Note: Descriptions are shown in the official language in which they were submitted.
WO 96123502 2 ~ ~ ~ 6 6 ~ PCT/1896/OOi7i
1
NOVEL IRON CHELATOR AS INHIBITOR OF IRON-MEDIATED OXIDATION
This invention was made in part with government support
from NIH Grant AI-33790 and NIH Grant HL-48177.
The present invention relates to the chemical structure
of a previously unidentified series of high affinity, iron-binding
compounds, referred to by prior investigators as exochefins, which are
released by mycobacteria. The invention also relates to modifications to
these newly identified compounds to vary their physiological properties
and applications of these newly identified and modified compounds.
!n acute myocardial infarction, cardiac tissue is damaged by
two sequential events, hypoxia in the ischemic phase and oxidative
damage in the reperfusion phase. Myocardium damaged in the ischemic
phase can be salvaged by reintroduction of blood to the ischemic area.
However, reperfusion can result in injury as a result of an inflammatory
response in the reperfused tissue caused by the migration of leukocytes
into the tissue and the production of reactive oxygen species. One of the
most reactive species is the hydroxyl species (OOH) which is generated in
the presence of iron and which results in cell death. Prevention of the
formation of (OOH) will prevent lethal cell damage from this cause. It is
known that the formation of (OOH) is dependent on the presence of free
iron and that iron chelators will prevent reperfusion injury. For example,
the iron cheiators deferoxamine, when administered prior to reperfusion,
prevent injury and reduces myocardial infarct size during coronary artery
occlusion and reperfusion. However, reperfusion injury occurs rapidly
after the reestablishment of blood flow to the ischemic myocardium.
The formation of the (OOH) radical is dependent on the
presence of free iron; iron chelators can scavenge the free iron and thus
suesrlrurE sHE~r ~~uu ~
WO 96/23502 ~., , PC1'IiB96100171
2
render the iron unavailable to catalyze the hydroxyl radical formation.
However, these prior known iron chelating materials either do not prevent
(OOH) production by the Fenton reaction (i.e., EDTA), or enter the cells
too slowly (i.e., desferoxamine) such that sufficient quantities are not'
available to act rapidly enough to chelate enough iron to prevent the
formation of (OOH) and the subsequent cell destruction. Desferoxamine
has been demonstrated to be effective if administered prior to occurrence
of the myocardial infarct but to be ineffective if administered at or after
the onset of reperfusion.
Similar injury to heart tissue can occur as a result of heart
bypass procedures, such as during open heart surgery, or to other body
organs when they are deprived of oxygenated blood as a result of surgery
or injury.
Exochelins were briefly described and their general function
in the growth of mycobacteria was discussed by Macham, Ratledge and
Barclay at the University of Hull in England (Lionel P. Macham, Coffin
Ratledge and Jennifer C. Nocton, "Extracellular Iron Acquisition by
Mycobacteria: Role of the Exochelins and Evidence Against the
Participation of Mycobactin", Infection and Immunity, Vol. 12 No. 6, p.
1242-1251, Dec. 1975; Raymond Barciay and Colin Ratlege,
"Mycobactins and Exochelins of Mycobacterium tuberculosis, M. bovis,
M. africanum and Other Related Species", Journal of General
Microbioloov, 134, 771-776, (1988); L.P. Macham and C. Ratledge, "A
New Group of Water-soluble Iron-binding Compounds from Mycobacteria:
The Exochelins", Journal of General Microbioloov, 89, 379-282, (1975)).
Macham identified the existence of a substance found in the extracellular
fluid, which he referred to as exochelin. He described exochelin as a
water and chloroform soluble compound which has the ability to chelate
free iron. According to Macham, this material has similarities to
W096123502 218 5 6 61 P~~96IOOI7i
3
mycobactin, which is located in the cell wall and functions to transmit
iron to the interior of the cell. However, in contrast thereto, mycobactin
is a lipophilic, water insoluble molecule which is unable to diffuse into,
and assimilate free iron from, the extracellular environment. Macham et
al recognized that exochelin functions at physiological pH to sequester
iron from other iron bearing compounds in the serum, such as transferrin
or ferritin, and present the iron in a form that can be transferred to
mycobactin. Macham et al. did not isolate or purify the exochelins but
identified them as a yenta- or hexapeptide, having a molecular weight of
750 to 800, containing 3 mol of s-N-hydroxylysine, sN-acetyl-sN-
hydroxylysine, or eN-hydroxyornithine and 1 mol of threonine. Also,
depending on the bacterial source of the exochelin, he disclosed that the
molecules may also include ,B-alanine or salicylic acid.
Barcfay (ibid) described the production of exochelins from
twenty-two different strains of M, tuberculosis and related species.
However, these prior investigators did not determine the specific structure
of exochelins or identify any applications of the exochelins other than
their function as a transport medium for iron to mycobactin located in the
cell wall.
Thus there is a need for a substance that can be easily
administered at the time of reperfusion and which will rapidly chelate the
free iron as it is formed or made available to prevent the formation of the
(OOH) radical. Further, there is a need to identify the specific structure of
exocheline so that its function can be more fully understood and its utility
as a diagnostic, treatment and preventive modality can be elucidated.
CA 02185661 2003-02-14
Cardioplegia solutions are used during cardioplegia
procedures, which is a procedure where the cardiac
mechanical activity is temporarily halted.
Cardioplegia solutions ere described in "Cardiopulmonary
Bypass: Principles and Techniques of Extracorporeal
Circulation" (1995 edition) - Chapter 2 pp. 21-39, the
requirements of cardioplegic solutions are to: (a) induce
myocardial diastolic relaxation (arrest); (b) maintain a
favorable metabolic mi~_ieu; (c) prevent interstitial and
intracellular edema; (d) prevent loss of cellular
metabolites; (e) maintain appropriate acid-base balance: and
(f) provide metabolic substrate (oxygen and glucose).
The Inova Health System explains that when a patient is
placed on the heart-lung machine, the heart and body are
cooled to reduce the heart's metabolic requirements and
oxygen consumption. Coi.d chemical agents such as potassium
chloride can be infused into the heart to further reduce the
metabolic requirements and oxygen consumption of the heart
by quickly causing the heart to stop beating, causing
cardioplegia (cardiac paralysis). Without these chemical
agents, the heart will continue to quiver or remain in a
state of ventricular fibrillation while the surgery takes
place. This situation wastes energy resources in the heart
that can be used when t:he heart needs to be stimulated to
start beating again.
Cardioplegia, or cardiac paralysis, allows the surgeon to
perform delicate procedures on a motionless heart. The
chemical agents are rei.nfused into the heart periodically,
in a solution of axyger~ated blood. Thus, repeat infusions of
blood cardioplegia solution provide oxygen to the heart
while it remains at a standstill or arrest.
CA 02185661 2004-06-11
SUMMARY
In accordance with one aspect of the present invention
there is provided a composition, for protecting live tissue in a
mammal from injury resulting from exposure to the hydroxyl free
radical formed following re-establishment of fluid flow to a
body organ after restriction of blood flow to that body organ,
the composition comprising in combination an effective amount of
at least one water and lipid soluble desferriexochelin, and a
pharmaceutically acceptable carrier, wherein the amount of
desferriexochelin is sufficient to protect the mammal on re-
establishment of flow of fluid to the tissue.
Another aspect of the present invention provides for
the use of exochelins to prevent damage to living tissue from
the formation or presence of the (OOH) radical. In particular,
this aspect of the invention is directed to the administration
of exochelins to infarcted myocardium prior to or coincidental
with reperfusion to prevent damage to myocardium from iron
mediated free radical formation. Also presented is the chemical
structure of exochelins and modified exochelins as well as other
applications of these materials in the treatment and diagnosis
of disease mammals.
T1 T'1 T T.7 T TT l~ C~
These and other features, aspects and advantages of
the present invention will become better understood with
reference to the following description, appended claims, and
accompanying drawings, where:
- 4 -
CA 02185661 2004-06-11
Figure 1 shows the chemical structure of an iron
chelate of exochelin (ferriexochelin) and the desferriexochelin
(iron free) molecule.
Figure 2 shows an elution profile of a culture
filtrate of M. tuberculosis monitored at 220nm and 450nm.
Figure 3 shows an elution profile of the same filtrate
monitored at 450nm with the molecular weight of each peak shown.
Figure 4 shows the mass spectrometer spectra of a
major serine-containing exochelin at m/z = 720.3 along with the
structure determined therefrom.
- 4a -
WO96J23502 218 5 b 61 p~J~96100171
Figure 5 is a graph showing the inhibition of cell injury as a
result of the use of an exochelin mixture on cardiac myocytes.
Figure 6 is a graph showing the inhibition of cell injury as a
5 result of the use of exochelin 758C on cardiac myocytes.
Figure 7, 8, and 9 are graphs comparing the inhibition of cell
injury as a result of the use of exochelin 758C, 772A and 772C on
cardiac myocytes.
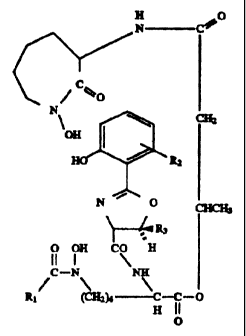
Figure 10 shows the chemical structure of an iron chelate of
exochelin (ferriexochelin) and the desferriexochelin (iron frees molecule
with cites for modification identified.
DETAILED DESCRIPTION OF THE INVENTION
It has been found that exochelins can block, or significantly
reduce, oxidative damage to tissue resulting from the iron-mediated
catalysis of tissue/free radicals reactions, such as the hydroxyl radical
t~OH), particularly hydroxyl radicals generated in the Fenton reaction,
commonly referred to as reperfusion injury. It has been further found that
the exochelins are effective to retard or prevent reperfusion injury when
administered at the start of or concurrent with reperfusion. Additionally,
it has been found that exochelins encompass a much broader class of
materials and have a different chemical structure then originally theorized
by Macham et al. and Barclay et al.
It has also been found that these materials can chelate a
broad range of metals to result in materials not previously known.
Besides preventing reperfusion injury, properly modified exochelins can be
used to treat certain diseases, attack certain cells, such as cancer cells,
R'O 96123502 2 '~ PCTIIB96100171
6
and be used to monitor the effectiveness of drug treatment and detect
the presence of certain disease states. In particular, it is known that the
growth of neuroblastoma cells can be negatively affected by the removal
of iron using the iron chelating compound desferrioxamine without
similarly affecting the growth of normal cells. Other applications of
exochelins include treatment of iron overload from transfusions or cancer
chemotherapy, particularly for leukemia.
As a result of isolating and purifying exochelins, it has been
found that exochelins are a family of molecules having a range of
molecular weights and various different side chains. Further, purified
exochelins have been prepared and their utility as scavengers of free iron,
such that they are effective in preventing the formation of tissue
damaging hydroxyl radicals (OOH), has been demonstrated for the first
time. In particular, purified exochelins of M. tuberculosis have been
isolated and have been shown to effectively remove iron from transferrin,
lactoferrin and ferritin at physiological pH without transmitting any of the
infectious properties of the bacteria from which they are derived. It has
also been demonstrated for the first time that these exochelins block
hydroxyl radical formation by the Fenton reaction and, based on the
response of cardiac myocytes, can be effective to prevent reperfusion
injury after myocardial infarction or vascular insults to other tissue when
administered after the attach occurs as well as for several hours after the
episode.
While mycobactins have been extensively studied, individual
exochelins had not been isolated or purified and their structure and
composition had not been previously defined. Further, we have found
that prior references have mischaracterized the exochelins, and thus have
failed to identify the structure of these compounds. In particular,
Macham (ibid.) identified them as a penta- or hexapeptide, having a
WO 96!23502 - ~ ~ ~ ~ p~/Ig96100I~I
molecular weight of 750 to 800, containing 3 mol of e-N-hydroxylysine,
sN-acetyl-eN-hydroxylysine, or sN-hydroxyornithine and 1 mol of
threonine. We have found that the exochelins have a much broader range
of molecular weights, constitute several series of compounds with an
identifiable difference in molecular weights, include only 2 mol of s-N-
hydroxylysine and are not peptides. A peptide is a polymer of an amino
acid (NHz CHR-COOH) formed by the condensation of the carboxylic
group of a first molecule with the amino group of another molecule to
form an amide linkage (-CO-NH-). Exochelins cannot be considered to be
peptides. Instead, they contain three amino acids and other structural
moieties (salicylic acid, dicarboxylic acids or monoester analogs, and
hydroxy carboxylic acids) formed by amide (-NH-CO-), hydroxymate
(-NH(OH)-CO-) and ester condensations (-CO-O-). The ferri- and desferri
forms are shown in Figure 1.
Preparation - Exochelins were generated and purified from a
virulent (Erdman) and avirulent (H37Ra) strain of M. tuberculosis. To
enhance the production of M. tuberculosis exochelins the bacteria were
cultured in an iron deficient medium. In particular, the Erdman strain of
M. tuberculosis (American type culture collection 35801 ) and H37Ra
(ATCC 25177) were grown on Middlebrook 7H11 agar plates at 37°C in
596 COa. After 14 days the bacteria were harvested, suspended in 150 ml
of modified Sauton's medium in culture flasks and incubated for 3 to 8
weeks. The modified Sauton's medium contained 0.12 mg/I ferric
ammonium citrate without added surfactant.
Iron rich exochelins (ferriexochelins) were then recovered by
filtering, saturating with iron and extracting with chloroform and purified
by high pressure liquid chromatography (HPLC): Specifically, the
supernatant fluid from the above suspension was filtered through
successive 0.8pm and O.ZUm low-protein binding filters. The exochelins
CA 02185661 2003-02-14
wo ~soz pcr~9~oom
8
were then loaded with iron by saturating the filtered supernatant fluid by
exposure to ferric chloride (150mg per liter of culture filtrate). The ferric-
exochelins were mixed with chloroform (1 volume of culture filtrate per
1.5 volumes of chloroform) ancl, after separation of the layers, the
exochelin rich chloroform layer was removed and stored under anhydrous
magnesium sulfate (2gll). The chloroform extract was then passed
through a fritted glass tiller and evaporated by rotary evaporation leaving
behind a brown residue.
The brown residue was further purified by suspension in 5ml
of a first buffered solution (0.1 % trifluoroacetic acid) which was
introduced into a liquid chromatography column (C-18 Sep-Pal' cartridge).
The brown band which formed near the top of the column was eluted
with a second buffer (0.1 % TFA, 50% acetonitrile). The partially purified
material was then diluted three-fold in 0.1 % trifluoroacetic acid and
subjected to reverse phase high pressure liquid chromatography at a rate
of 1 mllmin followed by exposure to a C-18 column. The presence of the
iron rich exochelins in the HPLC eluate was detected by simultaneous
monitoring of the UV absorbance of the 450nm peak (iron compounds)
and the 220nm peak which is indicative of amide and aromatic groups.
Approximately 5 major and 10 minor peaks, shown in Figure 2, eluted out
of the final C-18 column exhibited a high 4501220nm absorbance ratio.
These were confirmed to be exochelins by mass spectrometry. Major
peaks were further purified by a second reverse phase HPLC on an alkyl-
phenyl column. The exochelins recovered from the Erdman strain of M.
tuberculosis were identical to the exochelins recovered from the H37Ra
strain.
Characterization - Based on LSIMS and ESI-MS analysis of
the numerous peaks, in their ferri- (Fe3+) form, eluted from the column
(see Fig. 3), the iron-exochelins are not confined to the two specific
* - Trade Mark
1 W096123502 PCT'/IB96/OOI71
2185661
9
molecules detailed above but include a family of species ranging in mass
from 716 to 828 daltons. Each member of the family appears to differ
from its neighbor by 14 daltons, reflecting the number of CH2 groups in
the R~ alkyl side chain andlor 2 daltons, reflecting the presence of a
double bond in the R, alkyl side chain. Accordingly, the exochelins
appear to form two series with the subsequent members of each series
differing in mass by 14 daltons, the saturated series having masses of
approximately 716, 730, 744, 758, 772, 786, 800, 814 and 828 daltons
and the unsaturated series having masses of 742, 756, 770, 784 798,
812 and 826. Additionally, the presence or absence of a methyl group at
R3 (i.e., H or CH3) further defines an additional two series of molecules
referred to as the serine (R3=H) and the threonine series (R3=CH3), as
confirmed by amino acid analysis. The most polar compounds are to the
left of the figure (elute earlier) and the least polar (most soluble in lipid)
are to the right. However all the peaks are water soluble. Where more
than one peak was found to have the same molecular weight each peak is
further designated A, B or C (i.e., 758A, B and C) to indicate the level of
polarity with A representing the more polar compound and the C
representing the less polar form. The more polar forms are believed to
result from a methyl groups attached at different location in the molecule.
Structure of the Exochelin - Figure 4 shows the results of
tandem mass spectrometric analysis under induced dissociation (He
floated at 2 keV for a collision energy of 6 keV) of the major saturated
serine-containing desferriexochelin with (M+H)* at mlz 720.3. The
fragment ions were assigned to one of the six structural moieties A-F
resulting from the cleavage products generated about the amide or ester
bonds with the hydrogen transfer relative to the neutral molecule
- associated with each peak indicated on the spectrum shown in Figure 4.
Acid hydrolysis and methylation of the exochelins resulted in the
formation of salicylic acid and pimelic acid. The mass spectrographic
R'O 96~23s~? 21 8 5 6 6 1 FCT/DB96100t7t
,o
analysis indicates that the pimelic acid is present in the exochelin as a
methyl ester.
Based on this analysis the general structure of the
ferriexochelins and the desferriexochelins is shown in Figure 1. The
methyl group shown at the RQ position (as defined in Figure 10) may be in
the RS position. The iron-exochelin core molecule is circular with iron in
the center. It contains 3 amino acid moieties (two N-hydroxylysines and
1 serine or threonine, depending on whether R3 is a hydrogen or methyl
group). The major difference between exochelins and mycobactins of M.
tuberculosis is that R, in the exochelins exists as either a saturated adkyi
methyl ester ((CH2)NCOOCH3) or a singly unsaturated alkyl methyl ester
(CHz)xCH=CH(CHa)YCOOCH3 and exochelins have a much shorter alkyl
side chain than mycobactins with these shorter side chains terminating in
methyl ester moieties. These differences provide for the water solubility
of the exochelins and their ability to function in the extracellular
environment.
Clinical Utility - The clinical utility of the administration of
exochelins to prevent reperfusion injury was demonstrated by application
to adult rat myocytes.
In the Examples below the different exochefins, in both the
desferri- and fern- form, will be identified by the molecular weight as
shown in the elution curve in Figure 3.
Example 1
The heart of a male rat was excised after the rat was
anesthetized, a thoracotomy performed and the heart chilled in situ. The
excised heart was then placed on a Langendorff apparatus and perfused
with a collagenase and hyaiuronidase in a 50pM calcium in modified Krebs
CA 02185661 2003-02-14
wo 96n3soZ pcr~moom
Ringer buffer solution. The tissue was then finely divided and dispersed
in a collagenase/trypsin solution, filtered into a cold trypsin inhibitor
solution and exposed to increasing concentrations of calcium. After
removal of damaged cells, the remaining cell suspension was placed in
5 several larninin-coated plastic dishes along with a culture medium
containing 5% fetal bovine serum.
After the cultures were allowed to sit for 48 hours hydrogen
peroxide was added to each dish and the lactate dehydrogenase activity
(LDH), which is indicative of cell injury, was measured at variaus time
intervals. A cell injury index (CII) for comparison purposes was obtained
by measuring the LDH in a nonexposed cell culture in both an as is
condition (0 Index) and following exposure to a detergent that lysis 100%
of the myocytes (1% Tritori X-100) representing a CII of 100. The LDH
15 under specified treatment conditions for various periods of time was then
determined, the corresponding CII value determined and the individual
results were plotted against time (Figure 5).
Using the procedure described above, a mixture of the
desferri- farm of exochelins 772C and 784 (a 50:50 mixture of the 772C
peak and the 784 peak), a relatively non-polar substance, was isolated
and used to treat cell cultures. The exochelin were converted to the
desferriexochelin form by incubation for several days with 50 millimolar
EDTA at pH 6. The desferri- form was then repurified by chloroform
extraction.
Three samples of cells were exposed to either a) H202, b)
H202 and 50,uM of desferriexochelin (iron free exochelin) added
simultaneously or c) H~OZ added 2 hours after addition of 100,uM of
30 desferriexacheiin to the cell culture (preincubation). The untreated cell
cultured showed almost 62% cell injury over a 4 hour period. In contrast
- Trade Mark
wo 96I23soz 218 5 6 61 PCTIIB96100171
12
thereto, addition of exochelin simultaneously with, or 2 hours prior to,
peroxide addition substantially prevented or significantly reduced cell
damage, the cell injury being approximately 2 to 9%.
~xamole 2
The procedure of Example 1 was repeated with desferri -
exochelin 758C which is relatively more polar than exochelins 772C and
784. There was tittle or no difference between the effect when
desferriexochelin 758C was delivered along with or within 15 minutes of
delivery of H,Oz. In both instances after 2 hours the cell destruction was
substantially the same as in the control. However, delivery of the
desferriexochelin 758C 2 hours prior to H20z introduction cut the cell
destruction to a CII of about 20. The results are shown in Figure 6.
Example 3
The procedure set forth above was repeated using
desferriexochetin 772A, 772C and 758C. Plotted in Figures 7 - 9 are the
results for 2 hour predelivery, simultaneous delivery and 20 minute
delayed delivery of the exochelins. Only exochelin 772C shows
retardation of injury under all conditions while exochelin 772A is not
effective under any conditions. On the other hand, exochelin 758C
shows protection only if delivered 2 hours prior to peroxide introduction.
It is therefor concluded that the relatively non-polar, more lipid soluble
exochelins are effective when administered with or after formation of the
(OOH) radical, i.e., after injury occurs; the more polar exochelins must be
administered 1 to 2 hours prior to the free radical generating event to
prevent or reduce cell destruction.
wo vsra.~soa 218 5 6 6 i P~~~~~t71
13
Example 4
The capacity of exochelins to compete for iron with host
iron-binding proteins was determined by incubating desferriexochelin with
solutions of transferrin, lactoferrin, or ferritin at 4:1 and 1:1 molar ratios
of iron to exocheiin. The conversion of the exochelin from its desferri- to
its ferri- form was then determined by reverse phase HPLC. Within one
minute of exposure of desferriexochelin to 95% iron-saturated transferrin,
the exochelin had started to pick up iron from the transferrin and within
one hour the exochelin was fully saturated with iron. Iron was also
readily removed from 40% iron-saturated transferrin, which approximates
the iron level in transferrin as it exists in serum. Similar results were
obtained when desferriexochelin was exposed to iron-saturated
lactoferrin. Likewise, ferritin released iron to the exochelin but at a
slower rate than other iron binding proteins.
It has been discovered that exochelins are very effective in
scavenging free iron in a physiological system and withdrawing iron from
iron bearing protein. In particular it has been found that exochelin
effectively block the formation of the hydroxyl free radical (OOH) and thus
significantly reduce or prevent the injury to ischemic tissue when
circulation of blood to that tissue is reestablished with the higher
molecular weight, less polar exochelins being more effective in preventing
cell destruction. While the benefit to cardiac tissue has been
demonstrated, the benefit of the use of exochelins following interruption
of blood flow to other body organs, including but not limited to the brain,
kidney, liver, bowel, and skeletal muscle is now apparent.
Experimentation has shown that the affinity of the
exochelins is not limited to iron but that other metals can be chelated,
such as Na, K, Mn, Mg, AI and Zn. Therefore, the exochelins can be used
to deliver to the body various desirable metals or chelate various
WO96/23502 21' O 5 U 6 PCTIIB96100171
14
undesirable metals within the body. Additionally, certain cells, including
certain cancer cells are known to have a need for or affinity for certain
metals. This can be utilized to deliver to that cell reactive compounds
attached to the exochelins for destruction of the cell (chemotherapy) or to
target a diseased organ with a beneficial drug bound to the exochelin.
Conversely, since certain cancer cells have a high demand for iron, the
desferriexochelins can be used to bind free iron, thus preventing iron
delivery to the cancer cell, resulting in the destruction of the cancer cell.
While the structure of exochelins recovered from M.
tuberculosis is shown in Figure 1, it is known that other mycobacteria can
generate exochelins and that these exochelins may have different
structure and include different amino acids depending on the
mycobacteria from which they are derived. However, all exochelins will
behave in a similar manner and exist in similar series with subsequent
members thereof having a similar progression of molecular weights. The
effectiveness of the different members of the series will also depend on
the relative polarity of the molecules. Therefore, the invention
contemplates exochelins generated from other mycobacteria including,
but not limited to, M. tuberculosis, M. microti, M. bovis, M. africanum, M.
kansasii, M. marinum, M. gastri, M. nonchromogenicum, M. terrae, M.
trivale, M. maimoense, M. shimoidei, M. gordonae, M. asiaticum, M.
szulgai, M. simiae, M. scrofulaceum, M. avium, M. intracellulare, M.
xenopi, M. ulcerans, M. haemophilum, M. farcinogenes, M. lepraemurium,
M. paratuberculosis, M. chelonae subsp. chelonae, M. chelonae subsp.
abscessus, M. fortuitum, M. chitae, M. senegalense, M. agri, M.
smegmatis, M. phlei, M. thermoresistibile, M. aichiense, M. aurum, M.
chubuense, M. duvalii, M. flavescens, M. gadium, M. givum, M.
komossense, M. neoaurum, M. obuense, M. parafortuitum, M. rhodesiae,
M. sphagni, M. tokaiense or M. vaccae.
W096l23502 ~ ~ PCTIIB96/OOI7I
It is also contemplated that exochelins can be modified to
effect their solubility properties, metal chelating ability or cellular
absorption rates. Additionally, detection of modified exochelins or
exochelin in their metal chelated state, using monoclonal antibodies or
5 chemical analysis as diagnostic tools, by blood analysis, urinalysis or
noninvasive instrumental techniques, to monitor progress of a disease
state or effectiveness of treatment. In particular, referring to the
structures of the metal containing and metal free compounds shown in
Figure 10, the following substitutions are contemplated:
R, _ (CHZ)"CH3 as a linear or branched chain; (CHz)"COOH, a fatty
acid; (CHZ)"COOR, a fatty acid ester where R is an alkyl group;
(CHz)"CONH2;
R2 = a substitution at any of the 4 open ring sites of alkyl groups,
sulfonamides, hydroxyl, halogen, acetyl, carbamyl, amines, NOZ or
any combination thereof;
R3 - the H (serine) or CH3 (threonine) can be replaced by side chains
found on ~-hydroxy amino acids which are capable of forming
cyclic oxazofine structures.
R4, and R4b = H, CH3 or other alkyl or substituted alkyl groups;
Rs, and R5b = H, CH3 or other alkyl or substituted alkyl groups;
X = O, NH, S, CHZ;
M = mono-, di-, or trivalent metals such as Pb. Ai, Cd, Ni, Ag, Au,
As, Mg, Mn, Zn, Cu, Ru, Nb, Zr, Ta, V, Ga, Pt, Cr, Sc, Y, Co, Ti,
Na, K;
R'096/23502 ~'18 5 6 6.1 PCTI1896/00171
16
~ represents chiral centers which may be R or S;
The various hydroxyl groups (OH) involved in chelating the metal
can be replaced by various functional groups, such as H or a
halogen, to vary the affinity of the compound for the chelated
metal or to convert the molecule into a metal antagonist.
Although the present invention has been described in
considerable detail with reference to certain preferred versions and uses
thereof, other versions and uses are possible. For example, exochelins
can be used to attack infectious bacteria, such as M. tuberculosis, by
blocking access of the mycobacteria to iron, to remove toxic levels of
metals from the body or to deliver desirable metals to the body. Further,
modified metal containing exochelins can deliver appended active drugs or
chemicals to cites in the body which preferentially absorb the chelated
metaland preferentially absorbed exochelins with chelated metals can be
used as targets for treatment by other modalities, such as microwave
energy for hypothermia treatment of cancer cells. Therefore, the spirit
and scope of the appended claims should not be limited to the description
of the preferred versions contained herein.