Note: Descriptions are shown in the official language in which they were submitted.
WO 95129159 P~ J~.S/O 19S6
2 1 87932
- 1 -
. TITLE OF THE INVENTION
SUBSTITUTED SULFONAMIDES AS SELECTIVE ~3 AGONISTS
. ~Y FOR THE TREATMENT OF DIABETES AND OBESITY
5 CROSS RFFERENCE
This is a c.~ illu~lion-in-part of co-pending application
U.S.S.N. 08/233,166 filed April 26, 1994, which is hereby incorporated
by reference in its entirety.
BACKGROUND OF THE INVENTION
~ -Adrenoceptors have been ,~ c~ d as ~1 and ~2 since
1967. Increased heart rate is the primary congeq--~nl~e of ,131-receptor
stimulation, while brnn~hn~ tion and smooth muscle relaxation
typically result from ~2 stimlll~ltion Adipocyte lipolysis was initially
5 thought to be solely a ,~l-mediated process. However, more recent
results indicate that the receptor-mP~ tin~ lipolysis is atypical in
nature. These atypical receptors, later called ~3-adrenoceptors, are
found on the cell surface of both white and brown adipocytes where
their stimulation promotes both lipolysis (breakdown of fat) and energy
20 ~ rlont1ifl1re.
Early developments in this area produced c~ ")~,u-,ds with
greater agonist activity for the stimulation of lipolysis (,B3 activity) than
for stimulation of atrial rate (,~1) and tracheal relaxation (~2)- These
early developments disclosed in Ainsworth et al., U.S. Patents 4,478,849
and 4,396,627, were dGIiv~lLivGs of phenyl~th lnnl~min~g
Such sel~,Livi~y for ~3-adrenoceptors could make
;v",l,(Jul,d~ of this type potentially useful as antiobesity agents. In
addition, these C~JIII~OUIIdS have been reported to show
antil,y~G,~;ly~G",ic effects in animal models of non-insulin-dG~!G.IdGllL
3 diabetes mellitus.
A major drawback in treatment of chronic diseases with ,133
agonists is the potential for stimulation of other ~-receptors and
~"l,se~lllr"l side effects. The most likely of these include muscle tremor
(1~2) and increased heart rate (,~1)- Although these phenyleth~nnl~minf-
W095/29159 ~ 2 ~ ~ 7932 ~ ol~sG
- 2 --
derivatives do possess some ~3 selectivity, side effects of this type have .
been observed in human volunteers. It is reasonable to expect that these
side effects resulted from partial ~1 and/or ~2 agonism. .,
More recent developments in this area are disclosed in
5Ainsworth et ~.~ U.~. Patent 5,153,210, Caulkett et al., U.S. Patent
4,999,377, Alig et al., U.S. Patent 5,017,619, Lecount et al., European
Patent 427480 and Bloom et 1, European Patent 455006.
Even though these more recent developments purport to
describe compounds with greater ~3 selectivity over the ¦31 and ,B2
activities, this sclc~livily was rlr~ cd using rodents, in particular,
rats as the test animal. Because even the most highly selective
compounds, as (1~l. . ",;". d by these assays, still show signs of side
effects due to residual ~1 and ,B2 agonist activity when the compounds
are tested in humans, it has become apparent that the rodent is not a
5good model for predicting human ~3 s~lc~livily.
Recently, assays have been developed which more
ac, u-dl~ly predict the effects that can be expected in humans. These
assays utilize cloned human ~3 receptors which have been expressed in
Chinese hamster ovary cells. See Emorine et al, Science, 1989,
2245:1118-1121; and Liggett, Mol. Pharmacol.. 1992, 42:634-637. The
agonist and antagonist effects of the various ~;UIII~JUUII~Lt on the
cultivated cells provide an inrlir~tion of the amtiobesity and ~nti~ hetir
effects of the ~ uul~ds in humans.
SUMM~R Y OF THF. T~VENTION
The instant invention is concerned with substituted
sulfonamides which are useful as antiobesity and ~nti(li~hetic
compounds. Thus, it is an object of this invention to describe such
compounds. It is a further object to describe the specific preferred ,~
stereoisomers of the ~ub~lilul~d sulf~-n~nnifif ~ A still further object is
to describe processes for the preparation of such compounds. Another ,
object is to describe methods and compositions which use the
cu~ c.u.lds as the active ingredient thereof. Further objects will
become apparent from reading the following description.
WO 95129159 r ~ . Q .~JG
~ 2~ 87932
- 3 -
DESCRIPTION OF THE INVENTION
.. The present invention provides compounds having the
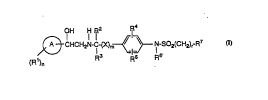
formula I:
. (R )n CHCH2N-C~~X)m~ ~ N-So2(CH2)r-R7
w
here O to 5;
mis Oorl;
ris Oto3;
A is (1) a 5 or 6-1llclllb~l~,d heterocyclic ring with from 1 to 4
h~ ua~ullls selected from oxygen, sulfur and nitrogen,
(2) a benzene ring fused to a 5 or 6-membered heterocyclic
ring with from 1 to 4 h~,t~,lu~ selected from oxygen,
sulfur and nitrogen,
(3) a 5 or 6-1ll.,lllb~l-,d heterocyclic ring with from I to 4
heteroatoms selected from oxygen, sulfur and nitrogen
fused to a 5 or 6-membered heterocyclic ring with from I
to 4 heteroatoms selected from oxygen, sulfur and
nitrogen,
(4) phenyl, or
(5) a benzene ring fused to a C3-Cg cycloalkyl ring;
R1 is (1) hydroxy,
(2) oxo,
(3) halogen,
(4) cyano,
(S) NR8R8~
WO 95/29159 r~ o :~56
~1 8~3~
-- 4 --
(6) SR8,
(7) trifluoromethyl,
(8) Cl-C1o alkyl,
(9) oR8,
(10) S02R9,
(11) OCOR9,
(12) NR8COR9,
(13) COR9,
(14) NR8S02R9,
o (15) NR8C02R8, or
(16) Cl-Clo alkyl s--hctit~ d by hydroxy, halogen, cyano,
NR8R8, SR8, I-ifluulolllethyl, oR8, C3-Cg cycloalkyl,
phenyl, NR8CoR9, CQR9, S02R9, OCOR9, NR8S02R9 or
NR8C02R8;
5 R2 and R3 are independentlY
(1) hydrogen,
(2) Cl-C1o alkyl or
(3) C1-C1o alkyl with 1 to 4 c~lhstitllPntc selected from
hydroxy, Cl-Clo alkoxy, and halogen;
X is (1) -CH2-,
(2) -CH2-CH2-,
(3) -CH=CH- or
(4) -CH20-;
R4 and R5 are in~ .p~n~1~ntly
(l)hydrogen,
(2) C1-C1o alkyl,
(3) halogen,
(4) NHR8,
(S) oR8,
(6) S02R9 or
(7) NHS02R9;
R6 is (1) hydrogen or
(2) C1-C1o alkyl;
R7 is Z-(R 1 a)n;
wo951~91~i9 PU~ c:95G
2 1 87932
- 5 -
.~ Rla is (1) Rl, with the proviso that when A is phenyl, Rla is not
Cl-Clo alkyl,
,~ (2) C3-Cg cycloalkyl,
(3) phenyl optionally substituted with up to 4 groups
independently selected from R8, NR8R8, oR8, SR8 and
halogen, or
(4) S or 6-membered heterocycle with from 1 to 4
heteroatoms selected from oxygen, sulfur and nitrogen,
optionally substituted with up to four groups independently
o selected from oxo, R8, NR8R8, oR8, SR8, and halogen;
Z is (1) phenyl,
(2) naphthyl,
(3) a 5 or 6-membered heterocyclic ring with from 1 to 4
heteroatoms selected from oxygen, sulfur and nitrogen,
(4) a benzene ring fused to a C3-Cg cycloalkyl ring,
(5) a benzene ring fused to a 5 or 6-membered heterocyclic
ring with from 1 to 4 hc;l~ludlollls selected from oxygen,
sulfur and nitrogen,
(6) a 5 o} 6-1llc;llll)el~,d heterocyclic ring with from 1 to 4
heteroatoms selected from oxygen, sulfur and rlitrogen
fused to a 5 or 6-membered heterocyclic ring with from 1
to 4 h~,t~,lualullls selected from oxygen, sulfur and
rlitrogen, or
2s (7) a S or 6-membered heterocyclic ring with from 1 to 4
heteroatoms selected from oxygen, sulfur and nitrogen
fused to a C3-Cg cycloalkyl ring;
R8 is (1) hydrogen,
(2) Cl-Clo alkyl,
(3) C3-Cg cycloalkyl,
(4) Z optionally having 1 to 4 ~ub~lilut;lll~ selected from
halogen, nitro, oxo, NRlOR10, Cl-clo alkyl, Cl-clo
alkoxy, Cl-Clo alkylthio, and Cl-Clo alkyl having 1 to 4
sllhstitll.ont~ selected from hydroxy, halogen, Co2H~ Co2-
Cl-Clo alkyl, So2-cl-clo alkyl, C3-c8 cycloalkyl, Cl-
WO 95129159 2 1 8 7 9 3 ~ G
- 6 -
Clo alkoxy, and Z optionally substituted by from l to 3 of
halogen, C1-Clo alkyl or C1-C1o alkoxy, or
(5) C1-C1o alkyl having 1 to 4 substituents selected from
hydroxy, halogen, Co2H~ Co2-cl-clo alkyl, S02-Cl-C10
alkyl, C3-Cg cycloalkyl, C1-C1o alkoxy, C1-Clo alkyl, and
Z optionally s~lhstit~t~d by from 1 to 4 of halogen, Cl-C10
alkyl or C1-C1o alkoxy;
R9 is (1) R8 or
(2) NR8R8;
lO is (1) Cl-Clo alkyl, or
(2) two RlO groups together with the N to which they are
attached formed a 5 or 6-membered ring optionally
h..l,:,lilll~d with Cl-C10 alkyl; or
a rh~rmsl~ellti~lly acceptable salt thereof.
In one embodiment of the instant invention A is a 5 or 6-
membered heterocyclic ring with from I to 4 heteroatoms selected from
oxygen, sulfur and nitrogen, a benzene ring fused to a 5 or 6~ lb~
heterocyclic ring with from 1 to 4 h~,t~,lu~l~.llls selected from oxygen,
sulfur and nitrogen, or a 5 or 6-membered heterûcyclic ring with from
1 to 4 h~telu~L~llls selected from oxygen, sulfur and nitrogen fused to a
5 or 6-membered heterocyclic ring with from l to 4 heteroatoms
selected from oxygen, sulfur and nitrogen.
In another embodiment of the instant invention A is phenyl
or benzene fused to a C3-Cg cycloalkyl ring.
Preferred cL)Ill~oul~ds of the instant invention are realized
when in the above structural formula I:
R2 and R3 are hydrogen or methyl;
X is -CH2-;
nis Oto3;
30 m is 1;
r is O to 2; and
R4, R5 and R6 are hydrogen.
Other preferred compounds of the instant invention are
realized when in the above structural formula I:
wo 9S/~9lS9 2 1 ~ 7 9 3 ~ JV C :956
-- 7 -
. A is phenyl or a 6-membered hete}ocyclic ring with I or 2
heteroatoms selected from nitrogen and sulfur;
Rl is hydroxy, halogen, cyano, trifluoromethyl, NR8R8,
NR8SO2R9, NR8COR9, NR8CO2R8, Cl-C6 alkyl
optionally substituted by hydroxy; and
ris Oor2.
More preferred compounds are represented by the formula
Ia:
OH H R2
(Rl)n ~ J CHcH2N-c-(x)m~ ;3NH=so2--Z (R1a)~
Ia
wherein
nis Oto3;
mis
Rl is (1) halogen or
(2) NR8R8;
R2, R3 are in-l.op~n~ nt1y hydrogen or methyl;
Rla is (1) halogen,
(2) Cl-Clo alkyl,
2s (3) NR8R8,
(4) NR8COR9,
(S) NR8C02R8
(6) COR9,
(7) OCOR9, or
(8) a S or 6-membered heterocycle with from I to 4
het~,lua~ullls selected from oxygen, sulfur and nitrogen,
optionally ~I-hstitllt~-rl with up to four groups independently
selected from oxo, halogen, R8, NR8R8, oR8, and SR8;
Z is (1) phenyl,
(2) naphthyl,
WO 95/29159 r~ c 155G
21 87~32
- 8 -
(3) a 5 or 6-membered heterocyclic ring with from l to 4 ,
heteroatoms selected from oxygen, sulfur and nitrogen,
(4) benzene ring fused to a S or 6-membered heterocyclic
ring with from l to 3 heteroatoms selected from oxygen,
sulfur and nitrogen, or
(S) a S or 6-membered heterocyclic ring with from l to 4
hc~ s selected from oxygen, sulfur and nitrogen
fused to a C3-Cg cycloalkyl ring;
X is -CH2-; and
R8 and R9 are as defined under formula 1.
Even more preferred compounds are those ~l~,sc.-L~d by
formula Id:
OH H R2 B
(R1)n--~ JCHCH2N- I cH2~NH-so2--~ (Rta)n
Id
nis Oorl;
Rl is NR8R8;
R2 and R3 are in~PpPn~ ntly
(l) hydrogen, or
(2) methyl;
B is (l) hydrogen,
(2) benzene fused to the benzene ring to form naphthyl, or
(3) a S or 6-membered heterocycle with l to 4 heteroatoms
selected from oxygen, sulfur and nitrogen atom fused to the
benzene ring;
Rla is (l) halogen,
(2) Cl-Clo alkyl,
(3) NR8R8~
(4) NR8CoR9,
(S) NR8C02R8
WO95/29159 1~I/u~'C.~
2 1 8 7~32
g
- (6) COR9~ or
(7) a ~ or 6-membered heterocycle with from 1 to 4
- hcilel~,dt~,llls selected from oxygen, sulfur and nitrogen,
optionally substituted with up to four groups independently
selected from oxo, R8, SR8, o~8, arld NR8R8;
when B and the benzene ring form a fused ring system, Rla
is attached to either ring;
R8 is (1) hydrogen,
(2) Cl-Clo alkyl,
(3) Z optionally having l to 4 sllhstitl-~nt~ selected from
nitro, oxo, and NR10Rl0, or
(5) Cl-Clo alkyl having 1 to 4 substituents selected from
hydroxy, halogen, Cl-Clo alkyl, C3-Cg cycloalkyl, and Z
optionally sllbstitllt~-d by from l to 4 of halogen, Cl-clo
alkyl or Cl-Clo alkoxy;
R9 is (1) R8 or
(2) NR8R8;
R10 is (1) Cl-Clo aLkyl, or
0 (2) two R10 groups together with the N to which they are
attached formed a 5 or 6-lll~.llb~ d ring optionally
bub~ ul~d with Cl-Clo alkyl; and
Z is (1) phenyl,
(2) a 5 or 6-membered heterocyclic ring with from 1 to 4
L~JIlls selected from oxygen, sulfur and nitrogen,
(3) a benzene ring fused to a 5 or 6-membered heterocyclic
rirlg with from 1 to 4 h~ u~al~ulls selected from oxygen,
sulfur and nitrogen, or
(4) a 5 or 6-membered heterocyclic ring with from 1 to 4
heteroatoms selected from oxygen, sulfur and rlitrogen
fused to a C3-Cg cycloalkyl ring.
.- Other more preferred compounds are l~ s~ t;d by
formula Ib:
wo ssnslss P~ o ;9s6
7932
- 10-
OH H R2 .
(R1)n ~--CHCHzN- I (X)m~NH-soz-z-(R1a)n
R3
Ib
wherein
nis Oto3;
mis
Rl is (1) hydroxy,
(2) cyano,
(3) NR8R8 or
(4) halogen;
Rla is (1) halogen,
(2~ NR8R8
(3) NR8COR9,
(4) NR8CO2R8,
(5) OCOR9, or
(6) a S or 6-membered heterocycle with from 1 to 4
.,L~Iv~llullls selected from oxygen, sulfur and nitrogen,
optionally substituted with up to three groups in~lPp~nrl~ntly
selected from ûxo~ halogen, R8, NR8R8~ oR8 and SR8;
Z is (1) phenyl,
(2) naphthyl or
(3) benzene ring fused to a S or 6-membered heterocyclic
rirlg with from 1 to 4 heteroatoms selected from oxygen,
sulfur and nitrûgen;
X is -CH2-; and
R2 and R3 are independently hydrogen or methyl.
Re~ live ~ntinb~ity and ~nti~ hetif~ compounds of
the present invention include the following: .
~-[4-[2-[[2-hydroxy-2-(6-allfill~ylidin-3-yl)ethyl]amino]ethyl]phenyl]-
4-(hexylamin~carbonylamino)l,~", r11~ 1 fO~ llidc
WO 95/29159 2 1 8 7 q 3 2 ~ . ;.c ,jSf
- 11 -
~-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethy1]pheny1]-
4-iodokçn7f nPsl-lfonamide
N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
b~ lfonamide
N-[4-[2-[[2-hyd}oxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
2-nslrhth~lPn~sl-lfonamide
N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
3-quinolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
o 5-bt;~ vlc-s-ll rvllal~lide
N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
4-[(h~yl~ yl~minoc~rbonyl)amino]l~r.~.r.~ lfonamide
N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
4-[(dimethyl~minvc~rbonyl)amino]~.r.,,.r.~f.~,llfonamide
5 N-[4-[2-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]ethyl]phenyl]-
4-(3-hexyl-2-imi~1 l7oli~10n-l-yl)br.~Y~ Ilfonamide
N-[4-[3-[[2-hydroxy-2-(6-~.1illv~ylidin-3-yl)ethyl]amino]propyl]-
phenyl]-4-~fhexyl:-minor~rbonylamino)~ .r"~jlllrollalllide
~[4-[3-[[2-hydroxy-2-(6-aminopyridin-3-yl)ethyl]amino]propyl]-
20 phenyl]-4-iocl~ . ,,.=. ,. . j"lfonamide
~-[4-[3-[[2-hydroxy-2-(6-alllillo~ylidin-3-yl)ethyl]amino]propyl]
phenyl]l,- .,,...lf ClllfOnamide
~!:-t4-t3-t[2-hydrvxy-2-(6-alllillv~ylidin-3-yl)ethyl]amino]propyl]
phenyl]-2-n~rhth~ n.o.slll rvllalllidf
25 N-[4-[3-[[2-hydroxy-2-(6-~--illvl,y-idin-3-yl)ethyl]amino]propyl]-
phenyl]-3-qllin~-lin~slllfonamide
~[-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
(hexyl~minoc~rbonylamino)l,~ ul rvllalllide
N-[4-t2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
30 isv~,lv~,ylll l~ ll]fonamide
. N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-2-
l ,r.l: .. ,..,. llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-3-
quinolinesuLfonamide
WO 95/29159 1~ .'C ;~5G
93:2
- 12 -
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
[(hexylmethylaminocarbonyl)amino]bf~n7~.n~s~1fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
hexyl-2-imidazolidinon- 1 -yl)b~ r . ,r.~. ll fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
iodObr.,,...~P~Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(3-
cyclopentylpropyl)-[1,2,4]-oxadiazol-3-yl]hPn7.~ .nc~1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[(1 -
oxoheptyl)amino]l ,..., ,.~ .. "~.clllfonamide
~!-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[(1 -
oxo-4-phenylbutyl)amino]l~r",...~ lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
[(propoAyl,d,bo"yl)amino]~çn7~onPs--lfonamide
5 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[[(fur-
2-ylmethyl)amino]carbonyl]amino]bf~n7.l nPc--lfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[[(2-
phenylethyl)amino]carbonyl]amino]b; ,,.., ,,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[[(2-
20 indol-3-ylethyl)amino]carbonyl]amino]l,r"~i..,..,.llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
[[(~ yl~~ lo)carbonyl]amino]~...,,.~., ~.Ilfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -
[(h~ ylalllillo)carbonyl]-s-inllnlin~slllfonamide
25 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-
[(octylamino)carbonyl]-S-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[(N-
methyl-N-octylamino)carbonyl] -5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-( I -
30 oxononyl)-s-inflnlinps~lfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
ia~lol-2-yl)-s-inl1Qlin~s~llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
octylthiazol-2-yl)-S-in~lolin~s--lfonamide
W0 95129159 r~ V' l~56
21 87932
- 13 -
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(4-
ethyl-S-methylthiazol-2-yl)-5-in~lnlinP.s-llfonamide
- N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
octyl-2-imidazolidinon-l-yl)bPn7PnPslllfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-
(4,4,4-trifluorobutyl)-2-imitl~7nli~1innn-l-yl]br.,ll~.r.,f.i,.llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
phenylpropyl)-2-imidazolidinon- l-yl]bF n7PnF s~llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-
o (4,4,5,5,5-pentafluoropentyl)-2-imidazolidinon-1-
yl]b~ l,rllF~ lfonamide
N-~4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(2-
cyclohexylethyl)-2-imidazolidinon- 1 -yl]l,r", rl IF-SI llfonamide
N-L4-[2-[[2-hydroxy-2-(3-pyridin~ ethyl]amino]ethyl]phenyl]-4-[3-[3-
15 (4-chlorophenyl)propyl]-2-imidazolidinon-l-yl]hP,n7.enP.~ulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
pentyl-2-imidazolidinon-1-yl)l~r",.~ P,~ fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
cyclo~ lyl~lu~)yl)-2-imi~1~7n~ inon-l-yl]hl~ rllp~ fonamide
20 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(2-
~;y~.lU~ ylethyl)-2-im~ 7~n~ innn- 1 -yl]l I ,r.l l~.rl IF ~. Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
cycloll~,AylL,Iu~yl)-2-imidazolidinon-l-yl]bFn7Pn~slllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(2,2-
25 dimethylhexyl)-2-imifl~7olil1inon-l-yl]l~"~r ~F~ lfonamide
~L-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
hexyl-2-imidazolon- 1 -yl)~r~ I ~.f -1l'.~ lfonamide
~[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-
(4,4,4-trifluorobutyl)-2-imidazolon-1-yl]b~ .r"P.~"lfonamide
30 ~_[4_[2_[[2_hydroxy-2-(3-pyridinyl)ethyl]amillo]ethyl]phellyl]-4-(3-
octyl-2-imitl~.nlnn-1-yl)l?r",F..~P,~,Ilfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
cyclo~..,.llyl~1luL~yl)-2-imidazolon-l-yl]hpn7enpslllfonamide
WO 95/29159 P~ .'C 1~6
932
- 14-
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
octyl-3-oxo-[1,2,4]-triazol-4-yl)ben7P,nPsl-lfonamide
N-~4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
hexyl-S-tetrazolon- 1 -yl)bPn7PnP.sl-lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
octyl-5-tetrazolon-1-yl)~ .17f 1If ~I llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[(3-
cyclopentylpropyl)-5-tetrazolon- 1 -yl]bpn7pnpslllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
10 pentyloxazol-5-yl)l, 1~ ~f ,~lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
octyloxazol-5-yl)bPn7f nP.~IIlfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(2-
cyclopentylethyl)oxazol-5-yl]~ lfonamide
5 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[(4-
ethyl-5-".~ ylil.i~ol-2-yl)amino]l,~ fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-
[(4,5,6,7-tetrallyd.~ .zullli~ul-2-yl)amino]bPn7P.nPslllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]-4-(2-
20 hexylimidazol-4-yl)lr",. l- ~ llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(1-
methyl-2-octylimidazol-S-yl)l,~ - f D~ ~I ru--Oll ide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[1 -
methyl-2-(2-cyclopentylethyl)imidazol-S-yl]b~ - '7~ ~ If slllfollamide
25 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[l-
methyl-2-[2-(4-flu~lu~ yl)ethyl]imidazol-5-yl]bPn7PnPsl~lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
pentyl-[l ,2,4]-oxadiazol-3-yl)b,~", ~. ~f ~ Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(2-
30 cyclopentylethyl)-[1~2~4]-oxadiazol-3-yl]l~ fD~Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
heptyl-[1,2,4]-oxadiazol-3-yl)~ . If D~ llfonarnide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
octyl-[1,2,4]-oxadiazol-3-yl)~r",.~ .llfonamide
WO 95/29159 P~, I/L~_,'U ~9~C
2 1 8 7932
- 15-
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
hexylthio-[1,2,4]-triazol-3-yl)~.r",l~ ,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[4-(4-
propylpiperidin- 1-yl)- 1,1 -dioxo-[ 1 ,2,5]-thi~ 7Ol-3-
5 yl]amino]br~ f-~,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[4-
(hexylmethylamino)- 1,1 -dioxo- [ 1,2 ,5] -thiadiazol-3 -
yl]amino]~r~ . "~,clllfonamide
N-[4-[2-t[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[[4-(N-
o heptyl, N-methylamino)- I ,1 -dioxo- [ 1,2,5] -thiadiazol-3-
yl]amino]bf n7f.nf c-l1fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]~(1 -
octyl-2,4-imi-i~7~ 1inf ~ion-3-yl)bf. n7f~nr-~ ~1 rullalllide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl~phenyl]-4-[3-(3-
5 llillu~ lyl)-s-pyrazolon-l-yl]b~ rllf~lllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(1 -
hydroxy-l-hexylheptyl)-5-methyl-[1,2,3]-triazol-2-yl]l,r..,,~.~f-.~..lrul~-
amide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]~[4-( 1 -
20 hydroxyheptyl)-5-methyl-[ 1 ,2,3]-triazol-2-yl]bf n7f nf s--1fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]-2-1ll.,lllyl~l~",y~]-
phenyl]-4-(3-hexyl-2-imi(1~7- lirlinon- 1 -yl)l: f n7f nf clllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]-2-m~lllyll,luL~yl]-
phenyl]~iodoh.~n7f nf s--1 rullallPidf
25 ~-l4-[2-[[2-hydrOXy-2-(3-pyridinyl)ethyl]amillo]-2~ lllyl~)lu~Jyl]-
phenyl]-4-[[(he~ylalllillo)carbonyl]amino]b~n7rnf,s~lfonamide
~-[4-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]phenyl]-4-iodub~ llf;-
sulfonamide
N-[4-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]phenyl]-2-n~rh~h~lf n~
3 Sulfollamide
N-14-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]phenyl]-3-quinoline-
~ullullalllide
N-[4-[2-[[2-hydroxy-2-(3-chlorophenyl)ethyl]amino]ethyl]phenyl]-3-
isopropylhr. ., ~ .. If ~. .lfonamide
woss/2slss ~1 ~3.7~32 P~,l/u~.~.~C l~s6
- 16 -
~-[4-[2-[[2-hydroxy-2-(3-chlorophenyl)ethyl]amino]ethyl]phenyl]-2-
n~rhth~lf n~slllfonamide
~-[4-[2-[[2-hydroxy-2-(3-chlorophenyl)ethyl]amino]ethyl]phenyl]-3-
quinolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(4-amino-3,5-dichlorophenyl)ethyl]amino]ethyl]-
phenyl]-4-(hexylaminocarbonylamino)b~n7f nf slllfonamide
N-[4-[2-[[2-hydroxy-2-(4-amino-3,5-dichlorophenyl)ethyl]amino]ethyl]-
phenyl]- I -[(octylamino)carbonyl]-S-inf1--1inPclllfonamide
~[-[4-[2-[[2-hydroxy-2-(4-amino-3,5-dichlolu,~ lyl)ethyl]amino]ethyl]-
10 phenyl]-4-(3-hexyl-2-imidazolidinon-l-yl)kf~n7fnpslllfonamide
~-[4-[2-[[2-hydroxy-2-(4-amino-3,5-dichlorophenyl)ethyl]amino]ethyl]-
phenyl]-4-(3-octyl-2-imi~1~7nli~1inrn- 1 -yl)~r~ l .1 rullalllide
N-~4-[2-[[2-hydroxy-2-(4-llydlu~y~ llyl)ethyl]amino]ethyl]phenyl]-
,- If ~ lfonamide
5 N-[4-[2-[[2-hydroxy-2-(4-hydroxyphenyl)ethyl]amino]ethyl]phenyl]-4-
iodo~ 11 rullalllide
~-[4-[2-[[2-hydroxy-2-(3-cyanophenyl)ethyl]amino]ethyl]phenyl]-4-
(hexyl~lminor~rbonylamino)kf n7f nf cl~lfonamide
~-[4-[2-[[2-hydroxy-2-(3-~:yallu~h~ll,yl)ethyl]amino]ethyl]phenyl]-3-
20 quin()linf.slllfollamide
4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4
hexyl-[1,2,4]-oxadiazol-3-yl)l,r.,i~ ,P~,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
heptyl-5-methyl-[1,2,3]-triazol-2-yl)~,....~.f ..f'~. lfonamide
25 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
hexyl-2,4-imidazoli~linf.-lit)n- 1 -yl)l,r., ,.... If.,"l rc,llalllide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
octyl-2,4-imidazolidinedion- 1 -yl)k~n7f.nf slllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
3 ,ycl~ yl~-lul!yl)-2,4-imidazolidinedion- 1 -yl]~f n7f nf slllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
pentyl-[1,2,4]-oxadiazol-S-yl)1~ lf~ fonamide
~[-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
hexyl-[ 1 72,4]-oxadiazol-5-yl)ben7f nf c~l1fonamide
W095129159 2 1 8 7 9 3 2 ~ u." ~04JsG
- 17 -
.~ ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
heptyl-[1,2,4]-oxadiazol-5-yl)br",.r,"P~lfonamide
N-[~[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
octyl- [ 1,2 ,4] -oxadiazol-5-yl)benzenesulfonamide
N-~4-[2-L[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(2-
cyclopentylethyl)-[1,2,4]-oxadiazol-S-yl]b.qn7P.nf~s-llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
cyclu~ yl~lul,yl)-[1,2,4]-oxadiazol-S-yl]ben7~n~-s--lfonamide
N-[4-t2-tt2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]4-(3-
pentyl-[1,2,4]-thiadiazol-5-yl)bf n7.~n~s--1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
hexyl-[1,2,4]-thi~ 701-S-yl)l~r.~f "~.~lllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
heptyl-[1,2,4]-thiadiazol-5-yl)ll. ~l~rll~lllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(3-
octyl-[1,2,4]-thi~ 7QI-S-yl)l~ zf .~e~ulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(2-
cyclopentylethyl)-[1,2,4]-thiadiazol-5-yl]l,r",r .,~.blllfonamide
I!!.-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[3-(3-
20 cyclo~.,.l~yl~lu~yl)-[1,2,4]-thiadiazol-5-yl]b~ ,f ,~lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]-4-(5-
pentyl-[l,2,4]-thiq~ 7--1-3-yl)br",~ .llfonamide
N-[4-[2-t[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]4-(5-
hexyl-[1,2,4]-thi~ 7-1-3-yl)llr.,,.~."f-s~lfonamide
25 1!~4-~2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(S-
heptyl-[1,2~4]-thiadiazol-3-yl)bf n7lonl~slllfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(S-
octyl-[ 1 ,2,4]-thiadiazol-3-yl)l.f . ~.f .~f~l llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]e~hyl]phenyl]-4-[5-(2-
3~ cyclopentylethyl)-[1,2,4]-thiadiazol-3-yl]hf n7f nf~s--lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(3-
cyclu~ yl~lu~yl)-[1,2,4]-thiadiazol-3-yl]b~n71-nlocl-lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
pentyl-3-oxo-[1,2,4] triazol-2-yl)l~PIl7r,~. ,lllfonamide
WO 95/29159 1~,11 ~J.,,_10 I~SG
7~ ~7~3~ --
- 18 -
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4- ,
hexyl-3-oxo-~1,2,4]-triazol-2-yl)iJc;."e~ ulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
heptyl-3-oxo-[1,2,4]-triazol-2-yl)l: Pn7.3nf~sll1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
octyl-3-oxo-[1,2,4]-triazol-2-yl)ben7~nr,s~l1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(2-
cyclopentylethyl)-3-oxo-[1,2,4]-triazol-2-yl]~,n7f~nl~.sll1fonamide
~-[4- [2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(3-
10 cyclopentylpropyl)-3-oxo-[1,2,4]-triazol-2-yl]hPn7Pn~slllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
pentyloxazol-2-yl)bPn7~n~.cll1fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
hexyloxazol-2-yl)b~n7~n~sll1fonamide
15 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]~(5-
heptyloxazol-2-yl)bPn7f~,n~.cll1fonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
octyloxazol-2-yl)l,~ lfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(2-
20 cyclopentylethyl)oxazol-2-yl]l ~r~ ,lfonamide
I!I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(3-
;y-,lu~ yll~ullyl)oxazol-2-yl]b...~ lfonamide
~!-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
pentyloxazol-2-yl)b~.n7P,n~..c~lfonamide
25 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
hexyloxazol-2-yl)k~,n7~nf~s~l1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
heptyloxazol-2-yl)~ P~Illfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]~(4-
30 octyloxazol-2-yl)b~n7..onl~.sll1fonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(2-
cyclopentylethyl)oxazol-2-yl]l-r- ~ ~.. "~ lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(3-
cyclo~ yl~lu~yl)oxazol-2-yl]~n7~n~os~lfonamide
WO 95/Z9159 F~
2 1 8 7932 ~ 556
- 19 -
I!~-L4-~2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2
hexyloxazol-S-yl)bf,n7enPslllfonamide
. N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
heptyloxazol-5-yl)L r.",r,~e~,llfonamide
1~1-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(3-
cyclup~ yl~lu~yl)oxazol-5-yl]~ .rl l. .~. llfonamide
N-L4-L2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(4-
cycloll~hylL,ulyl)oxazol-5-yl]L.. .,,r~,f.i,,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-[2-
(4-fluorophenyl)ethyl]oxazol-5-yl]L~r~ 17rl Ir~ lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
pentyloxazol-4-yl)bf n7f nf-slllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]~(2-
hexyloxazûl-4-yl)L,~" ~r:J)f ~l llfonamide
5 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
heptyloxazol-4-yl)Llrl 17f . If ~1 llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
octyloxazol4-yl)L,r, I,rl .... Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]4-[2-(2-
2 0 cyclopentylethyl)oxazol-4-yl]L~r ~ ~ ~ r ~ Ir~l ~1 ru~ lide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(3-
cyclu~ yl~luL,yl)oxazol-4-yl]br,~ foll~lllide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
p~ ylllliaz~JI-2-yl)lJ...,,.r"e~ fonamide
25 N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(5-
hehylllliaLul-2-yl)L~ .rl If.~. Ilfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]4-(5-
yl~lli~ol-2-yl)bf n7f.nesll1fonamide
~-[4-12-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(
30 o~yl~.lliazul-2-yl)L~r",r".,.llfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(2-
cyclopentylethyl)thiazol-2-yl]L~r~ I~.r.l If ~- Ilfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[5-(3-
cyclu~ yl~lu~l)thiazol-2-yl]~ 7r",~ lfonamide
WO 95/29159 r~ 'C ;~6
21 ~932
- 20 -
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyljphenyl]-4-(4-
pentylthiazol-2-yl)L,r.,,r".~ lfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
hexylthiazol-2-yl)L ~",r"t-~llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
heptylthiazol-2-yl)~.r.,,,~ lfonamide
~-[4-[2-~[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(4-
octylthiazol-2-yl)L r.,,~.. .f.~"lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(2-
10 cyclopentylethyl)thiazol-2-yl]br~~~P ,p~lllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[4-(3-
cyclo~clllyllJluluyl)thiazol-2-yl]kpn7pnp~lllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]~(2-
pentylthiazol-4-yl)kP,n7PnP,slllfonamide
5 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
hexylthiazol-4-yl)LP.~ f ,,llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
heptylthiazol-4-yl)bPn7PnPslllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
20 octylthiazol-4-yl)b .,~ ,lP~Illfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(2-
cyclopentylethyl)thiazol-4-yl]~ "1 rvll~llid~
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]-4-[2-(3-
cyclopentylpropyl)thiazol-4-yl]l,r",~ .~lp~lllfonamide
25 ~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
ylLllidL~)I-S-yl)l~ r lP~.llfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
hexylthiazol-5-yl)l,r.,, .... ,..1 r~ ide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
30 heptylthiazol-5-yl)l-,~..,..,f j..lfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-(2-
octylthiazol-S-yl)L,- ,~ lllfonamide
~I-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(2-
cyclopentylethyl)thiazol-S-yl]hPn7P,nPslllfonamide
WO95/2915g P~,I/u., ~ 35C
21 87932
- 21 -
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-4-[2-(3-
cyclopentylpropyl)thiazol-5-yl]ben7enes-llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
methylthiazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
pentylthiazol-2-yl)-5-in~ lin~s--lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
hexylthiazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
10 h~ yll~lia2c,l-2-yl)-5-in~ lin~slllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(5-
octylthiazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[5-(2-
cyclopentylethyl)thiazol-2-yl] -5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[5-(3-
cyclo~ lyl~lu~yl)thiazol-2-yl]-5-inrlnlines~lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
pentylthiazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(4-
20 hexylthiazol-2-yl)-5-in,l,~lin~slllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(4-
h~;L)Iyllllia~1-2-yl)-5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -[4-(2-
cyclopentylethyl)thiazol-2-yl]-5-in-101inesl-1fonamide
25 ~[-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[4-(3-
y~ ylpropyl)thiazol-2-yl]-5-infl~lineslllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
methyloxazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-(5-
3 ~ pentyloxazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-(5-
hexyloxazol-2-yl)-5-in-loline~- Il rOIIalllide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(5-
heptyloxazol-2-yl)-5-in~ lin~s--lfonamide
WO95/29159 r~~ ,5!019~G
~ ~7~3~ --
- 22 -
N-~4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
octyloxazol-2-yl)-S-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-[5-(2- - .
cyclopentylethyl)oxæol-2-yl] -5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[5-(3-
cycluy~ ylyluyyl)oxæol-2-yl]-S-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
methyloxazol-2-yl)-5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
pentyloxazol-2-yl)-5-in~ lin~slllfonamide
~[-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
hexyloxazol-2-yl)-5-inrl~lin~lllfonamide
;~-[4-[2-[[2-hydroxy-2-~3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(4-
heptyloxazol-2-yl)-5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(4-
octyloxazol-2-yl)-5-intll linf~.clllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -[4-(2-
cyclopentylethyl)oxazol-2-yl] -5-indolinesulfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[4-(3-
cycluy~ ylyluyyl)oxazol-2-yl]-5-in~ lin~oslllfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]- 1 -(3-
methyl-[l ,2,4]-oxadiæol-5-yl)-5-indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(3-
pentyl-[l ,2,4]-oxadiæol-5-yl)-5-in-lolin~slllfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]arnino]ethyl]phenyl]-1-(3-
hexyl-[1,2,4]-oxadiæol-5-yl)-5-in~lolinf~.slllfonamide
![-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-(3-
heptyl-[l ,2,4]-oxadiazol-5-yl)-S-indolinesulfonarnide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-(3-
octyl-[1,2,4]-oxadiæol-5-yl)-5-in~ lin~slllfonamide
~[-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -[3-(2-
cyclopentylethyl)-[1,2,4]-oxadiæol-5-yl]-5-inrl~linP~IIlfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[3-(3-
cycloy~ ylyl~lyyl)-[l,2,4]-oxadiazol-5-yl]-5-in-lolin~slllfonamide
WO 95/29159 r~ . v :556
8793~
- 23 -
N-L4-~2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(5-
methyl-[1,2,4]-oxadiazol-3-yl)-5-in~ linPslllfonamide
~-~4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -(5-
pentyl-[1,2,4]-oxadiazol-3-yl)-5-in~ linPs-llfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
hexyl-[ 1 ,2,4]-oxadiazol-3-yl)-5-indolinesulfonamide
~-[4-[2-~[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-(5-
heptyl-[1,2,4]-oxadiazol-3-yl)-5-in~ linPsl-lfonamide
N-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1-(5-
octyl- [ 1 ,2,4]-oxadiazol-5 -yl)-3 -indolinesulfonamide
~-[4-[2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]-1-[5-(2-
cyclopentylethyl)-[1,2,4]-oxadiazol-3-yl]-5-in~ lin~clllfonamide
~-[4-t2-[[2-hydroxy-2-(3-pyridinyl)ethyl]amino]ethyl]phenyl]- 1 -[5-(3-
cyclopentylpropyl)-[l ,2,4]-oxadiazol-3-yl]-5-in-lnlinPclllfonamide
The CU~ JUUII~S of the instant invention all have at least one
a~yll~"Gtlic center as noted by the asterisk in structural Formula I.
Additional a~yl,L~"~l~ic centers may be present on the molecule
depending upon the nature of the various sllhstitllPntc on the molecule,
in particular, R2 and R3. Each such a~yllull~llic center will produce
20 two optical isomers and it is intended that all such optical isomers, as
separated, pure or partially purified optical isomers or racemic
mixtures thereof, be included within the ambit of the instant invention.
In the case of the ~yllllll~lfic center l~l.,~ d by the asterisk in
Formula I, it has been found that the compound in which the hydroxy
2~ is above the plane of the structure, as seen in Formula Ic, is
more active and thus more preferred over the compound in which the
hydroxy sllhstitllPnt is below the plane of the structure.
The following stereospecific structure represents the
preferred stere~i~nmPr.c of the instant invention:
WO 95~29159 ~ C 15~G
27~J93~ --
- 24 -
H OH H R2 R4 --
~ C-CH2N-C-(X)~e ~ N-So2(CH2)r-R7
(R1)n R3 Rs R6
Ic
where n, m, r, A, Rl, R2, R3, R4, RS, R6, R7 and X are as defined
above under formula 1.
0 Throughout the instant application, the following terms
have the indicated m~ningS
The alkyl groups specified above are intended to include
those alkyl groups of the rll-ci~n~r-.cl Iength in either a straight or
branched configuration. Exemplary of such alkyl groups are methyl,
5 ethyl, propyl, isopropyl, butyl, sec-butyl, tertiary butyl, pentyl,
isopentyl, hexyl, isohexyl, and the like.
The alkoxy groups specified above are intended to include
those alkoxy groups of the lP~i~n~r-d length in either a straight or
branched configuration. Exemplary of such alkoxy groups are
20 methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, tertiary
butoxy, pentoxy, isopentoxy, hexoxy, isohexoxy and the like.
The term "halogen" is intended to include the halogen
atoms fluorirle, chlorine, bromine and iodine.
F-r~mrll~ of 5 and 6-~ llb~ ,d heterocycles and fused
25 h~,t~lu~,y~ of A, Z and Rla include pyridyl, quinolinyl, pyrimidinyl,
pyrrolyl, thienyl, imidazolyl, thiazolyl, ben7.imi~ rr~lyl7 thiadiazolyl,
benzu~ adidL~,lyl, indolyl, indolinyl, benzodioxolyl, benzodioxanyl,
benzothiophenyl, b~llY.,rul~lyl, benzoxazinyl, benzisoxazolyl,
benzothiazolyl, tetrahyr~ .d~ l-yl, dihydlol)t;llz~Jruldllyl,
30 tetrahydroquinolinyl, furopyridine and thienopyridine.
The preferred values of A and Z are phenyl, naphthyl,
benzene ring fused to a S or 6-membered heterocyclic ring with from I
to 4 heteroatoms selected from oxygen, sulfur and nitrogen, or
W0 95129159 1~ s~a .,.,v
2 1 87q32
- 25 -
heterocycles with from 1 to 4 heteroatoms independently selected from
one of oxygen or sulfur, and/or l to 4 nitrogen atoms.
The more preferred values of A are phenyl, pyridyl,
quinolinyl, pyrimidinyl, pyrrolyl, thienyl, imidazolyl, and thiazolyl.
The more preferred values of Z are phenyl, naphthyl,
quinolinyl, thienyl, kçn7imi~i~7olyl~ thiadiazolyl, benzothiadiazolyl,
indolyl, indolinyl, benzodioxolyl, benzodioxanyl, benzothiophenyl,
b~,l.zuruldllyl, benzoxazinyl, benzisoxazolyl, benzothiazolyl,
L~llahydlu~ JllLllyl, dihydrobenzofuranyl, triazûlyl, tetrazolyl,
oxadiazolyl, imidazolyl, oxazolyl, thiazolyl, imidazolidinyl, pyrazolyl,
isoxazolyl, pyridyl, pyrimidyl, pyrazolyl, tetrahydroh~n7ofhi~7--lyl and
tetrahydroquinolinyl. When Z is attached to -NSO2(CH2)r-, it is
preferably phenyl, naphthyl or a benzene ring fused to a 5 or 6-
~"llbelcd heterocyclic ring with from 1 to 4 heteroatoms selected from
5 oxygen, sulfur and nitrogen. When Z is part of the definition of R8, it
is preferably phenyl, a S or 6-1llt;lllb~lc;d heterocyclic ring with from 1
to 4 h~,Lt;lu~ullls selected from ûxygen~ sulfur and nitrogen, a benzene
ring fused to a S or 6-membered heterocyclic ring with from l to 4
h~,t1lu~1ullls selected frûm oxygen, sulfur and nitrogen, or a S or 6-
20 membered heterocyclic ring with from 1 to 4 h.,L~u~lullls selected from
oxygen, sulfur and nitrogen fused to a C3-Cg cycloaLkyl ring.
The preferred hc~l~-u-;ycles of Rla are thienyl, thiadiazolyl,
triazolyl, tetrazolyl, ~IY~ 7.~lyl, imidazolyl, oxazolyl, thiazolyl,
imi~iq7rtli-1inyl, pyrazolyl, isoxazolyl, pyridyl, pyrimidyl, and pyrazolyl.
Certain of the above defined terms may occur more than
once in the above formula and upon such occurrence each term shall be
defined independently of the other; thus for example, NR8R8 may
represent NH2, NHCH3, N(CH3)CH2CH3, and the like.
The following abbreviations are used throughout the
30 srte~ifi~:lti~ln
Boc : tert-butyloxycarbonyl
Cbz : carbobenzyloxy
DIP-CI : diisopinor:lmrhf ylchloroborane
DMF : dimethylformamide
WO 95/29159 1'~ .'0 ~5~
2187932
- 26 -
DMSO : dimethylsulfoxide
HPLC : high pressure liquid chromatography
Me : methyl
MPLC : medium pressure liquid chromatography
Ms : m~thAnPslllfonyl (mesyl)
NBS : N-bromosuccinimide
NCS : N-chlorosuccinimide
nHex : n-hexyl
TBAF : tetrabutylammonium fluoride
TBS (TBDMS) : t-butyldimethylsilyl
TFA : trifluoroacetic acid
THF : tetrahydrofuran
The compounds (I) of the present invention can be
prepared from epoxide in~Prm~rliAtf~c such as those of formula Il and
5 amine intr- "-. ~ .t~ ~i such as those of formula III. The preparation of
these i,,l~ .,,,P/l;AIrs is described in the following schemes.
~ H2N~C-(X)m{l~NI S2(CH2)r~R
(Rl)n
D m
where n, m, r, A, R1, R2, R3, R4, R~, R6, R7 and X are as dehned
25 aboYe.
Compounds II are known in the literature or may be
~ ,v~ ly prepared by a variety of methods familiar to those skilled
in the art. One common route is illll~trAtrd in Scheme 1. Acid chloride
30 L which may be culll~ ially available or readily prepared from the
corresponding acid by treatment with, for example, thionyl chloride or
oxalyl chloride, is treated with did2011lc;~ lC in a solvent such as diethyl
ether. The resultant diazoketone is then treated with hydrogen chloride
to give chloroketone ~ (X = Cl). The haloketone ~ is then reduced with
a reducing agent such as sodium borohydride. The resultant alcohol 3 is
,,,
_ _ _ . . . . . .. .. .
WO 95/29159 r~.,u~ r,
21~7932
- 27 -
. treated with base such as pOt~i~iiUIII carbonate in refluxing acetone to
provide the desired epoxide II. The enantiomerically enriched (R) and
,- (S) epoxides II are readily available by asymmetric reduction of
haloketones 2 using chiral reducing agents such as (-) or (+)-DIP-Cl,
5 (R) or (S)-Alpine borane or (R) or (S)-tetrahydro-l-methyl-3,3-
diphenyl-lH,3H-pyrrolo[1,2-c][1,3,2]oxazaborole-borane ((R) or (S)-
OAB-BH3).
SCHEMF. 1
o
~CI 1) CH2N2 ~J~,
(Rl)n 2) HCI (Rt)n~)
~ (X = Cl, Br)
OH
[H] ~~X base
1 ~)
(R )n 3 (X = Cl, Br)
0
,~
(R1)n II
An alternate route to the desired haloketones 2 is illustrated
in Scheme 2. Methylketone 4 may be converted to the ~ ldillg
haloketone using a variety of reagents known to those in the art and
,.", ~,;,. d in Larock Comprehensive Organic Transforfnations; VCH:
New York, 19~9, 369-372. Conveniently, methyLketone 4 is treated
WO 95129159 r~ o 1~6
~ ~7~32
- 28 -
with chlorine or N-chloros-lrrinimi(lr in acetic acid with an additional
acid source such as hydrogen chloride or ~ lnninllm chloride. For the
synthesis of ~ (X = Br), bromine, dibromobarbituric acid or NBS with
hydrogen bromide or ~IIlminllm bromide may be used. In some cases,
5 the chloro or bromokPton~c ~ may be commercially available.
SCHF.Ml~ 2
0
(R )n CH3 Cl2 or Br2 ~ _~ X
(R1)n
2 (X = Cl, Br)
Many of the methylketones _ are commercially available or
readily prepared by methods described in the literature and known to
those skilled irl the art. Rl substituents on the acid chlorides 1 or
20 methylketones 4 may need to be protected during the cllhseqll~nt
procedures. A description of such protecting groups may be found in:
Protective Groups in Or~nir Synthesis. 2nd Ed., T. W. Greene and P.
G. M. Wuts, John Wiley and Sons, New York, l991.
Compounds m can be conveniently prepared by a variety
25 of methods familiar to those skilled in the art. A ;OII~GII1.,II~ route for
their preparation when R6 is hydrogen is illustrated in Scheme 3.
Compound ~ is selectively protected as a suitable ~;~ub~lld~G derivative 6
with, for example, di-tert-butyl dicarbonate or ~;~I,ob~ yloxy
chloride. This compound is then treated with a sulfonyl halide,
30 ~ Glably the sulfonyl chloride 7. and a base such as pyridine in an
anhydrous solvent such as dichloromethane or chloroform for 0.5 to 24
hours at LGIIIIJGInLUIGS of -20 to 50C, preferably 0C, to provide the
sulfonamide 8. The protecting group is then removed with, for
WO9S/29159 r~ J. o
2 ~ ~7932
- 29 -
. example, trifluoracetic acid in the case of Boc or catalytic
hydrogenation in the case of Cbz, to give the desired amine 9.
SCHEM~ 3.
H2N-C~(X)m{ ~NH2 Boc20
R3 F~5 orCbzCVbase
2 R4
GNH--C~X)m~l~ N H2
6 R
G=BocorCbz
R2 R4
R7(CH2)rSO2cl CZ) GNH~C-(X)m~; ~Nso2(cH2)r-R7
pyridine, CH2CI2 R F;s H
8 4
TFA, CH2CI2 lR2 R
or H2/Pd catalyst F; Rs H
Cu~ uulld~ III where R6 is not hydrogen may be
conveniently prepared as illllctr~t.od in Scheme 4. Sulfonamide ~
3O prepared as described above, is aLkylated with an d,U~IU~Iidl~ alkylating
agent 10 in the presence of base to provide sulfonamide 1 1. Removal of
the protecting ~roup as above gives the desired compound ~.
WO 95/29159 ~ 7 9~ ~ c ;956
- 30 -
SCHEME 4
R2 R4 Alk-Y
C~X)m{l~ INso2(cH2)r-R
R4
~1~
GNH~Ç~X)m~ ~NSo2(CH2)r-R7 TFA, CH2CI2
10 R3 R5 Alk or H2/Pd catalyst
11
R2 R4
15 H2N~C~(X)m~NSO2(CH2)r~R G=BocorCbz
R3 15 Alk Y = Cl, Br, or I
R Alk = Cl-C6 alkyl
20 The sulfonyl chlorides 7, many of which are cullu~ i2illy
available, can also be readily prepared by a number of methods familiar
to those skilled in the art. One suitable method involves the addition of
an u~ lolillliulll reagent or a Grignard reagent to sulfuryl chloride
following the procedure of S. N. Bhattacharya, et. al., J. Chem. Soc.
25 (C), 1265-1267 (1969). Another convenient method involves the
treatment of a thiol with sulfuryl chloride and a metal nitrate according
to the procedure of Y. J. Park, et. al., Chemistry Letters, 1483-1486
(1992). Sulfonic acids are also conveniently converted to the
corresponding sulfonyl chloride by treatment with PC15, PC13 or SOC12
30 (J. March, Advanced Ore~ni~ (~hlomi~try~ 4th Ed., John Wiley and Sons,
New York: 1992, pl297 and references sited therein). Aromatic and
l;.,t~,lu~ullldLic compounds may be chlorosulfonylated directly by
treatment with Vilsmeier's reagent or chorosulfonic acid (Organic
Synthesis, I, 8).
WO95129159 P_ I/ u.. ,~ 56
2 1 8 7932
- 31 -
The diamines 5 are commercially available or readily
prepared by methods described in the literature or known to those
skilled in the art. Compound 5 where R2 or R3 is methyl can be
prepared from the co~ onding amino acid following the method of J.
D. Bloom, et. al., J. Med. Chem., 35, 3081-3084 (1992). As illustrated
in Scheme 5 for R3 = methyl, the a~lup.i~ (R) amino acid 12 is
cst~rifie-1 conveniently by treatment with methanolic hydrochloric acid,
and then treated with di-tert-butyl dicarbonate to give compound 13.
The ester group is reduced with a hydride source such as lithium
o borohydride and the resultant alcohol is converted to a leaving group
such as a mesylate. Removal of the Boc protecting groups gives
diamine 14. This compound is subjected to catalytic hydrogenation in
the presence of base such as sodium acetate to give the desired o~-methyl
amine 15. The other ~n~ntinmf-r is available through an analogous
sequence starting with the corresponding (S) amino acid.
WO 95/Z91~i9 . .,~ .'C :~6
~1 ~79~2
- 32 -
SCHEMF. 5
H2N (X)m~f/ 1) MeOH, HCI
2 R5~ NH2
BocNH (X)m ~ R4 1 ) LiBH
~ 2) MeSO2CI, Et3N
MeO2C Rs~ NHBoc 3) TFA, CH2C12
H2N (X)m ~ R4
~ ~ H2, NaOAc
~ 2CF3CO2H cat. Pd
H2N (x)m~¢~R4
Me /`~ NH
Diamines ~ or sulfonamide amines 2 where X is -cH2
and m is 1 are also readily prepared by methods described irl the
literature or known to those skilled in the art. For example, as shown
in Scheme 6, the sodium salt of 4-1liLIu~ ol 16 is alkylated with 1-
bromo-2-chl:>lu~ c, conveninetly in refluxing 2-butanone with a base
30 such as potassium carbonate to give chloro derivative 17. The chloride
is converted to the COll~ ulldillg amine by treatment with lithium azide
followed by reduction with, for example, kiphenylph~srhinp in aqueous
tekahydrofuran. Protection of the resultant amine, conveniently as its
t-butyl ~ by keatment with di-tert-butyldicarbonate, gives
derivative 18. The nitro group is then reduced, for example, by
... . . .. . ~
~O 951291S9 2 1 ~ 7 9 3 2 ~ c
- 33 -
catalytic hydrogenation to provide amine 19. Acylation of i"l~""P.l;~t~
19 with sulfonyl chloride 7, followed by deprotection with acid such as
trifluoroacetic acid gives the desired intermediate 20.
ScT IF~E 6
NaO~¢~ N2 KtCO ~, C1~~l3~ NO2
16
1. LiN3, DMF, 60
2. PPh3, THFIH2O, BocNH~ ~ H2, Pd/C
3. Boc annydride, 18 No2
BocNH~ `13~ 1. R7(CH2)rS02CI (~), pyridine, CH2CI2
19 NH2 2- TFAJCH2CI2 (1:3)
H2N ~ ~
NHSo2(CH2)r-R7
Alternatively, diamine 5 where X is -CH2O- and m is 1 is
available from i~ r~ lr 1~ by treatment with trifluoroacetic acid.
rhis diamine may then be modified as illustrated in Scheme 3.
wo 95/29159 , ~c
2 1 8 7 9 3 2 I ~ C ~
- 34 -
Diamines ~ and sulfonamide amines ~ where X is .
-CH2CH2- and m is 1 are also readily prepared by methods described in
the literature or known to those skilled in the art. For example, as
shown in Scheme 7, bromo derivative 21 is treated with sodium cyanide
5 to provide nitrile 22. The rlitro group is selectively reduced by
treatment with hydrogen and catalytic pr~ lm to provide arnine 23.
Amine ~ is acylated with sulfonyl chloride ~Z to give the ~u~ ulldillg
sulfonamide 24. Reduction of compound _ with cobalt chloride and
sodium borohydride provides the desired amine 25.
St~TlF.l\/lF 7
Br 3~ NaCN N C ~
NO DMSO 22 ~ No2
H2, Pd/C N C R7(CH2)rSO2CI ( Z)
' ~ ~
MeOH ~ `NH2 pyridine,CH2CI2
NC 3~ CoCl2 6H20
NHSo2(CH2)r-R7 NaBH4, MeOH
24
H2N 3~
NHSO2(CH2)r~R
W0 95/29159 2 ~ 8 7 9 3 2 P~ .'C :SS6
- 35 -
Alternatively, diamine 5 where X is -CH2CH2- and m is 1
is available from intPnn~ t~ 23 by reduction of the nitrile group with,
for example, cobalt chloride and sodium borohydride. This diamine
may then be modified as illustrated in Scheme 3.
Tntf nn~ t.os Il and III are coupled by heating them neat
or as a solution in a polar solvent such as methanol, ?,~etonitrilt~,
tetrahydrofuran, dimethylsulfoxide or N-methyl pyrrolidinone for 1 to
24 hours at l~ aiulc;s of 30 to 150C to provide compounds I as
shown in Scheme 8. The reaction is conveniently conducted in
refluxing methanol. Alternatively, a salt of amine III, such as the
trifluo.~,ac~,Lat~ or hydrochloride salt, may be used. In these cases, a
base such as sodium bicarbonate or diethylisopropylamine is added to
the reaction mixture. The product is purified from unwanted side
products by recrystallization, trituration, lJIC~udtiV~ thin layer
5 chromatography, flash chromatography on silica gel as described by W.
C. Still, et. ~., J. Org. Chem. 43. 2923 (1978), medium pressure liquid
L~ la~lly, or HPLC. Compounds which are purified by HPLC
may be isolated as the c~ ollding salt. Purification of int.onn
is achieved in the same manner.
W095/291!i9 ~ 1 .8;~93~7 P~1/~ ~CI~5G
- 36 -
S(~F.l~IF 8
O .
(~ +
(R )n
R3 ~I~ R6
III
~CHCH2N-C~X)m~l~NSo2(CH2),-R7
R
In some cases, the coupling product I from the reaction
described in Scheme 8 may be further modified, for example, by the
removal of protecting groups or the manipulation of ~ on, in
particular, Rl and R7. These manipulations may include, but are not
limited to, reduction, o~ AAtiO~ alkylation, acylation, and hydrolysis
reactions which are commonly known to those skilled in the art.
An alternate method for the synthesis of compound I is
illustrated in Scheme 9. Epoxide II is coupled to amine 5 as described
above for coupling ill~ ,,.f~llA . ~ II and III (Scheme 8) to giYe aniline
d~.liv~livt; ~Z. The secondary amine is selectively protected, for
example, as a carbamate by treatrnent with di-tert-butyldicarbonate to
provide C~llb~ 2. Alternatively, nitro amine 26 is used in the
coupling reaction to provide 28. Following protection as described
above, the nitro group is reduced, for example, by catalytic
hydrogenation with pAAllArli~ catalyst or raney nickel, to provide
i"l~ . ,,,f,liAIf. 29. In some cases, other group may be reduced
con~;ullliL~l~ly. For example, if Rl is halogen in intermediate 28, it may
wo 95129159 r~ 56
2 1 ~79~2
- 37 -
be converted to hydrogen in int~rm~ t~ 29. Treatment with a sulfonyl
chloride in the presence of a base such as pyridine followed by removal
of the protecting group with, in the case of a tert-butylcarbamate, acid
such as trifluoroacetic acid or mt-th~noli~ hydrogen chloride, provides
5 the sulfonamide I.
SCHEME 9
~<1
l A )
(R1)n>~ Il
R2 R4
H2N-C-(X)m{ ~Z
~i (Z = NH2)
2~ (Z = NO2)
20 (R )n R pS 2) H Pd/C
2:Z (Z = NH2)
2~ (Z = NO2)
,OH Boc R2
(R1)n R3 {¦~
- 30 1) R (CH2)r-s02cl~ base
2) TFA or HCI/MeOH
In some cases, compound I from the reaction sequence
illustrated in ~heme 9 may be further modified, for example, by the
WO95/291~9 2~ 87g3~ I~,J/I 3'/CI3~6
- 38 -
removal of protecting groups or the manipulation of sllhstitl-f nts on, in .
particular, Rl and R7, as described above. In addition, m:lnir~ tion of
~Ub~ U~ i on any of the intf~rmf ~ tfAs in the reaction sequence
illustrated in Scheme 9 may occur. One such example is illustrated in
5 Scheme 10. Compound 30, which is prepared as outlined in Scheme 9
from the collc~l~ullding epoxide, is subjected to reduction using tin(II)
chloride to provide compound ~1. Other examples of Sllhcfitll~ntc on
compound I which may be reduced to the corr~.sron~lin~ amine by
methods commonly known to those skilled in the art include nitro
groups, nitriles, and azides
SCHFMF 10
OH BOC R5
N~ R6
¦ SnCI
~ aq. HCI-MeOH
OH H ,=~, R
H2N~ R~ R~R6
31
The compounds (I) of the present invention can also be
prepared from amine intf rrnf ~ tf c such as those of formula III and
30 haloketone i.,~.. ".f.1i,~rs such as those of formula 2, as shown in
Scheme 11. Amine III is alkylated with haloketone derivative ~,
~.~llvclP~ y by treatment of a mixture of III and ~ with base such as
polaS:~;ulll carbonate or triethylamine in a polar solvent such as
:~retonitrilf~ ~etone or ~ llc~llylrllll~ . The resultant ~nnin~kfsone
~09S~29159 2 ~ 8 793~ r~.,.)~ ~c .)~G
- 39 -
. 32 is reduced wi~, for example, sodium borohydride in methanol to
give the desir~d aminoalcohol I.
SCHE~E 1 1
~X +
~R1~n ~ (X = Cl, Br)
~2 r~
H2N-~ (X)m~ NSo2(CH2)r-R7
m
~,4
~ ~~ H ~S2 ~l~
(Rl)n CCH2N-C,-(X)m~ NSO2(CH2);R
~2
[H]
~ - I
In some cases, the product I from the reaction described in
Scheme 11 may be further mc-~ifiçrl, for example, by the removal of
protecting groups or the n~nirll~tinn of cllbstitllPnfc on, in particular,
R1 and K7. These manipulations may include, but are not limited to,
reduction, oxi~l~tinn, alkylation, acylation, and hydrolysis reactions
3 D which are commonly known to those skilled irl the aIt
An alternate synthesis of key intPrm~ t~ 29 is shown is
Scheme 12. The alcohol of i~ ~t~ 3 is protected, for examp~e, as
its t-butyldimethylsilyl ether to give ll~S derivative 33. This compound
is then treated with amine 5 and a base such as diisopropylethylamine in
a solvent, typically polar aprotic such as ~c~tnnifril~, at temperatures of
WO 95/29159 2 1 8 7 9 3 2 P~ 56
-40 -
25 to 150 C for 1 to 72 hours. Typically, an iodide source such as .
sodium iodide is added to facilitate the reaction. The protecting group
is then removed, in the case of silyl ether, by treatment of the resultant
amine 34 with a fluoride source such as tetrabutylammonium fluoride.
5 Protection of the secondary amine as before gives key intermediate 29.
SCHF.l~F. 1?
OR
. ~X +
~J 3(R=H,X=Cl,Br)
tR1)n 33 (R = SiMe2tBu)
R4
~2
H2N-C (X)m~l ~ NH2
R5
R4
~OTBS Hl R2 rl=\ 1 ) TBAF
(R )n CHCH2N-C, (X)m~ kNH2
OH Boc ~2 ~4
~CHCH2N-CI ~X)m~l~ NH2
R
In some cases, compound I may be synthesized directly
from int~.rrn~ t~ ~ Z without protection of the secondary amine. For
example, when R2 and R3 are both methyl, aniline derivative 27 is
treated with sulfonyl chloride :Z and a base such as pyridine in a solvent
0 9SI291S9 ~ U~ C
ff~f ~ ~ ;i'f~f3
- 41 -
such as dichloromethane at a le~ dlulfc of -30 to 50 C, typically O
C, to provide compound I.
In some cases, the product I from the reaction described in
Scheme 13 may be further modified, for example, by the removal of
5 protecting groups or the manipulation of substituents on, in particular,
Rl and R7, as described above.
SCHF.MF 13
(R )n OH H R2
2~ (R2,R3 = Me
R7(CH2`f,-SO2CI (~,~f
base
2 o The compounds (I) of the present invention where R2 and
R3 are hydrogen can also be prepared from acid intff~nnfff~fi~tP.s of
formula 36 and aTninf ff~lrohols of formula 37, as shown in Scheme 14.
Acid 36 is available from the Cullc"~full.lillg ester 35, typically a methyl
or ethyl ester, by treatment with sulfonyl chloride 7 and a base such as
25 pyridine, followed by hydrolysis of the ester with aqueous acid or base.
Acid 36 is coupled to amine 37, which is known in the literature or
readily prepared by methoff1s known to those skilled in the art, using a
coupling agent such as benzotriazolyl-N-oxy-
tris(dimef,llylalllillo)phosphonium hexdlluulu~ o~rh~tff~ or 1-(3-
30 dimethyl~ulu~luL,lupyl)-3-ethylcarbodiimide methiodide to provide the
amide 38. This is treated with a reducing agent, typically borane, to
provide the desired compound I.
WO 95/29159 r~~ .'0155G
~ 8~3~ --
- 42 -
S(~TTF~IF. 14
R4
RO-C (X)m~ ~ NH 1 ) ClSO2(CH2)r-R~ (7), base
Rs 2) aqueous acid or base
~R = Me orEt, etc.
H-C'(X)m{l~ INSO2(CH2)r~R
OH
~J NH2
(R1)n 3;7
(R )n CCH N~C~(X)m~ ~ INso2(cH2)r-R
38
2S
[H]
Compounds of the general Formula I may be separated into
;lia~ oi~ ic pairs of enantiomers by, for example, fractional
crystallization from a suitable solvent, for exarnple methanol or ethyl
acetate or a mixture thereof. The pair of enantiomers thus obtained
~0 95129159 2 ~ 8 ~ 9 3 2 ~ 5~
- 43 -
. may be separated into individual sterer~ig~mer.s by conventional means,
for example by the use of an optically active acid as a resolving agent.
.. Alternatively, any enantiomer of a compound of the
general Formula I may be obtained by stereospecific synthesis using
5 optically pure starting materials of known configuration.
The instant compounds can be isolated in the form of their
. rlllirRlly acceptable acid addition salts, such as the salts derived
from using inorganic and organic acids. Examples of such acids are
hydrochloric, nitric, sulfuric, phosphoric, formic, acetic
trifluoroacetic, propionic, maleic, succinic, malonic and the like. In
addition, certain COIII1JOUIId~ contRinin~ an acidic function such as a
carboxy or tetrazole, can be isolated in the form of their inorganic salt
in which the counterion can be selected from sodium, pot~iulll,
lithium, calcium, Il~ l and the like, as well as from organic bases.
As previously inAirRt~.A the compounds of the present
invention have valuable rhRrmRr~logirRl properties.
The present invention also provides a compound of the
general Formula I or a pl~ UI;r Rlly acceptable salt thereof for use
as an active Ill. .,.l.~.l~;r sllhgtRnrr
In one aspect, the present invention provides a compound
of the general Formula I or a 1~ ;rRlly acceptable ester thereof:
or a rhRrmRrelltirRlly acceptable salt thereof for use in the treatment of
obesity in human or non-human animals.
The present invention further provides a cu---~Jou--d of the
general Formula I, or a l,llR.lll~.-rlll;~RIly acceptable ester thereof; or
;eRlly acceptable salt thereof, for use in the treatment of
hyperglycemia (diabetes) in human or non-human animals.
The disease diabetes mellitus is ~ ala~,t~ ,d by metabolic
defects in production and utilization of glucose which result in the
30 failure to maintain appropriate blood sugar levels. The result of these
defects is elevated blood glucose or hyperglycemia. Research on the
treatment of diabetes has centered on attempts to nr)rrnRli7e fasting and
pr~ 1 blood glucose levels. Tl~,a~ b have included parenteral
WO 95129159 I ~ ,,.,.'C I~S6
2~ ~7932
- 44 -
administration of exogenous insulin, oral administration of drugs and
dietary therapies.
Two major forms of diabetes mellitus are now recognized.
Type I diabetes, or insulin~ .p~.n(l~nt diabetes, is the result of an
5 absolute deficiency of insulin, the hormone which regulates glucose
lltili7~tic)n. Type II diabetes, or insulin-independent diabetes, often
occurs in the face of normal, or even elevated levels of insulin and
appears to be the result of the inability of tissues to respond
ul~liat~ly to insulin. Most of the Type II diabetics are also obese.
In addition the ~,u~ uulld~ of the present invention lower
triglyceride levels and cholesterol levels and raise high density
i~U~lUlCill levels and are therefore of use in cOIll~à~lillg medical
conditions wherein such lowering (and raising) is thought to be
beneficial. Thus they may be used in the treatment of hyper-
5 triglycP.ri~ mi~ hypercholesterolaemia and conditions of low HDL(high density li~u~u~ill) levels in addition to the treatment of
uscl~lu~ic disease such as of coronary, cerebrovascular and
peripheral arteries, cardiovascular disease and related conditions.
A-,~u~dil~ly, in another aspect the present invention
20 provides a method of lowering triglyceride and/or cholesterol levels
and/or increasing high density li~u~,~u~ill levels which .. " . ,1-, ;c~s
~Amini~t~orjn~, to an animal in need thereof, a ~ ui~ y effective
amount of a compound of the formula (I) or pharrn~e--ti~lly
acceptable salt thereof. In a further aspect the present invention
25 provides a method of treating atherosclerosis which comprises
~minict~rin~, to an animal in need thereof; a th~r~relltirsllly effective
amount of a compound of the formula (I) or phzlrmsl~ellti~ y
acceptable salt thereof. The compositions are formlllAtPd and
:~11mini~t~-.red in the same general manner as detailed below for treating
3 diabetes and obesity. They may also contain other active ingredients
known for use in the treatrnent of atherosclerosis and related conditions,
for example fibrates such as clofibrate, bezafibrate and gemfibrozil;
inhibitors of cholesterol biosynthesis such as HMG-CoA reductase
inhibitors for example lovastatin, ~ lva~la~ and pravastatin; inhibitors
WO 95/291!i9 P~ 'C 19~i6
~ ~ B7~
- 45 -
of cholesterol absorption for example beta-sitosterol and (acyl
CoA:cholesterol a~yl~ldll~r~lase) inhibitors for example mrlin~mi~if;
anion exchange resins for example cholestyramine, colestipol or a
dialkyl~mino~lkyl derivatives of a cross-linKed dextran; nicotinyl
5 alcohol, nicotinic acid or a salt thereof; vitamin E; and thyromimetics.
The compounds of the instant invention also have the effect
of reducing intestinal motility and thus find utility as aiding in the
treatment of various ga~Lluill~e~i"al disorders such as irritable bowel
syndrome. It has been proposed that the motility of non-grhinrt~ric
smooth muscle contraction is mediated by activity at ,~3
adrenu.~cc;~ol~. The availability of a ~3 specific agonist, with little
activity at ~1 and ~2 receptors will assist in the plldlllla~OlOgiC control
of intestinal motility without concurrent cardiovascular effects. The
instant compounds are ~(l,,,i,,i~l~ .~d generally as described below with
5 dosages similar to those used for the treatment of diabetes and obesity.
It has also been found Illlr,~llç~ r11y that the compounds
which act as agonists at J33 ~l~ Olt~ Ul~i may be useful in the
treatment of ~5d~ i"lr,li"~l disorders, especially peptic ulcerations,
~su~ha~i~is, gastritis and duodenitis, (including that induced by ~.
20 ~Ls~, intestinal Illrrr~tionc (including i~ y bowel disease,
ulcerative colitis, Crohn's disease and proctitis) and ~ Lluill~ ldl
Illrrr~tir,ng
In addition, ~3 receptors have been indicated to have an
effect on the inhibition of the release of n~ulu~ ides in certain sensory
25 fibers in the lung. As sensory nerves may play an illlllOl~dll~ role in the
nt;ulu~ ic infl~mm~tion of airways, including cough, the instant
specific ,B3 agonists may be useful in the treatment of neurogenetic
il~ll,.""",.lion, such as asthma, with minimal effects on the cardio-
~UllllUllaly system.
,B3 al.~ o.c;c~ u.~ are also able to produce selective
antid~ d..L effects by stimlll~tin~ the ~3 receptors in the brain and
thus arl additional ~-)"lr."l.l,.lrd utility of the uu---lJou--ds of this
invention are as antid~ s~a.ll agents.
WO 95/29159 P~ . 5'0 1~6
2 1 8 7~32
- 46 -
The active compounds of the present invention may be
orally administered as a rh,-l ",~ l composition, for example, with
an iner~ diluent, or with an assimilable edible carrier, or they may be
enclosed in hard or soft shell capsules, or they may be c,u~ .c~sed into
5 tablets, or they may be incorporated directly with the food of the diet.
For oral Ih~r~lre~ltic ~-lmini~tr~tion, which includes sublingual
administration, these active compounds may be iI~cul~ula~d with
excipients and used in the form of tablets, pills, capsules, ampules,
sachets, elixirs, suspensions, syrups, and the like. Such cornpositions
and preparations should contain at least O. l percent of active compound.
The percentage of active c--mro~ln-1 in these compositions may, of
course, be varied and may conveniently be between about 2 percent to
about 60 percent of the weight of the unit. The amount of active
compound in such ~ lly useful compositions is such that an
5 effective dosage will be obtained. The active Co~ oUIldS can also be
lmini~tl~.red intranasally as, for exarnple, liquid drops or spray.
The effective dosage of active ingredient employed may
vary r1~pPn~lin~ on the particular compound employed, the mode of
administration, the condition being treated and the severity of the
20 condition being treated.
When treating diabetes mellitus and/or hyL~ ly~lllià
generally soti~f~tory results are obtained when the culll~oulld~i of the
present invention are ~-l",i"i~ d at a daily dosage of from about 0.1
milligram to about 100 milligram per kilogram of animal body weight,
25 preferably given in divided doses two to six times a day, or in sustained
release form For most large m~mm~lg the total daily dosage is from
about 10 milligrams to about 1000 milligrams, preferably from about l
milligr;lmc to about 50 milli~r~mc In the case of a 70 kg adult human,
the total daily dose will generally be from about 7 milligrams to about
30 350 milligrams This dosage regimen may be adjusted to provide the
optimal thlqr~relltic response
When treating obesity, in conjunction with diabetes and/or
hyperglycemia, or alone, general!y ~ticf~t~ry results are obtained
when the compounds of the present invention are ~lmini~t~red at a daily
951~9159 P~ u~ Jl~
21 87q32
- 47 -
dosage of from 1 milligram to about lO00 milli~rAmc per kilogram of
animal body weight, preferably given in divided doses two to six times a
. day, or in sustained release form. For most large mAmmAls, the total
daily dosage is from about lO milligrams to about lO,000 milligrams,
5 preferably from about lO milligrams to about 500 milligrams. In the
case of a 70 kg adult human, the total daily dose will generally be from
about 70 milligrams to about 3500 milligrams. This dosage regimen
may be adjusted to provide the optimal therapeutic response.
The tablets, pills, capsules, and the like may also contain a
binder such as gum ~ a~ , acacia, corn starch or gelatin; excipients
such as ~lirAlrillm rhocrhAt~; a disiu.L~ i--g agent such as corn starch,
potato starch, alginic acid; a lubricant such as mA~n~ m stearate; and a
il.g agent such as sucrose, lactose or sArrh~rjn When a dosage
unit form is a capsule, it may contain, in addition to materials of the
5 above type, a liquid carrier such as a fatty oil.
Various other materials may be present as coatings or to
modify the physical form of the dosage unit. For instance, tablets may
be coated with shellac, sugar or both. A syrup or elixir may contain, in
addition to the active ingredient, sucrose as a b~vc;t;L~Ilillg agent, methyl
20 and ~ yllJalabens as ~ ,.v~livc;s, a dye and a flavoring such as
cherry or orange flavor.
These active compounds may also be ~l",i";~ d
parenterally. Solutions or s--~pPnci~m~ of these active ~ ou~d~ can ~e
prepared in water suitably mixed with a $1lrfAr~An~ such as hydroxy-
propylcellulose. Di~rrrsion~ can also be prepared in glycerol, liquid
polyethylene glycols and mixtures thereof in oils. Under ordinary
con~1ifi~-n~ of storage ar~a use, these preparations contain a plt;s~-v~liv~
to prevent the growth of microorganisms.
The rl~ ",;~ ir~l forms suitable for injectable use
30 include sterile aqueous solutions or dib~ i.,lls and sterile powders for
the ~t~ ulcul~)us preparation of sterile injectable s~lutions or
dispersions. In all cases, the form must be sterile anu must be fluid to
the extent that easy syringability exists. It must be stable under the
conditions of mAnllf~rtllre and storage and must be preserved against the
WO 951291~9 r~ ''O ;~iG
21 87932
- 48 -
cont~min~tin~ action of microorganisms such as bacteria and fungi.
The carrier can be a solvent or dispersion medium containing, for
example, water, ethanol, polyol (e.g. glycerol, propylene glycol and
Iiquid polyethylene glycol), suitable mixtures thereof, and vegetable
5 oils.
The following examples are provided so that the invention
might be more fully understood. They should not be construed as
limiting the invention in any way.
F~xAMpLE 1
OH
N~ N~13`NH
N=N 2
[2-[4-(aminophenyl)]ethyl]-2-hydroxy-2-(tetrazolo[ 1 ,5-a]pyrid-6-
yl)ethylamine
A solution of 1.62 g (10 mmol) of (R)-2-(tetrazolo[l,S-
a]pyrid-6-yl)oxirane (See Fisher and Wyvratt, European Patent
Application 0 318 092 A2 for the synthesis of this compound.) and 4.1 g
(30 mmol) of 2-(4-arninophenyl)ethylamine in 30 rnL of methanol was
heated at reflux for Sh. The reaction rnixture was cullc~ t~d and the
25 residue chromatographed on silica gel (2% methanol/98% methylene
chloride) to give 1.69 g (56%) of the title compound:lH NMR (400
MHz, CD30D) o 9.01 (d, lH, J =1.3 Hz), 8.02 (d,l H, J = 9.2 Hz), 7.82
(dd, lH, J = 1.3, 9.2 Hz), 6.94 (d, 2H, J = 6.3 Hz), 6.63 (d, 2H, J = 6.3
30 Hz), 4-91 (m, IH), 2.82 (m, 4H), 2.67 ~t,: H, J = 7.1Hz).
S'C4956
~V0951~9159 2 ~ ~ 79 32
- 49 -
EXAMPT T~ 2
.
OH
Boc
N ,N NH2
\N=N
ao-~-[2-[4-(a~ llyl)]ethyl]-2-hydroxy-2-(tetrazolo[l,5-a]pyrid-6-
yl)ethvl~rl?~mic acid 1.~--limerllylethvl ester
c . A solution of 1.69 g (56.7 mmol) of the amine from
~xample 1 and 1.23 g (56.7 mmol) of di-tert-butyl dicarbonate in 10
mL of t~tldl~ lluruldll (THF) at 0 C was stirred for 2 h. The reaction
mixture was ~,u~ t~dl~d and the residue chromatographed on silica gel
(4% methanoV96% methylene chloride) to afford 2.2 g (97%) of the
title compound: 1H NMR (400 MHz, CD30D) Q 8.96 (s, lH), 8.05 (m,
2H),7.85(m,2H),6.93(dd,2H,J=7.7,8.3Hz),6.66(d,2H,J=8.3
Hz), 4.99 (m, lH), 3.49 (m, 4H), 2.70 (t, 2H~ J =6.5 Hz), 1.26 (s, 9H).
ExAMpT F 3
nHex-NH H~
2s
4-(Hexyl~minQcarbonyl~mino)~.f..7~ -f-~ fonyl chloride
Hexylamine, 12.15 ml (9.2 mmol), was added dropwise to a
solution of 10 ml (9.2 mmol) of phenyl isocyanate iD T~TF (150 ml) at
0C, and stirring was continued for 1 h. The solvent was removed in
30 vacuo, and the resultant hexyl phenyl urea was used without further
purification.
A 6-g (2.7 mmol) portion was added over 20 min to
chlorosulfonic acid at 0C, followed by heating at 60C for 2h. After
cooling, the mixture was added to ice/water (lOOml) and the aqueous
WO 95/29159 1 ~ .,'0 :~SG
- 2~87~32
- 50 -
phase extracted with EtOAc (3x100 ml). The combined organic phase
was washed with brine (50 ml), dried with MgSO4, concentrated, and
purified by flash chromatography (silica gel, 75% hexane/ 25% ethyl
acetate) to give 6 g (70%) of the title compound: lH NMR (CDC13) o
7.85 (d, 2H, J = 9.6 Hz), 7.54 (d, 2H, J = 9.6 Hz), 6.79 (br.s, IH),
4.71(br. s, lH), 3.23 (t, 2H, J = 8 Hz), 1.54-1.44 (m, 2H), 1.33-1.20 (m,
6H), 0.91-0.79 (m, 3H).
EXAMPLE 4
OH Boc NH
N=N ~so2
nHex-NH NH~J
20 (O-N-[4-[2-[N-(l~l-dimethyleth~l~y~lb~llyl)-N-[2-hydroxy-2-(tetra
zolo[l,5-a]pyrid-6-yl)]ethyl]amino]ethyl]phenyl]-4-(l~ y~ ;"n~
vlamino)benzenesulfonamide
To a stirred solution of 0.200 g (0.502 rnmol) of the Boc-
compound from Example 2 in 3 mL of methylene chloride was adde~
25 80 mg (1.00 mmol) of pyridine followed by 0.16 g (0.75 rnmol) of the
sulfonyl chloride from Example 3. After being stirred for 5h, the
reaction mixture was concentrated and the residue chrom~to~phl~d on
silica gel (10% methanol/90% methylene chloride) to afford 0.303 g
(88%) of the title compound: lH NMR (400 Hz, CD30D) o 8.95 (s,
30 lH), 8.0-8.08 (m, lH), 7.75-7.87 (m, lH), 7.40-7.62 (m, 4H), 7.00 (m,
4H), 4.95 (m, 2H), 3.47 (m, 2H), 3.15 (m, 2H), 2.75 (m, 2H), 1.52 (t,
2H, J = 6.0 Hz), 1.33 (m, 8H), 1.21 (s, 9H), 0.90 (t, 3H, J = 60 Hz).
\
~0 95129159 2 ~ 8 7 9 3 2 ~ 3'~ 5~5
- 51 -
. FX~PT F S
~ OH H
H2N~ ,N 3`NH
J~ ~
o nHex NH H
(O-N-l4-[2-[[2-hydroxy-2-(6-~lllilloL~ylidin-3-yl)ethyl]amino]ethyl]
phenyll-4-(hexyl~min~c~rbonylamino)~.~,.,7r.,e.~.11fonamide
A mixture of 0.302 g (0.44 mmol) of the tetrazine from
Example 4, 0.20 g (0.88mol) of tin(II) chloride dihydrate and 0.3 ml of
c~,llL~ ed aqueous hydrochloric acid in 2 mL of methanol was heated
at reffux for S h. The reaction mixture was col-c~ d and the
residue purified by reverse-phase MPLC (C8, 47%methanol/53 0.1%
trifluoroacetic acid buffer) to give 0.32 g (78%) of the title compound
20 as its bistriflu~,loact;~c salt: lH NMR (400 MHz, CD30D) o 7.96 (dd,
lH, J = 2.0, 9.2 Hz), 7.86 (d, lH, J = 2.0 Hz), 7.59 (d, 2H, J = 8.8 Hz),
7.43 (d, 2H, J = 8.8 Hz), 7.14 (d, 2H, J = 8.4 Hz), 7.07 (d, 2H, J = 8.4
Hz), 7.03 (d, lH, J = 9.2 Hz), 4.92 (m, lH), 3.23 (m, 2H), 3.15 (m, 2H),
2.93 (m, 2H, 4.0 Hz), 1.49 (t, 2H, J = 6.0Hz), 1.32 (m, 8H), 0.91 (t, 3H,
25 J = 6-0 Hz); CI MS m/z 555(M+l).
Following the procedures outlined for Fx~mrl.os l-S, the
compounds listed in Table 1 were prepared.
WO 95/29159 . ~ 4~56
- 52 -
T.ART,F, 1
H2N ~ tl H O
Example R Selected lH NMR (CD30D) Data
o 6 Ph, trifluoroacetate salt 7.74 (m,2H), 7.53 (m, lH), 7.45
(m, 2H).
7 2-naphthyl, 7.93 (m, 4H), 7.75 (d, IH, J = 1.7
trifluoroacetate salt Hz), 7.61 (m, 2H)
8 3-quinolinyl, 9.00 (d, lH, J = 2.3 Hz), 8.06 (m,
trifluol~,act;~ salt 2H), 7.94 (m, 2H), 7.72 (t, lH, J =
7.2 Hz)
9 1,2-b.qn7i~o~ 7c)l-5-yl, 9.02 (s, lH), 8.30 (d, lH, J = 1.3
trifluoroacetate salt Hz), 7.90 (m, lH), 7.77 (m, lH)
4-iodophenyl, 7.83 (d, 2H, J = 8.6 Hz), 7.46 (d,
Llinuoloac~iL ~ salt 2H, J = 8.6 Hz)
11 4-[(N-hexyl,N-methyl- 7.62 (d, 2H, J = 4.6 Hz), 7.48 (d,
aminocarbonyl)amino]- 2H, J = 4.6Hz), 2.99 (s, 3H)
phenyl, trifluc,l~,ace
salt
25 12 4-[(N,N-dimethyl- 3.0 (s, 6H)
arninocarbonyl)amino] -
phenyl, Llinuol~,acetate
salt
13 4-(3-hexyl-2- 3.88-3.83 (m, 2H), 3.57-3.50 (m,
imidazolidinon-1- 2H), 2.89-2.95 (m, 2H), 1.61-1.52
yl)phenyl, (m, 2H), 1.37-1.30 (m, 6H), and
trifluoroacetate salt 0.93-0.88 (m,3H)
95129159 r_l~u,,. _/0 :956
~ ~793~
- 53 -
. E~;AMPLE 14
~J~ CI
N HCI
3-(2-Chloroacetyl)pyridine hydrochloride
To a solution of 12 g (11 mL, 100 mmol) of 3-
a~lyl~ylidil.c in 100 mL of ethyl ether was added 100 mL of 1 M
ethereal hydrogen chloride. The resultant ~Iu.,i~ lc was filtered and
15.0 g (95.2 mmol) was collected and placed in a 500-mL round bottom
flask equipped with a magnetic stir bar. To this was added 95 mL of 1
M hydrogen chloride in acetic acid. After the mixture was stirred until
5 all the solid had dissolved, 12.7 g (95.2 mmol) of N-chlol-,~uc~;illilllide
(NCS) was added in one portion. The solution turned yellow and the
NCS gradually di~solved. After 4 h, a white ~ iL~l~ had formed.
The mixture was allowed to stir for 2.5 days. It was then filtered. The
solid collected was washed with 10 mL of acetic acid and 200 mL of
20 ethyl ether to give 15.2 g (83%) of the title compound as a white solid:
IH NMR (200 MHz, d6-DMSO) o 9.22 (t, lH, J = 1 Hz), 8.29 (dd, IH, J
= 1.6, 5.1 Hz), 8.55 (td, lH, J = 2, 8.1 Hz), 7.82 (ddd, lH, J = 0.8, 5.1,
8.1 Hz), 5.27 (s, 2H).
EXAMPLE 15
OH
~
(R)-a-Chloromethyl - 3-pyridinemethanol
To a stirred solution of 3.67 g (11.5 mmol) of
(-)-B-chlorodiisopin-~r~mrh~ylborane [(-)-DIP-CI] in 11 mL of THF at
-25 C was added a slurry of 1.00 g (5.21 mmol) of the product from
.
WO95/29159 ~` 11IJ~--'~j-'(i
2 1 8 7q32
- 54 -
Example 14 in S mL of THF via a cannula. Following the addition of
0.80 mL (5.79 mmol) of triethylamine, the reaction mixture was stirred
at -25 C for 4 days. To the mixture was added 10 mL of water which
was then allowed to warm to room ~ ;ld~UI~. To the mixture was
5 added 20 mL of ethyl acetate and the organic phase separated. The
aqueous phase was neutralized with saturated NaHCO3 solution then
extracted six times with ethyl acetate. The combined organic phase was
~,u", ~.,l...~. d in vacuo to afford a yellow oil. Flash chromatography
(silica gel, 75 - 100% ethyl acetate-hexanes) afforded 561 mg (68~o) of
the title compound as a pale yellow oil: IH NMR (400 MHz, CD30D) o
8.58 (d, IH, J = 1.8 Hz), 8.46 (dd, lH, J - 4.9, l.S Hz), 7.90 (d, lH, J =
7.9 Hz), 7.44 (dd, lH, J = 7.9, 4.9 Hz), 4.93 (m, lH), 3.75 (m, 2H).
FXAMPT F 16
[~
(R)-(Pyrid-3-yl)oxirane
To a solution of 557 mg (3.55 mmol) of the product from
Example lS in 16 mL of acetone was added 1.80 g of ~ iul--
carbononate. The mixture was heated at reflux for 20 h then cooled to
25 room L~lllp~;ldlul~. The mixture was filtered and the filtrate evaporated
in vacuo. Flash chromatography (silica gel, 2% methanol-methylene
chloride) afforded 262 mg (61~o) of the title compound as a pale yellow
oil: lH NMR ~200 MHz, CDC13) o 8.54 (m, 2H), 7.52 (m, lH), 7.24
(m, lH), 3.86 (dd, lH, J = 4.0, 2.5 Hz), 3.17 (dd, lH, J = 5.4, 4.0 Hz),
30 2-80 (dd, lH, J = 5.4, 2.5 Hz).
95129159 ~ u.,,~ 'C 1~5C.
2 1 87932
- 55 -
EXAMPLE 17
OH
~ ,H NH2
(R)-N-r2-r4-(Aminophenvl)lethyll-2-hydroxy-2-(pyrid-3-yl)ethylamine
To a stirred solution of 377 mg (2.44 mmol) of
o 4-aminophenethylamine in 10 mL of methanol was added a solution of
300 mg (2.48 mmol) of the product from Example 16 in 15 mL of
methanol. The mixture was heated at reflux for 16 h then cooled to
room ~III~IdlUlC;. The methanol was removed in vacuo and the residue
chromatographed (silica gel, 6 - 8% m.-th~n-)l, 1% ammonia-methylene
5 chloride) to afford 101 mg (16%) of the title compound together with
279 mg of a mixture that was l~ lu~ld~ ed (5% methanol, 1%
ammonia-methylene chloride) to give a further 54 mg (9%) of the title
~_ullll~uulld as an off-white solid: lH NMR (500 MHz, CD30D) o 8.52
(d, lH, J = 1.8 Hz), 8.43 (dd, lH, J = 4.8, 1.4 Hz), 7.81 (m, lH), 7.40
20 (m,lH),6.95(d,2H,J=8.3Hz),6.67(d,2H,J=8.3Hz),4.81(m,
lH~, 2.90-2.65 (m, 6H).
FXAMPT.F 18
~I` BNC NH2
ao-N-[2-[4-(aminophenyl)]ethyl]-2-hydroxy-2-(pyrid
yl)ethylcarbamic acid l.l-dimethylethyl ester
A solution of 386 mg (1.77 mmol) of di-tert-butyl
dicarbonate in 3.5 mL of THF was added, via a cannula, to a stirred
slurry of 456 mg (1.77 mmol) of the product from Example 17 in 3.6
WO 95/29159 P~ '0 :9~G
2~ 87932
- 56 -
mL of THF cooled to 0 C. The yellow solution was stirred at 0 C for
3 h, then the THF was removed in vacuo. Flash chromatography (silica
gel, 10% ml-th~nol, 1% ammonia-methylene chloride) afforded 549 mg ~.
(87%) of the title compound as an off white solid: lH NMR (500 MHz,
CD30D, mixture of rotomers) o 8.45 (m, 2H), 7.83 (d, 0.6H, J = 7.4
Hz), 7.78 (d, 0.4H, J = 6.9 Hz), 7.41 (m, lH), 6.94 (d, 0.8H, J = 8.0
Hz), 6.89 (d, 1.2H, J = 7.8 Hz), 6.66 (d, 2H, J = 7.3 Hz), 4.89 (m, lH),
3.42-3.21 (m, 4H), 2.67 (m, 2H), 1.39 (s, 5.4H), 1.36 (s, 3.6H).
o An alternative synthesis of the aniline derivative in Example 18 is
illustrated in F.~ nnpl~ 19-23:
FX~ MPLE 19
O
CI~JI "Br
20 2-Chloro-5-(2-bromoacetyl)pyridine hydrochloride
A solution of 784 mg of 2-chloro-5-a~yl~,y~ .e in 10
mL of THF was added via canula to a solution of 1.44 g of
dibl.~lllo~ lic acid (DBBA) in 10 mL of THF. The resultan~
solution was heated at 50-55 C for 12 h, and then an additional 0.72 g
25 DBBA was added. After stirring at 50-S5 C for 2.5 more hours, 0.36
g DBBA was added. The mixture was allowed to stir for 2 h at which
point NMR analysis of an aliquot indicated 87% conversion. The
reaction mixture was cooled, diluted with ethyl acetate, washed with two
portions of saturated aqueous sodium bicarbonate, water, and brine,
30 dried over magnesium sulfate and concentrated. Purification by flash
chromatography (silica gel, 15% ethyl acetate~exane) provided 0.86 g
(73%) of the title compound as a white solid: lH NMR (400 MHz,
CDCI3) o 8.96 (d, lH, J = 2.6 Hz), 8.21 (dd, lH, J = 2.5, 8.3 Hz), 7.46
(d, lH, J = 8.4 Hz), 4.37 (s, 2H). The NMR also indicated the presence
~095129159 21 87q32 .~ o U36
- 57 -
.. of the cu~ ul~dillg 2-bromo derivative. The ~4:1 mixture was
carried on through the synthesis.
EXAMPLE 20
OH
Cl~ Br
(R)-cc-Bromomethyl-3-(6-chloropyridine)methanol
To a solution of 602 mg (1.88 mmol) of (-)-DIP-CI in 0.5
mL of THF at -25 C was added via canula 200 mg of ketone from
Example 19 in 1.5 mL of THF at -25 C. The reaction mixture was
5 allowed to stir at -25 C for 17 h. It was then quenched by the addition
of water and extracted with ether. The ether phase was diluted with
ethyl acetate, washed with two portions of saturated aqueous sodium
bicarbonate, water, and brine, dried over ", g~ sulfate and
cull~t;ll~la~d. Purification by flash chromatography (silica gel, 15 and
20 25% ethyl acetate/hexane) gave 170 mg (84%) of the title cu---~-uu--d.
H NMR (400 MHz, CDC13) ~ 8.38 (d, lH), 7.70 (dd, lH), 7.32 (d,
lH), 4.97 (m, IH), 3.61 (dd, lH), 3.50 (dd, lH), 2.85 (d, lH).
EXAMPLE 21
o
CI
(R)-(2-chloropvrid-5-yl)oxir~ne
- To a solution of 100 mg of bromoS~lr~-h- l from Example 20
in 2 mL of 1:1 THF:water was added 1 mL of 5 N aqueous sodium
hydroxide solution. The mixture was allowed to stir for 10 min. It was
then extract~d with three portions of dichlornmPth~n- The combined
wo 95/29159 . ~ C ~6
~7 8~3~ --
- 58 -
organic phases were washed with two portions of water and brine, dried .
over m~n~ m sulfate, and concentrated to give 98 mg (93%) of the
title compound which was used without further purification: IH NMR
(400 MHz, CDC13) o 8.34 (d, IH), 7.48 (dd, lH), 7.29 (d, lH), 3.86
(dd, IH), 3.18 (dd, lH), 2.78 (dd, lH).
FXAMPLE 22
Boc
Cl J~ , N--3~ N2
-[2-[4-(Nitrophenyl)]ethyl]-2-hydroxy-2-(2-chloropyrid-5-
5 yl)ethylcarbamic acid l.l-dimethylethyl ester
Following the procedure outlined in F~slmrlf c 17 and 18,
the title compound was prepared from the epoxide from Example 21
and 4-lliL~u~l~c;~ylethylamine: IH NMR (400 MHz, CDC13) o 8.32 (d,
lH, J = 1.3 Hz), 8.13 (d, 2H, J - 8.6 Hz), 7.66 (br m, lH), 7.30 (d, 2H,
20 J = 8.1 Hz), 7.27 (br m, lH), 4.94 (br m), 3.38 (br m, 4H), 2.84 (br m,
2H), 1.40 (s, 9H).
FXAMP! F 23
~1 ,N ¢~`NH2
3 ~-~-[2-[4-(aminophenyl)]ethyl]-2-hydroxy-2-(pyrid-3
yl)ethylcarbamic acid l.l-dimethylethyl ester
To a solution of 80 mg (0.19 mmol) of the nitro compound
from Example 22 in 2 mL of ethanol was added 0.114 mL (0.57 mmol)
of S N aqueous sodium hydroxide solution and 20 mg of rarley nickel.
0 95/29159 1 ~, I /IJ.. _ .'0 1,~
2~ ~7q32
59
~- The reaction mixture was shaken at room ~ laLul~ under 45 psi
hydrogen for 16 h. The mixture was neutralized with saturated aqueous
sodium phosphate monob~ir and extracted with three portions of ethyl
acetate. The combined organic phases were washed with water and
5 brine, dried (m:l~n-~gillm sulfate), and concentrated to give 40 mg (59%)
of the title compound which was identical to the sample prepared in
Example 18.
EXAMPLE 24
o ~SO2CI
nHex~NJ~NJ~J
4-(3-Hexyl-2-imidazolon-1-yl)phenylsulphonvl chloride
Hexyl iodide (SOmmol, 7.38ml) was added to a mixture of
2-amino acetaldehyde dimethyl acetal (lOOmmol, llml) and p~,l,.~;.....
carbonate (SOmmol, 6.9g) in DMF (lOml) at 0C. Stirring was
20 contin~ d for 16h before diluting with ethyl acetate (200ml), and
filtering the solution through a plug of celite. C.~.l.~ ..~I.;.I;~n in vacuo
was follwed by column chromatography (eluant ethyl acetate) to give N-
hexyl 2-amino acetaldehyde dimethyl acetal (7.39g, 78%) as a colourless
oil.
To the amine (38.6mmol, 7.3g) in methylene chloride
(lOOml) at 0C was added 4-(chlorosulphonyl) phenyl isocyanate
(38.6mmol, 8.4g). The reaction mixture was stirred for 20mins until a
clear solution had formed, and 1:1 water: trifluoroacetic acid (lOOml
total) was added. Vigorous stirring was continued for 16h., the layers
30 separated, the organic layer was diluted with ethyl acetate (SOOml) and
washed with saturated sodium bi~,allJull~l~ solution (4xSOml), brine
(SOml), dried with anhydrous mll~n.ocillm sulphate, and concentrated in
vacuo. Column chromatrography (eluant 3 hexane/ I ethyl acetate)
yielded the title compound as pale yellow crystals (8.8g, 67%).
WO9S/29159 r~~ o ~SG
2~87932
- 60 -
EX~MP! F 25
OH - .
~ "N NH
nHex-NH H)3,so2
(O-N-[4-[2-[[2-Hydroxy-2-(pyridin-3-yl)ethyl]amino]ethyl]phenyl]-4-
(hexylaminocarbonyl ~mino)benzenesulfonamide
To a solution of 302 mg (0.845 mmol) of the product from
Example 18 and 137 mL (1.69 mmol) of pyridine in 10 mL of
methylene chloride was added 296 mg (0.928 mmol) of
4-(hexylaminoca~bonylamino)l,r .~.~"- ,.~lfonyl chloride from Example
3. The reaction was stirred for 12 h then the solvent removed in vacuo.
Flash chromatography (silica gel, 6% m~th~ l, 0.5% ammonia-
20 methylene chloride) afforded 468 mg (87%) of the BOC-protected title
co...~ ,ou..d.
A solution of 468 rng (0.731 mmol) of BOC-protected title
compound in 5 mL of methylene chloride and 5 mL of trifluoroacetic
acid was stirred for 30 min then the volatile c~".,~ lr~ removed in
2~ vacuo. The residue was a~oL~ ed twice with 10% methanol/toluene,
twice with mP.th:ln(ll, then dried in vacuo to give 521 mg (93%) of the
title compound as its L.inu.~lac"t~e salt: IH NMR (400 MHz, CD30D)
8.8g (s, IH), 8.79 (d, lH, J = 5.5 Hz), 8.53 (d, lH, J = 8.2 Hz), 7.99
(m, lH), 7.59 (dd, 2H, J = 6.9, 1.9 Hz), 7.43 (dd, 2H, J = 6.9, 1.9 Hz),
30 7.15 (dd, 2H, J = 8.6, 2.1 Hz ~, 7.08 (dd, 2H, J = 8.6, 2.1 Hz), 5.23 (m,
lH), 3.40-3.10 (m, 6H), 2.94 (m, 2H), 1.49 (m, 2H), 1.32 (m, 6H), 0.90
(m, 2H).
~o 95/29159 , ~"I,.,,r~c :9s6
:~ 8~932
- 61 -
EXAMPLE 26
- CbzNH 13~
NH
NC J3'
10 ~ ~4 [2 ~(pl~ ly~ llo;~y~ llyl)amino]ethyl]phenyl]-4
cyanoben7f~n~ulfonamide
Following the procedure outlined in Example 4, the title
compound was prepared from 2-(4-dlllillu~llc;llyl)ethylcarbamic acid
phenylmethyl ester (see Fisher, et. al., Eur. Pat. Appl. 0 611 003 Al,
15 1994) and 4-cy~n- b~ , -lfonyl chloride: IH NMR (400 MHz,
CD30D) o lH ~MR (400 MHz, CDC13) o 7.81 (d, 2H, J=8.7Hz), 7.69
(d, 2H, J=8.7Hz), 7.32 (m, SH), 7.06 (d, 2H, J=8.4Hz), 6.96 (d, 2H,
J=8.4Hz), 6.75 (s, lH), 5.06 (s, 2H), 4.71 (t, br, lH), 3.38 (q, 2H,
J=6.9Hz), 2.74 (t, 2H, J=7.0Hz).
EXAMPT F. 27
CbZNH 3~
NH
~SO2
H2N ~l~
' 30 HO
(0-[4-[2-[(pll~,llyllll~ u~y.~ u''yl)amino]ethyl]phenyl]-4-
~minoo,lrimidomethyl)benzensulfonamide
A mixture of the nitrile from Example 26 (2.71g,
6.23mmol), absolute ethanol (65ml), finely divided K2co3 (5.17g,
WO g5/29159 P~ .,G
2~7932
- 62 -
37.4mmol), and hydroxylamine hydrochloride (2.17g, 31.2mmol) was
refluxed for 6 h. The ethanol was removed under reduced pressure.
The resulting solid was dissolved in ethyl acetate and washed with water
3 times. The organic phase was concentrated in vacuo to 2.87g (98%) of
5 the title ~u~ uulld as a white powder which was of sufficient purity to
be used in subsequent steps: 1H NMR (400 MHz, CD30D) o 7.71 (s,
4H), 7.31 (m, 5H), 7.04 (d, 2H, J=8.4Hz), 6.99 (d, 2H, J=8.4Hz), 5.02
(s, 2H), 3.25 (t, 2H, J=6.8Hz), 2.67 (t, 2H, J=6.7Hz).
o FXAMPLE 28
CbzNH 3~
,NH
~SO2
N /~)
/ 11
, / o~N
(rD-[4-[2-[(L~ lyllll~ ycarbonyl)amino]ethyl]phenyl]-4-[s-(3
L~y~lv~ lylyl ~,~yl)-rl .2.41-oxadiazol-3-ylll,. .,~ for~mi(l~o
To a solution of ~-)rnro--n~1 from Example 27 (0.468g,
l.OOmmol) in dry pyridine (5.0ml) was added 4-cyclopentylbutyryl
chloride (0.175g, l.OOmmol). The mixture was refluxed for 3.5 h. The
pyridine was removed under reduced pressure. The resulting residue
was purified by silica gel chromatography (35% ethyl acetate in
hexanes~ to give 0.152g (26%) of the title compound: 1H NMR (400
MHz, CDCL3) o 8.12 (d, 2H, J=8.7Hz), 7.81 (d, 2H, J=8.7Hz), 7.31 (m,
SH), 7.03 (d, 2H, J=8.1Hz), 6.97 (d, 2H, J-8.4Hz), 6.67 (s, lH), 5.05 (s,
2H), 4.70 (t, br, lH), 3.37 (q, 2H, J=6.5Hz), 2.91 (t, 2H, J=7.6Hz), 2.72
(t, 2H, J=7.0), 1.90-1.70 (m, 5H), 1.65-1.30 (m, 6H), 1.06 (m, 2H).
-
~0 95/79159 2 1 8 7 q 3 2 r~ 9~6
- 63 -
- l~XAMPLE 29
'- H2N 3~
NH
~SO2
N~
~</o~ N
N-[4-(2-aminoethyl)phenyl]-4-[5-(3-cycl~ lLyl~ l)-[1,2,4]-
5 oxadiazol-3-yllbenzensulfonamide
A mixture of Cbz amine from Example 28 (0.145g,
0.246mrnol), rq~ m hydroxide on carbon (0.02g), and glacial acetic
acid (5.0ml) was hydrogenated for 2 h. The acetic acid was removed
under reduced pressure. The residue was purified by silica gel
20 chromatography (1: 9 of 10% :~mm--ni--Tn hydroxide in methanol:
methylene chloride) to giYe 0.058g (52%) of the title l~ )UUlld. lH
NMR (400 MHz, CD30D) o 8.11 (d, 2H, J=8.6Hz), 7.87 (d, 2H,
J=8.5Hz), 7.06 (d, 2H, J=8.6Hz), 7.02 (d, 2H, J=8.7Hz), 2.97 (t, 2H,
J=7.5Hz)~ 2.84 (t, 2H, J=6.9Hz), 2.67 (t, 2H, J=7.5Hz), 1.90-1.75 (m,
25 5H), 1.70-1.40 (m, 6H), 1.12 (m, 2H).
WO 95/29159 r~ o l~SG
~ ~7~
- 64 -
FXAMPLE 30
-
OH H NH
~q~S02
N
~/ 11
15 a~)-N-[4-[2-[[2-Hydroxy-2-(pyridin-3-yl)ethyl]amino]ethyl]phenyl]~
[5-(3-cyclu~ yl~ ",yl)-rl.2.41-oxadiazol-3-ylll,...,~r..~.llfonamide
To a solution of arnine from Example 29 (0.053g,
0.117mmol) in dry methanol (30.0ml) was added 3-pyridine epoxide
from Example 16 (0.021g, 0.175rnmol). The resulting solution was
20 refluxed overnight. After c~ l;cn, the residue was purified by
silica gel ~ )".~ Y (13% methanol in methylene chloride) to
give O.Olg (15%) of ~e title CWII~OUU~. lH NMR (400 MHz, CD30D)
o 8.52 (d, lH, J=1.9Hz), 8.42 (dd, lH, J=1.5, 4.8Hz), 8.13 (d, 2H,
J=8.6Hz), 7.85 (m, 3H), 7.40 (dd, lH, J=4.8, 7.8Hz), 7.10 (d, lH,
25 J=8.6Hz), 7.03 (d, 2H, J=8.6Hz), 4.81 (dd, lH, J=4.9, 8.1Hz), 2.96 (t,
2H, J=7.5Hz), 2.93-2.70 (m, 6H), 1.90-1.72 (m, 5H), 1.68-2.48 (m,
4H), 1.42 (m, 2H), 1.11 (m, 2H).
0 95129159 ~ .'0 19~6
21 87932
- 65 -
FXAMPLE 31
OH H NH
~SO2
HO\ N_
--/~N
R = nHex
R=H Me
(O-_-[4-[2-[[2-Hydroxy-2-(pyridin-3-yl)ethyl]amino]ethyl]phenyl]-4-
[4-(1 -hydroxy- 1 -hexylheptyl)-5-methyl-[ 1 ,2,3]-triazol-2-
yl]br-I~.rl~r~l~1 ru~ lide and (O-N-[4-[2-[[2-Hydroxy-2-(pyridin-3-
yl)ethyl]amino]ethyl]phenyl]-4-[4-(1 -(R.S)-hydroxyheptyl)-5-methyl-
rl.2~31-triazol-2-vllbcl,7~.,. .~lllfonamide
To a solution of 180 mg of (O-~-[4-[2-[[2-Hydroxy-2-
(pyridin-3-yl)ethyl]amino]ethyl]phenyl]-4-(4-metho~yc~bvllyl-5-
methyl-[1,2,3]-triazol-2-yl)l,~ ,,r~ lfonamide (prepared ac-,vl-lillg to
the procedures outlined in examples 14-19) in 2 mL of distilled THF
under argon at OoC was added, dropwise, 2 mL of a 2.0M solution of
n-hexylm~gn--cillrn bromide in ether. After S min, the reaction was
quenched with cautious addition of 5 mL of aqueous ;1l,,,,,..,,;-,...
chloride followed by ethyl acetate extraction of the aqueous layer. The
combined organic extracts were dried over sodium sulfate, filtered, and
~;vll.;~ l.,t~,d in vacuo to yield the crude products. ~ ~dliv~ layer
chromatography (PLC) on 2X0.5 mm thick silica gel plates eluted in 9:1
(v/v) dichlol~",..~ ,- Illr~ l gave two bands A (20 mg) and B
(60mg). IH NMR (500 MHz, CD30D) of A: o 8.51 (d, lH, J=2 Hz),
8.41 (dd, lH, J=1.5, 5 Hz), 8.01 (dd, 2H, J=2.5, 6.5Hz), 7.81 (m, lH),
7.78 (dd, 2H, J=2.0, 9.0 Hz), 7.37 (m, lH), 7.07;7.02 (ABq, 4H, Jab=
8.5 Hz), 4.86 (s, CD30H), 4.79 (dd, lH, J= 7.5, 8 Hz), 2.9-2.7 (m, 6H),
2.44 (s, 3H), 1.85 (m, 4H), 1.40-1.15 (m, 16H), 0.83 (t, 6H, J=7 Hz)
WOgS/29159 2 ~ 8 7932 1~ 56
- 66 -
in~lic~ting the dihexyl tertiary alcohol adduct, mass spec. expected 677
found 677. IH NMR (500 MHz, CD3~D) of B: 8.51 (d, lH, J=2 Hz),
8.41 (dd, lH, J=1.5, 5 Hz), 8.03 (d, 2H, J=9 Hz), 7.78 (d, 2H, J=9 Hz),
7.37 (dd, lH, J= 4.8, 7.7 Hz), 7.07;7.02 (ABq, 4H, Jab=8 Hz), 4.86 ~s,
CD30H), 4.80 (m, 2H), 2.9-2.7 (m, 6H), 2.38 (s, 3H), 1.87 (m, 2H),
1.44 (m, lH), 1.4-1.2 (m, 7H), 0.87 (t, 3H, J=7 Hz) in~ir~ting the
mono-hexyl adduct. Mass spec expected 591 (for the hexyl ketone)
found 593 (hexyl alcohol, int~rmf~ t~ ketone reduced by Grignard
reagent in situ).
Following the procedures outlined for F.x:lmpll~c 14-31, the
compounds listed in Table 2 were prepared.
T~R! F 2
OH H
¢~, N `13` H ' lo~ R
Example R Selected 1H NMR (CD30D) Data
32 4-isc,~I~J~yllJhenyl 7.64 (d, 2H, J = 8.0 Hz), 7.33 (d,
2H, J = 8.0 Hz), 4.80 (m, lH), 2.95-
2.70 (m, 7H), 1.22 (d, 6H, J = 6.7
Hz)
33 4-iodopheny1 7.84 (d, 2H, J = 8.6 Hz), 7.47 (d,
10.1, 3.0 Hz), 3.40-3.20 (m, 4H),
2.96 (m, 2H)
34 2-naphthyl 8.28 (s, lH), 7.94 (m, 3H), 7.72
(dd, lH, J = 8.7, 1.9 Hz), 7.60 (m,
2H)
3-quinolinyl, 9.01 (d, lH, J = 2.3 Hz), 8.76 (d,
bistrifluoroacetate salt lH, 1 8 Hz), 8-08 (d, lH, J - 8j7
(m, lH), 7.73 (m, lH)
0 9S/29159 r~ r l,~G
2~ ~793~
- 67 -
-- 36 4-[(N-hexy1,N-methy1- 5-12 (d, IH, J - 8.7 Hz), 3.40-3.10
aminocarbonyl) (m, 6H) 2 99 (s, 3H), 2 95 (m, 2H),
.- amino]phenyl, (m, 3H)
bistrifluulu~;c;~ salt
37 4-(3-hexyl-2- S.lS (m, lH), 3.85 (m, 2H), 3.53
imi(i~7o~ inon-l- (m, 2H), 3.40-3.15 (m, 6H), 2.94
yl)phenyl, (m, 2H), 1.55 (m, 2H), 1.32 (m,
bistrifluoroacetate salt 6H), 0.89 (m, 3H).
o 38 4-[(1-oxoheptyl)- 2.35 (tr, 2H, J=7.5 Hz), 1.65
amino]phenyl, (quint., 2H, J=7. 1 Hz), 1.32 (m,
b~ inuuludc~l~t~ salt 6H), 0.892 (tr, 3H, J=6.8Hz).
39 4-[(1-oxo-4-phenyl- 7.34-7.25 (m, 4H), 7.15-7.05 (m,
butyl)amino]phenyl, SH), 2.71 (tr, 2H, J=7.7Hz), 2.36
bi~LIinuulua~ salt (tr, 2H, J-7.4 Hz), 1.96 (m, 2H).
4-[(propoxycarbonyl)- 4.07 (tr, 2H, J=6.6 Hz), 1.67
amino]phenyl (sextet, 2H, J=7.0 Hz). 0.968 (tr,
3H, J=7.4 Hz).
41 4-[[[(fur-2- 7.40 (d, lH, J = 0.9 Hz), 6.32 (dd,
ylmethyl)amino]car- lH, J = 2.9, 1.8 Hz), 6.23 (d, lH, J
bonyl]amino]phenyl, = 2.9 Hz), 4.34 (s, 2H)
hi~rifl~ u~c~ salt
42 4-[[[(2- 7.38-7.02 (m, 9H), 3.50-3.15 (m,
phenylethyl)amino]car- 6H), 2.80 (m, 2H)
bonyl]amino]phenyl,
bistrifluoroacetate salt
43 4-[[[(2-indol-3- 7.58-7.53 (m, 3 H), 7.42-7.30 (m, 4
ylethyl)amino]carbon- H), 7.08-6.94 (m, 7H), 3.48 (tr, 2
yl]amino]phenyl H, J=6.9 Hz) 2.94 (tr, 2H, J=6.8
3 o Hz).
44 4- 2.94 (m, 2H), 1.51 (tr, 2H, J=6.8
[[(octylamino)carbonyl] Hz), 1.30 (m, lOH), 0.884 (tr, 3H,
amino]phenyl, J=6.9 Hz).
bistrifluoroacetate salt
WO 95/~9 159 P~ I I ~1,,,~'0 1356
- 2 ~ ~ 7932
- 68 -
45 1- 7.83 (d, 2H, J = 9.2 Hz), 7.48 (m, .
[(hexylamino)carbonyl] 2H), 3.92 (t, 2H, J = 8.8 Hz), 3.1-
indolin-5-yl 3.2 (two overlap-ping t, 4H), 1.54
(m, 2H), 1.30 (m, 6H), 0.90 (t, 3H,
J=6.8Hz).
46 1- 7.83 (d, 2H, J = 9.2 Hz), 7.48 (m,
[(octylamino)carbonyl]- 2H), 3.92 (t, 2H, J = 8.8 Hz), 3.1-
indolin-5-yl 3.2 (two overlap-ping t, 4H), 1.63
(m, 2H), 1.30 (m, lOH), 0.89 (t, 3H,
o J=6.9Hz)
47 1-[(N-methyl-N- 7.53 (m, 2H), 6.90 (d, lH, J = 8.3
octylamino)carbonyl]- Hz), 3.89 (t, 2H, J = 8.4 Hz), 3.26
indolin-5-yl (t, 2H, J = 7.6 Hz), 3.04 (t, 2H, J =
8.4 Hz), 2.91 (s, 3H), 1.60 (m, 2H),
1.27 (m, lOH), 0.87 (t, 3H, J = 6.8).
48 l-(1-oxononyl)indolin- 7.49 (m, 2H), 8.09 (d, lH, J=9.1),
5-yl 4.04 (t, 2H, J=8.5), 3.07 (t, 2H,
J=8.5), 2.41 (t, 2H, J=7.5), 1.62 (m,
2H), 1.30 (m, lOH), 0.88 (t, 3H,
J=6.8)
49 1-(4-mt;lllyl~ .Lvl-2- 7.87 (d, lH, J= 8.6 Hz), 7.58 (lH,
yl)indolin-5-yl dd, J = 2.0, 8.6 ~z), 7.52 (d, lH, J
= 2.0 Hz), 6.48 (s, lH), 4.08 (t, 2H,
J=8.7Hz),3.25(t,2H,J=8.7Hz),
2.30 (s, 3H).
1-(4-octylthiazol-2- 7.97 (d, lH, J= 8.6 Hz), 7.57 (lH,
yl)indolin-5-yl dd, J = 2.0, 8.6 Hz), 7.53 (d, lH, J
= 2.0 Hz), 6.49 (s, lH), 4.06 ~t, 2H,
J=8.8Hz),3.24(t,2H,J=8.8Hz),
2.62 (t, 2H, J = 7.5 Hz), 1.68 (m,
2H), 1.2-1.4 (m, lOH), 0.88 (t, 3H,
J = 7.0 Hz).
95/29159 P~ l.. _.'C ~5~i
2 1 ~3~
- 69 -
. Sl 1-(4-ethyl-S- 7.87 (d, lH, J= 8.5 Hz), 7.54 (lH,
methylthiazol-2- dd, J = 2.0, 8.5 Hz), 7.50 (d, lH, J
- yl)indolin-S-yl = 2.0 Hz), 4.02 (t, 2H, J = 8.7 Hz),
3.20 (t, 2H, J = 8.7 Hz), 2.56 (q,
2H, J = 7.7 Hz), 2.26 (s, 3H), 1.20
(t, 3H, J = 7.7 Hz).
52 4-(3-octyl-2- 4.78 (m, IH), 3.83 (m, 2H), 3.52
imi~l~7- 1i(1int-n-l- (m, 2H), 3.24 (t, 2H, 8Hz), 1.60-
yl)phenyl l.S1 (m, 2H), 1.35-1.25 (m, lOH),
o 0.88 (t, 2H, 8Hz).
53 4-[3-(4,4,4- 3.86 (m, 2H), 3.54 (m, 2H), 3.40-
trifluorobutyl)-2- 3.20 (m, 6H), 2.19 (m, 2H), 1.82
imidazolidinon-l- (quin, J = 7.9 Hz, 2H)
yl]phenyl,
bistrifluoroacetate salt
54 4-[3-(3-phenylpropyl)- 7.20 (m, 4H), 7.10 (m, lH), 5.15
2-imi(l~7~ 1innn-1- (dd, IH, 9.6,4Hz), 3.75 (m, 2H),
yl]phenyl, 3.46 (m, 2H), 3.36-3.20 (m, 6H),
bistrifluoroacetate salt 2.95-2.91 (m, 2H), 2.65 (t, 2H,
8Hz), 1.90 (qu, 2H, 8Hz).
SS 4-[3-(4,4,5,5,5- 3.87 (m, 2H), 3.56 (m, 2H), 3.40-
pentafluoropentyl)-2- 3.20 (m, 6H), 2.14 (m, 2H), 1.86
imit1~7--1itlinon-1- (quin, J = 7.8 Hz, 2H)
yl]phenyl,
bistrifluoroacetate salt
56 4-[3-(2- 3.82 (m, 2H), 3.50 (m, 2H), 2.87-
cyclohexylethyl)-2- 2.70 (m, 6H), 1.78-1.63 (m, SH),
imi~7oli(1in-)n-l- 1.41 (quartet, 2H, J=7.2 Hz), 1.30-
30 yl]phenyl, 1.18 (m, 4H), 0.949 (m, 2H).
bistrifluoroacetate salt
57 4-[3-[3-(4- 7.19 (s, 4H), 4.79 (m, IH), 3.74 (m,
chlo~ ;.lyl)propyl]- 2H), 3.47 (m, 2H), 3.30 (m, 2H),
2-imidazolidinon-1- 2.63 (t, 2H, 7.6Hz), 1.91-1.83 (m,
yl]phenyl 2H).
.. . . . ..
woss/2slss i~l ~ 793~ r~ oi~c ~
- 70 -
58 4-(3-pentyl-2- 3.82 (m, 2 H), 3.53 (m, 2H), 2.94
imidazolidinon-l- (m, 2H), 1.57 (quintet, 2H, J=7.4
yl)phenyl, Hz), 1.39-1.28 (m, 4H), 0.916, (tr, -.
bistrifluoroacetate salt 3H, J=7.1 Hz).
S9 4-[3-(3- 3.81 (m, 2H), 3.51 (m, 2H), 3.23 (t,
cyclopentylpropyl)-2- J = 7.3 Hz, 2H), 1.78 (m, 3H), 1.57
imidazolidinon-l- (m, 6H), 1.33 (m, 2H), 1.17 (m,
yl]phenyl 2H)
o 60 4-[3-(2- 3.83 (m. 2H), 3.53 (m, 2H), 2.94
cyclopentylethyl)-2- (m, 2H), 1.81 (m, 4H), 1.65-1.53
imi~l~7o~ in~-n-l- (m, SH), 1.16 (m, 2H).
yl]phenyl,
bistrifluoroacetate salt
61 4_[3_(3_ 3.83 (m, 2H), 3.51 (m, 2H), 3.22 (t,
cyclohe~yl~lu~yl)-2- J = 7.3 Hz, 2H), 1.71 (m, SH), 1.56
imi~l~7~-lidin~-n-l- (m, 2H), 1.20 (m, 6H), 0.88 (m,
yl]phenyl 2H)
62 4-[3-(2,2- 3.82 (m, 2H), 3.60 (m, 2H), 3.03 (s,
dimethylhexyl)-2- 2H), 1.28 (m, 6H), 0.93 (m, 3H),
imidazolidinon-l- 0.91 (s, 6H)
yl]phenyl
63 4-(3-hexyl-2- 6.93 (d, lH, 4Hz), 6.70 (d, lH,
imida_olon-l-yl)phenyl 4Hz), 4.79 (m,lH), 3.64 (t, 2H,
8Hz), 1.71-1.64 (m, 2H), !.35-1.28
(m, 6H), 0.91-0.86 (m, 3H).
64 4-[3-(4,4,4- 6.97 (d, lH, 3Hz), 6.73 (d, lH,
trifluorobutyl)-2- 3Hz), 3.73 (t, 2H, 7Hz), 2.23-2.19
imidazolon-l-yl]phenyl (m, 2H), 1.98-1.92 (m, 2H).
30 65 4-(3-octyl-2- 6.93 (d, lH, 4Hz), 6.69 (d, lH,
imidazolon-l-yl)phenyl 4Hz), 3.64 (t, 2H, 7Hz), 1.70-1.63
(m, 2H), 1.33-1.23 (m, lOH), 0.90-
0.85 (m, 3H).
0 95/29159 r~l~u . ~ 5
2~ ~37932
- 71 -
- 66 4-[3-(3- 6.93 ~d, IH, 3Hz), 6.69 (d, lH,
cyclopentylpropyl)-2- 3Hz), 3.63 (t, 2H, 7Hz), 1.80-1.47
imidazolon- l -yl]phenyl (m, l l H), 1.35-1.29 (m, 2H), 1.13-
1.02 (m, 2H).
67 4-(2-octyl-3-oxo- 8.25 (s, lH), 3.79 (t, 2H, 7Hz),
[1,2,4]-triazol-4- 1.80-1.70 (m, 2H), 1.36-1.25 (m,
yl)phenyl lOH), 0.91-0.86 (m, 3H).
68 4-(4-hexyl-5- 3.9~ (t, 2H, J=7.1Hz), 2.9-2.7 (m,
0 tetrazolon-l-yl)phenyl 6H), 1.82 (q, 2H, J=7 Hz), 1.4-1.27
(m, 6H), 0.89 (t, 3H, J=7Hz )
69 4-(4-octyl-5-tetrazolon- 3.98 (t, 2H, J=7.1Hz), 2.9-2.7 (m,
l-yl)phenyl 6H), 1.83 (m, 2H), 1.4-1.2 (m,
lOH), 0.87 (t, 3H, J=7 Hz)
4_[(3_ 3.97 (t, 2H, J-7.1Hz), 2.9-2.7 (m,
cyclopentylpropyl)-5- 9H), 1.9-1.7 (m, 5H), 1.6 (m, lH),
tetrazolon-l-yl]phenyl 1.5 (m, lH), 1.37(m, 2H), 1.07(m,
lH)
71 4-(2-pentyloxazol-5- 7.48 (s, lH), 4.82 (m, lH), 2.92-
yl)phenyl 2.70 (m, 8H), 1.80 (m, 2H), 1.39
(m, 4H), 0.92 (m, 4H)
72 4-(2-octyloxazol-5- 7.52 (s, IH), 5.09 (m, IH), 3.01-
yl)phenyl 2.82 (m, 8H), 1.77 (m, 2H), 1.37-
1.27 (m, lOH), 0.87 (m, lH)
73 4-[2-(2- 7.52 (s, !H), 4.80 (m, lH), 2.94-
cyclopentylethyl)oxazol 2.70 (m, 8H), 1.79 (m, 5H), 1.62
-5-yl]phenyl (m, 2H), 1.54 (m, 2H), 1.12 (m,
2H)
74 4-[(4-ethyl-5- 7.62 (d, 2H, J = 9 Hz), 7.58 (d, 2H,
~0 mc;lllylll.iaz~l-2- J = 9 Hz), 2.53 (q, 2H, J = 7.5 Hz),
yl)amino]phenyl 2.23 (s, 3H), 1.18 (t, 3H, J = 7.5
~h)
WO95129159 I'~ J,, S'C:9~C
21 ~7932
- 72 -
75 4-[(4,5,6,7- 7.54 (d, 2H, J = 9 Hz), 7.48 (d, 2H,
tetrahydrobenzo- J = 9 Hz), 2.54 (m, 2H), 2.50 (m,
thiazol-2- 2H), 1.75 (m, 4H) ~.
yl)amino]phenyl
76 4-(2-hexylimidazol-4- 7.75 (s, lH), 5.04 (m, lH), 3.29-
yl)phenyl 3.20 (m, 4H), 2.97-2.90 (m, 4H),
1.82 (m, 2H), 1.40-1.30 (m, 6H),
0.9 (m, 3H)
77 4-(1-methyl-2- 7.92 (s, lH), 5.30 (m, lH), 4.84 (s,
o octylimidazol-S-yl)- 3H), 3.48-3.25 (m, 4H), 3.05-2.95
phenyl (m, 4H), 1.80 (m, 2H), 1.50-1.26
(m, lOH), 0.89 (m, 3H)
78 4-[1-methyl-2-(2- 7.41 (s, lH), 3.64 (s, 3H), 2.96-2.68
cyclopentylethyl)- (m, 8H), 1.90-1.79 (m, 9H), 1.16
imidazol-S-yl]phenyl (m, 2H)
79 4-[1-methyl-2-[2-(4- 7.40 (s, lH), 7.10-6.95 (m, 4H),
nuvluL,ll~,~lyl)ethyl]- 4.91 (m, lH), 3.39 (s, 3H), 3.0 (bs,
imidazol-S-yl]phenyl 4H)
20 80 4-(S-pentyl-[1,2,4]- 2.96 (t, 2H, J=7.6Hz), 1.84 (t, 2H,
oxadiazol-3-yl)phenyl . J=7.4Hz), 1.39 (m, 4H), 0.92 (t, 3H,
J=7.1)
81 4-[S-(2- 2.98 (t, 2H, J=7.5Hz), 1.84 (m, SH),
cyclopentylethyl)- 1.70-l.S0 (m, 4H), 1.16 (m, 2H)
2s [1 ,2,4]-oxadiazol-3-
yl]phenyl
82 4-(S-hexyl-[1,2,4]- 2.96 (t, 2H, J=7.5Hz), 1.84 (quin,
oxadiazol-3-yl)phenyl 2H, J=7.4Hz), 1.48-1.28 (m, 6H),
0.90 (t, 3H, J=7.0Hz)
3D 83 4-(S-heptyl-[1,2,4]- 2.96 (t, 2H, J=7.5Hz), 1.84 (quin,
oxadiazol-3-yl)phenyl 2H, J=7.0Hz), 1.46-1.26 (m, 8H),
0.89 (t, 3H, J=6.9Hz)
j~095~291S9 71 B7~32 ~ 6
- 73 -
84 4-(5-hexylthio-[1,2,4]- 3.11(t,2H,J=7.3Hz), 2.98-2.84
triazol-3-yl)phenyl (m,4H), 2.76 (t,2H,J=7.3Hz), 1.65
~ (q,2H,J=7.3Hz), 1.37
(q,2H,J=7.1Hz), 1.28-1.23 (m,4H),
0.84 (t,3H,J=6.9Hz)
4-[[4-(4- 8.84 (s, lH), 8.75(d, lH, J=5.07
ul~ylpi~eridin-l-yl)- Hz), 8.46(d, lH, J=8Hz), 7.15 &
1,1-dioxo-[1,2,5]- 7.08 each (d, 2H, J=8Hz), 0.92(t,
thi~ 7~1-3- 3H, J=7Hz)
o yl]amino]phenyl
86 4-[[4- 7.15(d, 2H, J=8.5Hz), 7.12(d, 2H,
yl~ ylamino)- J=8.5Hz), 5.19(dd, lH, 3.1Hz, 9Hz),
1,1-dioxo-[1,2,5]- 2.93(m, 2H), O.90(t, 3H, 6.8Hz)
thi~ 7-)l_3
yl]amino]phenyl
87 4-[[4- 7.16(d, 2H, J=8.8Hz), 7.11(d, 2H,
(h~yllllc;lllylamino)- J=8.8 Hz), 5.01(dd, J=3.2Hz,
1,1-dioxo-[1,2,5]- 9.9Hz), 2.92(m, 2H), 1.68(m, 2H)
thi~ 7~1-3-
yl]amino]phenyl
88 4-(1-octyl-2,4- 4.09 (s, 2H), 3.41 (t, 2H, 7hz),
imi-l~7~ iin~ n-3- 1.65-1.56 (m, 2H), 1.30-1.25 (m,
yl)phenyl lOH), 0.91-0.86 (m, 3H).
25 89 4-[3-(3-l~ pll~llyl)-5- 8.55 (t,lH,J=1.9Hz), 8.47 (d,
pyrazolon-l-yl]phenyl lH,J=2.0Hz), 8.37 (dd,lH,
J=3.2Hz), 8.14 (d,2H,J=8.9Hz), 8.08
(t,2H,J=8.5Hz), 7.74(d,
3H,J=8.9Hz), 7.56 (t,lH,J=8.0 Hz),
3o 7.33 (dd,lH,J=4.8Hz), 7.04
(dd,4H,J=6.6Hz), 4.75 (t,
lH,J=2.1Hz), 2.83-2.69 (m,6H)
W095/291~9 ~1 87~3~ r ~ C~55G ~
- 74 -
Starting with commercially available (R)-styrene epoxide
and following the procedures outlined for Examples 17,18 and 25, the
compounds listed in Table 3 were prepared.
TAp~T F. 3
OH
~N N,SOI~R
H O
Example R Selected lH NMR (CD30D) Data
4-iodopheny1 7.84 (d, 2H, J = 8.6 Hz), 7.45 (d,
trifluoroacetate salt 2H, J = 8.5 Hz)
91 2-naphthyl, 8.31 (s, lH), 7.96-7.90 (m, 3H),
uoloac~ salt 7.74 (dd, lH, J = 1.8, 8.7 Hz), 7.63
(t, lH), 7.58 (t, lH)
92 3-quinolinyl, 9.01 (d, lH, J = 2.2 Hz), 8.75 (d,
trifluoroacetate salt IH, J = 2.1 Hz), 8.07 (d, lH, J = 8.4
Hz), 8.03 (d, lH, J = 8.3 Hz), 7.92
(t, lH, J = 7.0 Hz), 7.72 (t, lH, J =
7.1 Hz)
EXAMPT F 93
OH
3 o ~Me~` NH
nHex-N N~--S02
-I I
wo 95r~9159 1 ~ ~ 1936
21 87~32
- 75 -
(O-N-[4-[2-[[2-Hydroxy-2-(pyridin-3-yl)ethyl]amino]-2-
methylpropyl]phenyl]-4-(3-hexvl-2-imidazolidinon- 1 -
vl)benzenesulfonamide
A solution of pyridine epoxide (160mg,1.32 mmol) from
example 16 and 4-amino-a,a-dim~ yl~ ethylamine (1.2g, 7.3 mmol),
prepared according to J. Biol. Chem. 1981, 256, 11944-50, in methanol
(8 ml) was warmed at reflux for 16 hours. After cooling, the reaction
mixture was c. ll. r~ lrd and purified by flash chromatography (silica
gel, 95:5 CH2C12: 10% NH40H/CH3OH) to give 23 mg (0.080 mmol)
o Of product as an oil.
The above product (18 mg, 0.063 mmol) was dissolved in
CH2C12 (1 mL) and pyridine (0.05 mL). The resulting solution was
cooled to 0C and treated with 4-(3-hexyl-2-imidazolidinon-1-
yl)l,~"~r.l~ lfonyl chloride (22 mg, 0.063 mmol). The mixture was
5 allowed to stir at 0C for 20 hours and was then purified by flash
chromatography (silica gel, 95:5 CH2C12: 10% NH40H/CH30H) to give
the desired product (21 mg, 0.035mmol) as an oil: IHNMR (CD30D) o
8.53 (s, lH), 8.44 (d,lH, J=5.0), 7.83 (d~ lH~ J=7.9), 7.63 (m, 4H), 7.40
(dd, lH, J=5.0, 7.9), 6.98 (m, 4H), 4.72 (dd, lH, J=4.0, 8.4), 3.80 (m,
20 2H), 3.49 (m, 2H), 3.22 (t, 2H, J=7.2), 2.78 (m, 2H), 2.62 (m, 2H), 1.55
(m, 2H), 1.31 (m, 6H), 1.01 (s, 3H), 0.99 (s, 3H), 0.89 (m, 3H).
Following the ~loccdul~; outlined above, the compounds in
Table 4 were prepared.
TABT F 4
OH
¢~e~ NH'lo`R
Example ¦ R ¦ Selected IH NMR (CD30D) Data
WO 9S/29159 1 ~ .'C 1~56
~ ~793:2
- 76 -
94 4-iodophenyl 7.82 (d, 2H, J=8.6), 7.42 (d, 2H,
J=8.6)
4-[[(ll~yl~ )car- 7.55 (d, 2H, J=8.8), 7.42 (d, 2H,
bonyl]amino]phenyl J=8.8), 3.11 (t, 2H, J=7.0), 1.49 (m,
2H), 1.30 (m, 6H), .089~}n, 3H)
F.XAMPLE 96
o-Si(tBU)Me2
Cl~,Br
H2N ~
CI
(R)-4-amino-a-(l,lulllolll~,lllyl)-3 ,5-dichluluhr . 17r. Ir . "~th~lnoI, dimethyl-
1,1--limPthylethylsilyl .othPr
A solution of t-butyldimethylsilyl chloride (1.67 g, 11.1
mmol) in DMF (15 mL) was added slowly to a stirred solution of (R)-4-
20 amino-a-(l,lulllu;llyl)-3,5-dichlu.~ ,r~,,r.II.,..,ol (2.1 g, 7.4
mmol, see Judkins, et. al, European Patent Arrlit~ )n 0 460 924) and
imidazole (0.75 g, 11.1 mmol) in DMF (6 mL) with an ice-water bath
cooling. After being stirred at RT for 3h, the reaction mixture was
poured into water (300 mL) arld the product was extracted with ether.
25 The organic phase was washed with saturated aqueous sodium
bicarbonate solution, brine, dried(MgSO4) and evaporated to dryness.
The crude product was purified on silica (95/5 hexane/ethyl acetate) to
give the title culll~uulld (2.73 g, 93 %): IH NMR (400 MHz, CDC13) o
7.14 (s, 2H), 4.67 (dd, lH, J=2.1, 6.4 Hz), 3.33 (m, 2H), 0.87 (s, 9H),
30 0.89(s, 6U)
WO 95129159 P~ '.556
21 87932
- 77 -
EXAMPLE 97
O,Si(tBu)Me2
5 Cl~ N ~ 3~ NH2
Cl
o !R)-N-[2-[4-(Alllillu~l~cllyl)]ethyl]-2-[(dimethyl- 1,1-
dimethylethylsilyl)ûxy]-2-(4-amino-3.5-dichlorophenyl)ethylamine
O-TBDMS bromo ;u~ uulld from Example 96 (2.73g,
6.86 mmol) was dissolved in CH3CN (50 mL) and 4-amino-
phenethylamine (1.86 g, 13.72 mmol) was added, followed by the
addition of N,N'-diisopropylethylamine (3.58 mL, 20. 6 mmol) and
sodium iodide (1.03 g, 6.86 mmol). After being heated at reflux for 48
h, the reaction mixture was cullccllLIi~L~d and the residue
~luulllalu~,ld~llcd on silica (50/50 ethyl acetate/hexane) to provide the
title compound (2.3 g, 75 %): lH NMR (400 MHz, CDC13) o 7.08 (s,
20 2H), 6.94 (AA', 2H, J=8.4 Hz), 6.60 (BB', 2H, J=8.4 Hz), 4.63 (m, lH),
4.37 (s, 2H), 3.53 (br s, 2H), 2.87-2.60 (m, 6H), 0.80 (s, 9H), -0.03 (s,
6H)
FXAMPT F. 98
OH H
Cl~l`~ N--3~ NH2
. Cl
(R)-N-[2-[4-(Aminophenyl)]ethyl]-2-hydroxy-2-(4-amino-3,5-
dichlorophenyl)ethylamine
To a stirred solution of silyl compound from Example 97
(2.2 g, 4.8 mmol) in THF (20 mL) at RT was added
.
WO 9!;/291~9 . ~ .'0135G
~ 8193~ ~
- 78 -
tetrabutylammonium fluoride (10 mL of 1.0 M solution in THF) in one ..
portion. After being stirred at RT for 2h, the reaction mixture was
~ollc~llt,dl~d and chromatographed on silica (10/90 CH3OH~CH2C12) to
give the title compound (1.59 g, 97 %): lH NMR (400 MHz, CD30D) o
7.15 (s, 2H), 6.92 (AA', 2H, J=8.3 Hz), 6.60 (BB', 2H, 8.3 Hz), 4.58
(m,lH), 2.83-2.65 (m, 6H)
EXAMPLE 99
01 I H ,NH
-NJ~N~J3'SO2
H H
(O-N-[4-[2-[[2-Hydroxy-2-(4-amino-3,5-
20 dichlorophenyl)ethyl]amino]ethyl]-phenyll-4-
(hexvlaminocarbonyl~mino)b~llz~ c~ulfon~mi~f~
Following the procedure outlined in Example 18 and 25,
the title culll~uulld was prepared from the aniline dc;liva~iv~ from
Example 98: NMR (400 MHz, CD30D) 7.57 (AA', 2H, J=2.7 Hz), 7.42
25 (BB', 2H, J=2.7 Hz), 7.16 (s, 2H), 7.04 (AA', 2H, J=2.0 Hz), 7.00 (BB',
2H, J=2.0 Hz), 4.58 (t, IH, j=7.1 Hz), 3.14 (t, lH, J=7.0 Hz), 2.80 (m,
2H), 2.73 (m, 4H), 1.49 (m, 2H), 1.32 (m, 6H), 0.90 (t, 3H, J=6.7 Hz).
ESI MS n~/z 622 (M).
Following the l~luccdult; outlined in Examples 96-99, the
~;c,lll~oullds in Table 5 were p ~,~ed.
W~951291~9 2 l ~7932 P._l"~
- 79 -
TABT F 5
H2N~ N`^13~H'So~R
Cl
Example R Selected 1H NMR (CD30D) Data
o 100 1- 7.82 (d, lH, J=9.2 Hz), 7.47 (m,
[(octylamino)carbonyl]- 2H), 3.93 (t, 2H, J=9.0 Hz), 3.18
indolin-5-yl (m, 4H), 1.53 (m, 2H), 1.31 (m,
IOH), 0.88 (t, 3H, J=7.1 Hz)
101 4-(3-hexyl-2- 7.68-7.60 (AA'BB', 4H), 3.82 (t,
imidazolidinon-1- 2H, J=6.2 Hz), 3.52 (t, 2H, J=6.2
yl)phenyl Hz), 3.30 (t, 2H, J=6.0 Hz), 1.54
(m, 2H), 1.31 (m, 6H), 0.89 (t, 3H
J=6.0 Hz)
102 4-(3-octyl-2- 7.65-7.60 (AA'BB', 4H), 3.82 (t,
imi~iA7~ inon-1- 2H, J=6.2 Hz), 3.52 (t, 2H, J=6.2
yl)phenyl Hz), 3.29 (t, 2H, J=6.0 Hz), 1.54
(m, 2H), 1.30 (m, lOH), 0.87 (t, 3H,
J=6.1 HZ)
FXAMPLE 103
OH
HO~N 3`NH
[3,S2
,
WO95/29159 `? ~ 3~ F~,l/.l ,. /01~56
- 80 -
(E~)-N-[4-[2-[[2-Hydroxy-2-(4-
hydroxyphenyl)ethyl]amino]ethyl]phenyl]-b~n7Pn~ -lfonamide
A solution of 5 g of 4-aminophenethyl alcohol in 50 mL of
DMF was silylated with 5.5 g of t-butyldimethylsilyl chloride (TBDMS-
5 Cl) and 2.5 g of imidazole overnight at room ~ alulc. Extractionof the product following an aqueous ~mmonillm chloride workup
afforded 6.6 g of the O-TBDMS ether. This aniline derivative was then
coupled to bl~n7tont sl~lfonyl chloride in pyridine-dichlololll.,lllalle to
give the sulfonamide in greater than gO% yield after chromatographic
purification. The TBDMS group of the sulfonamide was removed with
methanolic HCI at room t~ lalul~; for 30 min. The crude alcohol was
oxidized to the corresponding carboxylic acid with Jones reagent in
acetone (RT 30 min, ethyl acetate extraction).
To a solution of 180 mg of (R)-octoparnine and 300 mg of
5 the resultant 4-N-br~ ,llfonamidophenylacetic acid in 7 mL of DMF
was added 0.5 mL of triethylamine and 490 mg of b~ o~ lyl-N-
oxy-tris(dimethylamino)rh--grh~ m hexafluororhosrh~t~ The
reaction mixture was stirred at RT 2h, flash chromatography over silica
gel eluting with 95:5 chloroform-methanol gave 322 mg of purified
20 amide.
A solution of 220 mg of this amide in 13 mL of 1.0 M
borane-THF was refluxed under argon for 2h followed by the addition
of 3 mL of N,N-dimethyl~minoethsm~l and further reflux for another
hour. The solvent and excess volatiles were removed in vacuo and the
2s residual solid was taken up in acetone and purified by PLC on silica gel
(9:1 ethyl ;I(~t;~ ) to yield 61 mg of the title compound: IH
NMR (500 MHz, CD30D) o 7.73 (dt, 2H, J=2.1, 8.2Hz), 7.53 (tt, lH,
J=1.4, 7.6Hz), 7.44 (t, 2H, J=8 Hz), 7.18 (d, 2H, J=8.4 Hz), 7.05 (ABq,
4H, Jab=8.5 Hz), 6.76 (d, 2H, J=8.4Hz), 4.75 (dd, lH, J=7.5, 7.6 Hz),
30 3.05-2.90 (m, 4H), 2.81 (t, 2H, J=7.6 Hz). Mass spec calcd. 412.5 found
413.2.
WO 95/29159
~ 2187932 r~ J. o .. JJC
- 81 -
FXAMPLE 104
OH H
HOJ~ N 3` NH
SO2
ao-N-[4-[2-[[2-Hydroxy-2-(4-
h~dlu~y~ lyl)ethyl]amino]ethyl]phenyl]-4-iodob~ lfonamide
Following the procedure outlined in Example 103, the title
cu~ uulld was prepared: IH NMR (500 MHz, CD30D) o 7.77 (d, 2H,
J=8.5 Hz), 7.43 (d, 2H, J=8.5 Hz), 7.15 (d, 2H, J=8.5 Hz), 7.02 (ABq,
4H, Jab=8.7 Hz), 6.75 (d, 2H, J=8.5 Hz), 4.67 (dd, lH, J=4.4, 6.6 Hz),
2.90-2.66 (m, 6H). Mass spec calcd. 538.4 found 538.9.
EXAMPLE 105
2s NC~ Br
3-(2-bromoacetyl)benzonitrile
To a solution of 1.02 g (7.04 mmol) of 3-acetylb~,.lz~lliLIile
in 70 mL of ethyl ether was added 1.02 g (3.52 mmol, 0.5 equiv) of
30 dibromobarbituric acid. The mixture was allowed to stir at room
I~IlllJc;l~Lult; overnight. The resultant white slurry was filtered and the
filtrate was concentrated. Purification by flash chromotography (silica
gel, 20% ethyl acetate/hexane) gave 1.28 g (81%) of the title compound
as a white solid: IH NMR (400 MHz, CDC13) o .8.26 (t, IH, J = 1.4
WO 95/29IS9 1 ~ .'C ~6
2~ 87q~2 ~
- 82-
Hz), 8.20 (td, lH, J = 1.5, 8.0 Hz), 7.87 (dd, lH, J = 1.3, 7.8 Hz), 7.64
(t, lH, J = 7.9 Hz), 4.40 (s, 2H).
EXAMPLE 106
OH
NC ~ ~, Br
(R)-a-Bromomelhyl-3-cyanophenylmethanol
To a suspension of 181 mg (0.623 mmol) of (O-
tetrahydro- l-methyl-3,3-diphenyl- lH,3~I-
pyrrolo[l,2~[1,3,2]oxazaborole-borane (~OAB catalyst) in 6 mL of
THF at 0 C was added dropwise 6.24 mL (6.24 mmol) of a I M
solution of borane in THF. The resultant clear solution was allowed to
stir for 5 min, and then a solution of 1.27 g (5.67 mmol) of
bromn~tot~nf from Example 105 in 6 mL of THF was added slowly
over 1 h. After the reaction was allowed to stir for 30 min more, it was
20 quenched by the dropwise addition of 6 mL of methanol and
cullccllLl~t~d~ pllrifir~ril~n by flash chromatography (silica gel, 20-25%
ethyl acetate/hexane) provided 944 mg (74%) of the title cu~ uul,d as a
clear oil which crystaUized: IH NMR (400 MHz, CDC13) o 7.70 (d, lH,
J = 1.5 Hz), 7.62-7.60 (m, 2H), 7.48 (t, lH, J = 7.7 Hz), 4.95 (dd, lH, J
25 = 3.4, 8.4 Hz), 3.63 (dd, lH, J = 3.4 Hz), 3.49 (dd, lH, J = 8.4 Hz).
FXAMPLE 107
NC~
1~
(R~-(3-cyano~henyl)oxirane
~ o 95129159 2 ~ ~ 7 9 3 2 P~ .'ûS~3u
- 83 -
To a solution of 937 mg (4.14 mmol) of bromohydrin
from Example 106 in 8 mL of methanol was added 601 mg (4.35 mmol,
. 1.05 equiv) of potas~iu-l. carbonate. The reaction mixture was allowed
to stir at room ~ pC;ldlUl~ for 7 h. It was then diluted with ethyl
5 acetate, washed with water, dried over m lgnPcillrn sulfate, and
c-)nr~.ntr~t.--1 Purification by flash chromatography (silica gel, 20%
ethyl acetdte/hexane) provided 573 mg (95%) of the title compound: IH
NMR (400 MHz, CDCI3) o .7.59-7.55 (m, 2H), 7.49 (dd, lH, J = 1.6,
7.9 Hz), 7.44 (t, lH, J = 7.7 Hz), 3.87 (dd, lH, J = 2.5, 4.0 Hz), 3.17
(dd, lH, J = 4.1, 5.5 Hz), 2.74 (dd, lH, J = 2.5, 5.4 Hz).
EXAMPLE 108
OH
Boc
NC ~, N ~¢~ NH2
a~)-N-[2-[4-(alllillo~hc;llyl)]ethyl]-2-hydroxy-2-(3-
20 ~;yallu~ lyl)ethylcarbamic acid l.l-dim~thylethyl ester
Following the ~luc~dulu~ outlirled in F.Y~mrlf-~ 17 and 18,
the title culll~,uulld was prepared from the epoxide from Example 107:
IH NMR (400 MHz, CDC13) o 7.58-7.52 (br m, 3H), 7.41 (t, lH, J =
7.5 Hz), 6.89 (br d, 2H, J = 7.6 Hz), 6.65 (br d, 2H, J = 7.8 Hz), 4.82
25 (br dd, lH, J = 2.7, 7.9 Hz), 3.42-3.05 (br m, 4H), 2.75-2.55 (br m,
2H).
WO 95129159 P~ o Ij5C
~ 879~
- 84 -
EXAMPLE 109 r~
H
5 NO~ ,N ¢~ ~NH
nHex-NH NJ3,so2
H
(O-~-[4-[2-l[2-Hydroxy-2-(3-~;ydllo~ yl)ethyl]amino]ethyl]phenyl]-4-
~h~xylaminocarbonylamino)benzenesulfonamide
Following the procedure outlined in Example 25, the title
compound was prepared from the Boc aniline derivative from Example
108: IH NMR (400 MHz, CD30D) o 7.70 (s, lEI), 7.63-7.57 (m, 4H),
7.48 (t, lH7 J - 7.7 Hz), 7.43 (d, 2H, J = 8.9 Hz), 7.06 (d, 2H, J = 8.5
Hz), 6.99 (d, 2H, J = 8.5 Hz), 4.77 (dd, lH, J = 3.9, 8.5 Hz), 3.15 (t,
2H, J = 7.0 Hz), 2.86-2.69 (m, 6H), 1.49 (br m, 2H), 1.31 (br m, 6H),
20 0-90 (br t, 3H).
EXAMPLE 110
OH
NC~ ~NH ~NH
~,SO2
(O-N-[4-[2-[[2-Hydroxy-2-(3-cyanophenyl)ethyl]amino]ethyl]phenyl]-3-
quinolinesulfonamide
Following the ~lOce-lu-~ outlined in Example 25, the title
compound was prepared from the Boc aniline derivative from Example
.
wo gS/29159 r~ ) w~su
21 87932
- 85 -
108 and 3-quinolinesulfonyl chloride: IH NMR (400 MHz, CD30D) o
9.02 (d, lH, J = 2.3 Hz), 8.68 (d, lH, J = 1.9 Hz), 8.06 (d, lH, J = 8.3
,. Hz), 8.02 (d, lH, J = 7.9 Hz), 7.90 (ddd, lH, J = 1.4, 7.0, 8.4 Hz), 7.72-
7.69 (m, 2H), 7.62-7.58 (m, 2H), 7.47 (t, lH, J = 7.7 Hz), 7.07 (d, 2H, J
= 8.7 Hz), 7.03 (d, 2H, J = 8.7 Hz), 4.76 (dd, lH, J = 4.0, 8.5 Hz), 2.85-
2.68 (m, 6H).
Following the procedures outlined for F.~mrlf-.s 14-31, the
compounds listed in Table 6 were prepared.
TABLE 6
¢~ ~~ N 'o`R
Example R Selected IH NMR (CD30D) Data
111 4-(3-hexyl-2,4- 4.40 (s,2H), 3.54 (m, 2H), 1.68-
imi~l~7~ 1in~-rlit)n-1- 1.59 (m, 2H), 1.37-1.28 (m, 6H),
yl)phenyl 0.91 (m, 3H).
112 4-(3-octyl-2,4- 4.40 (s,2H), 3.52 (m, 2H), 1.68-
imidazoli-lin~ inn-l- 1.59 (m, 2H), 1.38-1.23 (m, lOH),
yl)phenyl 0.89 (m, 3H).
113 4-[2-(4- 7.66 (s, lH), 5.35 (m, lH), 3.22-
cyclohexylbutyl)- 3.32 (m, SH), 2.95 (m, 2H), 2.90 (t,
oxazol-S-yl]phenyl, J=6.5Hz, 2H), 1.8 (m, 2H), 1.69 (m,
trihydrochloride SH), 1.45 (m, 2H), 1.24 (m, 6H),
0.89 (m. 2H)
114 4-[2-[2-(~ 7.49 (s, lH), 7.2 (m, 2H), 6.99 (m,
fluorophenyl)ethyl]- 2H), 4.90 (m, lH), 3.05 (m, 4H),
oxazol-5-yl]phenyl 2.70-2.85 (m, 6H)
WO 95/291~9 A ~ .0 1~56
- 7 ~ 8793~ --
- 86-
115 4-[2-(3- 7.51 (s, lH), 4.90 (m, lH), 2.65-
cyclopentylpropyl)- 2.90 (m, 8H), 1.80 (m, 5H), 1.46-
oxazol-5-yllphenyl 1.62 (m, 4H), 1.05 (m, 2H)
116 4-(4-hexyl-3-oxo- 8.04 (s, lH), 3.69 (m, 2H), 1.78-
[1,2,4]-triazol-2- 1.69 (m, 2H), 1.39-1.28 (m, 6H),
yl)phenyl 0.90 (m, 3H).
117 4-(4-octyl-3-oxo- 8.03 (s, lH), 3.69 (m, 2H), 1.77-
[1,2,4]-triazol-2- 1.69 (m, 2H), 1.38-1.25 (m, lOH),
o yl)phenyl 0.89 (m, 3H).
118 4-(4-heptyl-5-methyl- 2.28 (s,3H), 1.67 (t, 2H, J=6.9 Hz),
[1,2,3]-triazol-2- 1.36-1.34 (m, 4H), 1.31-1.29 (m,
yl)phenyl 2H), 1.18 (d, 4H, J=2.5 Hz), 0.88 (t,
3H, J=7.0 Hz)