazin-1-yl)carbonyl]phenoxy]-((3,3-
diethyl-N-[1 -(3,4-methylenedioxyphenyl)butyl-4-oxo- 1 -
azetidinecarboxamide
A solution of 250 mg of material from Example 26, Step A
in 10 ml of ethanol was hydrogenated at 40 p.s.i. over 50 mg of 10%
WO 95/24207 PCT/US95/02938
21 84385
- 88 -
Pd/C for 16 hrs. The reaction was filtered and evaporated. The residue
was purified by preparative TLC eluting with 2% TEA/10% methanol/-
88% ethyl acetate to afford 150 mg of title product.
Analysis: C30H38N4o6-3H2o
Calc: C, 59.59; H, 7.33; N, 9.29
Found: C, 59.66; H, 7.65; N, 9.61.