Note: Descriptions are shown in the official language in which they were submitted.
WO 96/25088 PCT1CTS96101672
2~a~aa~
-1-
4
mRANSCUTANEOUS SENSOR INSFRmroN aFm
BACKGROUND OF THE INVEN rnN
This invention relates generally to devices
and methods for placing a sensor at a selected site
within the body of a patient. More specifically,
this invention relates to an improved and relatively
simple insertion set for quick and easy
transcutaneous placement of a flexible thin film
sensor of the type used, for example, to obtain
periodic blood glucose readings.
In recent years, a variety of
electrochemical sensors have been developed for a
range of applications, including medical applications
for detecting and/or quantifying specific agents in a
patient s blood. As one example, glucose sensors
have been developed for use in obtaining an
indication of blood glucose levels in a diabetic -
patient. Such readings can be especially useful in
monitoring and/or adjusting a treatment regimen which
typically includes regular administration of insulin
to the patient. In this regard, blood glucose
readings are particularly useful in conjunction with
semiautomated medication infusion pumps of the
external type, as generally described in U.S. Patents
~ 4,562,752; 4,678,408; and 4,685,903; or automated
implantable medication infusion pumps, as generally
~ described in U.S. Patent 4,573,994.
CA 02186886 2004-12-21
-2-
Relatively small and flexible
electrochemical sensors have been developed for
subcutaneous placement of sensor electrodes in direct
contact with patient blood or other extracellular
fluid, wherein such sensors can 'be used to obtain
periodic readings over an extended period of time.
In one form, flexible transcutaneous sensors are
constructed in accordance with thin film mask
techniques wherein an . elongated sensor includes thin
film conductive elements encased between flexible
insulative layers of polyimide sheet or similar
material. Such thin film sensors typically include
exposed electrodes at a distal end for transcutaneous
placement in direct contact with patient Mood or the
like, and exposed conductive contac s at an
externally located proximal end for convenient
electrical connection with a suitable monitor
device. Such thin film sensors hold significant
promise in patient monitoring applications, but
unfortunately have been difficult to place
transcutaneously with the sensor electrodes in direct
contact with patient blood or other extracellular
fluid. Improved thin film sensors and related
insertion sets are described in commonly assigned
copending U.S. Patent Nos. 5,390,671, issued February 21,
1995; 5,391,250, issued February 21, 1995; and 5,482,473;
issued January 9, 1996. See also U.S. Patent 5,299,571.
The present invention relates specifically
to an improved adapted for
sensor
insertion
set
quickly and easily placing a thin film sensor on
a
patient with sensor electrodes in direct contact with
patient blood or other extracellular fluid.
SUMMARY OF THE INVENTION
In accordance with the invention, a
subcutaneous insertion set is provided for placing a
WO 96/25088 21 B 6 8 ~,b PCT/US96/01672
-3-
flexible sensor such as a thin film electrochemical
sensor at a selected site within the body of a
patient. The insertion set comprises a slotted
insertion needle extending through a mounting base
adapted for seated mounting onto the patient's skin.
A flexible thin film sensor includes a proximal
segment carried by the mounting base, and a distal
segment protruding from the mounting base and having
one or more sensor electrodes thereon. The distal
segment of the sensor is carried within a protective
catheter which extends from the mounting base with a
portion of the catheter being slidably received
within the insertion needle. One or more windows
formed in the catheter are positioned in general
alignment with the sensor electrodes on the sensor
distal segment.
When the mounting base is pressed onto the
patient's skin, the insertion needle pierces the skin
to transcutaneously place the catheter with the
sensor distal segment therein. The insertion needle
can be withdrawn from the mounting base, leaving the
catheter and sensor distal segment within the
patient, with the sensors electrodes thereon exposed
through the window or windows for direct contact with
to patient fluid at the selected position within the
patient, such as a subcutaneous, intravascular,
intramuscular, or intravenous site. Conductive
contacts on the sensor proximal end can be
electrically connected to a suitable monitor device
so that appropriate blood chemistry readings can be
taken.
In the preferred form, the insertion needle
has a cross-sectional shape which is somewhat greater
than 180° in arcuate cross section. This part-circle
needle construction protrudes downwardly from the
mounting base of the insertion set, and terminates in
a sharp tip for piercing the patient's skin. A first
portion of the protective cannula has a cross
W 0 96!25088 PCTlU596101672
2 o8~s~s6
-4-
sectional shape for nested reception within the
insertion needle, to extend from the mounting base to
a distal end which terminates at least slightly
before the needle tip. This first portion of the
cannula is sized for longitudinal sliding movement
within the part-circle profile of the insertion
needle, but to prevent lateral dislocation of the
cannula from the insertion needle. A second portion
of the cannula extends longitudinally in parallel
with said first portion and defines a lumen for
receiving and guidably supporting the distal end of
the thin film sensor. At least one window is formed
at or near the distal end of the lumen, in general
alignment with the sensor electrodes, to expose said
electrodes to patient body fluid. In the preferred
form, the cannula is constructed from a resilient
medical grade plastic or elastomer, and the second
portion of the cannula cooperates with the insertion
needle to define a substantially circular cross
sectional shape for facilitated insertion into the
patient's skin.
During insertion, the insertion needle and
the protective cannula cooperatively protect and
guide the sensor to the desired transcutaneous
placement position. The insertion needle can then be
withdrawn, whereupon the slotted needle geometry
permits the insertion needle to slide over and
longitudinally separate from the second portion of
the cannula, thereby leaving the cannula and sensor
therein at the selected insertion site.
Other features and advantages of the present
invention will become more apparent from the
following detailed description, taken in conjunction
with the accompanying drawings which illustrate, by
way of example, the principles of the invention.
WO 96/25088 2 ~ g ~ g g 6 PCT/US96101672
-5-
~TEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings illustrate the
invention. In such drawings:
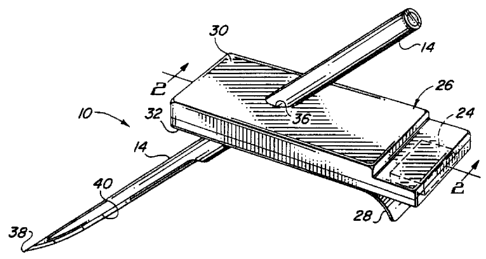
FIGURE 1 is a perspective view illustrating
a transcutaneous sensor insertion set embodying the
novel features of the invention;
FIGURE 2 is an enlarged longitudinal
vertical section taken generally on the line 2-2 of
FIG. 1;
FIGURE 3 is an enlarged longitudinal
sectional of a slotted insertion needle used in the
insertion set of FIGS. 1 and 2;
FIGURE 4 is an enlarged transverse section
taken generally on the line 4-4 of FIG. 3;
FIGURE 5 is an enlarged transverse section
taken generally on the line 5-5 of FIGS. 3;
FIGURE 6 is an enlarged fragmented sectional
view corresponding generally with the encircled
region 6 of FIG. 2; and
FIGURE 7 is an enlarged transverse section
taken generally on the line 7-7 of FIG. 2.
DETAILED DESCRIpTT~N OF THE PRFFFRRF
D EI4BODTMFNT
As shown in the exemplary drawings, an
improved sensor insertion set referred to generally
in FIGURE 1 by the reference numeral 10 is provided
for transcutaneous placement of a flexible sensor 12
(FIG. 2) at a selected site within the body of a
patient. The insertion set 10 includes a rigid
hollow slotted insertion needle 14 for quick and easy
transcutaneous placement of a cannula 15 with a
distal segment 16 of the sensor 12 therein, wherein
the distal segment 16 has one or more sensor
electrodes 18 exposed to patient fluid through a
window 19 in the cannula 15. The insertion needle 14
W0 96125088 2 ~ ~ 6 8 8 b. P~T~S96101672
-6-
is then withdrawable to leave the cannula 15 with the
sensor distal segment 16 and the sensor electrodes 18
in place at the selected insertion site.
The transcutaneous sensor insertion set 10
of the present invention is particularly designed for
facilitating accurate placement of a flexible thin
film electrochemical sensor of the type used for
monitoring specific blood parameters representative
of patient condition. The insertion set 10 is
designed to place the sensor subcutaneously or at
another selected site within the body of a patient,
in a manner minimizing patient discomfort and
trauma. In one preferred application, the sensor 12
may be designed to monitor blood glucose levels, and
may be used in conjunction with automated or
semiautomated medication infusion pumps of the
external or implantable type as described in U.S.
Patents 4,562,751: 4,678,408; 4,685,903 or 4,573,994,
to deliver insulin to a diabetic patient.
In a preferred form, the flexible
electrochemical sensor 12 is constructed according to
so-called thin film mask techniques to include
elongated thin film conductors embedded or encased
between layers of a selected insulative material such
as polyimide film or sheet. The sensor electrodes 18
(shown in exaggerated form in the drawings) at a tip
end of the sensor distal segment 16 are exposed
through one of the insulative layers for direct
contact with patient blood, when the sensor is
transcutaneously placed. The distal segment 16 is
joined to a proximal segment 20, (FIG. 2) the end of
which terminates in suitable conductive contact pads
or the like which are also exposed through one of the
insulative layers. As is known in the art, and
illustrated schematically in FIG. 2, the proximal
segment 20 and the contact pads thereon are adapted
for electrical connection to a suitable monitor 22
CA 02186886 2005-02-04
WO X6125088 PCTIUS96101672
for monitoring patient condition in response to
signals derived from the sensor electrodes 18.
Further description of flexible thin film sensors of
this general type may be found in copending U.S.
Patent No. 5,391,250, issued February 21, 1995, entitled
METHOD OF FABRICATING THIN FILM SENSORS
The proximal
segment 20 may be conveniently connected electrically
to the monitor 22 by means of a connector block 24 as
shown and described in copending U.S. Patent No.
5,482,473, issued January 9, 1996, entitled FLEX CIRCUIT
CONNECTOR
The sensor 12 is carried by a mounting base
26 adapted for placement onto the skin of a patient.
As shown, the mounting base 26 comprises an enlarged
and generally rectangular pad having an underside
surface coated with a suitable pressure sensitive
adhesive layer, with a peel-off paper strip 28
normally provided to cover and ~ protect the adhesive
layer, until the insertion set 10 is ready for use.
As shown in FIGS. 1 and 2, the mounting base
comprises upper and lower layers 30 and 32, with the
proximal segment 20 of the flexible sensor 12
sandwiched therebetween. The proximal sensor segment
20 has a forwardmost end joined to the distal segment
16 which is folded angularly to extend downwardly
through a slot 34 formed in the lower base layer 32.
The insertion needle 14 is adapted for
slide-fit reception through a needle port 36 formed
in the upper base layer 30 and further through the
lower slot 34 in the lower base layer 32. As shown,
the insertion needle 14 has a sharpened tip 38 and an
open slot 40 which extends longitudinally from the
tip 38 at the underside of the needle to a position
at least within the slot 34 in the lower base layer
32. Above the mounting base 26, the insertion needle
WO 96125088 ~ ~ PCT/CTS96/01672 t
_g-
14 may has a full round cross sectional shape and is
desirably closed at a rear end thereof. In the
i
preferred form, the slotted needle 14 has a
part-circular cross sectional shape, with an arcuate
dimension or span greater than 180°, such as on
arcuate dimension of about 210°. This leaves a
longitudinal slot in the needle with an arcuate
dimension of about 150°.
The cannula 15 is shown best in FIGS. 6 and
7, and comprises a first portion 44 of part circular
cross section fitted within the insertion needle 14
to extend downwardly from the mounting base 26. This
cannula 15 is constructed from a suitable medical
grade plastic or elastomer, such as
polytetrafluoroethylene, silicone, etc., to define an
open lumen 42 in a second portion thereof for
receiving, protecting and guidably supporting the
distal segment 16 of the sensor 12. The cannula 15
has one end fitted into the slot 34 formed in the
lower layer 32 of the mounting base 26, wherein the
cannula 15 is desirably secured to the mounting base
by a suitable adhesive or other selected attachment
means. From the the mounting base 26, the cannula
extends angularly downwardly with the first portion
44 nested within the insertion needle 14, terminating
slightly before the needle tip 38. Importantly, at
least one window 19 is formed in the lumen 42 near
the distal end thereof, in general alignment with the
sensor electrodes 18, to permit direct electrode
exposure to patient body fluid when the sensor is
transcutaneously placed.
In the preferred form, as shown in FIG. 7,
the second portion 42 of the cannula 15 has a
part-circular cross sectional shape which cooperates
with the part-circular shape of the insertion needle
14 to define a substantially full-circle geometry for
facilitated insertion through the patient's skin.
WO 96125088 ~ ~ ~ PCT/US96101G72
_g_
The first portion 44 of the cannula 15 has a smaller
cross sectional profile than the second portion 42,
for sliding nested reception into the needle 14. The
needle 14 and first cannula portion 44 are thus
mechanically interlocked to prevent lateral
dislocation of the cannula 15 from the insertion
needle, while permitting longitudinal sliding motion
of the needle over the cannula first portion 44. The
distal or free end of the cannula second portion 42
is appropriately cut or otherwise set at an oblique
angle, as viewed in FIG. 2, to form a continuation of
the angle-cut tip 38 of the insertion needle.
In use, the insertion set 10 permits quick
and easy transcutaneous placement of the sensor
distal segment 16 at a selected site within the body
of the patient. More specifically, the peel-off
strip 28 (FIG. 1) is removed from the mounting base
26, at which time the mounting base 26 can be pressed
onto and seated upon the patient's skin. During this
step, the insertion needle 14 pierces the patient's
skin and carries the protective cannula 15 with the
sensor distal segment 16 therein to the appropriate
transcutaneous placement site. During insertion, the __
cannula 15 provides a stable support and guide
structure to carry the flexible sensor to the desired
insertion site.
When the sensor 12 is transcutaneously
placed, with the mounting base 26 seated upon the
patient's skin, the insertion needle 14 can be
slidably withdrawn from the patient. During this
withdrawal step, the insertion needle 14 slides over
the first portion 44 of the protective cannula 15,
leaving the sensor distal segment 16 with electrodes
18 thereon at the selected insertion site. These
electrodes 18 are directly exposed to patient body
fluid via the window 19. The sensor proximal segment
20 is appropriately coupled to the monitor 32, so
WO 96125088 ~ ~ . ~ PC'd'IUS96/01672
-10-
that the sensor 12 can then be used over a prolonged
period of time for taking blood chemistry readings,
such as blood glucose readings in a diabetic
patient. If desired, the first portion 44 of the
cannula 15 can be hollow as shown to form a second
lumen available to deliver medication and/or sensor
calibration fluid to the vicinity of the electrodes
18, or alternately to withdraw patient fluid such as
blood for analysis.
The transcutaneous sensor insertion set of
the present invention thus provides a relatively
simple device for quickly and easily placing a
flexible thin film electrochemical sensor at a
selected position within a patient.
A variety of modifications and improvements
to the transubcutaneous sensor insertion set of the
present invention will be apparent to those skilled
in the art. Accordingly, no limitation on the
invention is intended by way of the foregoing
description and accompanying drawings, except as set
forth in the appended claims.