Note: Descriptions are shown in the official language in which they were submitted.
wo ss/306s3
2~8~8~7
Ar)ENOSINE DERIVATIVES
This invention relates to adenosine derivatives, and in particular, provides a new use
of A1 recepl:or agonis~s.
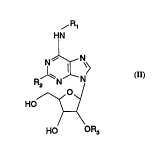
In particular the present invention relates to a new use of compounds of fonmula (I):
,R1
HN
~ N>
/\~~
HO ~<
HO OR3
wherein
R1 is Ihydrogen, (C, ,)alkyl, allyl; methallyl; a straight-chain or branched
(C3.7)alkinyl, (C3~)cycloalkyl, hydroxy(C~3)cycloalkyl, phenyl being mono-, or
i"dep~, Id~ y of each other di-s~ ~hstitl l'^d by halogen with an atomic number
of 9 to 35, (Cl8)alkyl, (C,.,)alkoxy or CF3; or phenyl(Cl ,)alkyl wherein the
phenyl ring is ur~ hstit~ d or mono- or i"dt:~,ellcle"~ly of each other di-
s~ stit~ ~ ' by halogen with an atomic number of 9 to 35, (Cl ,)alkyl, (Cl ,)
3 o a~koxy or CF3; (Cl ,)alkyl having at least one hydroxy group or at least two
phenyl groups, a bicycloalkyl such as endo- or exo-bicyclo[2,2,1]heptyl, a
naphthyl (Cl.4)alkyl-group, an acenapthylenyl (Cl ,)alkyl-group or a group of
formula A or fommula B
wo gs/30683
2I868q 7
. . ~
Z ~ or ~3
Y Q Y
(A) Q (B)
wherein
Z is hydrogen, a hydroxy group or a (C,.")alkoxy group
Q is hydrogen or hydroxy,
A is -CH2-, -0-, -S- or a direct bond,
Y is -(CH2)n- or a direct bond,
n is a whole number from 1 to 3
and the broken line in formula A ~ st"~ts an optional bond,
R2 is hydrogen, (Cl.,)alkyl, amino, (C3s)cycloalkyl or halogen with an atomic
number of 9 to 35 and
R3 is (C1~)alkyl.
Some of these compounds e.g. those wherein Rl is other than hydroxy cycloalkyl
are known and disclosed, for example, in published British Patent Application 2 226
027 A and European Patent ~!, " ' ls EP-0-378 518 and EP-0-269 574, and
USP 4,843, 066 and 4,985,049.
3 o In the formulae described herein the 2', 3', 4' and 5' substituents of the
tetrahydrofuran ring have the configuration as in adenosine.
wo 95/30683 2 1 ~ 6 8 4 7
-3-
ln one group of compounds under fommu~a 1 R, is other than hydroxycycloalkyl. Inanother group of compounds under fommula 1, R1 is hydroxy(C~ 3)cycloalkyl.
Preferably the compound of fonnula I is of 6-cy~ xyl 2-0-methyl-adenosine,
he,t,i,,drl~l referred to as Compound M.
Typically the A adenosine activlty in the preferred compounds of fomnula I may be
dt,l~""i"~d as follows:-
Affinity for adenosine receptors
Pig striatal " ~IllLld~ ,es are prepared as previously described by H. Bruns et al. in
Molecular Pl,al",acoloyy 29 (1986) pages 331-344.
3H-NECA, a non-selective adel~o:.i"e receptor agonist is used to label both A, and
A2 receptors. ICso values are derived from the dis~,lact:",~"l curves by weighted
non-linear least-square curve fitting to the Langmuir equation and pKD values
r::llcl ll~tF~rl
T~h~ ffinity of adenosine receptor ligands for A, and A2 receptors
A, A2 A,: A2
(KD;nM) (KD;nM) selectivity n
CPA 0.74 + 0.08 930 + 110 1260 6
25 CV 1808 2460 + 757 269 + 70 0.1 5
CGS 21680 4360 +1080 18.6 + 4 0.004 4
Compoulnd M 23 + 2 24500 + 5160 1090 5
1.5 H2O
3o CPA = Cyclopentyla~el10s;"e
CV 18013 = 2-Phenyld",i"oadel~o:,i"e (Carbohydrates vol. 81
.
WO9!i/30683 218684 7
1974) ref. 91898 K)
CGS 21680 = 2-lp-(2-Carboxyethyl)phenethylamino]-5'-N-ethyl
ca, i~OXd~ -acll~"osi"e (FASEB J, 1989; 3 (4) Refs. 4770/3)
In ay,~a",t",I with the literature CPA proves to be a potent and highly selective
displacer of binding to the A1 receptor CV 1808 is a relatively weak but selective
A2 receptor ligand and CGS 21680 shows high potency and selectivity for the A2
receptor.
Compound M in the form of its 1.5 hydrate shows good affinity and high selectivity
for the A, receptor vis à vis the A2 receptor.
The known compounds of the fommula I are known as antihype,It" .;~ agents and
coronary vaso, ni.
Furthermore it is known that the compounds of fommula I protect the vascular
endothelium by inhibiting both Illlu~ ocyt~ ayylt:y ~ and activation of
leucocytes. They also lower the blood lipid leYels. Further compounds of formulaI have a protective effect against diseases caused by hypeltel~sioll such as
congestive heart failure Illy~cdrdidl infarction or sudden cardiac death and renal
insufficiency (see Europ. Pat. Appl. EP-0-378 518 and EP-0-269 574).
These compounds are also known for the treatment of neu,.,dey~"t:,dIiol1
disorders, peripheral neuropathies such as diabetic neuropathy and of disorders
~Cso. i ,l.-d with peripheral vascular diseases and/or disorders ~~~ ' with
neuronal dey~"~,dIiol~, hypertriglyceridemia/low HDL ~l,olt~ ,ul levels, lipid
dysfunction, elevated free fatty acids or type I or type ll diabetes including
non-insulin depel-d~"I diabetes arrhythmias in particular paroxysmal
supraventricular tachycardia and tachycardiac atrial fibrillation and for protection
3 0 against myocardial infarction.
W0 95/30683~
~ 21868q7
-5-
The present Applicants have found that the compounds of fommula I are particularly
i"~ "dlyesics for example for the treatment of pai~, e.g. acute or chronic
pain.
The analgesic activity of the compounds of the fommula I are indicated by their
analgesic activity in standard animal tests, e.g. illlld~llllld~vly and neuropathic
models e.g. in reducing persistent i"" Illlldluiy ",eul,a"i..al l,;p~,dl~aly~:~id [(tests
a) and b)] and persistent neuropathic themmal hy,o~,alye:.id [test c)] indicative of
chronic lleuropathic pain.
ld"", IdlUIy hyp~,dlu~aid
Test a~ Freund's adjuvant-induced hy~ ldlyesid
Rats are injected i~tra-articularly in one knee joint with Freund's complete adjuvant
(100 microlitres). The load that the rat will tolerate on that leg de,;,~ases and
remains d~ ssed for up to 5 days. This effect is indicative of ",e~l,a"i~;al
hyperalgesia, and is responsive to NSAlD's and opiates. The compounds of the
formula I are adl "i"i~ rt:d by injection and preferably orally at doses of from about
3 to 60 Illi~;lUyldll~y animal body weight. The increased load tolerated on the
injected side is measured to determine the reversal of hyperalgesia.
Compound M shows particularly illt~.~alillg activity on p.o. a.l~"i"i:.l,d~ioll from
about 3 to about 60 ~iu~uyldllllkg with a duration of action of about 1 hour. There
is no significant difference in response between the doses of 3 30 and 60
Illi~ luyldll~kg suggesting that the maximum effect had been reached in the range
3 to 30 I l li~;l uyl dl l l/l~y.
Test b) Turpentine-induced ",e~;l,a,~ical hy~61alUe:~id
A local intra-plantar injection of h~ ,6/~,ardl~i" in rat paw (left hind) results in
WO 95/30683
218~847 -6- ~
a local ill~ldlllllldlUIy response and a reduction in the ~ 'Idlcl~vdl threshold (cut-off
threshold 340 9) for a ",~uI,d,~ical stimulus (paw pressure). The compounds of
fommula I are active at doses from about 1 to 100 Illicluylall~y p.o. or s.c.
adl"i"i~ rt,d three days after the injection, further threshold readings being taken
1 and 3 hours later.
Compound M shows significant activity at doses of 30 and 60 Illiuluyldll~ky orally,
with the maximum effect at 30 Illi~lUyldll~y. Morphine has an EDso value of 1.2
mg/kg s.c. in this test.
Neuropathic hy~ldl,U,esid
Test c) Neuropathic thermal hy~eldl,u,t:aid (according to the principles of Z. Seltzer
et ~I Pain. 1990. 43. 205-218)
Unilateral partial ligation of the sciatic nerve eliminates fibres throughout the
innervation of a paw of a rat. The rats develop II~ ldlyt:aia to ",eul,anicdl and
thermal stimuli and allodynia of the partially denervated paw without the induction
of autotomy. The animals are placed in a perspex box on a thin glass plate and aramp-shaped heat stimulus is applied to the planter surface of a paw. Latency topaw ~;.lldlCIvvdl is measured. The compounds of the fommula I are active at doses
from about 1 to about 100 ~ uyldllJky injection (s.c. or preferably orally),
ad" ,i"i~l~rt:d 12 to 15 days after nerve ligation. Compound M is particularly active
against thermal hyp~ldlgesid. Compound M shows significant activity at doses of
30 and 60 Illi.. lugldlll/kg, with the maximum effect at 30 ",iuluyldlll/kg. The EDso
is around 60 IlliUlUyldlll /kg p.o. Morphine has an EDso of around 3 mg/kg in this
test when a~l"i"ial~,~d subcutaneously.
~ini~l trials _ _ _0
Clinical trials may be effected as follows:-
W09~30683 ~ 8~8~7
-7-
Subjects suffering from pain, post-operative pain or post herpetic neuralgesia, are
a.i",i"ial~,~d with compounds of the fommula 1, especially compound M, i.v. at adose of ~rom 0.02 to 5 mg. The alleviation of pain is noted.
The compounds of the invention are therefore useful as al ,dl~siua, e.g. againstacute or chronic pain, e.g. chronic neuropathic pain.
In one aspect, therefore, the present invention provides a method forthe treatment
of pain which comprises a~",i"i~ ri"g a therapeutically effective amount of a
compoulld of fommula I as defined above, to a subject in need of such treatment.
In another aspect, this invention provides the use of a compound of formula I asdefined above as an analgesic in the ,UIt:pdldtiOI~ of ",edi-,d",~"l suitable for the
treatment of pain.
In anothler aspect, this invention provides a compound of formu~a I as defined
above for use in tlle treatment of pain.
In a furtller aspect, this invention provides an analgesic c~,,,,uo:,iliu,, c~",,uri~i"g a
compound of fommula I as defined above as an analgesic in A.c5or~ n with a
phanmaceutically ;Irc:ul~ carrier or diluent.
Il Idi~dliuns include pain, for example acute pain Accor iAtr~d with tissue damage and
dl 1111 IClliUI~ (e.g. post operative pain, bum pain, injuries, etc.) chronic i, I~ldl 111 l IdlUIy
pain (e.g. arthritis) and chronic neuropathic pain (e.g. diabetic neuropathy, post-
herpetic neuralgia, multiple sclerosis, causalgia, etc.).
The doses of the compound used in the uses of the invention indicated above will,
- of course, vary depending with the compound employed, the seriousness of the
disorders, the host, the weight of the patient, the mode of ad~"i"i~l,dliol, and the
relative efficacy of the compound. However, in general, Sdli:~d~;~UIy results in
W0 95130683
21~6~ 8- --
animals are indicated to be obtained at 3 daily doses from about 1 to about 100
;lUyldll~y animal body weight, e.g. 3 to 60, such as 10 to 60, IlliClu"ldllllk~.In iarger mammals, for example humans, an indicated daily dose is from about 0.1to about 10 mg, conveniently a~l"i"i~ d in unit doses from about 0.02 to about
5 mg, and such unit doses may be adl"i"i~ d more than once a day, for
example 2, 3, 4, 5 or 6 times a day, but preferably 1 or 2 times per day.
In test a) above, the NSAID ibuprofen has an ED60 of about 4 mg/kg p.o. and
i"d~",tltl,a-,i" 13 mg/kg p.o.. Compound M is about 3ûû times more active. For
compound M the preferred dose range is from about 0.1 mg/day to about 10
mg/day, e.g. 3 - 30 ~ uy~d~l~kg for a 70 kg adult, cle~ di"g on the severity of
the indication(s) and frequency of ad",i"i:,l~ ).
Compound M has been shown to have no serious adverse effects in man after
single doses up to 5 mg p.o.
When used herein the term "~JI,ar",~l~el~ y ~cept~ cu"".asses materialssuitable for both human and veterinary use.
The compounds of the invention may be fommulated for a~lllilli:illdli~ll by any
suitable route, the preferred route d~uel ,di"9 upon the disorder for which treatment
is required, and preferably in unit dosage fomm or in a form that a human patient
may a,ll"i"iblel to himself in a single dosage. Advantageously, the cu"",osit;o~ is
suitable for oral, rectal, topical, parenteral, intravenous or intramuscular
a~ dliu~.
The co" ,yosit;ul la of the invention may be in the fomm of tablets, capsules, sachets,
vials, powders, granules, lozenges, s~,u,uosilo, it:5, reconstitutable powders, or liquid
p,~parnliol~s such as oral or sterile parenteral solutions or Su:"Jt",:,iol~s. Topical
fommulations are also envisaged where a,o,u~u,uli~
WO g5/30683
~1 868~ 7 -9-
ln order to obtain col)s~ cy of adl l lil lia~ liù~) it is preferred that a cul l lpûailion of
the invention is in the form of a unit dose.
Unit dose ~ "ldLiùn fonms for oral a.i",i"i~ may be tab~ets and capsules
5 containing 0.02 - 5 mg ûf a compûund of the invention and may containco" ~ tivnal excipients such as binding agents, for example syrup, acacia, gelatin,
sorbitol, I,dgacd"tl" or polyvinylpyrrolidone; fillers, for example lactose, sugar,
maize-starch, calcium ,ul,o~ullal~, sorbitol, or glycine; tabletting lubricants, for
example magnesium stearate; diaill ~ ,.s, for example starch, cross-linked
polyvinyll~yrrolidone, sodium starch glycollate or microcrystalline cellulose; or
pl)di",ac,~utically A~c~lJ~ wet~ing agents such as sodium lauryl sulphate.
The solid oral cur"p~ )s may be prepared by conventional methods of blending,
filling, tabletting or the like. Repeated blending ùperdliu,,s may be used to
distribute the active agent throughout those cu,, ,,u~ ~s employing large quantities
of fillers.
Such oper~t;J,)s are of course conventional in the art. The tablets may be coated
according to methods well known in normal ,ul,al",aceutical practice, in particular
2 o with an enteric coating.
Oral liquid ~,~par 15 may be in the fomm of, for example, emulsions, syrups, or
elixirs, or may be presented as a dry product for reconstitution wlth water or other
suitable vehicle before use. Such liquid ~ UdldliU~)s may contain conventional
2 5 additives such as suspending agents, for example sorbitol, synup, methyl cellulose,
gelatin, llydroxyethylcellulose, caluu~y",~ ,ylcellulose, aluminium stearate gel,
h~,~,uy~,,dlt:d edible fats; emulsifying agents, for example lecithin, sorbitan
I"u,)o~ledl~:, or acacia; non-aqueous vehicles (which may include edible oils), for
example almond oil, i, d~;tiUI~dl~d coconut oil, oily esters such as esters of glycerine,
propylen~ glycol, or ethyl alcohol; preservatives, for example methyl or propyl
p-hydroxybenzoate or sorbic acid; and if desired conventional flavouring or
WO 95/30683 P~
2186~7 -10-
colouring agents.
Examples for solid oral c~ll r- ~s, e.g. tablets and capsules, are as follows:
The tablets are c~," ",r~sed from the capsule granulation of the c~" ~.olldi"g dose
strength. Size #3, yellow capsules are used for the er~rs~ of compound M,
and the respective fommulation for the 0.1 and 5 mg dose strengths are shown
below.
0.1 mQ Tab/Cap 5.0 mg Tab/Cap
Compound M 0.1 5.0
Microcrystalline Cellulose 76.92 74.96
Lactose, hydrous 87.63~ 84.69
Polyvinylpyrrolidine 7.4 7.4
Crospovidone 7.4 7.4
Magnesium Stearate 0.925 0.925
Moisture Retained 4.625 4.625
Size Three Capsule 50 50
Theoretical Capsule Fill
Weight or Tablet Weight 185 185
Theoretical Capsule Weight 235 235
~ The amount of lactose is adjusted to CUIII~ ,dl~ for the amount of moisture in the drug substance.
The compounds may also be adl"i"i~ d in the fomm of a sustained release form,
in order to provide a prolonged duration of action. Conventional drug delivery
systems may be used to provide sustained release, for example, coated pellets or push-pull osmotic systems.
WO 95/30683 1 ._ 1 / L ~, C,
218 6 8 ~
For parenteral ad",i"i:,l,dli~l), fluid unit dosage forms are prepared utilizing the
compound and a sterile vehicle, and, .It~ "~i"g on the col ~C~"~d~ used, can be
either suspended or dissolved in the vehicle. In preparing solutions the compound
can be dissolved in polyethyleneglycol or ethanol and diluted with water for injection
and filtel sterilized before filling into a suitable vial or ampoule and sealing.
Advanta~eously, adjuvants such as a local m ,a~ t;_, a preservative and buffering
agents can be dissolved in the vehicle. Parenteral su:.~e":.iol1s are prepared in
S~u~ldll 'Iy the same manner, except that the compound is suspended in the
vehicle instead of being dissolved, and ~lt:,ili~d~ion cannot be accu,,,,uli~.,,ed by
filtration. The compound can be sterilized by exposure to ethylene oxide before
suspending in the sterile vehicle. Advantageously, a surfactant or wetting agent is
included in the cu,,,,uo:,iliu,, to facilitate uniform distribution of the compound.
The compositions may contain from about 0.1% to about 99% by weight, preferably
from 1û to 6û% by weight of the active material, depending on the method of
ad" ~i"iall dliùn.
Compou~1ds M of the invention, or a hydrate or an addition product with other
solvents or salts thereof, may also be adl"i"isl~ d as a topical formulation in
COIlluilldliul~ with conventional topical excipients.
Topical formulations may be presented as, for instance, ointments, creams or
lotions, illl,ultlylldlt:d dressings, gels, gel sticks, spray and aerosols, and may
contain ~.,u, up, idl~ conventional additives such as preservatives, solvents to assist
drug pt~ ldliOI1 and emollients in ointments and creams. The formulations may
contain c~""~dlil,le conventional carriers, such as cream of ointment bases and
ethanol or oleyl alcohol for lotions.
Suitable cream, lotion, gel, ointment, spray or aerosol fommulations that may be
3 o used for compounds of formula I or a hydrate or an addition product with other
solvents or salts thereof, are conventional fommulations well known in the art, for
WO 95/30683
2186847 -12- -
example, as described in standard text books of pl,al",aceutics and cosmetics,
such as Harry's Co:""~lk"~luy,r published by Leonard Hill Books, P~ l,u,~ull's
Pl,d""aceutical Sciences, and the British and US rlldlll.~:OI)OF~;~C
In another aspect, the present invention provides adenosine~t"i:. ".rcc, of fomnula
la
/R~'
HN
R 1`~ N~
H 0/~~ ~1
H O = =
wherein
R1~ is (C~3)cycloalkyl ~llh'~ ' by a hydroxy group,
R2 iS as defined above or preferably hydrogen, (Cl.,,)alkyl or halogen with an atomic number of 9 to 35, and
R3 is as defined above.
Compounds of formula la can exist in fomn of el~d,,liu,,,e,a or ~lid~ u~eli~
mixtures.
In formula la, halogen with an atomic number of 9 to 35 is fluorine, chlorine orbromine; (C,,,)alkyl is methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, tert.-butyl,
especially methyl; and (C~ 8)cycloalkyl is cyclobutyl, cyclopentyl, cy~luhex~l,
WO ~5/30683 2 1 8 6 8 4 7
-1 3-
cycloheptyl or cycloctyl, especially cyclohexyl or cy~,lu~t:"tyl.
The compounds of formula la may be prepared by a process .,I,d,c.~,Lt~ ,ed in that
comp~unds of fomnula lla
x
1~ >
~o¦ ~a
~1 .
HO OR3
wherein
R2 and R3 possess the :,iyll ' ,ces given above and
X is chlorine or bromine,
are reacted with a compound of fomnula Illa
Rl'-NH2 Illa
5
wherein
Rl' poss~s~s the siy"iri~;d"ces given above.
3 o The above process is conveniently effected by heating a compound of formula lla
together ~Nith a compound of formula Illa in the presence of a solvent such as
W09~/30683 ~ , I~l/~i,, '
218fi8~7 -14-
dioxane to a temperature of between about 80C to and 1 20C, preferably to boiling
temperature.
In the compounds of fommula lla, which are used as starting compounds in this
process, X is especially chlorine. The compounds of fommula lla, as well as a
process for the production thereof, are described in published European Patent
Application 269 ~74.
A further process for p,e:ualdliùn of compounds of fommula la comprises treatingcompounds of fommula IVa
,Rl
~ ' ` IVa
Si OR3
~ ~
wherein R,' , R2 and R3 possess the ~iyl," )ces given above with
tetrabutylammonium fluoride trihydrate.
The above reaction may be performed in an organic solvent e.g. tetrahydrofuran at
room temperature under stirring. Compounds of fommula IVa can be obtained by
treatment of compounds of formula lla with 1,3-dichloro-1,1,3,3-Le~ uulu,u;l
disiloxane in an alkaline solvent e.g. pyridine, and reacting the so obtained
compounds of formula Va
WO 95130683 1 ~, 11 J.. '. ~
2~868~7 -15- -
RJ~ N
~ / \~~/ Va
1' \o o~
\ / OR3
wherein X, R2 and R3 have ths ~iy~ ical1ces given above with compounds of
formula Illa.
In the following Examples 1 to 8, all temperatures are in degrees Celsius and are
2 o uncorrected.
Exampl~2 1: 6-[(trans~-4-Hydrox~/cyclohexyl]-2'-0-methyl adenosine
19 of 6-[(trans)-4-hydroxycyclohexyl]-9-purinyl-2'-0-methyl-3',5'-0-(1,1,3,3-tetraiso-
2 5 propyldisilox-1 ,3-diyl)-D-ribose is stirred during 15 minutes at room temperature with
2 9 of tetrabutyl ammonium fluoride trihydrate in 20 ml of tetrahydrofuran. The
mixture is subsequently evaporated to dryness and the residue is eluted on siiica
gel witll a mixture of ethyl 2C~Idl~ IlldllOI 85:15. The purified product is
crystallised from methanol/ethyl acetate. The title compound crystallises with one
equivalent of methanol and has a melting point of 121-124C.
[a]D2~ = -50.2 (c = 1 in dimethyl~,,,,d,,,ide)
WO 95130683 . ~I/ I
q 7 -16- ~ --
The compound may aiso be IC~ y " 3r' from ethanol and crystallises with one
equiYalent of water after standing in moist air. Melting point:114-117.
[a]D20 = -46.9 (c = 1 dimethy:~,,,,c.,,,ide).
The 6-[(trans)-4-hydroxycyclohexyl]-9-purinyl-2'-o-methyl-3',5'-0-(1,1,3,3-tetraiso-
propyl- disilox-1 ,3-diyl-)-D- ribose used in the above process as starting material
may be produced as follows:
2gof6-chioro-9-purinyl-2'-0-methyl-3',5'-0-(1,1,3~3-~ ;C~UIU~JY ' ' -1,3-diyl)-D-
ribose are stirred together with 1.32 g of trans-4-hydroxycyclohexylamine and 1,4
ml of N-ethyl-diisopropylamine in 80 ml of dioxane during 6 hours in an oil bath of
105C. The mixture is .sllhseq~lently cooled to room l~"".e, ~re, filtered and the
filtrate col ,c~ ldl~d to dryness. For purification the so obtained mixture is eluted
on silica gel, first with a mixture of hexane/ethyl acetate 7:3 and then with a mixture
of ethyl act:ldt~,,'~, le:ll ,al~ol 95:5. The so purified oily compound has a R, value of 0,3
(from ethyl acetate / methanol 95:5).
Exarnple 2: 6-[(cis)-4-Hydroxycyclohexyl]-2'-0-methyl adenosine
2 0 The compound named in the title is obtained analogously to example 1 when cis~
hydroxyc~,.,lol ,~,~ylnmine is used instead of trans-4-hydroxycyclohexylamine.
The so obtained compound named in the title crystallises with a half of an
equivalent of ethyl acetate and has a melting point of 110-111C. []D20 = -48,6
2 5 (c = 1 in dimethyl~", Idl "i.le).
EYSIIT~31P 3 6-[(1S,trans)-2-Hydroxycy11u~Jt"~yl] 2'-0-methyl adenosine
The compound named in the title is obtained analogously to example 1 when 1 S,
trans-2-hydroxycyclopentylamine is used instead of trans-4-
hydrox~",y.iloh~,~ylnmine. The so obtained compound named in the title has the
w0 95/30683 r~ oC
21868~7-17'
following ~ dld~.luli~ . Foam, R, in ethyl~retr'-l~,t',a,~ol 75:25 = 0,44, []D20 =
25,5 ~c = 1 in dimethyl~v,,,,d,,,idt,)
Example 4: 6-[(1R,trans)-2-Hydroxy~.y~,lop~"~1]-2'-0-methyl adenosine
The compound named in the title is obtained analogously to example 1 when 1 R,
trans-2-hydroxycyclopentylamine is used instead of trans-4-
hydroxycy~;lùi ,~Aylnmine. The so obtained compound has the following
1 0 ~.1 Idl dl,lC~ s.
Melting p~oint: 164-166, R, in ethylacetate/ethanol, 75:25 = 0,44, [a]D20 = -80,2 (c
=1 in dimethyl~ul",a",id~).
FY-~PIP 5: 6-[(1S,trans)-2-Hyd,uAy.,y~ilul,t,Ayl]-2'-0-methyl adenosine
The compound named in the title is obtained analogously to example 1 when 1 S,
trans-2-hydroxycyclohexylamine is used instead of trans~-hydroxycyclohexylamine.The so obtained compound named in the title has the following .,I,d,d.,leri~l;.,s.
Foam, R, in ethylacetate / Ethanol 75:25 = 0,42; [a]D20 = -26.2 (c = 1 in
dimeth~,llv""d",ide).
EY~-rple 6: 6-[(trans)-4-hydroxycyclohexyl]-2'-0-ethyl adenosine
Prepared analogously to Example 5. Obtained as 0.4 hydrate M.pt. 98C
(liquefies). [a]DZ = - 58 (c = 1 in dimethyl ~VIIIIdllli(le).
EY_~PIP 7: 6-[lS,(trans)-2-hydroxycyclohexyl]-2'-0-ethyl avel~osi"e
Prepared analogously to that described in Example 5.
FY~mple 8: 6-[(1R,trans)-2-Hydroxycyclohexyl]-2'-0-methyl avel,osi"e
W0 95130683 j l
218681t7 -18-
The compound named in the title is obtained analogously to examp~e 1 when 1 R
trans-2-~ Jd UAy~ :~u~ 2m~ne ~s used ~nstead of trans~-hydroxycyc~ohexylam~ne.
The so obtained compound named ~n the t~t~e has the fol~owing :I a a k i~ti~s:
Foam; R in ethyl~At~ t. a~ol 75:25 = û.42; [a]D20 = -69.5 (c = 1 in
dimethy;~u Idl ici~).
The compounds according to the invention have i t li g ~ dl~ lC..J;. .Ipropert~es. They are therefore usefu~ as ~ di-idlllt:llts. For this compounds
according to the invention can be used as such as hydrates or addition products
with organic so~vents e.g. methano~ ethano~ or ethy~ acetate. The most i L~ . g
compound of fommu~a ~a is the compound of Examp~e 1 above wh~ch ~s in the
fo~owing named compound No. 1.
The compounds of fommu~a la are of interest as ana~gesic as described above. They
are a~so of interest for other activities. Among other activities the compounds
according to the invention have anti-hypertensive activ~ty as indicated from theresu~ts of the fo~owing inve~liydliùl: .
Measurement of the b~nding to adenos~ne A1 and A2 receptors in t~ d~es from
2 0 the rat s cortex or from the cortex or striatum of the pig s brain using the method
of R.F. BRUNS G.H. LU and T. A. PUGSLEY wh~ch is described in MOLEC.
PHARMACOL. 29 331-346 (1986) . Further testing of the activity of the compounds
is effected in the iso~ated perfused rats k~dney for the fo~lowing pdldlll~l~la.
- renin secretion
- rena~ haemodynam~cs
- ~nh~b~t~on of the re~ease of noradrena~ne from the nerve endings fo~owing
3 o e~ectro-stimulation of the rena~ nerves according to the method of P. M.
VANHOUTTE D. BROWNING E. COEN T. J. VERBEUREN L. ZONNEKEYEN
wos~/306s3 21 8fi8~ 7 r~
-1 9-
and Ml. G. COLLIS described in HYPERTENSION 4, 251-256 (1982).
- Measurement of blood pressure, heart rate, urine production and renin activityin plasma of wake, NaCI-depleted and repleted, n~", lUt~ C or :"u~ dl ,eously
I,ype~tel,:,ive rats with catheters implanted in the clLdu~ al aorta and in the
vena cava, following i.v. a.ll"i"i~ , or a.l",i,~ l,d~iul1 of the compounds
according to the invention as an infusion or bolus according to the method of
J.F.M. SMITS and J. M. BRODY described in Am. J. Physiol. 247, R1003-R1008
(1984).
It is deduced from the results of the t:AdlllilldliUils that both the inhibition of renin
secretionl and of release of noradrenaline from nerve endings, and the direct
VnSOI "' " I, take part in the anti-hypertensive activity of the compounds of fommula
la. In contrast to the lowering of the blood pressure, urine production and
electrolyte excretion remain u~ l Idl ,ged. It results from this that the compounds of
formuia la can not only be used as a"lil,j~.e,lti,sive agents, but also effect
coronary \~dsouildliull. Furthermore, they protect the vascular endothelium by
inhibiting both thrombocyte agy,~d~ and activation of leucocytes. They also
lower blood lipid levels.
For the above illdk.dli~l1s, of the compounds of formula la, the compound of
Example 1 is preferred. The ca,~io~-,ult:~.live and analgesic ill~iudliolls are
preferred.
Furthermore the compounds of formula la are also active in the treatment of
arrhythmias as indicated by their adenosine A1 receptor agonist activity, which is
selective via-a-vis their activity against the A2 receptor, as stated e.g. in test a)
described ht:l ~i"dtler and for example their capability to prolong conduction through
the atrio-ventricular (A-V)node of the heart as indicated in test b) described
he,~i,,drl~l. Thus, they restore sinus rhythm in supraventricular tachycardias, in
particular paroxysrnal supraventricular tachycardia, reduce cardiac rate in
WO 95/30683 ~ s
21~6847 -20-
tachycardic atrial fibrilation and retum ventricular tachycardias induced by
~-a ilt"~yiu stimulation to nommal rhythm.
The compounds of formula la mimic the effect of "~ col, iilivl ,i"y", the procedure
wherein a brief period of ischaemia renders the heart resistant to infaretion from
sllhse~ nt ischaemia. They are, therefore, useful to protect the heart during
unstable angina, against myocardial infaretion with or without adjunctive
thrombolytic therapy, or ischaemic injury in patients Ull i~lyuilly (e.g.) coronary
artery bypass graft surgery, d~lyiu~-ld~ly, eardiac ~Idllb,uldllldliun or non-cardiac
surgery.
The compounds of fommula la are especiaily useful for a il"i"i~ ,i"g to subjectsprone to myocardial i"~drutivlls, e.g. as result of diagnosis or those who have
already suffered from a myocardial infaretion.
The compounds of fommula la lower further plasma insulin without influencing
glucose tolerance. They potentiate the insulin effect to increase glucose uptake and
decrease lipolysis in adipose tissue leading to lower plasma free fatty acid
collcelllldlivlls (see test d) described ll~,~i,,d~l~l). Lower piasma free fatty acids in
tum lead to lower triglycerides, which leads to increased HDL .. I,~le~ ,ul.
The insulin sparing effect and/or action to lower free fatty acids makes the
compounds of formula la useful for the treatment of Type I diabetes and Type ll
diabetes i.e. non-insulin d~pellde"l diabetes.
The compounds of formula la lower plasma triglycerides and free fatty acids (seetest e) lle,~i, Id~ l) and raise HDL ~:I lol~:,t~, ul and hence, are useful in treating lipid
dysfunction, and conditions AccO~ ;..l.-d with elevated free fatty acids and
triglycerides and where an increase in HDL .I,~leal~,ul is desirable.
The compounds of fommula la show good metabolic stability.
wo 95/30683 2 1 8 68 4 7
-21 -
In another aspect, the invention provides the use of compounds of fommula la for
thetreatmentof~orinthepl~:iJaldliunof~lledi~dlll~l~tasuitableforthetreatmentof~
pain.
In another aspect, this invention provides a compound of formula la as defined
above for use in the treatment of pain.
In a further aspect this invention provides an analgesic cull,uuailiul) c~,,,,uriaillg a
compound of fommula la as defined above in ~C50; ~ .., with a i l~a""ac~utically~C~ carrier or diluent. The present applicants cu"l~",~ the use of
compounds of formula la in the ii ~di. .~t;o,~s as described above for compounds of
formula 1.
The following i l,a""~ lo.J;~I tests illustrate the above ",~"~;~",ed activity of the
compounds of formula la.
a) ~lfini~y for a~ aill~ reoeptors
Pig striatal Illc:lllLldiles are prepared as previously described by H. Bruns et al. in
Molecular Pl,a""acolùgy 29 (1986) pages 331-344. 3H-NECA a non-selective
d~el1oai"e receptor agonist is used to label both A, and A2 receptors. ICso values
arederiv~dfromthedia~ula- ~",_"~curvesbyweightednon-linearleast-squarecurve
fitting to the Langmuir equation and pKD values ~ tPrl
The compounds of fommula la show affinity for the A, receptor e.g. at a range offrom 1 to 500 nM.
b) A~ ;a~
3 o Adenosine A, receptor activation reduces the incidence of, e.g. paroxysmal
supraventricular tachycardia, Id :l "/_~l diL atrial fibrillation and other arrhythmias by
WO 95/306U , I ~
21868~7 -22- 1--
slowing conduction through the atrio-ventricular (AV) node of the heart detected by
increases in the P-R interval. The test method involves recording of ECG changesin conscious adult Rhesus monkeys. The compounds of formula la are
a~i~"i"i~ d at dosage ranges from about 0.1 mg/kg to about 1000 mg/kg p.o. or
0.03 to 30 mg/kg i.v. in the test referred to by C. Clarke et. al. in The
Pl,d""ac~utical Joumal 244, 595-597 (1990). For example in this test compound
No. 1 prolongs P-R interval of the surface ele~.l,u- d, iiuy,d,,, (ECG), indicating
siowing of conduction through the AV node at doses~ 'of 0.1, 0.3 and 0.6 mg/kg
p.o. As noted above such an action leads to ltlllllilldli~o of AV nodal reentrant
tachycardia and reduction in heart rate in tachycardic atrial fibrillation.
c) Protec~Yon against infar~Yon by r ~ Y( ~ 9
P,~con ,il,g (5 minutes of ischaemia followed by 10 minutes of recovery)
renders the heart very resistant to infarction from s~ ~hsequent ischaemia (30 min)
and reperfusion (3h). The test method is disclosed in the article of G. S. Liu et. al.
1991 - Circulation (~4) 1 350 - 356 and J. D. Thomton et al. in 1992 - Circulation
(85), 659-665. The compounds of fommula la are a~i~"i"i~l~,t,d i.v~ at a dose of from
about 0.01 to 10 mg/kg to rabbits. In this test rabbits given 100 ug/kg of compound
No. 1 were protected to neariy the same extent as elldo~e~ous plc:co,.~ g
from infarction induced by a 30 min. period of coronary occlusion.
d) Glucose Tmnsi~ort and Lipoiysis Ll Rst A i;~
2 5 i) Adipocytes are isolated f rom the epididymal fat pads of normal chow-fed rats
by digestion with c~lldg~l~ase. Cells (final cul lu~ dtiùl~ 2% v/v) are
pre-incubated with ad~"o~i"e cied",i"as~ (1 U/ml) and with test compounds
e.g. compound No. 1 and other additions as indicated to measure glucose
transport at 37C.
[3-3H]Glucose (final c~l~c~ ldliol~ 50 uM 0.5 uCi/ml) is then added after 30
W095/30683: r~
- -23- 21868~ 7
minutes, and incubation is continued for a further 60 min. lllcOl~Olaliui~ of
radioactivity from [3-3H]glucose into cell lipids (a measure of glucose transport) is
evaluated by extraction of the cell suspension (0.5 ml) with 5 ml of toluene-based
scintillant, followed by liquid 5..i~ counting; water-soluble " ~ i and
residual [3-3H] glucose remain in the aqueous phase and are not detected.
Lipolysis is measured in a conventional manner. In one method cells are incubated
with and ~Nithout 1 uM isop,v~ ol and then the incubation continued for 60 min.
The supsmatant is separated from the cells after b~ll' '' l~ptinn through
dinonylphthalate and lipolysis estimated by enzymatic d~l~llllilld~iuil of giycerol
released into the supematant.
The compounds of formula la are active at col~ct:lllldlio~ls from 0.1 to 10ûO nM.
Compound No. 1 suppressed lipolysis in rat adipocytes at col~c~"I,dliu11s from 0.1
to 100 niM.
In the presence of adenosine ded",i"ase (1 U/ml), compound No. 1 has no
significan~: effect on the il)co~uordl;v,, of rddiua-;.iJity from [3-3H]glucose into cell
lipids in tlle absence of insulin and only a marginal effect in the presence of a
maximally stimulating insulin cullc~ dliol~ (8 nM), but siy"iri-;d,.:!y increases the
stimulatiol~ of i"~,uOrdli~l1 of ~ddiua~ ;.y in a cu"~ ~"l~dliol~-dep~"dt",~ manner,
at cu,1ce,,l,dliul,s from 0.1-50 nM insulin. These observations indicate that the
compound increases the sensitivity of glucose transport to insulin. In the presence
of anear-l~aximallyeflectivecc,,~c,:,,lldliu,, of compoundNo.1,theECsOforinsulinstimulatioll of [3-3H]glucose illC~lUOIdliUII into lipids is d~ ased between 2 and 3
fold. In the presence of ad~l,osi"e dea",i"ase and 1 uM isuj r- ~"ol, 10-50 nM
of compound No. 1 increases both the sensitivity and the magnitude of the
response to insulin; the EC50 for insulin is reduced up to 5- fold and the stimulation
by a maximal insulin CullC~Itldtiull is ~iy" Illy increased.
WO 95/30683 1
2186,8 ~ 24-
ii) Lipid and G~ucose in Nommal 18 hr Fasted Piats
The rats, 2 to 3 months of age,~weighing ca. 250 grams are kept in a room
at a controlled ambient temperature of 22 C and a 12/12 hour lighVdark
cycle for seven days.
Purina rat chow and water are available ad libitum. Following an 18 hour
fast, rats (5/group) are given test compounds by gavage in 0.5% CMC. The
animals receive 1.0 mU100 9 body wt. Three hours after a~ d~i~o~ the
rats are a~,e~l,t,li~t,d with CO2 and blood collected via cardiac puncture.
Sera are collected and used for glucose, free fatty acid and
~-hydroxybutyrate d~ ,;"at;o,~. Free fatty acids are measured by acyl-
COA peruA;ddse cdlulilll_~ic enzyme assay, glucose is measured by the
glucose oxidase method (YSI Model 27, Yellow Spring, Oh) and
~-hydroxybutyrate is assayed with a ~-hydroxybutyrate
dehy.l,ugellase-linked en_yme assay (Sigma Kit 310-A - St. Louis, Mo.).
The compounds of fommula la are active at a dose of from about 1 to about
5û00 ,ug/kg.
The doses for lowering free fatty acids (the primary result of the effect on
adipocytes) for the compound No. 1 are between 5 and 100 ug/kg at 2 hr
post dose. This results in dose d~pt:llltlt:lll decrease in ,I~-hydroxybutyrate
and blood glucose levels.
iii) Effects in non-insulin Dependent Diabetic (NIDD) Rats
In the NIDD screen test, the rats (200-220 9) are fed a high fat diet ad
Ji~ihlm. At fed state, 40 mg of a~ 71~t~ /kg of body weight are injected
via the tail vein. One week later, those rats are cull~idt,rt,d to be diabetic
3 o which have fed blood glucose of greater than 200 mg/dl and, following an
ovemight fast, when given an oral glucose tolerance test, have blood
WO 95/306~3 -25- 1 868~ 7
glucose of 40 to 80 mg/dl three hours after the test. Four days later, animals
are used in the screen, if fed blood glucose levels are greater than 180
mg/dl. Blood glucose is ~ d with a YSI Glucose Analyzer. The
cllronic screen test is carried out as follows:
On Day 1, food is removed from rats at 9:00 a.m.; and after an initial blood
glucose reading is taken via the tip of the tail, vehicle (control) or compound
(9 Idla/tl~dLIllt:l ll) is a~",i"ia~ d orally. Six hours later blood glucose level
is measured; and illllll~didl~ly thereafter the rats are refed.
The same rats are given either vehicle or drug once a day for 11
consecutive days. Blood glucose is d~ l lil ,ed at 0 hour and after a 6-hour
fast post dosing on days 4, 8, and 11. 100 Illi~;luyldll~kg of a compound
oF fommula la e.g. compound No. 1 for 11 days results in a significant
: reduction in plasma free fatty acids that produces a significant decrease in
b ood glucose.
e) Dy 'i~ ~,i~dbyelevateda-erumbi,~ .i.l~s
Several studies have shown a positive co"~' l between serum
triglyceride levels (and an ~.so,,; U~d cl~ ased HDL l,l ,uleal~,ul level) and
the risk for coronary heart disease (CHD) (Grundy, in Cholesterol and
Alllelusc~leluais~DiagnosisandTreatment~Lippincott~pll;ldd~l~Jllid(199o))~
The value of reducing elevated triglyceride levels as an approach to
reducing the risk of CHD emerged from the Helsinki Heart Study where,
following treatment with y~lll"' u,;l, the greatest reduction in serious
coronary events occured in Type IIB hyp~,li,u;d~,,,;c patients in whom both
LDL-~I loleal~, ul and total serum triglycerides are elevated and HDL
- cholesterol levels are generally reduced. The compounds of formula la are
3 o a~tive in the Rhesus monkey at doses from about 0.03 to 30 (e.g. 0.1 to 30)mg/kg i.v. and 0.1 to 100 (e.g. 0.1 to 10) rng/kg p.o. Compound No. 1
WO 95/30683 . . J ~ -1~)'''~ '
2i~8~ -26-
produces doc,0..~,1.2~d and long-lasting falls in plasma free fatty acids and
triglycerides in the Rhesus monkey at doses from 0.03-0.6 mg/kg i.v. and
0.1-1.2 mg/kg p.o.. Re,,u, ~s~"tdlive results for Compound No. 1 at 0.6 mg/kg
p.o. show a lowering of free fatty acids by ca. 60 % compared to a control
after 300 minutes and of l~i~u,~ycelid~s by 40%.
The compounds of fommula la are also active in tests for mean arterial blood
pressure, ~,d~y~ar,lid and peripheral v~ in d"a~ d rats.
ThQ ~ ue, i" ,~"ts are carried out on male Wistar rats, body weight 300-350
g under Pentothal anaesthesia (120 mg/kg i.p.), according to the method of
Salzmann et al. (J. Cardiovasc. rl,d""acol. 12, 451-460, 1988). Catheters
are placed in the right jugular and right femoral veins, in the left ventricle
(inserted via the right carotid artery), left femoral artery, and aorta ~inserted
through the right femoral artery). The following variables are measured or
calculated: systolic, diastolic, and mean arterial blood pressure (mm Hg; left
femoral artery, Statham pressure transducer P 23 Gb), pulse pressure (mm
Hg), heart rate (bedt~~ l, triggered from the blood pressure curve), rate of
rise of left ventricular pressure (dP/dtmaX, mm Hg/s; Statham pressure
transducer P 23 Gb), cardiac output (mUmin/100 g body weight,
" ""- lllti~,n method, right jugular vein and aorta), total peripheral
resistance (dynes s.cm ~/100 9 body weight), superficial ECG. Arterial blood
pressure, left ventricular pressure, dP/dt m~X~ heart rate, and
ele.:~,u..a,diug,d,,, are continuously recorded with a Schwarzer polygraph.
The pdldlll~t~l~ are measured 30, 20, 10 and 2 min before ad,,,i,,ial,dliùn
of the test substance and 1, 5, 10 and 15 min after injection of the drug into
the right femoral vein. The compounds of formula la are injected at dosage
ranges from about 0.001 to about 1 û mg/kg animal body weight. Compound
No. 1 was tested in cumulative doses of 0.003, 0.010 and 0.03 mg/kg, three
animals per dose being used. Compound No. 1 induced falls in blood
pressure [EDso = 49 Illi.;lUyldll~ u, iv] and heart rate and an increase in
WO 9~il30683 1' ~, I I 1~... _ .
2l8684 7
-2~-
systemic vascular conductance.
The doses of the compound of fommula la used in the above illdk.dliulls will vary
in the usual way with the seriousness of the disorders, the weight of the sufferer,
and the relative efficacy of the compound. Hûwever, as a general guide suitable
unit doses may be 0.1 to 1 ûOO mg, such as 0.1 to 10, 0.5 to 200, 0.5 to 100 or 0.5
to 10 mg, for example 0.1, 0.5, 1, 2, 3, 4 or 5 mg; and such unit doses may be
adl"i"iale,~d more than once a day, for examp!e 2, 3, 4, 5 or 6 times a day, butpreferabl~l~ 1 or 2 times per day, so that the total daily dosage for a 70 kg mammal
including humans is in the range of about 0.1 to 1000 mg e.g. per os or 0.0û3 to300 mg i.~., that is in the range of about 0.001 to 20 mg/kg/day, such as 0.007 to
3, 0.007 to 1.4, 0.007 to 0.14 or 0.01 to 0.5 mglkg/day, for example 0.01, 0.02,0.04, 0.05, 0.06, 0.08, 0.1 or 0.2 mg/kg/day; and such therapy may extend for a
number of weeks or months. For compound No. 1 the preferred dose range for all
the illli~;d~iUlls of the invention is from 0.1 mg/day to 10 mg/day for a 70 kg adult.
For the preferred indication non-insulin depe~ "l diabetes and for
hypertrigl~/ceridemia the indicated dose for humans is 0.2 to 2 mg per day per os
and for treating heart failure and other cardiac disorders 0.2 to 10 mg per day in
particular 0.5 to 5 mg/day per os or 0.25 to 5 mg i.v. for arrhythmias e.g.
tachycardic atrial fibrillation.
The compounds of formula la may be fommulated for adl "i, liall dliUO by any suitable
route, the preferred route ~ept:"-li"g upon the disorder fûr which treatment is
required, and preferably in unit dosage fomm or in a form that a human patient may
administer to himself in a single dosage. Advantageously, the compositiûn is
suitable for oral, rectal, topical, parenteral, intravenous or intramuscular
a.llllill;a~ldLiol~ as described above with respect to the analgesic use.
- Unit dose ,ul~a~ dliull forms for oral adlllillialldliui~ may be tablets and capsules
co"ldi"i"g 0.05-20 mg of a compound of formula la.
W0 95/30683 ,~
- 218~8~7 -28~
Suitabiy, the compounds according to this invention, or a hydrate or an additionproduct with organic solvents, will comprise from about 0.5 to about 20% by weight
of the fommulation, preferab~y from about 1 to about 10%, for example 2 to 5%.
Compounds of forrnula la wherein Rl' is hydroxy (C4.8)cycloalkyl and R3 is alkyl, are
detected as ,,, ~c~L " - on a~"i"ibl, , of com~pounds of forrnula I wherein R,
is (C4.")cycloalkyl and R3 is alkyl to warm blood~3d animals, e.g. rats, dogs and
humans.
Thus a-l~"i, lialldliU~ of Compound M leads to the production of hydrox~ ,lul,~Ayl
2'-O-methyl adenosine.
In a further aspect, the present invention provides a method of ad~llillibIt~lillg N6-
cyclohexyl-2'-O-methyladt31~0~i"e to a warm blooded animal in order to produce N6-
hydroxycyclohexyl-2'-O-methyl adenosine.
The present invention provides in another aspect N6-hydroxycycloalkyl-2'-O-alkyladel10si"e in pure form, e.g. greater than 95% pure. The eAt:",, ' ' 3~ compounds
described herein meet this criterion.
The present invention provides in a further aspect N6-hydroxycyclohexyl-2'-O-alkyl
adenosine free from cyclohexyl-2'-O-methyl adenosine.
In another aspect, the present invention provides a process for the production of
2'-0-alkyldd~:llObille5 which comprises treating adenosine with an a,u,uluplidIc: alkyl
sulphate in the presence of a phase transfer catalyst.
Preferably the 2'0-alkyl adenosine is any compound defined above.
3 o In a further aspect, the present invention provides a process for the production of
compounds of formula I as defined above, which comprises reacting a compound
WO 95/30683 1~ Jr ~,
' 29 2186847
of fommula Vl
R2 ~\ $N
N ,~
H O--~/
HO O H
Vl
with a basic solution of a compound of formula
(R3)2SO4
in the pre~ence of tetrabutylammonium hydrogen sulfate and a non-polar solvent
and purifying the product by recry: ' " ' ~.
If necessary reactive groups may be It~ Ial;ly protected.
Preferably the alkyl sulphate is di(C1 4)alkyl sulphate.
3 o Preferably the phase transfer catalyst is tetrabutyl ammonium hydrogen sulphate.
WO 95/30683 2 1 8 6 8 4 7
~ .3~ ~
Preferably the reaction is effected in a non-polar solvent, e.g. as described
he~ ~i, la~lar.
A group of compounds of the formula l is defined wherein
R1 is (C3 s )cycloalkyl, R2 iS hydrogen or (Cl ,)alkyl, and R3 is (Cl, )alkyl.
Hitherto, the pl~,udldlioO of compounds of formula i typically involved six steps,
which includethe pl~:pdl " .1 of 2',3',5'~ tyli"~si"e; cl~ illdlioll and hydrolysis
to 6-chloro-9-13-D-ribofu,d, l~all ~1 I purine; protection of the 3'-0- and 5'-0-positions
with ItslldiSoplupy; ' ' ,e (TIPDS-CLz); 2'-0-alkylation and purification by silica
gel ~ l ul l ldluyla~ul ly; d~ul ut~ ivl~ of the 3l-o- and 5l-o-positions; and reaction with
R1NH2 and ,~."y~ " ~Atinn to obtain the compound of fommula 1.
The present applicants have found that the compounds of forrnula I can be
prepared in good yields and purity without the need for 3'-0- and 5'-0-disiloxane
protection and dt:,ulul~ul;ol~ or for silica gel ~IIlullldluyld~lly~ The applicants have
found that the inosine-2',3,'5'-triacetate starting material of the prior art process may
be advantageously replaced with the lipophilic inosine-2',3',5'-l,i,u,u,uiul~dl~, which
pemmits the doubling of throughput and the ~i.llilldliul~ of u"d~si,dule pyridine
2 0 solvent.
In the present invention the compounds of fomnula I are prepared under phase
transfer catalysis conditions in accoldal~ce with the following reaction scheme:
W0 95/30683 21 ~ 68~ 7
-31- , .
\ / (R3)2SO~ N
5 R~ $~ N
H O/~ N Bu~N~HSo4- HO /~N~
HO OH HO Ol~
Vll
' '
where R1 R2 and R3 are as defined above.
The compounds of formula I may be prepared by reacting a basic aqueous solution
of a~compound of fommula (V~) with a di-(C1 1)alkyl sulfate in the presence of
tetrabutylammonium hydrogen sulfate and an organic water-i"""is.i~le solvent.
The base used to prepare the basic aqueous solution is preferably an alkali metal
hydroxide such as sodium hydroxide or potassium hydroxide. The solvent can be
any water i"""i:,.;il,le or esst",~i..lly i"""is~i~le solvent in which the compound of
formula I is soluble such as ",t,tl,yl~.~e dichloride or t-amyl alcohol. It is also
preferred that the reaction be run at temperatures between about -20C to about
50C in particular, room temperature. The time of the reaction is not critical but
it is pref~rably carried out over a period of from about 5 to 10 hours especially
about 7 to 8 hours. The crude compound of formula I is isolated by evaporation
2 5 followed by stinring with a 1:1 mixture of water and inert solvent, preferably toluene,
at room temperature for 5 to 7 hours, then filtering and drying. Pure compound may
be obtail1ed by fractional cry~ldlli~dliv,), preferably by:
1) recry~t~ ti~n from an inert solvent, such as toluene, by dissolving the crudecompound in the solvent at 80C, heating at about 55C for d~J~lU~ y 1 hour
cooling to room temperature, seeding with pure compound and filtering;
2) repeating the recr~,: 1 procedure of step 1) but heating at about 65C
WO 9S/30683 218 6 8 4 i F~
-32-
before cooling to room temperaturQ; and
3) recrystallizing from 100% ethyl alcohol by dissolving at reflux, diluting with water,
seeding, then filtering and drying.
Many of the compounds of fommula Vl are known and may be prepared by methods
described in the literature such as the rcvrt~ tio~led USP 4,843,066 and USP
4,985,409. The compound formula Vl may also be prepared advantageously, as
indicated above, in acc-,,dd,~ce with the following preferred reaction scheme:
N$ (C~HsCO)20 )~ G~/N
H O O H H5C C2Hs
2~
O O
Vll Vlll
WO 9~/30683 21 ~8 g 7
-33-
Cl
2 --<\ $ N
5 ,, ~ HsC2 )~ ~ N~ Vl
H5C2 ~ O ~C2H5
O O
IX
where R1 and R2 are as defined above.
The compounds of fommula Vl may be prepared by acylating inosine of formula Vll
with propionic anhydride to form the 2-O-, 3-O- 5-O-protected compound of
formula \1111; llalogel,dli"y the compound of fonnula Vlll with thionyl chloride to
form the i~ lll of formula IX; and simultaneously aminating and hydrolyzing
the compound of formula IX. Acylation of inosine is preferably carried out in a
mixture oi toluene tributylamine and 4-dimethylaminopyridine at a temperature offrom 100 to 110C over a period of 4 to 5 hours. The compound of formula Vlll is
isolated by prt~ dliOI~ with heptane. HdlOg~lld~iOIl of the compound of formula
Vlll is preferably carried out in a mixture of toluene and N N-dimethy;'u, ",a",i~e at
a temper~ture of from about 60 to 70C over a period of 3 to 4 hours, and the
solution of the compound of formula IX may be used after washing with water and
brine. Arnination a~d simultaneous hydrolysis of the illl~lllledidlt: of formula IX is
preferably effected by adding the amine R1NH2 and reacting at a temperature of
from 100 to 11 0C over a period of 15 to 20 hours. The compound of formula Vl
is isolated by filtration at room temperature and purified by recr~
The proc~ss of this invention is illustrated by the following examples.
Process IExample 1:
,, A ~ ~:
WO 9~i/30683
2186847 _34-
N -cy,,l~ Ayl 2 -O-meth~,ld~t" ,o~i"~
Step A. Inosine-2',3',5'-l,i,u,u,uiu,,dl~
A miAture of 271.2 9 of inosine, 966 ml of tributylamine, 3.30 9 of
4-dimethylaminopyridine and 600 ml of toluene is heated to an intemal temperature
of 104-105C; and oYer a period of 35 minutes, 453 ml of propionic anhydrida areadded at a rate which maintains the intemal temperatùre between 104-1 05C. After
stirring the mixture at this temperature for an additional 4 hours, the miAture is
cooled to 5-10C with an ice bath; and 1000: ml of heptane are added. The
resulting suspension is stirred at room temperature (20-22C) for 30 minutes andthen filtered e.g. on a Buchner funnel. The solids are washed with a total of 450
ml of heptane in three equal portions of 150 ml each and dried at 45-50C (25 mmHg) ovemight (14 hours) to yield 425.9 9 of inosine-2',3',5'-l,i~ ,u,ùio~,dlt: as a white
solid. (MP 171-172C; yield 96.5%).
Ste~ B. N6-cyclohexylafiello~i"e
A mixture of 270.6 9 of inosine-2~,3~,5~-l,i,u,uyioll , 240 ml of
N,N-dimeth~l~u""a",i~t, and 600 ml of toluene is heated to 65C; and over a period
of 1 hour, 67.84 ml of thionyl chloride are added at a rate which maintains the
intemal temp~rature between 62-65C. The mixture is stirred at this temperature
for an additional 2.5 hours and then cooled to 1 0C with an ice bath. After addition
of 60û ml of water precooled to 10-15C in an ice bath at a rate which maintainsthe temperature below 20C, the organic layer is separated and washed with a
total of 800 ml of 10% aqueous sodium chloride in four equal portions of 200 mi
each. The organic layer containing crude 6-chloro-9-(2,3,5-tri-O-
,ulupiu~yl B-ribofuranosyl)-9H-purine is added with stirring to 620 ml of
cyclohexylamine heated to 105C over a period of 2 hours at a rate which
maintains the intemal temperature at 105C. After this mixture is stirred at this
3 o temperature for an additional 17 hours, it is cooled to room temperature (25C) over
d,u,uluAi,lldl~ly 2 hours with efficient stirring and then filtered e.g. in a Buchner
WO 95130683 1 ~_l~.l.. ~'
_35, ~`~8~ 7
funnel with suction. The solid containing N6-cyclohexyladt" ,o~i"e and
cyclohexylamine hydlu~ uricl~ is washed with a total of 46û ml of toluene in four
equal po:~tions of 115 ml. each and ~, m la~ d damp to a 5-liter flask equipped with
a ~ ulldllicdl stirrer. After addition of 2 liters of aqueous saturated sodium
bi-,dl bUIl~dlt~ solution and 2.5 liters of ~thyl acetate, the mixture is stirred until all the
solid dissolve (d~ ll U)~il l ' ~y 1 û-15 min.). The oryanic layer is s~ and theaqueous layer is extracted with 1.5 iiters of ethyl acetate in two portions of 1 liter
and 500 ml-, ~ -r ~ ~'IY
The organic layers are combined and evaporated until about 3 liters of ethyl acetate
(at 40C, 1 00-2ûO mbar) is removed. To the residue, 500 ml. of heptane is added;
and the Iresulting mixture is stirred for 30 minutes. The solids are separated on a
Buchner funnel, and washed with a total of 300 mi of heptane in three equal
portions of 100 ml each. The solids are then dried at 45-50C (30-35 mbar) for
a~J,ulu.~illlut~ly 3 hours to yield 148 9 of crude N6-cyclohexylad_noai"e as a white
solid. This is lldt~F~ d to a 1 liter round-bottomed flask equipped with a
",e~,l,a"iual stirrer, and 175 ml. of 95% ethanol are added. The suspension is
stirred for 15-20 min and 175 ml of tert-butyl ethyl ether are added. After stirring for
5 min, tlle suspension is cooied in an ice bath and stirred for an additional 152 0 minutes. This suspension is filtered in a Buchner funnel and washed with a total of
50 ml. oF tert-butyl methyl ether in two equal portions of 25 ml. each.
The filtered solid is dried at 45-50C (30-35 mbar) for 14 hours to yield 130 9 of
N5-cyclohex~,ldd~"osi"e as a white solid (m.p. 185-187C; yield 60.0%).
Step C. N6-cyclohexyl-2'-O-meth),ldd~"osi"e
A suspension of 94.33 9 of N6-cyuloll~ ,lu~t"~:,i"e and 720 9 of 5% aqueous
sodium hydroxide is stirred at room lt:"",~, ~re (24-25C) until all of the solids are
dissolved (d,UIJlU~ lldl~ly 5 min.). Using an addition funnel, 850 ml. of
di~ rul"_;1,al~e and 5.5 9 of tetrabutylammonium hydrogen sulfate are added
WO 95/30683
~ ~ . ,t '~ i ''
21~8~7 -36- ~
followed by 61.3 g of dimethyl sulfate over 5-10 minutes while l"ai"lai"i"g the
intemal temperature between 24-25C. The addition funnel is washed with an
additional 50 ml of di.;l,luru",_ll,a"e which is added to the reaction vessel. After
stirring the biphasic mixture at 24-25C (intemal temperature) for 7.5 hours theorganic layer is separated and evaporated àt 40C,(270-290 mbar) until no further
solvent distills. The residue is dissolvrid in 200 ml of toluene and evaporated at
45-50C (30 mm Hg) until again no further solvent distills.
A mixture of the above-",~, ,li~l ,ed crude material and 2470 ml of toluene is stirred
at room temperature for 10 minutes and then 2470 ml of water are added over a
period of 22 min. The resulting suspension is stirred at room temperature for anadditional 6 hours and solids are collected by filtration e.g. on a Buchner funnel
with suction. After washing the solids with 114 ml of toluene and a total of 285 ml.
of water in three equal portions of 95 ml each the solids are dried at 48-50C (25
mm Hg) ovemight (14 hours) to yield 53.0 9 of a white solid. A suspension of this
solid in 397 ml. of toluene is heated to 80C with stirring to form a clear solution
which is cooled to 56C over 45 min and seeded with 10 mg of pure product. The
mixture is stirred at 55-56C for 45 minutes and then cooled to room temperatureover l hour. After stirring at this temperature for an additional 1 hour the solid are
2 0 collected by filtration e.g. on a Buchner funnel with suction. The solids are washed
with a total of 75 ml. of toluene in three equal portions of 25 ml. each to yield 60
g of a white solid. A suspension of this solid in 159 ml of toluene is again heated
to 80C and the above seeding procedure is carried out at 65 to 66C.
2 5 After cooling to room temperature over 1 hour and stirring at the same temperature
for an additional 1 hour, the solids are filtered and washed with 42 ml. of toluene
in three equal portions of 14 ml. each and dried at 48-50C (25 mm Hg) ovemight
(14 hours) to yield 57.7 9 of a white solid. The solid and 122 ml. of 100% ethanol
are heated to reflux with stirring to obtain a clear solution, and 288 ml. of water
3 o pre-wamned to 55C are added over 25 minutes. The mixture is cooled to 55Cand seeded with 20 mg of pure product. This mixture is cooled to room
wo 95/30683 _ I ~ . I .~... .
21888~7 37
temperature over 1 hour and stirred at this temperature ovemight (16 hours). Thesolids are collected by filtration on a Buchner funnel with suction and washed with
a total of 57 ml. of 1:2.36 mixture (Y/Y) of 100% ethanol and water in three equal
portions of 19 ml each. The solids are dried at room temperature (29 mm Hg) to
yield 62.2 9 of product as a white solid in 1.5 hydrate form (m.p. 88-91C; yield
42.5%).
Process Exam~le 2:
Following the aboYe procedure but using an equiYalent amount of
N6-cy~;lope"tylade"osi"e there is obtained N6-cyHui,t "~yl 2'-O- methyl adenosine.
Process FY~rn~le 3
The steps of process Example 1 are followed except that the di~-l,lc,,u,,,~tl,a~le in
step C) is replaced by tert-amyl alcohol, and subsequent to the initial t:~l ,c" Inll~dl~l
cryst~'- " the product is recrystallised from ethanol and water. 32.19 (yield
approx. 30%) N6-cyclohexYI-2-O-methylddt:llosi"e are obtained in excess of 98%
purity.
The process of this inYention is more economic in time and cost than hitherto
known p,ucesses. The process provides a convenient method for selective 2-O-
methylation of N6-cyclohex~,lddel~o~ e under phase-transfer conditions, and a
method of purification of the 2'-O-methyl derivative. Expensive protecting groups
and the use of ~.lllollldL~yld,ully are aYoided.