Note: Descriptions are shown in the official language in which they were submitted.
CA 02185394 2006-02-02
26474-384
- 1 -
Biotinderivate
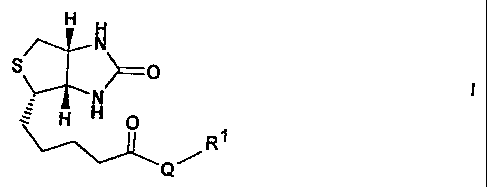
The invention relates to biotin compounds of the
formula I
"a
~o ,
~N
H H 0
R~
Q
in which
Q 1.8 absent, -NH- (CHZ) a-C~- Or -N8- (CH2) n-NH-,
R1 is X-Arg-Gly-Asp-Y, A-Cys(R2)-H-U or
cyclo-(Arg-Gly-Asp-Z), where Z is bonded in the
side chain to Q or, if Q is absent, to biotin,
X and Y are each, independently of one another, an
amino-acid residue or a di-, tri-, tetra- or
pentapeptide residue, where the amino acids are
selected, independently of one another, from a
group consisting of Ala, Asn, Asp, Arg, Cys,
Gln, Glu, Gly, 4-Hal-Phe, His, homo-Phe, IIe,
Leu, Lys, Met, Nle, Phe, Phg, Pro, Ser, Thr,
Trp, Tyr or Val,
and the said amino acids can also be derivatized,
A is absent, Asp or a peptide fragment selected
from a group consisting of Ala-Asp,
Thr-Ala-Asp, Lys-Thr-Ala-Asp, Lys-Thr-Ala-Asn,
Lys-Thr-Gly-Asp, Lys-Ala-Ala-Asp,
Arg-Thr-Ala-Asp, Ser-Ala-Asp, Gln-Ser-Ala-Asp,
Glp-Ser-Ala-Asp, Gly-Lys-Thr-Ala-Asp,
Asn-Gly-Lys-Thr-Ala-Asp, Ile-Ser-Ala-Gly,
Arg-Ser-Ala-Gly, Cys-Asn-Gly-Lys-Thr-Ala-Asp,
_ 2 _ 2185394
-
Tyr-Cys-ASn-Gly-Lys-Thr-Ala-Asp,
Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Ala-Asp,
Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Ala-Asp,
Gly-Lys-Thr-Cys(Trt)-Asp,
Met-Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Ala-Asp,
Asp-Met-Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Ala-Asp,
B is absent, OH, Ala, Arg, Asn, Asp, Cys, Gln,
Glu, Gly, His, Ile, Leu, Lys, Met, Orn, Phe,
4-Hal-Phe, Pro, Ser, Thr, Trp, Tyr, Val or an
N-methylated derivative of the said amino-acid
residues, or a peptide fragment selected from a
group consisting of Pro-Arg, Pro-Arg-Asn,
Pro-Arg-ASn-Pro, Pro-Arg-Asn-Pro-His,
Pro-Arg-Asn-Pro-His-Lys,
Pro-Arg-Asn-Pro-His-Lys-Gly,
Pro-Arg-Asn-Pro-His-Lys-Gly-Pro,
Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-Ala,
Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-Ala-Thr,
ao
where, if R1 is A-Cys(R2)-B-U, only one of the radicals
A or B can be absent,
RZ is H, alkyl with 1-6 C atoms, Trt, Dpm or Bzl,
U is OH, OR9, NHz, NHR9 or N(R9)z
Z is in each case, independently of one another,
an amino-acid residue or a di-, tri- or tetra-
peptide residue, where the amino acids thereof
can also be selected, independently of one
another, from a group consisting of Ala, Asn,
Asp, Arg, Cys, Gln, Glu, Gly, His, Ile, Leu,
Lys, Met, Phe, Pro, Ser, Thr, Trp, Tyr, Val or
M,
where the said amino acids can also be deriva-
tiled,
- 2185394
and the amino-acid residues can be linked
together in the manner of a peptide via the
a-amino and a-carboxyl groups, with M always
being present,
M is NH(RB)-CH(R;)-COOH,
R3 is -R5-R~, -R6-R~. -R~-Rt.
R4 is OH, NHs, SH or COOH,
20
RS is alkylene with 1-6 C atoms,
R6 is alkylenephenyl with 7-14 C atoms,
R' is alkylenephenylalkylene with 8-14 C atoms.
RB is H, alkyl with 1-6 C atoms or alkylenephenyl
with 7-12 C atoms,
R9 is alkyl with 1-6 C atoms,
Hal is F, C1, Br or I and
n is 1, 2, 3, 4. 5, 6, 7, 8, 9 or 10,
where both the D and the L forms are included if
radicals of optically active amino acids and amino-acid
derivatives are present, and the salts thereof.
Similar compounds of biotinylated peptides are
described, for example, in WO 9415956 (biotinylated
endothelia receptor antagonists), in WO 9413313 (bio-
tinylated LHRH antagonists) or WO 9418325 (biotinylated
necrosis factor).
The biotinylatioa of peptides during solid-phase syn-
thesis on a resin for the purpose of improving the
possibility of purification is described by T.J. Lobl
et al. in Anal. Biochem. 170, 502 (1988). Similar com-
pounds of cyclic and linear peptides are disclosed in
= ~~ - 4 - 218594
DE 43 10 643, DE 43 36 758, EP 0 406 428 and
WO 89/05150.
The invention was based on the object of finding
novel compounds with valuable properties, especially
those which can be used to produce pharmaceuticals.
It has been found that the compounds of the
formula I and their-salts have, while being well tole-
rated, very valuable pharmacological properties. In
particular, they sat as integrin inhibitors, and they
inhibit in particular the interactions of the a", (33 or
[35 integrin receptors with ligands, such as, for
example, the binding of fibrinogen to the (i3 integrin
receptor. The compounds show particular activity in the
case of the integrins a~~i3, axis, a=~(33 and a"[31, ao[36 and
a~,(3a. This effect can be detected, for example, by the
method described by J.W. Smith et al. in J. Biol. Chem.
265, 12267-12271 (1990).
The dependence of the beginning of angiogenesis on the
interaction between vascular integrins and extra
cellular-matrix proteins is described by P.C. Brooks,
R.A. Clark and D.A. Cheresh in Science ~~~, 569-71
(1994).
The possibility of inhibiting this interaction
and thus of initiating apoptosis (programmed cell
death) of angiogenic vascular cells by a cyclic peptide
is described by P.C. Brooks, A.M. Montgomery,
M. Rosenfeld, R.A. Reisfeld, T.-Iiu, G. Rlier and
D.A. Cheresh in Cell ZQ, 1157-64 (1994).
Compounds of -the formula I which block the
interaction of integrin receptors and ligands, such as,
for example, of fibrinogen on the fibrinogen receptor
(glycoprotein IIb/IIIa), prevent as GPIIb/IIIa antago
nists the spread of tumour cells by metastasis. This is
proved by the following observations:
The spread of tumour cells from a local tumour into the
vascular system takes place by the formation of
microaggregates (microthrombi) by interaction of the
tumour cells With blood platelets. The tumour cells are
- ~~ - 5 - 218594
- shielded by the protection in the microaggregate and
are not recognized by the cells of the immune system.
The microaggregates may become attached to
vessel walls, which facilitates further penetration of
tumour cells into the tissue. Since the formation of
microthrombi is mediated by fibrinogen binding to the
fibrinogen receptors on activated blood platelets, the
GPIIa/IIIb antagonists can be regarded as effective
inhibitors of metastasis.
The compounds of the formula I can be employed
as pharmaceutical active substances in human and
veterinary medicine, in particular for the prophylaxis
and/or therapy of- thrombosis, myocardial infarct,
arteriosclerosis, inflammations, stroke, angina
pectoris, oncoses, osteolytic diseases such as oateo-
poroais, pathologically angiogenic disorders such as,
for example, inflammations, ophthalmological disorders,
diabetic retinopathy, macular degeneration, myopia,
ocular histoplasmosis, rheumatoid arthritis,
osteoarthritis, rubeotic glaucoma, ulcerative colitis,
Crohn's disease, atherosclerosis, psoriasis, restenosis
after angioplasty, viral infection, bacterial
infection, fungal infection, for acute kidney failure
and for wound healing to support the healing processes.
The compounds of the formula I can be employed
as antimicrohially active substances in operations
where biomaterials, implants, catheters or cardiac
pacemakers are inserted. In this case, they act as
antiseptics. The efficacy of the antimicrobial activity
can be demonstrated by the method described by
P. Valentin-Weigund et al. in Infection and Immunity,
2851-2855 (1988).
Since the compounds of the formula I are
inhibitors of fibrinogen binding and thus ligands of
the fibrinogen receptors on blood platelets, they can
be used as diagnostic aids for detecting and locating
thrombi in the vascular system in vivo, because the
biotinyl radical is a W-detectable radical.
~
- 6 - 2185394
The compounds of the formula I can, as
inhibitors of fibrinogen binding, also be used as
effective aids for studying the metabolism of blood
platelets in various stages of activation or of intra-
cellular signal mechanisms of the fibrinogen receptor.
The detectable unit of the °biotin label° makes it
possible to investigate the said mechanisms after
binding to the receptor.
The abbreviations of amino-acid residues
employed hereinbefore and hereinafter represent the
radicals of the following amino acids:
Abu 4-Aminobutyric acid
Aha 6-Aminohexanoic acid, 6-aminocaproic acid
Ala Alanine
Asn Asparagine
Asp Aspartic acid
Arg Arginine
Cys Cysteine
Dab 2,4-Diaminobutyric acid
Dap 2,3-Diaminopropionic acid
Gln Glutamine
Glp Pyroglutamic acid
Glu Glutamic acid
Gly Glycine
His Histidine
homo-Phe homo-Phenylalanine
Ile Isoleucine
Leu Leucine
Lys Lysine
Meth Methionine
Nle Norleucine
Orn Ornithine
Phe Phenylalanine
Phg Phenylglycine
4-Hal-Phe 4-Halo-phenylalanine
Pro Proline
Ser Seriae
Thr Threonine
CA 02185394 2006-02-02
26474-384
Trp Tryptophan
Tyr Tyrosine
Val Valine
and
H H
N
S ~O
Hit
_ a
Further meanings are the following:
BOC tert-Butoxycarbonyl
CHZ Benzyloxycarbonyl
DCCI Dicyclohexylcarbodiimide
DMF Dimethylformamide
Et Ethyl
F~noc 9-Fluorenylmethoxycarbonyl
HOHt 1-Hydroxybenzotriazole
Me Methyl .
MBHA 4-Methylbenzhydrylamine
Mtr 4-Methoxy-2,3.6-trimethyiphenylsulfonyl
OBut tert-Butyl eater
OMe Methyl ester
OEt Ethyl ester
POA Phenoxyacetyl
TFA Trifluoroacetic acid
Trt Trityl (triphenylmethyl).
Whea the abovementioned amino acids can occur in
several
enantiomericv
forms,
all
these
forms,
and
mixtures
thereof
(for
example
the
D and
the
L forms),
are
also
included
hereiabefore
and
hereinafter,
for
example
as
constituent
of
the
comp4unds
of
the
formula
I.
Further-
more, the amino acids can be provided~with appropriate
protective
groups
which
are
known
per
se,
for
example
as constituent
of
compounds
of
the
formula
I.
CA 02185394 2006-02-02
26474-384
_ g _
The compounds according to the invention also
include so-called prodrug derivatives, that ie to say
compounds of the formula I which have been modified,
for example, with alkyl or acyl groups, sugars or
oligopeptides and which are rapidly cleaved in the body
to the active compounds according to the invention.
The invention furthermore relates to a process
for the preparation of compounds of the formula I
according to Claim 1, and the salts thereof,
characterized in that
(a) a compound of the formula II
H-Q-R1 II
in which
Q and R1 have the meaning stated in Claim 1, is reacted
in an acylation reaction with a compound of the formula
III
N
H
H O '
L
in which
L is C1, Hr, I or a free or reactively
functionally modified OH group,
or
b) a compound of the formula IV
H-R1 IV
CA 02185394 2006-02-02
264x4-384.
_ g _
in which
R1 has the meaaing stated in Claim 1, is reacted in an
acylation reaction with a compound of the formula V
s ~o
H V
.' O
Q~L
in which
Q has the meaning stated in Claim 1, and
L is H, C1, Br, I or a free or reactively
functionally modified 08 group,
or
c~ they are liberated from one of their
functional derivatives by treatment with a solvolysing
or hydrogenolysiag agent, ,
and/or in that a basic or acidic compound of the
formula I is converted by treatment with sa acid or
base into one of the salts thereof.
8ereinbefore and hereinafter, the radicals Q, Rl
and L have the meanings stated for formulae I, II aad
III unless expressly stated otherwise.
In the above formulae, alkyl is preferably
methyl, ethyl, isopropyl~or tent-butyl.
Alkylene is preferably methylene, ethylene,
propylene, butylene. pentylene or hexylene.
Alkylenepheayl is preferably benzyl or phenethyl.
Alkylenepheaylalkyleae is preferably 4-methyleaebeazyl
or 4-ethylenebenzyl.
- 1° - 2 ~ 85394
The radical -R6-R4 is preferably 2-, 3- or
4-hydroxybenzyl, 2-, 3- or 4-aminobenzyl, 2-, 3- or
4-mercaptobenzyl, 2-, 3- or 4-carboxybenzyl, further-
more preferably 2-, 3- or 4-hydroxyphenethyl, 2-, 3- or
4-aminophenethyl, 2-, 3- or 4-mercaptophenethyl or 2-,
3- or 4-carboxypheaethyl.
Q is preferably 6-aminohexanoic acid (6-amino-
caproic acid) or is abseat.
M is preferably Dap, Ser, Cys, Asp, D-Asp, Dab,
homoserine, homocysteine, Glu, D-Glu, Thr, Orn, Lys,
D-Lys, 4-aminomethyl-Phe or 4-aminomethyl-D-Phe.
X is preferably Ala, Asn, Asp, Arg, Cys, Gln,
Glu, Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr,
Trp, Tyr or Val, furthermore preferably
Lys-Gly, Lys-Ala, Lys-~i-Ala, Tyr-Gly, Tyr-Ala, Tyr-(3-Ala, Phe-Gly, Phe Ala,
Phe-(3-Ala, Tyr-Gly-Gly, Phe-Giy-Gly, Lys-Gly-Gly, Tyr-Giy Ala,
Phe-Gty-Ala, Lys-Gty-Aia, Arg-Giy-Asp, Lys-Gly-Gly-Gly, Tyr-Giy-Gly-Gly,
Phe-Gly-Gly-Gly, Lys-Gly-Gly-Ala, Tyr-Gty-Gty-Ala, Phe-Gfy-Gly-Ala,
Lys-Giy-Giy-(3-Ata, Tyr-Gly-Gty-(i-Ala, Phe-Giy-Giy-[3-Ala,furthermore also
Lys-Giy-Gly-Gly-Gly, Tyr-Giy-Gly-Giy-Gly, Phe-Gly-Giy-Gly-Gly,
Lys-Gly-G(y-A!a-Gly, Tyr-Giy-Giy-Ata-Giy, Phe-Gly-G!y-Ala-Gly,
Lys-Gly-Gly-(3-Ala-Gly, Tyr-Gly-Gly-E3-Ala-Gly or Phe-Gly-Gly-(3-Ala-Gly.
Y is preferably Ala, Asa, Asp, Arg, Cys, Gln,
Glu, Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr,
Trp, Tyr or Val, furthermore preferably Tyr-Ala,
Tyr-Asn, Tyr-Asp, Tyr-Arg, Tyr-Cys, Tyr-Gln, Tyr-Glu,
Tyr-Gly, Tyr-His, Tyr-Ile, Tyr-Leu, Tyr-Lys, Try-Met,
Tyr-Phe, Tyr-Pro, Tyr-Ser,
- li. - 2185394
Tyr-Thr, Tyr-Trp, Tyr-Tyr,Tyr-Val, Phe-Ala, Phe-Asn, Phe Asp, Phe-Arg,
Phe-Cys, Phe-Gin, Phe-Glu, Phe-Gly, Phe-His, Phe-Ile, Phe-Leu,
Phe-Lys, Phe-Met, Phe-Phe, Phe-Pro, Phe-Ser, Phe-Thr, Phe-Trp,
Phe-Tyr, Phe-Val, Trp Ala, Trp-Asn, Trp-Asp, Trp-Arg, Trp-Cys, Trp-Gln,
Trp-Glu, Trp-Gly, Trp-His, Trp-Ile, Trp-Leu, Trp-Lys, Trp-Met, Trp-Phe,
Trp-Pro, Trp-Ser, Trp-Thr, Trp-Trp, Trp-Tyr, Trp-Val, Asp Ala, Asp-Asn,
~P-ASP, ~P-~9, ~P-CYs. AsP-Gln, Asp-Glu, Asp-Gly, AsP-His, Asp-Ile,
Asp-Leu, Asp-Lys, Asp-Met, Asp-Phe, Asp-Pro, Asp-Ser, Asp-Thr,
Asp-Trp, Asp-Tyr, Asp-Vat, Ser-Pro-Lys, Tyr-Pro-Lys, Phe-Pro-Lys,
Trp-Pro-Lys, Asp-Pro-Lys, Ser-Gly-Lys, Tyr-Gly-Lys, Phe-Gly-Lys,
Trp-Gly-Lys, Asp-Giy-Lys, Ser-Ala-Lys, Tyr-Ala-Lys, Phe Ala-Lys,
Trp-A!a-Lys, Asp-Ata-Lys, Ser-Pro-Ala, Ser-Leu-Lys, Tyr-Leu-Lys,
Phe-Leu-Lys, Trp-Leu-Lys, Asp-Leu-Lys, Ser-Ile-Lys, Tyr-Ile-Lys,
Phe-ile-Lys, Trp-Ile-Lys, Asp-Ile-Lys, Ser-Pro-Aia-Ser, Tyr-Pro-Ala-Ser,
Phe-Pro-Ala-Ser, Trp-Pro-Ala-Ser, Asp-Pro-Ala-Ser, Ser-Gty Ala-Ser,
Tyr-Gty-Ala-Ser, Phe-Gly-Ala-Ser, Trp-Gly Ala-Ser, Asp-Gly Ala-Ser,
Ser Ala Ala-Ser, Tyr-Afa Ala-Ser, Phe-Ala-Ala-Ser, Ttp Ata-Ala-Ser,
Asp-Ala-Ala-Ser, Ser-Vat Ala-Sec, Tyr Val-Afa-Ser, Phe-Vat-Ata-Ser,
Trp Val-Ala-Ser, Asp-Val-Ala-Ser, Ser-Leu Ala-Ser, Tyr-Leu-Ala-Ser,
Phe-Leu-Ala-Ser, Trp-Leu-Afa-Ser, Asp-Leu Ala-Ser, Ser-Ile lUa-Ser,
Tyr-tle Ala-Ser, Phe-Ile-Ala-Ser, Trp-ile Ala-Ser, Asp-tle Ata-Ser, further-
more also Ser-Pro-Ata-Ser-Ser,Tyr-Pro Ala-Ser-Ser, Phe-Pro Ala-Ser-Ser,
Trp-Pro-Ala-Ser-Ser, Asp-Pro Ala-Ser-Ser, Ser-Gly-A!a-Ser-Ser,
Tyr-Gly-Ala-Ser-Ser, Phe-Gly-Ata-Ser-Ser, Trp-Gly-Ala-Ser-Ser,
Asp-Giy-Ala-Ser-Ser, Ser-Als-Ala-Ser-Ser, Tyr-Afa-Ala-Ser-Ser,
Phe-Ala-Ala-Ser-Ser, Trp Ala Ala-Ser-Ser, Asp Ala-Ala-Ser-Ser,
Ser-Val Ala-Sec-Ser, Tyr Val Ala-Ser-Ser, Phe-Val-Ala-Ser-Ser,
Trp Val-Ata-Ser-Ser, Asp-Va!-Ata-Ser-Ser, Ser-Leu Ala-Ser-Ser,
Tyr-Leu Ala-Ser-Ser, Phe-Leu-Ata-Ser-Ser, Trp-Leu Ala-Ser-Ser,
Asp-Leu-Ala-Ser-Ser, Ser-Ile Hla-Ser-Ser, Tyr-Ile AIaSer Ser,
Phe-Ile Ala-Ser-Ser, Trp-Ile AIa-Ser-Ser or Asp-Ile-Ala-Ser-Ser.
The amino acids and amino-acid residues
mentioned in the meanings for X, Y and Z can also be
derivatized, with the N-methyl, N-ethyl, N-propyl, N-
benzyl or Ca-methyl derivatives being preferred.
Additionally preferred are derivatives of Asp
and Glu, in particular the methyl, ethyl, propyl,
butyl, tert-butyl, neopentyl or benzyl esters of the
side-chain carboxyl groups, furthermore also
- 12 - 2185394
derivatives of Arg which can be substituted on the -NH-
C(=NH)-NHz group by an acetyl, benaoyl, methoxycarbonyl
or ethoxycarbonyl radical.
Furthermore, the amino acids and amino-acid
residues mentioned in the meanings for X and Y can be
provided with appropriate protective groups known per
se.
Z is preferably M, furthermore preferably
D-Phe-M, D-Trp-M, D-Tyr-M, D-Phe-Lys, D-Phe-D-Lys,
D-Trp-Lys, D-Trp-D-Lys, D-Tyr-Lys, D-Tyr-D-Lys,
D-Phe-Orn, D-Phe-Dab, D-Phe-Dap, D-Phe-D-Orn,
D-Phe-D-Dab, D-Phe-D-Dap, D-Phe-4-aminomethyl-Phe,
D-Phe-4-aminomethyl-D-Phe, D-Trp-4-aminomethyl-Phe,
D-Trp-4-aminomethyl-D-Phe, D-Tyr-4-aminomethyl-Phe,
D-Tyr-4-aminomethyl-D-Phe, D-Phe-Asp, D-Phe-D-Asp,
D-Trp-Asp, D-Trp-D-Asp, D-Tyr-Asp, D-Tyr-D-Asp,
D-Phe-Cys, D-Phe-D-Cys, D-Trp-Cys, D-Trp-D-Cys,
D-Tyr-Cys, D-Tyr-D-Cys, Phe-D-Lys, Trp-D-Lys,
Tyr-D-Lys, Phe-Orn, Phe-Dab, Phe-Dap, Trp-Orn, Trp-Dab,
Trp-Dap, Tyr-Ora, Tyr-Dab, Tyr-Dap, Phe-4-aminomethyl-
D-Phe, Trp-4-aminomethyl-D-Phe, Tyr-4-aminomethyl-
D-Phe, Phe-D-Asp, Trp-D-Asp, Tyr-D-Asp, Phe-D-Cys,
Trp-D-Cys, Tyr-D-Cys, D-Phe-Lys-GIy, D-Phe-M-Gly,
D-Trp-Lys-Gly, D-Trp-M-Gly, D-Tyr-Lys-Gly, D-Tyr-M-Gly,
D-Phe-Val-Lys, D-Phe-Gly-Lys, D-Phe-Ala-Lys, D-Phe-Ile-
Lys, D-Phe-Leu-Lys, D-Trp-Val-Lys, D-Trp-Gly-Lys,
D-Trp-Ala-Lys, D-Trp-Ile-Lys, D-Trp-Leu-Lys, D-Tyr-Val-
Lys, D-Tyr-Gly-Lys, D-Tyr-Ala-Lys, D-Tyr-Ile-Lys,
D-Tyr-Leu-Lys, furthermore also M-Pro-Ala-Ser-Ser.
The compounds of the formula I may have one or
more chiral centres and therefore occur in various
stereoisomeric forms. Formula I embraces all these
forms.
Accordingly, the invention relates in particular
to those compounds of the formula I in which at least
one of the said radicals has oae of the meanings
indicated above as preferred. Some preferred groups of
compounds can be represented by the following part-
formulae Ia to If which correspond to the formula I and
- is - 2$5394
in which the undefined radicals have the meanings
stated for formula I, but in which
in Ia Q is absent and
R1 is X-Arg-Gly-Asp-Y;
in Ib Q is -NH-(CH2)5-CO- and
Rl is X-Arg-Gly-Asp-Y;
in Ic Q is -NH-(CHi)5-CO- and
R1 is cyclo-(Arg-Gly-Asp-Z);
in Id Q is -NH-(CHz)5-CO- and
R1 is cyclo-(Arg-Gly-Asp-M);
in Ie Q is -NFi-(CH~)5-CO- and
R1 is A-Cys (R2) -BD
and
i.n If Q is -NH- (CH2) n-CO-,
RI is X-Arg-Gly-Asp-Y and
n is 1, 2, 3, 4, 5 or 6.
The compounds of the formula I, and the starting
materials to prepare them, are moreover prepared by
methods known per se, as described in the literature
(for example in the standard works such as Houben-Weyl,
Methoden der organischen Chemie [Methods of organic
chemistry], Georg-Thieme-Verlag, Stuttgart),
specifically under reaction conditions which are known
and suitable for the said reactions. It is moreover
possible to make use of variants which are known per se
but which are not mentioned here in detail.
The starting materials can, if required, also be
formed in situ so that they are not isolated from the
reaction mixture but immediately converted further into
the compounds of the formula I.
- 2185394
Compounds of the formula I can preferably be
obtained by reacting compounds of the formula II with
compounds of the formula III.
The compounds of the formula II and III are
known as a rule. If they are unknown, they can be
prepared by methods known per se.
The radical -CO-L is the compounds of the
formula III is a preactivated carboxylic acid,
preferably a carbonyl halide, symmetrical or mixed
anhydride or an active ester. Radicals of this type for
activating the carboxyl group in typical acylation
reactions are described in the literature (for example
in the standard Works such as Houben-Weyl, Methodea der
organischea Chemie, Georg-Thieme-Verlag, Stuttgart).
Activated esters are preferably formed is situ, for
example by adding HOBt or N-hydroxysuccinimide.
L is preferably H, C1, Br or -ON-succinimide.
The reaction takes place, as a rule, in as inert
solvent in the presence of an acid-binding agent,
preferably an organic base such as triethylamine,
dimethylaniline, pyridine or quinoline or an excess of
the carboxyl component of the formula III.
It may also be beneficial to add as alkali metal or
alkaline earth metal hydroxide, carbonate or
bicarbonate or another salt of a Weak acid of the
alkali metals or alkaline earth metals, preferably of
potassium, sodium, calcium or caesium.
The reaction time depends on the conditions used and is
between a few minutes and 14 days, and the reaction
temperature is between about -30 and 140, normally
between -10 and 90, is particular between about 0
and about 70.
Examples of suitable inert solvents are hydro-
carbons such as hexane, petroleum ether, benzene,
toluene or xylene; chlorinated hydrocarbons such as
trichloroethylene, 1,2-dichloroethane, tetrachloro-
methane, chloroform or dichloromethane; alcohols such
as methanol, ethanol, isopropanol, n-propanol,
n-butanol or tert-butanol; ethers such as diethyl
- y - 15 - 2185394
ether, diisopropyl ether, tetrahydrofuran (THF) or
dioxane; glycol ethers such as ethylene glycol
monomethyl or monoethyl ether (methylglycol or
ethylglycol), ethylene glycol dimethyl ether (diglyme);
ketones such as acetone or butanone; amides such as
acetamide, dimethylacetamide or dimethylfo~am~de
(DMF); nitriles such as acetonitrile; sulfoxides such
as dimethyl sulfoxide (DMSO); carbon disulfide;
carboxylic acids such as formic acid or acetic acid;
vitro compounds such as nitromethane or nitrobenzene;
esters such as ethyl acetate, water or mixtures of the
solvents mentioned.
Compounds of -the formula I can furthermore be
obtained by reacting compounds of the formula IV with
compounds of the formula V. The starting compounds of
the formula IV and Y are, as a rule, known. If they are
not known, they can be prepared by methods known per
se.
The radical -CO-L in the compounds of the
formula V is a preactivated carboxylic acid, preferably
a carbonyl halide, symmetrical or mixed anhydride or an
active ester. Radicals of this type for activating the
carboxyl group in typical acylation reactions are
described is the literature (for example in the
standard works such as Houben-weyl, Methoden der
organischen Chemie, Georg-Thieme-Verlag, Stuttgart).
L is preferably C1, Br or -ON-succinimide.
Reaction of compounds of the formula IV with
compounds of the formula V takes place under the same
conditions relating to the reaction time, temperature
and solvent as described for the reaction of the
compounds of the formula II with compounds of the
formula III. -
Linear open-chain compounds of the formula I in
which
R1 is X-Arg-Gly-Asp-Y or A-Cys(R~)-B
can furthermore be obtained by coupling, in the last
step of the solid-phase synthesis, biotin in the same
cycle as a normal N-terminally protected amino acid as
CA 02185394 2006-02-02
26474-384
- 16 -
'
last component, and cleaving the biotin-pegtide off the
resin under normal conditions.
The solid-phase synthesis, cleavage off and
purification are carried out as described by A. Joaczyk
and J. Meieahofer in Peptides, Proc. 8th Am. Pept.
Symp., Eds. 9. Hruby and D.H. Rich, Pierce Comp. III,
p. 73-77 (1983) or in analogy to the techniques
described fa Aagew. Chem, ~, 375-391 (199x).
Open-chain linear compounds of the formulae II
' and IV can moreover be prepared by conventional methods
of amino acid and peptide synthesis as described, for
example, is the standard works and patent applications
mentioned, for example also by the solid-phase
synthesis of Merrifield (B.F. Gysin and
R.e. Merrifield, J. Am. Chem.. Soc. 9~, 310Z ff.
(1972) ) .
Cyclic compounds of the formula II and IV is
which Rl is cyclo-(Arg-Gly-Asp-Z) can be prepared by
cyclizing the linear compounds as described, for
example, in D8 43 10 643 or is Houben-Weyl, l.c.,
Volume 15/II, pages 1 to 806 (1974).
The compounds of the formula I can
furthermore be obtained by liberating them from their
functional derivatives by solvolysis, is particular
hydrolysis, or by hydrogenolysis.
Preferred starting materials for the solvolysis
or hydrogeaolysis are those which comprise is place of
one or more free amino and/or hydroxyl groups corres-
ponding protected amino and/or hydroxyl groups, pre-
ferably those which have is place of an H atom which is
connected to as N atom an amino protective group, for
example those which correspond to the formula I but
comprise in place of an NH= group an NHR' group (in
which R' is an amino protective group, for example HOC
or CHZ) .
Further preferred starting materials are those
which have in place of the H atom of a hydroxyl group a
hydroxyl protective group, for example those which
correspond to the formula I but comprise in place of a
v - 1~ - 2185394
hydroxyphenyl group an R"O-phenyl group (in which R" is
a hydroxyl protective group).
It is also possible for several - identical or
different - protected amino and/or hydroxyl groups to
be present in the molecule of the starting material. If
the protective groups which are present differ from one
another, they can in many cases be cleaved off
selectively.
The term °amino protective group° 3s generally
known and refers to groups which are suitable for pro
tecting (blocking) as amino group from chemical
reactions but which can easily be removed after the
required chemical reaction has been carried out else
where in the molecule. Typical groups of this type are,
in particular, uasubstituted or substituted acyl, aryl,
aralkoxymethyl or aralkyl groups. Since the amino pro-
tective groups are removed after the required reaction
(or sequence of reactions), their nature sad size are
not otherwise critical; however, those with 1-20, in
particular 1-8 C atoms are preferred. The term "acyl
group" is to be interpreted in its widest sense is con-
nection with the present process. It includes acyl
groups derived from aliphatic, araliphatic, aromatic or
heterocyclic carboxylic acids or sulfoaic acids, and,
in particular, alkoxycarbonyl, aryloxycarbonyl and,
especially, aralkoxycarbonyl groups. Examples of acyl
groups of these types are alkanoyl such as acetyl, pro-
pionyl, butyryl; aralkanoyl such as phenylacetyl; aroyl
such as beazoyl or toluyl; aryloxyalkanoyl such as POA;
alkoxycarbonyl such as methoxycarbonyl, ethoxycarbonyl,
2,2,2-trichloroethoxycarbonyl, BOC, 2-iodoethoxy-
carbonyl; aralkyloxycarbonyl such as CBZ
("carbobenzoxy°), 4-methoxybenzyloxycarbonyl, FMOC;
arylsulfonyl such as Mtr. Preferred amino protective
groups are BOC and -Mtr, also CBZ, Fmoc, benzyl and
acetyl.
The term °hydroxyl protective group" is likewise
generally known and refers to groups which are suitable
for protecting a hydroxyl group from chemical reactions
- I8 - 2 ~ 85394
but which can easily be removed after the required
chemical reaction has been carried out elsewhere in the
molecule. Typical groups of this type are the above-
mentioned unsubstituted or substituted aryl, aralkyl or
acyl groups, as well as alkyl groups. The nature and
size of the hydroxyl protective groups is not critical
because they are removed again after the required
chemical reaction or sequence of reactions; groups with
1-20, in particular 1-I0, C atoms are preferred.
Examples of hydroxyl protective groups include benzyl,
p-nitrobenzoyl, p-toluenesulfonyl, tert-butyl and
acetyl, with benzyl and tert-butyl being particularly
preferred. The COOH groups in aspartic acid and
glutamic acid are preferably protected in the form of
their tert-butyl esters (for example Asp(O But)).
The liberation of the compounds of the formula I
from their functional derivatives takes place, depend-
ing on the protective group used, for example with
strong acids, preferably with TFA or perchloric acid,
but also with other strong inorganic acids such as
hydrochloric acid or sulfuric acid, strong organic
carboxylic acids such as trichloroacetic acid or
sulfonic acids such as benzene- or p-toluenesulfonic
acid. The presence of an additional inert solvent is
possible but not always necessary. Suitable and pre-
ferred inert solvents are organic, for example
carboxylic acids such as acetic acid, ethers such as
tetrahydrofuraa or dioxane, amides such as DMF, halo-
genated hydrocarbons such as dichloromethane, also
alcohols such as methanol, ethanol or isopropanol, and
water. Also suitable are mixtures of the abovementioned
solvents. TFA is preferably used is excess without
addition of another solvent, perchloric acid in the
form of a mixture of acetic acid and 70% perchloric
acid in the ratio 9:1. The reaction temperatures for
the cleavage are preferably between about = [sic] and
about 50°, preferably between 15 and 30° (room
temperature).
- 19 - 2185394
The groups BOC, OBut and Mtr can be cleaved off,
for example, preferably with TFA in dichloromethane or
with approximately 3 to 5N HC1 in dioxane at 15-30°,
the FMOC group with an approximately 5 to 50% solution
of dimethylamine. Diethylamine or piperidine in DMF at
15-30°.
The trityl group is employed to protect the
amino acids histidine, asparagine, glutamine and
cysteine. The cleavage off takes place, depending on
the required final product, with TFA/10% thiophenol, in
which case the trityl group is cleaved off all the
amino acids mentioned, and when TFA/anisole or
TFA/thioanisole is used, only the trityl group on His,
Asn and Gln is cleaved off, whereas it remains on the
Cys side chain.
Protective groups which can be removed by hydro-
genolysis (for example CBZ or beazyl) can be cleaved
off, for example, by treatment with hydrogen is the
presence of a catalyst (for example of a noble metal
catalyst such as palladium, preferably on a support
such as carbon). Suitable solvents in this case are
those indicated above, especially, for example,
alcohols such as methanol or ethanol or amides such as
DMF. The hydrogenolysis is, as a rule, carried out at
temperatures between about 0 and 100° and under
pressures between about 1 and 200 bar, preferably at
20-30° and 1-10 bar. Hydrogenolysis of the CBZ group
takes place, for example, well oa 5 to 10% Pd/C is
methanol or with ammomium [sic] formate (in place of
hydrogen) on Pd/C in methanol/DMF at 20-30°.
A base of the formula I can be converted with an
acid into the relevant acid addition salt, for example
by reacting equivalent amounts of the base and the acid
in an inert solvent such as ethanol and subsequently
evaporating. Acids particularly suitable for this
reaction are those which provide physiologically accep-
table salts. Thus, it is possible to use inorganic
acids, for example sulfuric acid, nitric acid, hydro-
halic acids such as hydrochloric acid or hydrobromic
.- ~ _ 20 _ 2 ~ 8394
acid, phosphoric acids such as orthophosphoric acid,
sulfamic acid, also organic acids, in particular ali-
phatic, alicyclic, araliphatic, aromatic or hetero-
cyclic monobasic or polybasic carboxylic, sulfonic or
sulfuric acids, for example formic acid, acetic acid,
propionic acid, pivalic acid, diethylacetic acid,
malonic acid, succinic acid, pimelic acid, fumaric
acid, malefic acid, lactic acid, tartaric acid, malic
acid, citric acid, gluconic acid, ascorbic acid,
nicotinic acid, isonicotinic acid, methane- or ethane-
sulfonic acid, ethanedisulfonic acid, 2-hydroxy-
ethanesulfonic acid, benzenesulfonic acid, p-toluene-
sulfonic acid, naphthalenemono- and -disulfonic acids,
laurylsulfuric acid. Salts with physiologically
unacceptable acids, for example picrates, can be used
to isolate and/or purify the compounds of the
formula I.
On the other hand, an acid of the formula I can
be converted by reaction with a base into one of its
physiologically acceptable metal or ammonium salt.
Particularly suitable salts in this connection are the
sodium, potassium, magnesium, calcium and ammonium
salts, also substituted ammonium salts, for example the
dimethyl-, diethyl- or diisopropylammonium salts, mono-
ethanol-, diethaaol- or diisopropylammoaium salts,
cyclohexyl, dicyclohexylammonium salts, dibenzyl-
ethylenediammonium salts, furthermore, for example,
salts with arginine or lysine.
The invention furthermore relates to the use of
the compounds of the formula I and/or their physio
logically acceptable salts for producing pharmaceutical
preparations, in particular by non-chemical means. For
this purpose they can be converted into a suitable
dosage form together with at least one solid, liquid
and/or semiliquid vehicle or ancillary substance and,
where appropriate, is combination With one or more
other active substances.
The invention furthermore relates to
pharmaceutical preparations comprising at least one
21 - z ~ 85394
compound of the formula I and/or one of its
physiologically acceptable salts.
These preparations can be used as pharma
ceuticals is human or veterinary medicine. Suitable
vehicles are organic or inorganic substances which are
suitable for enteral (for example oral), parenteral,
topical administration or for administration in the
form of an inhalation spray and which do not react with
the novel compounds, for example water, vegetable oils,
benzyl alcohols, alkylene glycols, polyethylene
glycols, glycerol triacetate, gelatin, carbohydrates
such as lactose or starch, magnesium stearate, talc,
petrolatum. Used for oral administration are, in
particular, tablets, pills, coated tablets, capsules,
powders, granules, syrups, solutions or drops, for
rectal administration are suppositories, for parenteral
administration are solutions, preferably oily or
aqueous solutions, furthermore suspensions, emulsions
or implants, for topical administration are ointments,
creams or dusting powders. The novel compounds can also
be lyophilized and the resulting lyophilizates used,
for example, to produce injection products. The indi-
cated preparations can be sterilized and/or comprise
ancillary substances such as lubricants, preservatives,
stabilizers and/or wetting agents, emulsifiers, salts
to influence the osmotic pressure, buffer substances,
colorants, flavourings and/or several other active
substances, for example one or more vitamins. The
sprays which can be used for administration as inha-
lation spray comprise the active substance either
dissolved or suepeaded is a propellant gas or mixture
of propellant gases (for example COs or chlorofluoro-
carbona). In this case,, the active substance is pre-
ferably used is micronized form, it being possible for
one or more additional physiologically tolerated sol-
vents to be present, for example ethanol. Inhalation
solutions can be administered using conventional
inhalers.
- 285394
The compounds of the formula I and their physio-
logically acceptable salts can be used as integrin
inhibitors for controlling diseases, in particular
pathologically angiogenic disorders, thromboses,
myocardial infarct, coronary heart diseases, arterio-
sclerosis, tumours, osteoporosis, inflammations and
infections.
For this purpose, the substances according to
the invention can be administered as a rule in analogy
to other known peptides which are commercially avail
able, but in particular in analogy to the compounds
described in US-A-4 472 305, preferably in doses bet-
ween about 0.05 and 500 mg, in particular between 0.05
and 100 mg, per dosage unit. The daily dose is pre-
IS ferably between about 0.01 and 2 mg/kg of bodyweight.
The specific dose for each patient depends, however, on
a wide variety of factors, for example on the activity
of- the specific compound employed, oa the age, body-
weight, general state of health, sex, on the diet, on
the time and route of administration, on the rate of
excretion, medicinal substance combination and severity
of the particular disorder for which the therapy is
applied. Parenteral administration is preferred.
The novel compounds of the formula I can
furthermore be used in analytical biology and molecular
biology. This entails utilization of the ability of the
biotinyl radical to form a complex with the
glycoprotein avidin.
Baes of the biotin-avidin complex are disclosed in
E.A. Bayer and M. Wilchek in Methods of Biochemical
Analysis ~ø, 1-45 (1980) (Lit. 1).
The novel compounds of the formula I can be used
as integrin liganda for .producing columns for affinity
chromatography to prepare pure iategrias. The complex
of an avidin-derivatized support material, for example
Sepharose, and the novel compounds of the formula I is
formed by methods known per se, as described, for
example, in Lit. 1.
- 23 - 2185394
For this reason, no further details of this method are
given at this point, and reference is made to the
corresponding literature, for example Lit. 1.
Suitable polymeric support materials are the
polymeric solid phases which are known in peptide
chemistry and preferably have hydrophilic properties,
for example crosslinked polysaccharides such as cellu
lose, Sepharoae or SephadexR, acrylamidea, polymers
based oa polyethylene glycol or teatacular polymersR.
The novel compounds of the formula I can also be
used as diagnostic markers for anti-biotin antibody
reactions in ELISA-type assays and is FACS
(Fluorescence Activated Cell Sorter) analysis.
The use of antibiotin antibodies for detecting biotin
is disclosed by M. Berger, Biochemistry ~, 2338-2342
(1975). The use of immunoglobulin IgG derivatized with
biotin is an enzyme immunoassay (ELISA) is described by
B. Holmkov-Nielsen et al. in Journal of Chromatography,
2.22, 225-233 (1984).
J. Gao and S.J. Shattil describe in J. Tmmuaol.
Methods 1$l, 55-64 (1995) an ELISA test which detects
substances which inhibit iategria a=Ib(3==I activation. In
this case, biotinylated fibrinogen is employed for
detection.
The use of flow cytometry in clinical cyto-
diagnosis is described by G. Schmitz and G. Rothe in DG
Kliniache Chemie Mitteiluagen 24 (1993) No. 1, page
1-14.
The compounds of the formula I can furthermore
be employed in force field microscopy (atomic force
microscopy AFM) to measure the strength of ligand-
receptor interactions.
Ligand preferably means, acomplex of avidia and the
novel compounds of the formula I.
Receptor preferably means an integrin receptor.
E.-L. Florin et al. describe measurements of adhesion
forces between an avidin-functionalized force field
microscope and biotinylated agarose in Science
415-417 (1994).
CA 02185394 2006-02-02
264'14-384
- 24 -
All temperatures are stated in °C hereinbefore
and hereinafter. In the following examples ~usual
working up" means: if necessary, water is added, if
necessary, depending oa the constitution of the final
product, the pH is adjusted to between 2 and 10, ex-
traction is carried out with ethyl acetate or dichloro-
methane, the organic phase is separated off, dried over
sodium sulfate and evaporated, and purification is
carried out by chromatography on silica gel and/or by
crystallization. Rf values oa silica gel; mobile phase:
ethyl acetate/methanol 9:1.
RT = retention time (minutes) on HPLC in the following
systems:
[A]
TM
Column: Nucleosil 7C18 250 x 4 mm
Eluent A: 0.1% TFA in water
Eluent B: 0.1% TFA in acetonitrile
Flow rate: 1 ml/min
Gradient: 20-50% B/30 min
[H]
50-minute gradient 0-80% 2-propanol in water with 0.3%
TFA at 1 ml/min oa a LichrosorbR RP Select H (7 Ecm)
250 x 4 mm column
[C]
TM
Column: Lichrospher (5 fcm) 100RP8 125 x 4 mm
Eluent A: 0.01 M Na phosphate pH 7.0
Eluent B: 0.005 M Na phosphate pH 7.0/60% by
volume 2-propanol
Flow rate: 0.7 ml/min
Gradient: 1-99% B/50 min.
Mass spectrometry (MS):EI (electron impact ionization)
Mr .
FAH (Fast Atom Bombardment)
(M+H)'
~. _ 25 _ ~ 18539
DMPP-resin represents 4-(2',4'-dimethoxyphenylhydroxy-
methyl)phenoxy-resin [sic], a super acid-labile resin
which permits the synthesis of side-chain protected
peptides.
E~nle 1
0.6 g of Fmoc-Lys(Boc)-OH is dissolved in 100 ml
of dichloromethane, 1.2 equivalents of DMPP-resin, 1.4
equivalents of HOBt and 1.4 equivalents of DCCI are
added, and the mixture is stirred at room temperature
for 12 hours. Removal of the solvent results in Fmoc-
Lys(Boc)-DMPP-resin. In a peptide synthesizer,
Fmoc-Pro-OH is condensed With H-Lys(BOC)-DMPP-resin
[liberated from Fmoc-Lys(BOC)-DMPP-resin with
piperidine/DMF (20%)] by employing a three-fold excess
of the protected proline. The coupling is carried out
with DCCI/HOBt at room temperature. Fmoc-Pro-Lys(BOC)-
DMPP-resin is obtained. Analogously, subsequent
cleavage off of the Fmoc protective group and
consecutive couplings with Fmoc-Ser(BUt)-OH,
Fmoc-Asp(OBut)-OH, E~oc-Gly-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Gly-OH and Bit-OH under
reaction conditions repeated for each coupling
- Liberation of the a-amino group with
piperidine/DMF (20%)
- Washing with dimethylacetamide
- Reaction with the Fmoc-amino acid or Bit-OH
Bit-Gly-Gly-Gly-Arg(Mtr)-Gly-Asp(OBut)-Ser(BUt)-Pro-
Lys(BOC)-DMPP-resin.
The resin is washed With CF3S03H/CH=Clz/Ha0 to result in
Bit-Gly-Gly-Gly-Arg(Mtr)-Gly-Asp(OBut)-Ser(But)-Pro-
Lys(BOC)-OH.
The protective groups are cleaved off with 2N HC1 in
dioxane, the solvent if removed, the residue is taken
up in TFA/CHzClz and precipitated with EtzO, and then
purification is carried out by RP-HPLC.
Bit-Gly-Gly-Gly-Arg-Gly-Asp-Ser-Pro-Lys-OH x 2 TFA;
_ 26 _ 2185394
RT [B] = 12.14 is obtained; FAB 1056.
E~lpe2
In analogy to Example 1, consecutive couplings
of the DMPP-resin with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, Fmoc-Thr(But)-OH,
Fmoc-Lys(BOC)-OH, Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Gly-OH
and Bit-OH result in:
Bit-Gly-Gly-Gly-Lys{BOC)-Thr(But)-Ala-Asp(OBut)-
Cys(Trt)-Pro-DMPP-resin.
Cleavage off from -the resin, cleavage off of the
protective groups and purification result in
Bit-Gly-Gly-Gly-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-OH x2 TFA;
RT [B] 27.6; FAB 1273.
The following are obtained analogously by
condensing the DMPP-resin
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and
Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ala-OH, Fmoc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Ala-Ala-Asp-Cys(Trt3-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Arg(Mtr)-OH and
Bit-OH:
Bit-Arg-Thr-Ala-Aap-Cys(Trt)-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH and Bit-OH:
Bit-Ser-Ala-Asp-Cys(Trt)-Pro-OH;
~ . ~ _ 2, _ 2185394
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH, Fmoc-Gln(Trt)-OH and
Bit-OH:
Bit-Gln-Ser-Ala-Asp-Cys(Trt)-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH, Fmoc-Glp-OH and Bit-OH:
Bit-Glp-Ser-Ala-Asp-Cys(Trt)-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gly-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH, Fmoc-Ile-OH and Bit-OH:
Bit-Ile-Ser-Ala-Gly-Cys(Trt)-Pro-OH;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gly-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH, Fmoc-Arg(Mtr)-OH and
sit-ox:
Bit-Arg-Ser-Ala-Gly-Cys(Trt)-Pro-OH;
with Fmoc-Pro-oH, Fmoc-cys(Trt)-oH, FMOC-Asp(oBut)-ox,
Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH and Bit-OA:
Bit-Lys-Gly-Gly-Asp-Cys(Trt)-Pro-OH;
with Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH,
Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-OH;
with Fmoc-Thr(BUt)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Floc-Ala-OH aad Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiophenol;
Bit-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-
Ala-Thr-OH;
b) When the protective groups are cleaved off With
TFA and 10% thioanisole:
Bit-AIa-Asp-Cys(Trt)-Pro-Arg-Asn-Pro-His-Lys-Gly-
Pro-Ala-Thr-OH;
2185394
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH, Fmoc-
Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH, Fmoc-Pro-
ox,
Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-oH, Fmoc-Pro-ox,
Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH, Fmoc-Gly-OH and
Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiophenol;
Bit-Gly-Lys-Thr-Cys-Asp-Cys-Pro-Arg-Asn-Pro-His-
Lys-Gly-Pro-Ala-Thr-OH;
b) When the protetive groups are cleaved off with
TFA and 10% thioanisole:
Bit-Gly-Lys-Thr-Cys(Trt)-Asp-Cys(Trt)-Pro-Arg-Asn-
Pro-His-Lys-Gly-Pro-Ala-Thr-OH;
with FmOC-Gly-OH, FEIOC-Lys(BOC)-OH, FEIOC-His(Trt)-OH,
FmoC-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, FmoC-Lys(BOC)-OH and
Bit-OH:
a) When the protective groups are cleaved off with
TFA and 10% thiophenol:
Bit-Lys-Thr-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-
Gly-OH;
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asn-Pro-His-
Lys-Gly-OH;
with FmoC-Thr(But)-OH, FmoC-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, FmoC-Lys(BOC)-OH and
Bit-OA:
a) When the protective groups are cleaved off With
TFA and 10% thiophenol:
. .. ~ 2185394
- 29 -
Bit-Lys-Thr-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-
Gly-Pro-Ala-Thr-OH;
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asn-Pro-His-
Lys-Gly-Pro-Ala-Thr-OH;
with Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH,
Fmoc-Gly-OH aad Bit-OH:
a) when the protective grougs are cleaved off with
TFA and 10% thiopheaol:
Bit-Gly-Lys-Thr-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-
Lys-Gly-OH:
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Gly-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-ASn-Pro-
His-Lys-Gly-OHp
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-oH, Fmoc-Lys(BOC3-oH, Fmoc-His(Trt)-oH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH and Bit-OH:
Bit-Thr-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-
Pro-Ala-Thr-OH;
with Fmoc-Thr(But)-OH, Pmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asa(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH and Bit-OH:
Bit-Ala-Asp-Cys-Pro-Arg-Asa-Pro-His-Lys-Gly-Pro-
Ala-Thr-OH;
with Floc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asa(Trt)-OH, Fmoc-Arg(Mtr).-OH,
2185394
- - 30 -
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Floc-ASp(OBut)-OH,
Fmoc-Ala-OH and Bit-OH:
Bit-Ala-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-OH;
with E'moc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OA, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Emoc-Lys(BOC)-OH and
Bit-OH:
Bit-Lys-Thr-Ala-Aep-Cys(Trt)-Pro-Arg-Asn-Pro-OH;
with Fmoc-Lys(BOC)-OH, ~noc-His(Trt)-OA, Fmoc-Pro-OH,
Fmoc-ASn(Trt)-OH, P'moc-Arg(Mtr)-OH, Fmoc-Pro-OH,
Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH,
Fmoc-Thr(But)-OH, P'moc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asn-Pro-His-
Lys-OH;
with Fmoc-His(Trt)-OH, Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH,
E'moc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, l~oc-Thr(But)-OH,
Fmoc-Lys(BOC)-OH aad-Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-His-
OH;
with Fmoc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, Fmoc-Thr(But)-OH,
Fmoc-Lys(BOC)-off ana sit-oH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-OH;
with Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH, Fmoc-Pro-OH,
Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH, Fmoe-Pro-OH,
Fmoc-Cys(Trt)-OH, Floc-Asp(OBut)-OH and Bit-OH:
Bit-Asp-Cys(Trt)-Pro-Arg-Asn-Pro-His-Lys-OH;
with Fmoc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH and Bit-OH:
Bit-Ala-Asp-Cys(Trt)-Pro-Arg-OH;
CA 02185394 2006-02-02
26474-384
- 31 -
with Floc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, Fmoc-Thr(Hut)-OH, and
Hit-OH:
Hit-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-OH;
with Fmoc-Pro-OH, F~noc-Cys (Trt) -OH, Fmoc-Asp (OHut) -OH,
Fmoc-Ala-OH, Fmoc-Thr (But) -OH, Fbnoc-Lye (BOC) -OH and
Bit-OH:
Hit-Lys-Thr-Ala-Aap-Cys(Trt)-Pro-OH;
with Pbnoc-NMeAla-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OHut)-OH, Fmoc-Ala-OH, Fmoc-Thr(8ut)-OH,
F'moc-Lys(BOC)-OH and Hit-OH:
Hit-Lys-Thr-Ala-Asp-Cys(Trt)-NMeAla-OH;
Ex~
1.7 g of (+)-biotinyl-N-auccinimidyl
ester, which is inexpensive, and 0.5 g of triethylamine
are added to a aolutioa of 3.05 g of cyclo-(Arg-Gly
20. Asp-D-Phe-Lye) [obtainable by cyclizing 8-Arg(Mtr)-Gly
Asp(OBut)-D-Phe-Lys(BOC)-OH to cyclo-(Arg(Mtr)-Gly-
Asp(OHut)-D-Phe-Lys(BOC)) and subsequently cleaving off
the protective groups] in 100 ml of dichloromethane.
The mixture is stirred at room temperature for 5 hours,
and the usual working up results in cyclo-(Arg-Gly-Asp-
D-Phe-Lys (NE-Hit) ) x TFA; RT [H] 11.32; FAB 830.
Example 4
In analogy to Example 3, cyclo-(Arg-Gly-Asp-D
Phe-Lys (N"-Hit-Aha) ) x TFA; RT [C] 23 .67; FAH 943, is
obtained from 3.05 g of cyclo-(Arg-Gly-Asp-D-Phe-Lys)
and 2.3 g of 'N-auccinimidyl (+)-biotinyl-6
aminocaproate (°A"), . which can be purchased
inexpensively, and 0.5 g of triethylamine.
There are obtained analogously from "A" and the
following cyclic compounds
Cyclo-(Arg-Gly-Asp-D-Trp-Lys)
Cyclo-(Arg-Gly-Asp-D-Tyr-Lys)
- 32 - 2 i 85394
Cyclo-(Arg-Gly-Asp-D-Phe-D-Lys)
Cyclo-(Arg-Gly-Asp-D-Phe-Cys)
Cyclo-(Arg-Gly Asp-D-Phe-Dab)
Cyclo-(Arg-Gly-Asp-D-Trp-D-Cys)
Cyclo-(Arg-Gty-Asp-D-Tyr-D-Cys)
Cyclo-(Arg-Gly-Asp-Phe-D-Lys)
Cycto-(Arg-Gly Asp-Trp-D-Lys)
Cyclo-{Arg-Gly-Asp Tyr-D-Lys)
Cyclo-(Arg-Gly-Asp-Phe-D-Cys)
Cyclo-(Arg-Gly-Asp-Phe-Dab)
Cyclo-(Arg-Gly-Asp-Trp-D-Cys)
Cyclo-(Arg-Gly-Asp-Tyr-D-Cys)
Cyclo-(Arg-Gly-Asp-D-Trp-Om)
Cyclo-{Arg-Gly-Asp-D-Tyr-Om)
Cyclo-(Arg-Gly-Asp-D-Phe-Om)
Cyclo-(Arg-Gly-Asp-D-Trp-D-Orn)
Cyclo-(Arg-Gly-Asp-D-Tyr-D-Orn)
Cycto-(Arg-Gly-Asp-D-Phe-D-Orn)
Cyclo-(Arg-Gly-Asp-D-Trp-Dab)
Cyclo-(Arg-Gly-Asp-D-Tyr-Dab)
Cyclo-(Arg-Gty-Asp-D-Trp-Dap}
Cyclo-(Arg-GIyAsp-D-Tyr-Dap)
Cyclo-(Arg-Gly-Asp-D-Phe-Dap}
Cyclo-(Arg-Gly-Asp-D-Trp-D-Dap)
Cyclo-{Arg-Giy-Asp-D-Tyr-D-Dap)
Gyclo-(Arg-Gfy-Asp-D-Phe-D-Dap)
the following compounds
Cyclo-{Arg-Gly-Asp-D-Trp-Lys(N'-Bit-Aha))
Cyclo-(Arg-Gty-Asp-D-Tyr-Lys(N'-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Phe-D-Lys(N'-Bit-Aha))
Cycto-(Arg-Gly Asp-D-Phe-Cys(S-Bit-Aha))
Cycto-(Arg-GIyAsp-D-Phe-Dab(N'-Bit-Aha))
Cycio-(Arg-Gly Asp-D-Trp-D-Cys(S-Bit Aha})
Cyclo-(Arg-G(y-Asp-D-Tyr-D-Cys(S-Bit Aha))
- 2185394
Cyclo-(Arg-Gly-Asp-Phe-D-Lys(N'-Bit-Aha))
Cyclo-(Arg-Gly-Asp-Trp-D-Lys(N'-Bit-Aha))
Cyclo-(Arg-Gly-Asp-Tyr-D-Lys{N'-Bit Aha))
Cyclo-(Rrg-Gly-Asp-Phe-D-Cys(S-Bit Aha))
Cyclo-(Arg-Gly Asp-Phe-Dab(NY-Bit-Aha))
Cyclo-(Arg-Gly-Asp-Tip-D-Cys(S-Bit Aha))
Cyclo-(Arg-Gly-Asp-Tyr-D-Cys(S-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Trp-Om(Na-Bit-Aha))
Cycio-(Arg-Gly Asp-D-Tyr-Om(Na-Bit Aha))
Cyclo-(Arg-Gly-Asp-D-Phe-Om(Na-Bit Aha))
Cyclo-(Arg-Gly-Asp-D-Trp-D-Om(Na-Bit Aha))
Cyclo-(Arg-Gly Asp-D-Tyr-D-Om(Na-Bit-Aha))
Cyclo-(Arg-Gty Asp-D-Phe-D-Om(Na-Bit-Aha))
Cyclo-(Arg-G!y-Asp-D-Trp-Dab(N'-Bit-Aha))
Cycio-{Arg-Gly-Asp-D-Tyr-Dab(N'-Bit-Aha))
Cyclo-{Arg-Gly-Asp-D-Trp-Dap(N°-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Tyr-Dap(Na-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Phe-Dap(N°-Bit-Aha))
Cycto-(Arg-Gly-Asp-D-Trp-D-Dap(N~-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Tyr-D-Dap(N°-Bit-Aha))
Cyclo-(Arg-Gly-Asp-D-Phe-D-Dap(Na-Bit-Aha))
E
6 g of Boc-Aha N-succinimidyl ester are added to
a solution of 3.05 g of cyclo-(Arg-Gly-Asp-D-Phe-Lys)
in 40 ml of 5$ aqueous NaHC03 and 40 ml of THF. Stirring
for 4 hours and the usual working up result in cyclo-
(Arg-Gly-Asp-D-Phe-Lys(BOC-Aha)); RT [C) 27.7; FAB 817.
Cleavage off of the BOC group in HC1/dioxane and the
usual working up result is cyclo-(Arg-Gly-Asp-D-Phe-
Lys(N'-Aha)) x 2 TFA; RT [C] 14.76; FAB 717.
In analogy to Example 1, subsequent reaction with
(+)-biotinyl-N-succinimidyl [sic? ester results in
cyclo-(Arg-Gly-Asp-D-Phe-Lys(N'-Bit-Aha)) x 2 TFA; RT
[C] 23.67; FAB 943.
,~ - 34 - 2185394
Exam, 1~
In analogy to Example 4 there is obtained from
cyclo-(Arg-Gly-Asp-D-Phe-Lys-Gly) [obtainable by
cyclizing H-Arg(Mtr)-Gly-Asp(OBut)-D-Phe-Lys(BOC)-Gly-
S OH to cyclo-(Arg(Mtr)-Gly-Asp(OBut)-D-Phe-Lys(BOC)-Gly)
and subsequently cleaving off the protective groups]
and N-succinimidyl (+)-biotinyl-6-aminocaproate
cyclo-(Arg-Gly-Asp-D-Phe-Lys(N'-Bit-Aha)-Gly) x TFA; RT
(A] 10.97; FAB 1000.
There is obtained analogously from cyclo-(Arg-
Gly-Asp-D-Phe-Val-Lys) [obtainable by cycliziag
H-Arg(Mtr)-Gly-Asp(OBut)-D-Phe-Val-Lys(BOC)-O8 to
cyclo-(Arg(Mtr)-Gly-ASp(OBut)-D-Phe-Val-Lys(BOC)) and
subsequently cleaving off the protective groups] and
N-succinimidyl (+)-biotinyl-6-aminocaproate cyclo-(Arg-
Gly-Asp-D-Phe-Val-Lys(N'-Bit-Aha)) x TFA; RT (A] 16.11;
FAB 1042.
There is obtained analogously from cyclo-(Arg-Gly-ASp-
D-Phe-N-Ne-Lys) and N-succinimidyl (+)-biotinyl-6-
aminocaproate cyclo-(Arg-Gly-Asp-D-Phe-N-Me-Lys(N'-Bit-
Aha)).
ale 7
Ia analogy to Example l, successive couplings
onto an MBHA-resin with the addition of 1.4 equivalents
of HOBt and 1.4 equivalents of DCCI with Fmoc-Pro-OH,
Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH, Fmoc-Ala-OH,
Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH, Fmoc-Gly-OH,
Fmoc-Gly-OH and Bit-OH result ia:
Bit-Gly-Gly-Gly-Lys(BOC)-Thr(But)-Ala-Asp(OBut)-
Cys(Trt)-Pro-MBSA-resin.
Cleavage off from the resin with TFA, cleavage off of
the protective groups with piperidine/DMF and puri
fication result is
Bit-Gly-Gly-Gly-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-NH,
There are obtained analogously by condensation
of the NBHA-resin
35 - 21 t35~94
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and
Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-NH2:
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ala-OH, Fmoc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Ala-Ala-Asp-Cys(Trt)-Pro-NHa
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBUt)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Arg(Mtr)-OH
and Bit-OH:
Bit-Arg-Thr-Ala-Asp-Cys(Trt)-Pro-NH2
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(08ut)-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH and Bit-OH:
Bit-Ser-Ala-Asp-Cys(Trt)-Pro-NH2s
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH,
L~'moc-Ala-OH, Fmoc-Ser(8ut)-OH, Fmoc-Gln(Trt)-OH, aad
Bit-OH:
Bit-Gla-Ser-Ala-Asp-Cys(Trt)-Pro-NHz;
with Pmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Ser(But)-OH, Fmoc-Glp-OH, and Bit-OH:
Bit-Glp-Ser-Ala-Asp-Cys(Trt)-Pro-NHz:
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gly-OH,
Fmoc-Ala-OH, Pmoc-Ser(But)-OH, Fmoc-Ile-OH and Bit-OH:
Bit-Ile-Ser-Ala-Gly-Cys(Trt)-Pro-NHz;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Gly-OH,
Fmoc-Ala-O$, Fmoc-Ser(8ut)-OH, Fmoc-Arg(Mtr)-OH
and Bit-OH:
Bit-Arg-Ser-Ala-Gly-Cys(Trt)-Pro-NH2:
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(08ut)-OH,
Fmoc-Gly-OH, Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, and Bit-OH:
Bit-Lys-Gly-Gly-Asp-Cys(Trt)-Pro-DiHzs
2185394
- 36 -
with Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH, Fmoc-Ala-OH,
Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-NHst
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(MtrD-OH, Fmoc-
Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH, Fmoc-Ala-
OH and Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiophenol:
Bit-Ala-Asp-Cys-Pro-Arg-ASa-Pro-His-Lys-Gly-Pro-
Ala-Thr-DTHs s
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Ala-Asp-Cys(Trt)-Pro-Arg-ASa-Pro-His-Lys-Gly-
Pro-Ala-Thr-NHa;
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-ASn(Trt)-OH, Fmoc-Arg(Ditr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Cys(Trt)-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH,
Fmoc-Gly-OH and Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiophenol:
Bit-Gly-Lys-Thr-Cys-Asp-Cys-Pro-Arg-ASn-Pro-His-
Lys-Gly-Pro-Ala-Thr-NHis
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Gly-Lys-Thr-Cys(Trt)-Asp-Cys(Trt)-Pro-Arg-Asa-
Pro-His-Lys-Gly-Pro-Ala-Thr-NH2s
with Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-ASa(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Aap(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH
and Bit-OH:
' ~ - 3~ - 21 ~5~94
a) when the protective groups are cleaved off with
TFA and 10% thiophenol:
Bit-Lys-Thr-Ala-Asp-Cys-Pro-Arg-ASn-Pro-His-Lys-
Gly-NHz ;
b) when the protective groups are cleaved off with
TFA and 10% thioaaisole:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-His-
Lys-Gly-NH=;
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH,
Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-ASa(Trt)-OH, FMoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-ASp(OBut)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and
Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiophenol:
Bit-Lys-Thr-Ala-Asp-Cys-Pro-Arg-ASn-Pro-His-Lys-
Gly-Pro-Ala-NHs;
b) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-ASa-Pro-His-
Lys-Gly-Pro-Ala-Thr-IZHs;
with Pmoc-Gly-OH, Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Pmoc-Cys(Trt)-OH, Fmoc-Asp(OBUt)-OH,
Fmoc-Ala-OH, Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH,
Fmoc-Gly-OH and Bit-OH:
a) when the protective groups are cleaved off with
TFA and 10% thiopheaol:
Bit-Gly-Lys-Thr-Ala-Asp-Cys-Pro-Arg-ASn-Pro-His-
Lys-Gly-NHs;
a) when the protective groups are cleaved off with
TFA and 10% thioanisole:
Bit-Gly-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-
His-Lys-Gly-NSs;
- 3$ - 218394
with Fmoc-Thr(But)-OH, Fmoc-Ala-OH, Fmoc-Pro-OH, Fmoc
Gly-OH, Fmoc-Lys(BOC)-OH. Fmoc-His(Trt)-OH, PSmoc-Pro
OH, P'moc-ASa(Trt)-OH, Fmoc-Arg(Mtr)-OH, Fmoc-Pro-OH,
Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH. Fmoc
Thr(But)-OH aad Bit-O8:
Bit-Thr-Ala-Asp-Cys-Pro-Arg-Asa-Pro-His-Lys-Gly-
Pro-Ala-Thr-NHsp
with Fmoc-Thr(BUt)-OH, Fmoc-Ala-OH, Rmoc-Pro-OH,
Fmoc-Gly-OH. hoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH,
Fmoc-Pro-OH, Fmoc-ASn(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH and Bit-OH:
Bat-Ala-Asp-Cys-Pro-Arg-Asa-Pro-His-Lys-Gly-Pro-
Ala-Thr-NHat
with Fmoc-Gly-OH, Fmoc-Lys(BOC)-OH. Fmoc-His(Trt)-OH,
Pmoc-Pro-OH, Pmoc-Asn(Trt)-OH, Fmoc-Arg(Bitr)-OH,
Fmoc-Pro-OH. hoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH.
Fmoc-Ala-OH and Bit-08:
Bit-Ala-Asp-Cys-Pro-Arg-Asa-Pro-8is-Lys-Gly-NH2;
with Fmoc-Pro-OH, Fmoc-ASa(Trt)-OH, Fmoc-Arg(Mtr)-OH,
Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBUt)-OH,
Fmoc-Ala-OH. Fmoc-Thr(But)-OH, Fmoc-Lys(BOC)-OH and
Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-NHs;
with Fmoc-Lys(BOC)-OH, Fmoc-His(Trt)-OH, Fmoc-Pro-OH,
Fmoc-Asa(Trt)-OH, P'moc-Arg(Mtr)-OH, P'moc-Pro-OH,
Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH,
Pmoc-Thr(BUt)-OH, Fmoc-Lys(BOC)-OH and Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-ASn-Pro-His-
Lys-NHs:
with Fmoc-His(Trt)-OH, Fmoc-Pro-OH, Fmoc-ASa(Trt)-OH,
Fmoc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, Fmoc-Thr(But)-OH.
Fmoc-Lys(BOC)-OH aad Bit-OH:
CA 02185394 2006-02-02
26474-384
- 39 -
Bit-Lys-Thr-AIa-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-His-
NS~:
with Floc-Arg(D~tr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-O8,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, Fmoc-Thr(But)-OH,
Fmoc-Lys(BOC)-O8 aad Hit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-NSz;
with P~oc-Lys(BOC)-OH, Fmoc-His(Trt)-OH, Pmoc-Pro-OH,
Fmoc-Asa(Trt)-OH, Fmoc-Arg(8tr)-OH, Fmoc-Pro-OH,
Fmoc-Cys(Trt)-O8, Fmoc-Asp(OBut)-O8 and Bit-OH:
Bit-Asp-Cys(Trt)-Pro-Arg-Asa-Pro-His-Lys-NHz;
with Floc-Arg(Mtr)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(08ut)-OH, F'moc-Ala-OH aad Bit-OH:
Bit-Ala-Asp-Cys(Trt)-Pro-Arg-NBs;
with F'moc-Arg (8tr) -OH, Fmoc-Pro-OH, F'moc-Cys (Trt) -OH,
Fmoc-Asp(OBut)-OH, Fmoc-Ala-OH, F'moc-Thr(But)-08 aad
Bit-OH:
Bit-Thr-Ala-Asp-Cys(Trt)-Pro-Arg-NHz;
with Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asp(OBut)-OH,
Fmoc-Ala-OH, Floc-Thr(But)-OH, Fmoc-Lys~(BOC)-OH aad
Bit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-Pro-NBz;
with F'moc-NMeAla-OH, Fmoc-Cys(Trt)-OH,
Fmoc-Asp(08ut)-OH, Pmoc-Ala-OH, Fmoc-Thr(But)-OH,
Fmoc-Lys(80C)-08 aad Hit-OH:
Bit-Lys-Thr-Ala-Asp-Cys(Trt)-NMeAla-NHs;
Example 8
Production of a material suitable for affinity
chromatography to purify iategrias:
The activatioa of Sepharose takes place as
described is Lit. l, page 14. They 20 mg of avidia is
20 ml of 0.1 M sodium bicarbonate solution are added to
TM
10 g of activated Sepharose.
- 40 - ~ ~ 85394
The suspension is stirred at 4° for 12 hours and then
washed. The material is they suspended is water With a
few crystals of sodium azide.
The avidia complex with the biotinylated compounds
according to the invention, for example cyclo-(Arg-Gly
Asp-D-Phe-Lys(N'-Bit)) x TFA, is formed by dissolving
1.1 equivalent of peptide in sodium acetate buffer,
adding the solution to the suapeasioa of avidin
Sepharose and stirring at 4° for 10 hours. Excess
peptide is removed by washing.
The following examples relate to pharmaceutical
preparations:
Example A: Vials
A solution of 100 g of an active substance of
the formula I and 5 g of disodium hydrogen phosphate is
3 1 of double-distilled water are (sic] adjusted to pH
6.5 with 2 N hydrochloric acid, sterilized by
filtration, dispensed into vials and lyophilized and
sealed under sterile conditions. Each vial comprises
5 mg of active substance.
Example B: Suppositories
A mixture of 20 g of an active substance of the
formula I with 100 g of soya lecithin and 1400 g of
cocoa butter is melted, poured into moulds and left to
cool. Each suppository comprises 20 mg of active
substance.
Example C: Solution
A solution is prepared from 1 g of as active
substance of the formula I, 9.38 g of NaHaP04 - 2 H20,
28.48 g of NazHPO~ ~ 12 Ha0 and 0.1 g of benzalkoaium
chloride is 940 ml of double-distilled water. The pH is
adjusted to 6.8, the volume is made up to 1 1 and the
solution is radiation-sterilized. This solution can be
used in the form of eye drops.
Example D: Ointment
.r - 41 - 2185394
500 mg of as active substance of the formula 3
are mixed With 99.5 g of petrolatum under aseptic
conditions.
Example 8: Tablets
A mixture of 1 kg of active substance of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
0.2 kg of talc and 0.1 kg of magnesium stearate is
compressed to tablets is a conventional way so that
1D each tablet comprises 10 mg of active substance.
example F: Coated tablets
Tablets are compressed is analogy to example 8
and are then provided in a conventional Way with a
coating of sucrose, potato starch, talc, tragacanth and
colorant.
example G: Capsules
2 kg of active substance of the formula I are
packed into hard gelatin capsules in a conventional way
so that each capsule comprises 20 mg of the active
substance.
example H: Ampoules
A solution of 1 kg of active substance of the
formula I in 60 1 of double-distilled Water is
sterilized by filtration, dispensed into ampoules and
lyophilized and sealed under sterile conditions. each
ampoule comprises 10 mg of active substance.
8xample I: Lahalation spray
14 g of active substance of the formula I are
dissolved is 10 1 of isotonic NaCl solution, and the
solution is used to fill commercial spray vessels with
a pump mechanism. The solution can be sprayed into the
mouth or nose. One puff (about 0.1 ml) corresponds to a
dose of about 0.14 mg.